1. Introduction
The thalamus in mammals can serve as a relay station for the transmission of information between cortical areas. Although an earlier view posited that thalamic nuclei passively relay information to the neocortex, recent studies suggest that the thalamus might play a role in actively modulating cortical areas via reciprocal connections [
1,
2,
3,
4,
5]. Based on emerging anatomical and electrophysiological evidence, there are two types of thalamic relays [
6,
7,
8]. The first-order thalamic relays include core nuclei, such as the lateral geniculate nucleus and the medial geniculate nucleus, receive primary afferents from the periphery (eyes and ears, respectively), and connect with sensory cortical areas. The higher-order thalamic relays include matrix nuclei, such as the pulvinar, and connect reciprocally with neocortical association areas [
2,
6].
In the primate brain, the pulvinar is the largest nucleus of the thalamus and is purportedly involved in visual attention [
1,
9]. Unilateral lesions of the pulvinar in patients are associated with spatial neglect and visual attention deficits [
10,
11]. Recent studies hypothesized that the pulvinar serves as a modulator for coordinating neuronal activity across multiple cortical areas involved in visual perception and attention [
1,
12,
13,
14]. Several monkey studies provided evidence for this view by showing that pulvinar activity modulates cortico-cortical interactions, including fronto-parietal and V4 with temporal occipital (TEO) interactions, when monkeys performed visual attention tasks [
3,
15,
16]. More studies are needed to understand how thalamocortical interactions support visual attention.
The pulvinar in rodents, as well as its putative homolog in primates, receives inputs from the superior colliculus (SC) [
17,
18,
19] and connects heavily with the visual cortex and the posterior parietal cortex (PPC) [
17,
20,
21]. The role of the PPC in spatial attention is well established [
22,
23,
24,
25,
26], but there are open questions about the contribution of its thalamic input from the pulvinar. In addition, although the pulvinar in the rat is thought to support attention [
26], to our knowledge there is no electrophysiological evidence exploring the role of the rat pulvinar in visuospatial attention.
We previously examined the behavioral correlates of PPC cells in rats performing a visuospatial attention task (VSA). This task was adapted from the five-choice serial reaction time task [
27] for use in our floor projection maze apparatus [
28,
29]. In the VSA task, rats were required to visually monitor multiple locations in space in order to make a correct response for a food reward. PPC activity showed a variety of correlates in the VSA task, including stimulus onset, spatial location of the target, target choice, and trial outcome [
30]. Our prior findings provided evidence that the PPC engages top–down control in the translation of perception to action when visuospatial attention is engaged [
30]. Because the PPC is strongly and reciprocally connected with the pulvinar in the rat [
20,
31,
32] and the pulvinar is considered a higher-order thalamic relay nucleus, we hypothesized that the functions of the pulvinar go beyond relaying visual sensory information to include higher-order cognitive processes.
To address our hypothesis, we recorded neuronal activity in the rat pulvinar during performance on the VSA task. In this task, rats are required to use controlled attention at the beginning of the trial when monitoring multiple locations for the onset of a target stimulus. Stimulus-driven attention is engaged by the onset of the stimulus. Rats are then required to make a decision about the target location. Correct decisions are followed by a food reward. The VSA task thus engages visual attention and perception, decision-making, and reward learning. In addition, we used a version of the VSA task that allows dissociation of neuronal activity correlated with egocentric and allocentric reference frames.
2. Methods
2.1. Subjects
Subjects were five male Long–Evans rats (Charles Rivers Laboratories, Wilmington, MA) individually housed in a temperature-regulated colony maintained on a 12:12 h light:dark cycle. Experiments were carried out in the light phase. All procedures using animals were conducted in accordance with the Animal Welfare Act and were approved by the Brown University Animal Care and Use Committee (protocol #18-12-000, approved on 02/05/2019).
2.2. Apparatus
Rats were tested on the floor projection maze, an apparatus that exploits the natural tendency of rats to attend to items located on or close to the ground and that permits automated control over visual stimuli [
28,
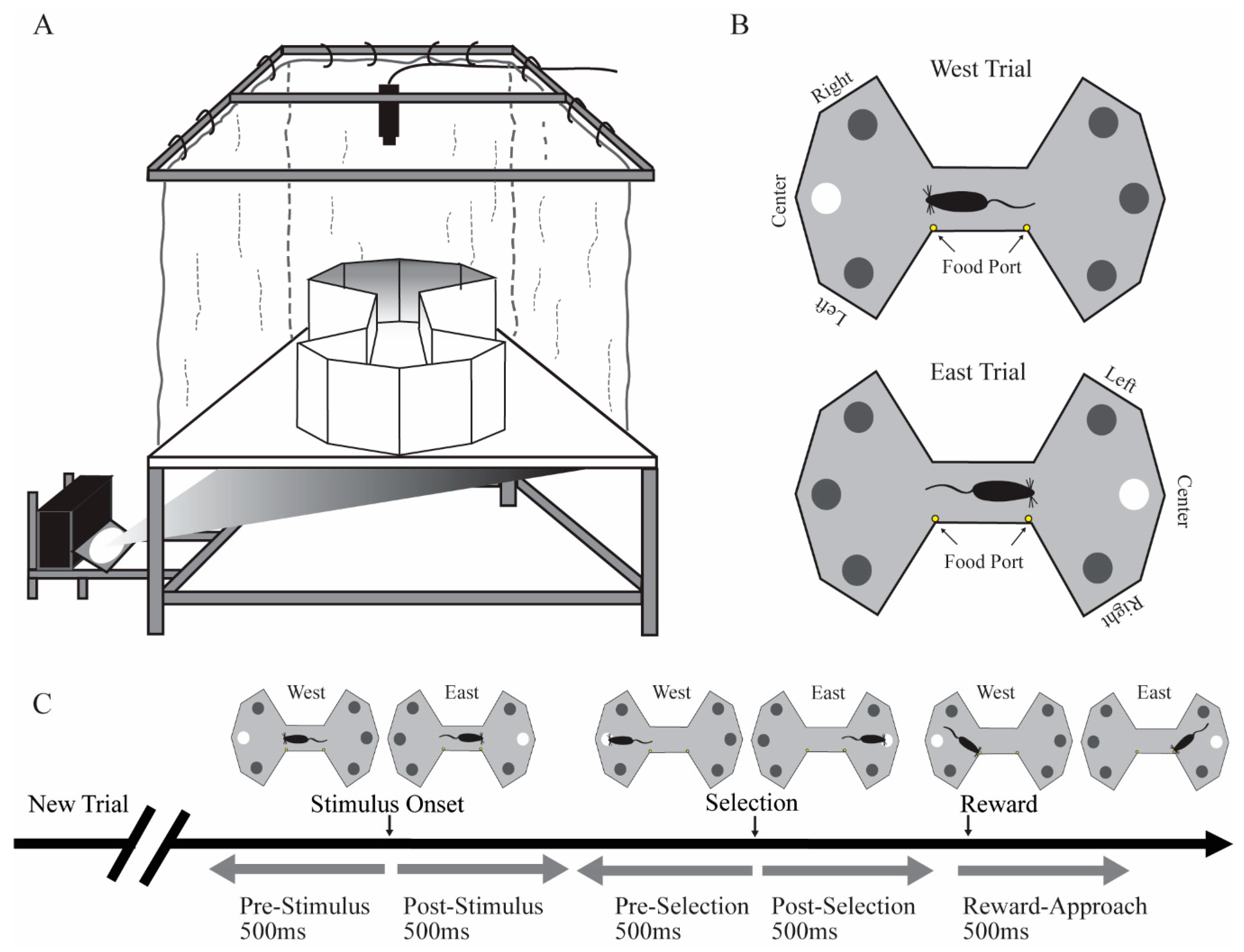
29]. The floor projection maze is a horizontal rear projection screen, which serves as a floor to any shaped arena and allows back-projection of visual stimuli from underneath (
Figure 1A). The apparatus has a clear Plexiglas subfloor (147.32 cm × 111.80 cm and 1.25 cm thick) covered by Dual Vision Fabric (Da-Lite Screen Company, Warsaw, IN), a unity gain flexible fabric designed for rear screen projection. A thin Plexiglas sheet (0.32 cm) covered the fabric for protection. Visual stimuli were projected onto the unity gain fabric from below the subfloor using an LCD projector (WT610 projector, NEC Corporation). In this experiment, the enclosure was a bowtie-shaped arena for the presentation of stimuli. Food reward (milk with various flavors) was delivered by two automated pumps (Med Associates, Inc., St. Albans, VT, USA) to stainless steel food ports located at the middle region of the maze. Auditory stimuli were controlled by an automated auditory stimulus generator (ANL926, Med Associates, Inc.) and delivered through a speaker located above the maze.
The floor projection maze was interfaced with three Windows PC systems, for location tracking, behavioral control, and neuronal data acquisition. Tracking was accomplished with a single camera using CinePlex Studio and Editor (v3.4.1) with Tracking and Basic Behavior modules (Plexon, Inc.). The position and body movements of the rat were recorded by calculating and tracking the centroid. Position data were analyzed online and saved in a data file for offline analysis, if needed. Based on the location of the rat, this system presented visual stimuli, collected behavioral data, and controlled delivery of reward. A Multichannel Acquisition Processor (MAP, Plexon Inc.) and SortClient (Plexon, Inc.) recorded real-time neuronal activity and behaviorally relevant event timestamps for later analysis. The MAP system was interfaced with the Med Associates system (DIG-713A SuperPort TTL Input Module and a DIG-726 SuperPort TTL Output module) used for controlling the projector, reward pumps, and audio signals.
2.3. Behavioral Training
Rats were put on food schedules to maintain body weight at 85%–90% of free feeding weight. After handling for at least 7 days, rats were habituated to the behavioral room for 10 min/day for three days. Rats were shaped and trained in the VSA task to a behavioral criterion prior to implantation. In the shaping sessions, rats were first trained in a 30 min session to approach a visual target stimulus for a food reward (a drop of flavored milk). In the initial shaping sessions, we adopted an errorless shaping procedure such that when the rat moved toward one of the three locations in one side of the maze, the visual stimulus at that location would illuminate and a tone would signal a correct choice. A new trial on the other side of the maze would be initiated after the rat entered the ready position of the other side of bowtie maze. After this initial shaping phase, rats were trained to stop in the ready position zone located in the middle of the bowtie shaped maze facing the side of the maze on which the target stimulus would be presented (
Figure 1B). After a variable delay to wait for a stimulus presentation, a visual stimulus would illuminate in one of three randomly chosen locations. There was a short response window for rats to approach the location of the visual stimulus. Approach to the correct location was signaled by a brief tone and presentation of a drop of flavored milk as a food reward. If the rat approached an incorrect location, no reward was given, the trial was terminated, and a new trial would begin immediately. Two food ports were in the middle of the maze. One food port was closer to the east side of the maze that would provide a drop of flavored milk after the animal made correct selection in east trials. The other food port was closer to the west side of the maze that would offer a drop of flavored milk after a correct selection in the west trials. Rats were gradually trained in a series of steps culminating in the final parameters of the task. The duration for rats to stay in the ready position was gradually increased from 0.1 to 1.6 s. Visual stimulus duration was gradually decreased from 20 to 0.5 s. The response time window was gradually decreased from 20 to 5 s. In the final stage, rats were required to stay in the ready position for a variable pre-stimulus interval (1.2–1.6 s) until stimulus onset. The 0.5 s stimulus presentation was followed by a 5 s response time window.
The behavioral performance criterion was 70%–80% accuracy. Chance on the VSA task is 33.33%. Rats required 2–3 months of training to reach the behavioral criterion on the final stage of the task. After rats had reached criterion for 5 to 7 consecutive days, we conducted surgery to implant a hyperdrive for electrophysiological recording.
2.4. Surgery
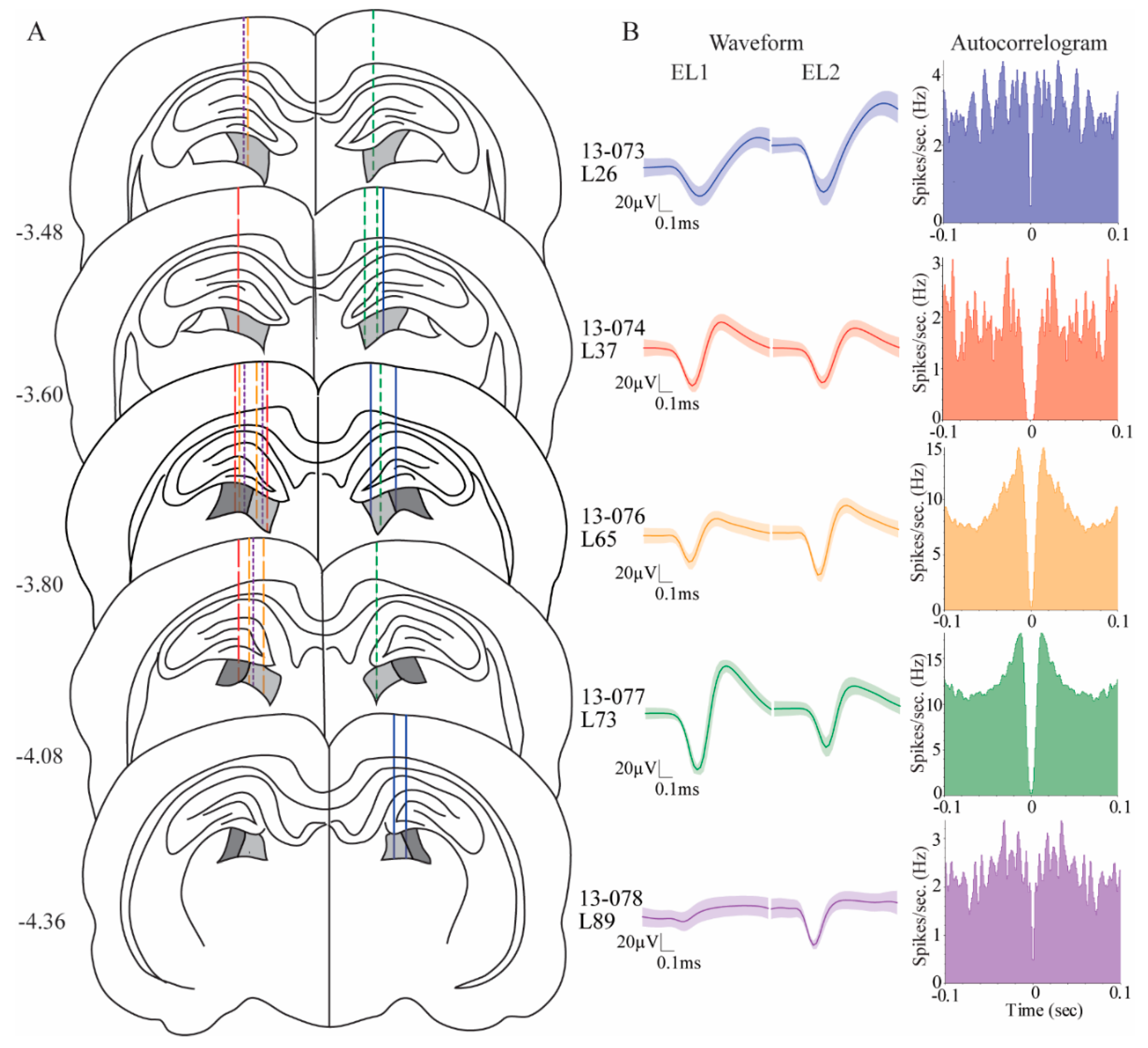
Animals were premedicated with diazepam (2–5 mg/kg; i.p.), glycopyrrolate (0.05 mg/kg; s.c.), carprofen (5 mg/kg; s.c.), and butorphanol tartrate (0.5 mg/kg; s.c.) to counteract respiratory effects of anesthesia, to control pain, and to decrease risk of seizures. They were brought to a surgical level of anesthesia with isofluorane (1.0%–2.5%). Using a stereotaxic apparatus (Kopf, Tujunga, CA), rats were unilaterally implanted with a custom hyperdrive into the pulvinar at −3.9 mm AP, ± 1.8 mm ML, and −4.0 mm DV relative to bregma. Three rats were targeted in the left pulvinar; two rats were targeted in the right pulvinar. The hyperdrive had fifteen microdrives, each consisting of a drivable screw with guide tubing containing one stereotrode. Five microdrives of each hyperdrive were implanted in the pulvinar. Stereotrodes were made of two 12 μm twisted, formvar-insulated nichrome wires (A-M systems, Sequim, WA, USA). A full turn of the screw advanced the stereotrode by 350 μm. Two silver ground wires were wrapped around anchor screws in the skull. The hyperdrive was secured to the skull by the ground screws, small anchor screws, grip cement (Dentsply Caulk, Milford, DE, USA), and dental cement (Coltene/Whaledent Inc., Cuyahoga Falls, OH, USA).
2.5. Histology
After the last recording session, the rats were deeply anesthetized with an overdose of Beuthanasia-D (100 mg/kg, i.p.), and the final recording site was marked with an electrolytic lesion. The rats were then perfused with normal saline, followed by 4% formalin. The brains were post-fixed for 24 h in 4% formalin and then transferred to a 30% sucrose solution until sectioning. The brains were sectioned at 40 μm and stained for Nissl material with thionin.
2.6. Single-Neuron Recording
Neuronal activity recorded from stereotrodes, was amplified with a gain of 2 through a 31-channel wireless head stage (Triangle BioSystems Inc., Durham, NC, USA). Signals were passed through a high-gain amplifier (total gain = 10,000, MAP system, Plexon, Inc., Dallas, TX, USA). Single-unit activity was filtered between 0.8 and 6 Hz. The signal was then digitized at 40 kHz for single-unit activity. These signals were extracted through real-time thresholding (Sort Client, Plexon, Inc). The final waveforms were stored with timestamps of relevant events and position information for later analysis.
2.7. Single-Neuron Activity Analysis
Spikes associated with putative individual cells were isolated offline based on waveform characteristics and using a variety of partially automated and manual techniques (Offline Sorter, Plexon, Inc.). The result was a dataset for each cell containing timestamps corresponding to spike times and behaviorally relevant event markers. These datasets were further analyzed using Neuroexplorer (NEX, Nex Technologies, Madision, AL, USA), SPSS (IBM Corporation, Somers, NY, USA), and Matlab (Mathworks, Natick, MA, USA).
Firing rates for each cell were analyzed for behavioral correlates using two methods. The primary method was factorial analysis of variance (fANOVA), but we confirmed those findings with the bootstrapping approach described below. Firing rate was the dependent variable. For each cell, we first computed the mean firing rate (spikes/s) for each of five epochs on each trial. The five epochs included the following: the pre-stimulus and post-stimulus epochs were the 500 ms periods immediately before and after stimulus onset, respectively; the pre-selection and post-selection epochs were the 500 ms periods immediately before and after the rat selected a target by approaching the location, respectively. Lastly, the reward-approach epoch was the first 500 ms after the animal entered the middle of the maze to collect a food reward. The stimulus event was the onset of the target stimulus (500 ms illumination the possible target locations). The selection time event was the moment the rat entered a zone just in front of the location in which the target stimulus had appeared (
Figure 1C). Entry of the ready position of the other side of bowtie maze triggered the next trial. Firing rate was the dependent variable for the fANOVAs. In the first set of analyses, the between-trial variable was outcome (correct response vs. incorrect response), and the two within-trial variables were stimulus onset (pre-stimulus vs. post-stimulus) and selection time (pre-selection vs. post-selection). The outcome was analyzed for the reward-approach epoch.
In a second set of analyses, we examined neural correlates associated with the location of the target stimulus. Based on the location of stimulus presentation, we pooled trials in which the target stimulus was at the same side of the maze (east vs. west) for analyzing allocentric location correlates. We then pooled trials in which the target stimulus was at the same egocentric location (left, right, and center) for analyzing egocentric location correlates. Only correct trials were used in this series of analyses. Thus, the between-trial variable was allocentric location (east vs. west) or egocentric location (left, right, and center). Sessions were analyzed for location only if there were at least three correct trials on each side. Allocentric location was analyzed separately for the post-stimulus, pre-selection, post-selection, and reward-approach epochs. The pre-stimulus epoch was not analyzed for trial location because there was no information about the allocentric location. Egocentric location was analyzed for the post-stimulus, preselection, and post-selection epochs without pre-stimulus and reward-approach epochs.
To confirm the results of the first series of analyses (fANOVAs), we used a bootstrapping procedure. For each cell in each recorded session, we randomly shuffled the firing rates for epochs analyzed across all trials 1000 times to create 1000 shuffled datasets. For example, if the reward approach epoch was the epoch under analysis and there were 100 trials, the 100 firing rates for the reward epoch were shuffled to create one new dataset, and this was done 1000 times. We then compared the original F value to the F values from the shuffled datasets. The cell was considered to be selective if the observed F value was higher than 95% of the distribution of the F values from the shuffled datasets.
The level of significance for all analyses was p < 0.05.
4. Discussion
Using the VSA task, we previously reported that neuronal correlates in the PPC signal stimulus onset, target selection, and outcome, suggesting a role in multiple cognitive functions during visuospatial attention [
30]. In the present study, we used a similar VSA task to study the function of the rat pulvinar, the major thalamic input of the PPC. Our double-sided version of the VSA task allowed the presentation of multiple stimuli on two sides of the maze permitting identification of both egocentric and allocentric spatial correlates. We report that pulvinar cells show selectivity for stimulus onset, choice, and outcome. In addition, more than half of the cells exhibited some type of task-relevant location selectivity. These findings support our hypothesis that the pulvinar is engaged in multiple, high-level cognitive functions, including both bottom–up and top–down attention, and that the pulvinar shows correlates of multiple spatial frames of reference. To our knowledge, this is the first study to examine neuronal correlates of pulvinar cells in a visuospatial attention task in rats.
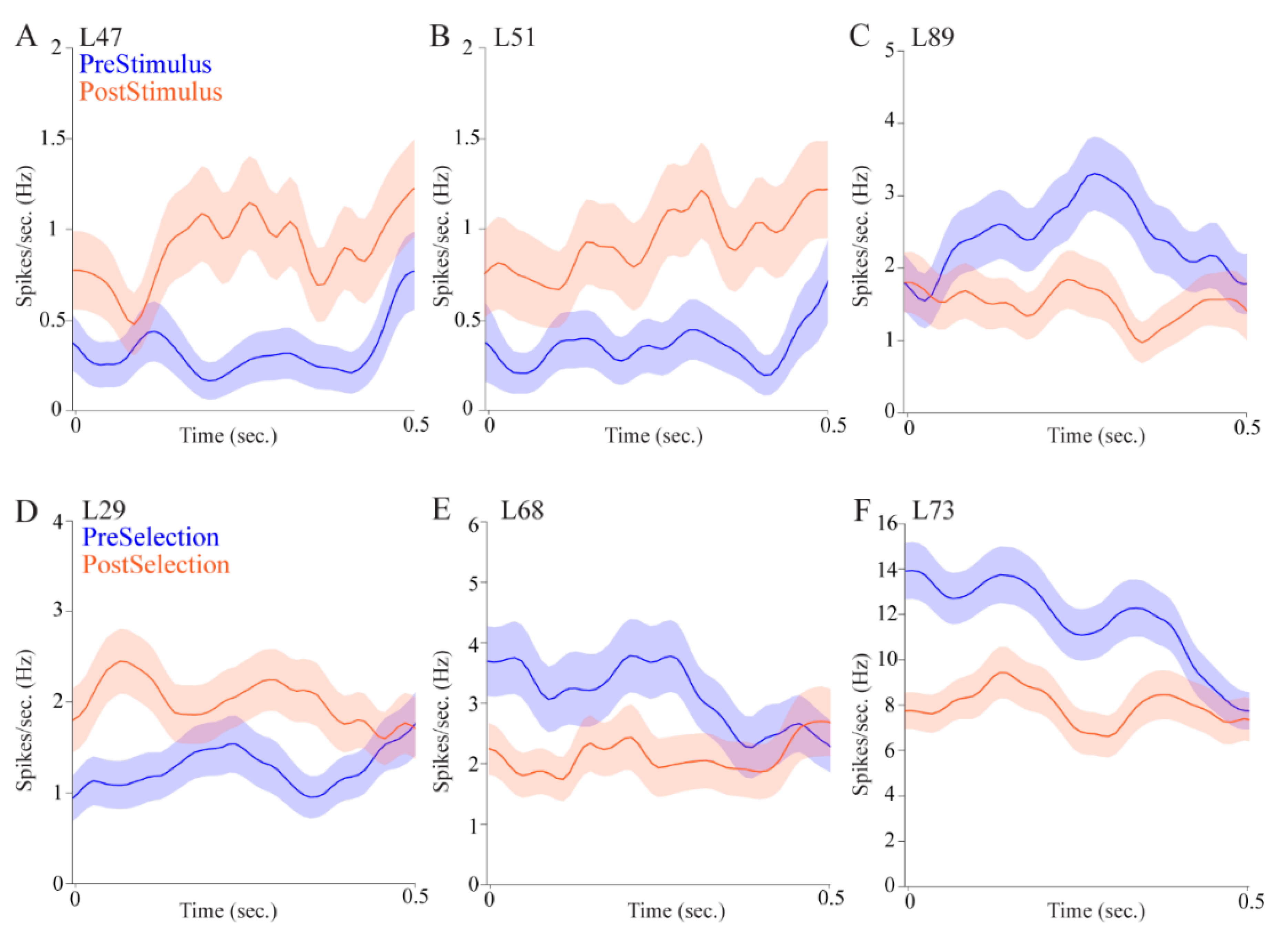
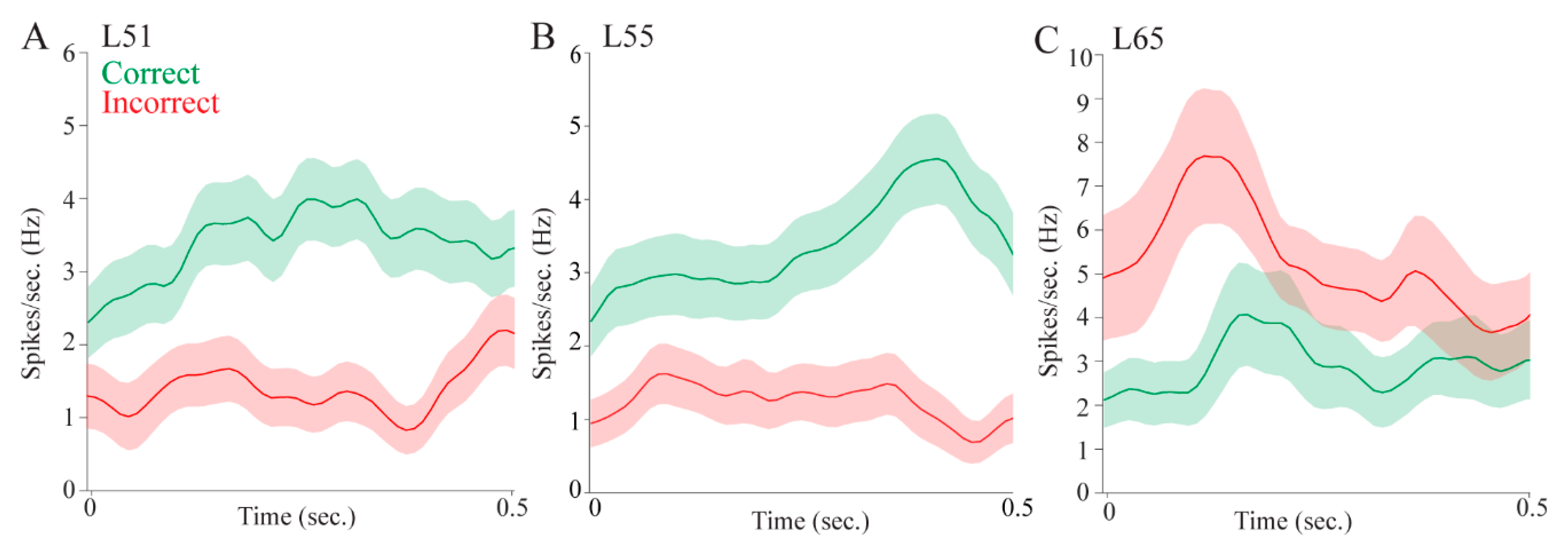
Regarding attention, pulvinar cells fired differentially in the pre- and post- stimulus epochs. This could mean that pulvinar cells signaled stimulus onset, showing that the pulvinar is involved in stimulus-driven attention. Some cells, however, fired more during the pre-stimulus interval, which could be a correlate of controlled attention as the rat is monitoring the possible target locations. Other cells fired more during the post-stimulus interval which, as we have suggested, could be a correlate of stimulus onset. Pulvinar cells also signaled target selection and reward, providing further evidence for a role in reward-guided decision-making.
Over a third of the cells displayed allocentric location selectivity and nearly a third showed egocentric location selectivity, indicating that the pulvinar is involved in processing information about different spatial reference frames. Some cells had both allocentric and egocentric selectivity, providing further evidence that the pulvinar is involved in translating spatial correlates across different frames of reference. Interestingly, the magnitude of the firing rate differences appears to be larger for allocentric cells compared with egocentric cells (
Figure 5, compare upper panels to lower panels). This may have to do with the particular epochs in which cells tended to show location correlates. Allocentric cells were more likely to show selectivity in the post-stimulus epoch immediately after stimulus onset, when the rat was in the center of the maze facing the fan-shaped part of the maze where stimuli appear on either the east or the west side. In contrast, egocentric cells were more likely to be selective in the epoch after a choice was made, when the animal was physically located in the left, center, or right in one or the other of the fan-shaped parts of the maze. This suggests that allocentric cells may be more sensitive to visual information, whereas egocentric cells may be more sensitive to spatial information. Alternatively, the egocentric cells could show lesser differences across conditions because they are more likely to show mixed selectivity given that three locations are analyzed. Indeed, the cell shown in
Figure 5F fires more to the right and left locations than to the center locations.
Our evidence that the rodent pulvinar is engaged under visuospatial attentional demands and signals task-relevant locations is consistent with a recent monkey study reporting that neuronal activity in the pulvinar, similar to the parietal cortex, significantly increased when a target appeared at the cued location indicating spatial correlates to behaviorally relevant locations [
3]. Thus, similar to the monkey pulvinar, the rat pulvinar can be considered a subcortical hub that supports visuospatial attention [
12,
14].
The pulvinar in both primates and rats is widely considered part of a visual attention circuit [
1,
26,
33,
34], but there have been disagreements about homology across rodents and primates. Two recent papers reviewed the evidence that the pulvinar is homologous across species [
19,
35]. An examination of nine species of rodents, tree shrews, and primates suggests that at least some subregions of the pulvinar are homologous across species based on molecular markers and connectional similarities, particularly connectivity with the SC, visual cortex, and visual association areas [
35]. In the rat, the pulvinar is divided into the medial rostral pulvinar, the medial caudal pulvinar, and the lateral pulvinar based on cytoarchitecture. The medial rostral pulvinar receives heavy inputs from the visual cortex and almost no SC inputs. The medial caudal pulvinar receives the strongest projections from the SC and almost no inputs from the visual cortex. The lateral pulvinar is further subdivided into the rostral and caudal portion based on its connectivity with the SC. The rostral portion of the lateral pulvinar receives weak SC projections and heavy projections from the visual cortex, whereas the caudal portion of the lateral pulvinar receives input from the superficial layers of the ipsilateral SC and weak projections from the visual cortex [
17].
Our electrodes were located in the medial rostral and lateral rostral pulvinar (
Figure 2). These two subareas of the pulvinar both receive heavy inputs from the visual cortex and weak projections from the SC [
17]. Cellular correlates of stimulus-driven attention and target selection behavior did not differ across these subregions. We did, however, observe that cells in the rostral lateral pulvinar had significantly higher proportions of egocentric cells compared to the cells in the rostral medial pulvinar. There were no differences between these two areas in proportions of allocentric cells. Our findings are in line with the notion that the medial pulvinar may be more involved in ventral visual stream functions and the lateral pulvinar may be more involved in dorsal visual stream functions, as has been suggested for the primate brain [
36]. Future research is needed to understand how these subregional differences in the rat pulvinar map onto subregional differences in the primate pulvinar.
To summarize, the present findings were aimed at understanding the function of thalamocortical connections in visuospatial attention. We previously reported neuronal correlates of the PPC in multiple cognitive processes when rats performed a VSA task [
30]. Here, we provided the first evidence that the rat pulvinar—the major thalamic input to the PPC—is engaged in tasks that have visuospatial attentional demands. We further demonstrated that the pulvinar is involved in multiple cognitive functions, as might be expected from a higher-order thalamic relay. These functions include top–down and bottom–up attention, decision-making, and processing of information about spatial reference frames. The present work, together with our previous findings, implicates both the PPC and pulvinar in the multiple cognitive processes tapped by the VSA task. This research lays the groundwork for understanding the role of thalamocortical interactions in visuospatial attention.