Abstract
Sea buckthorn (Hippophae rhamnoides L.) represents a valuable source of biologically active compounds such as carotenoids and polyphenols. High amounts of these substances are found in its fruits, bark, and leaves. However, their bioavailability is limited and must be increased in order to benefit from the properties they exert. Therefore, the purpose of this study was to increase the stability and bioavailability of sea buckthorn fruit’s bioactives. The sea buckthorn’s bioactive compounds were extracted with a solvent combination between glacial acetic acid, acetone, and water on one side and water only on the other side. Afterward, the phytochemicals from the extracts were encapsulated using the coacervation technique, followed by freeze-drying in order to obtain stable powders. The powders were characterized in terms of antioxidant activity, total carotenoids, β-carotene, lycopene, total polyphenol, and total flavonoid content, color, structure, and morphology. The phytochemical stability of the powders and their antioxidant activity was assessed during 270 days of storage at 4 °C. Moreover, the bioavailability of phytochemicals was measured during in vitro simulated digestibility. Our findings provide insights to promote carotenoids and polyphenols from sea buckthorn as bioactive ingredients with multiple purposes.
1. Introduction
Sea buckthorn (Figure 1) represents an ancient versatile plant that has been used for centuries in the daily life of people for a variety of purposes (animal fodder, treatment for several diseases, firewood, or even decorative elements), according to Olas [1].

Figure 1.
Sea buckthorn fruit [2].
Its fruits, leaves, seeds, and oils are sources of many biologically active substances, such as flavonoids, carotenoids, organic acids, unsaturated fatty acids, vitamins, proteins, amino acids, and minerals [3,4]. The average composition of sea buckthorn biologically active compounds is presented in Table 1, according to Ciesarova et al. [4].

Table 1.
Composition of sea buckthorn.
These substances and their free radicals scavenging activity endow sea buckthorn with health benefits. There are various studies suggesting that the consumption of sea buckthorn bioactives can promote health effects, such as neuroprotective effects, inhibition of cardiac cell destruction, anticoagulant effects, protection of the liver from oxidative stress, antiulcer, anticancer, antimicrobial effects, etc. [1,4,5].
The major compounds of scientific interest from sea buckthorn fruits are the carotenoids and flavonoids. Carotenoids represent lipophilic pigments with tetraterpenoid structure and colors including yellow, orange, and red. The most important biological activity associated with the carotenoids is provitamin A [6]. Flavonoids are secondary sea buckthorn metabolites and are a part of the polyphenols class, also having yellow and orange colors. Due to their colors and biological activities that can promote health effects, these compounds are suitable for use in food, nutraceutics, cosmeceutics, and pharmaceutics industries. However, the use of carotenoids and flavonoids as functional ingredients is limited due to their chemical instability, poor bioavailability, and bioaccessibility. Therefore, alternatives should be developed to increase the stability and bioavailability of these valuable bioactive compounds. The microencapsulation technique represents a valuable solution to these problems.
Nowadays, many encapsulation techniques are available, each with its advantages and disadvantages. The encapsulation method choice depends on both the properties of the bioactive compounds and encapsulating material, but also the desired final product [7]. Moreover, the encapsulation time, cost, and steps required, the volume of production, market requirements, and current legislation must be taken into account [7].
Coacervation is a superior technique to other microencapsulation techniques due to the advantages provided [8]. Depending on the amounts of polymers applied in the process, the coacervation technique could be simple or complex. The complex coacervation is based on the electrostatic attraction between the oppositely charged molecules. This attraction produces a complex with two distinct phases. One represents a polymer-rich phase (also named coacervate) and the other one is the solution solvent [9]. The biopolymer’s interaction is influenced by the biopolymer’s type, the pH, the ionic strength, the polymer’s concentration, and the ratio between the biopolymers [10]. Natural biopolymers, such as proteins and polysaccharides, are widely used to create a variety of delivery systems to protect the biologically active compounds from environmental factors. The encapsulation of the biologically active compounds leads to the production of functional ingredients that can then be used in a variety of ways [11].
This study aimed to extract and microencapsulate by coacervation the biologically active compounds from sea buckthorn fruits. Biopolymer composite matrices were formed by whey protein isolate (WPI) in combination with carboxymethyl cellulose (CMC). Two resulting powders, coded P1 and P2, were characterized for their phytochemical profile and encapsulation efficiency, with emphasis on carotenoids, polyphenols, antioxidant activity, and in vitro digestibility. Further, the structure and morphology of the powders were analyzed using the confocal laser scanning microscopy technique (CLSM). The obtained results could provide evidence concerning how targeted biologically active compounds from sea buckthorn can be used in developing functional ingredients for multiple applications. The powders that we have obtained exert biological activities demonstrated in this study and these make them suitable for multiple applications as natural antioxidants. In addition to the role of natural antioxidants, our powders can also act as natural dyes. They are suitable for the food industry, textile, nutraceuticals, cosmetics, and pharmaceutics industries.
2. Materials and Methods
2.1. Chemicals
Hexane, acetone, glacial acetic acid, ethanol and methanol HPLC-grade, sodium carbonate (Na2CO3), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, gallic acid solution, hydrochloric acid (HCl), sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium hydroxide (NaOH), Trolox solution, whey protein isolate (WPI), and carboxymethyl cellulose (CMC) were purchased from Sigma Aldrich (Steinheim, Germany).
2.2. Materials
Sea buckthorn fruits were purchased from a local market named “December 30 market” in Galati, Romania, September 2018. The sea buckthorn berries were washed, frozen, and then lyophilized at −42 °C with a pressure of 0.1 mBar for 48 h (Alpha 1-4 LD Plus, Christ, Osterode am Harz, Germany). Dried sea buckthorn fruits were stored in plastic bags with a lid at room temperature until extraction.
2.3. Extraction of the Biologically Active Compounds from Sea Buckthorn
The biologically active compounds from sea buckthorn fruits were extracted with different solvent mixtures, using the ultrasound-assisted extraction method (UAE). Polar solvents like acetone, glacial acetic acid, and water were used. Two separate extractions with different solvent mixtures were performed using the same lyophilized matrix. On one hand, 5 g of sea buckthorn was mixed with 20 mL of glacial acetic acid/acetone/water (0.35/20/80 ratio), with the extract encoded E1. On the other hand, 5 g of sea buckthorn was mixed with 20 mL of water, with the extract encoded E2. Both extractions took place using an ultrasonic water bath (Smart MRC LTD, Holon, Israel) for 20 min at 40 °C and a frequency of 40 kHz. Further, the extracts were centrifuged for 10 min at 5000 rpm and 4 °C. The supernatant was collected and concentrated to dryness under reduced pressure at 40 °C (AVC 2-18, Christ, Osterode am Harz, Germany). Finally, the obtained extracts were phytochemically analysed by solubilization in the extraction solvent.
2.4. Phytochemical Profile of the Sea Buckthorn Extracts
The phytochemical profile of both extracts was analysed in terms of lipophilic compound and hydrophilic compound contents, using spectrophotometric methods. The lipophilic compounds content quantified in our extracts were the lycopene (LC), β-carotene (βC), and total carotenoids (TC). The hydrophilic compounds analysed were flavonoids (TFC) and polyphenols (TPC). For both extracts, the antioxidant activity against ABTS and DPPH free radicals was measured.
2.4.1. Total Carotenoid Content
The lycopene, β-carotene, and total carotenoid contents were measured and calculated using the spectrophotometric method described by Rodriguez-Amaya and Kimura [12]. Briefly, the extracts were solubilized in the same extraction solvent and the absorbance of the mixture was read at λ = 470 nm for total carotenoids, λ = 450 nm for β-carotene, and λ = 503 nm for lycopene. The results were expressed as mg/g of dry weight (dw).
2.4.2. Total Polyphenol Content (TPC)
The colorimetric Folin–Ciocâlteu method, described by Turturica et al. [13], was used for the TPC quantification. Briefly, 0.20 mL of extract solution were mixed with 15.8 mL of deionized water and 1 mL of Folin–Ciocâlteu reagent. After 10 min of rest, 3 mL of Na2CO3 20% was added to the mixture and vigorously shaken. The resulting mixture was kept for 60 min in the dark at room temperature. Finally, the absorbance of the mixture was measured at 765 nm. The results were expressed as mg Gallic acid equivalents (GAE)/g dw, using a calibration curve.
2.4.3. Total Flavonoid Content (TFC)
The total flavonoid content was determined using a spectrophotometric method based on the reaction between the aluminum chloride (AlCl3) and phenolic compounds, described by Turturica et al. [13]. Briefly, 0.25 mL of extract solution was mixed with 2 mL of distilled water and 0.075 mL of NaNO2 5% solution. After 5 min of rest, 0.15 mL of AlCl3 10% solution were added, and the mixture was allowed to rest for another 6 min. Finally, 0.5 mL of NaOH (1 M) solution was added and the absorbance of the mixture was immediately measured at 510 nm. The results were expressed as mg catechin equivalents (CE)/g dw, using a calibration curve.
2.4.4. Antioxidant Activity
The antioxidant activities of the extracts were tested against ABTS [14] and DPPH free radical [13], the results being expressed as radical scavenging activity percentage (%).
For the DPPH scavenging activity, 3.9 mL of free radical solution 0.1 M in methanol were mixed with of 0.20 mL of extract solution (Af) and kept for 30 min at room temperature in the dark. Finally, the absorbance was recorded at λ = 515 nm against a blank (methanol instead of extract—A0).
For the ABTS scavenging activity, 1 mL of free radical stock solution 7 mM (in ethanol, mixed with 2.45 mM potassium persulfate) were mixed with 0.10 mL of extract solution (Af). The absorbance of the solution was measured at 734 nm after 1–6 min against a blank (ethanol instead of extract—A0).
The radical scavenging activity percentage (%) was calculated as follows:
2.4.5. Chromatographic Analysis of the Carotenoids
The separation, identification, and quantification of the carotenoids from sea buckthorn extracts was performed using High-performance liquid chromatography (HPLC) as described by Mihalcea et al. [15] with slight modifications. Briefly, a Thermo Finnigan Surveyor HPLC system with Xcalibur software (Finnigan Surveyor LC, Thermo Scientific, Waltham, MA, USA) was used. The carotenoids were analyzed at 450 bn and 30 °C on a Lichrosorb RP-18 (5 lm) Hibar RT 125-4 column. The elution solvents used were 90% acetonitrile (A) and 100% ethyl acetate (B). Thus, 0.02 mL of extract was injected into the column and the flow was maintained at 0.500 mL/min. The identification and separation of the compounds were achieved based on their standard curves using ethyl acetate as solvent.
2.5. Microencapsulation of the Bioactive Compounds from Sea Buckthorn and Powder Characterization
Both extracts were encapsulated in a matrix formed by 2% WPI and 2% CMC, using the complex coacervation method, as described by Mihalcea et al. [15]. The wall materials were dissolved in distilled water in a 1:1 ratio (w/w) on a magnetic stirrer at 300 rpm and 25 °C, until fully hydrated. Prior to the addition, 5 g of the concentrated extract were mixed with 5 mL of sunflower seed oil and subjected to ultrasound for 1 h at 40 kHz. To begin the coacervation process, the pH of the solutions was adjusted to 3.75 with 1 N HCl solution, under constant mechanical stirring at 300 rpm. Subsequently, the reaction mixture was allowed to separate in a funnel. The separated phases were collected and stored at −20 °C for 24 h. Subsequently, the frozen coacervates were lyophilized (Alpha 1-4 LD plus, CHRIST, Osterode am Harz, Germany) at 42 °C, under a pressure of 10 Pa for 48 h. Finally, the powders were collected, packed in metallic bags, and stored in the refrigerator at 4 °C until further analysis.
2.5.1. Encapsulation Efficiency and Powder Characterization
To determine the carotenoids encapsulation efficiency, the slightly modified method of Mihalcea et al. [15] was used. For the total carotenoids, 100 mg of encapsulated powder was dissolved in 6 mL of 10% NaCl: methanol (1:1 ratio), followed by 30 min of rest to break the microcapsules. A volume of 30 mL of hexane was further added. The samples were treated with ultrasounds for 40 min at 50 °C and then centrifuged at 6000 rpm for 10 min. The TC of the supernatant were analysed. The LC, βC, TFC, TPC, and antioxidant activity were also quantified from the same supernatant, using the methods described above to characterize the powders.
The same procedure was used for the surface carotenoids. Approximately 100 mg of powder was weighed, and 30 mL of n-Hexane was added. This mixture was vortexed for 2 min and then centrifuged at 6000 rpm for 10 min at 10 °C. Finally, the resulting supernatant was analysed in terms of SC.
EE (%) = (TC−SC)/TC × 100
EE—encapsulation efficiency, %;
TC—total carotenoids, mg/g dw;
SC—surface carotenoids, mg/g dw.
The encapsulated powders were evaluated regarding the encapsulation efficiency during storage at 4 °C in hermetically closed glass tubes with light protection after 180 days and 270 days of storage.
2.5.2. Colorimetric Analysis
For the color analysis of powders, a CR 410 Chroma Meter (Konica Minolta, Hino, Tokyo Japan) was used to appreciate the selected coordinates such as:
L*—clarity (=0 black)/(=100 white);
a*—shade of green (<0)/red (>0);
b*—shade of blue (<0)/yellow (>0).
2.5.3. Confocal Laser Scanning Microscopy (CLSM)
The CLSM technique has been used to observe the morphology and structure of the powders at a high resolution [15]. A Zeiss confocal laser system (LSM 710, (Carl Zeiss, Oberkohen, Germany) with a diode laser (405 nm), Ar-laser (458, 488, 514 nm), DPSS laser (diode-pumped solid-state e 561 nm), and HeNe-laser (633 nm) were used. The powders were stained with Red Congo (40 μM) and the distribution of the sea buckthorn bioactives into the matrices was observed using a Zeiss Axio Observer Z1 inverted microscope with a 40× apochromatic objective (numerical aperture 1.4). Moreover, for the analysis, the FS49, FS38, and FS15 filters (Carl Zeiss, Oberkohen, Germany) were used. Finally, ZEN 2012 SP1 software (black edition, (Carl Zeiss, Oberkohen, Germany) was used to analyse the 3D images.
2.5.4. In Vitro Digestion
The method described by Oancea et al. [16] has been used to evaluate the in vitro digestibility of the carotenoids from the obtained powders. The digestion was simulated using simulated gastric fluids (SGF) and intestinal fluids (SIF). Briefly, 200 mg of the powders mixed with 20 mL SGF (20 mg pepsin in 0.1 M HCl, pH 2.0) were incubated at 37 °C using an orbital shaking incubator (Medline Scientific, Oxon, UK) at 150 rpm, for 2 h. Afterward, in addition to the 15 mL of SGF which remained after digestion, 20 mL of SIF (40 mg pancreatin in 0.9 M baking soda, pH 7.8) were added and incubated for another 2 h at 150 rpm. Aliquots (1 mL) of each sample were taken every 30 min, diluted with 2 mL of n-hexane, and the absorbance was read at 450, 470, and 503 nm, respectively. Samples were prepared in duplicate (n = 2).
2.6. Statistical Analysis
The statistical analysis of the data was performed using Minitab 17 and the One-way ANOVA test. The experimental data were expressed as the mean of duplicate ± standard deviation.
3. Results and Discussion
3.1. Evaluation of the Phytochemical Profile of the Sea Buckthorn Extracts
The bioactive compounds from the freeze-dried sea buckthorn were extracted with different mixtures of solvents using the UAE method. Polar solvents, such as acetone and water, were used. Two extracts were obtained and the phytochemical contents of both are presented in Table 2.

Table 2.
Phytochemical profile of the sea buckthorn extracts.
The addition of acetone and glacial acetic acid into water led to a significant increase in the phytochemical content from sea buckthorn (p < 0.05) for both lipophilic and hydrophilic compounds (Table 2). This may be due to the stronger breaking of the cell walls. Thus, E1 presented a 1.69 ± 0.01 mg LC/g dw and E2 0.87 ± 0.01 mg LC/g dw lycopene content. In terms of β-carotene, E1 showed a 2.79 ± 0.02 mg βC/g dw and E2 1.38 ± 0.02 mg βC/g dw. Our results obtained are comparable to other studies. Ursache et al. [17] reported 38.34 ± 5.71 mg βC/g dw in the sea buckthorn extract.
Regarding the hydrophilic compounds, 310.06 ± 6.01 mg CE/g dw were found in E1 and 127.80 ± 2.41 mg CE/g dw in E2. The TPC of the extracts was 1023.50 ± 5.51 mg GAE/g dw and 368.12 ± 5.37 mg GAE/g dw for E1 and E2, respectively. Lower polyphenol contents in the sea buckthorn were reported by researchers like Korekar et al. [18], with values ranging from 9.64 to 107.04 mg GAE/g dw.
The antioxidant activity of the evaluated extracts was also different, the best results being obtained with acetic acid, acetone, and water for both free radicals. Thus, E1 presented a 4.36 ± 0.01 % inhibition against the ABTS free radical and 92.00 ± 0.42 % inhibition against DPPH. For E2, significantly lower inhibition results were highlighted (p < 0.05) such as 3.81 ± 0.02 % inhibition against ABTS and 61.01 ± 0.42 % inhibition against DPPH. Ursache et al. [17] reported lower DPPH inhibition with values of 33.7 ± 0.29 % for sea buckthorn extracts.
The extraction solvent choice represents a decisive step in the extraction of biologically active compounds from sea buckthorn, from both contents of the bioactive compounds and antioxidant activity point of view. In our study, the combination of acetic acid, acetone, and water provided the extract with the highest content of biologically active compounds and antioxidant activity.
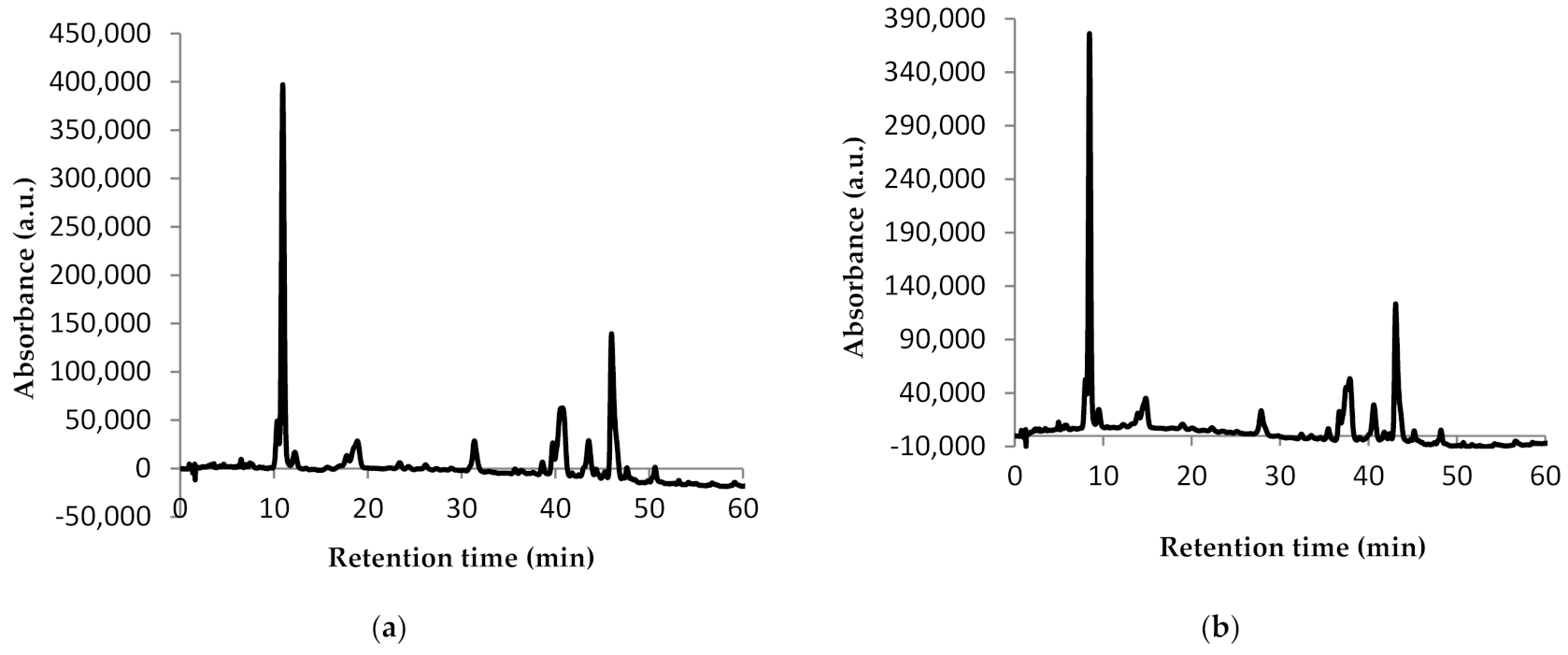
In order to characterize the carotenoid profile from the extracts obtained, the analysis of the chromatographic profile was performed by HPLC (Figure 2).
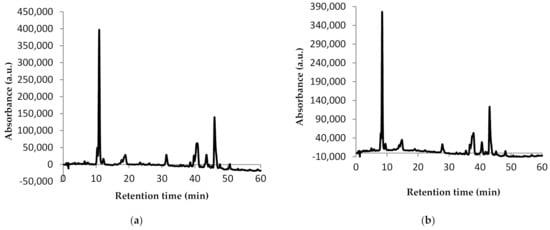
Figure 2.
Chromatographic profile of carotenoids from sea buckthorn E1 (a) and E2 (b): peak 1—astaxanthin; peak 2—zeaxanthin; peak 3—β-cryptoxanthin; peak 4—lycopene; peak 5—β-carotene.
The HPLC technique allowed the identification of five compounds in both extracts (Figure 2). The difference between the chromatographic profiles of the two extracts consists in the amount of compounds quantified. Thus, E1 presented 0.81 mg/g dw astaxanthin, 5.11 mg/g dw zeaxanthin, 0.43 mg/g dw β-cryptoxanthin, 0.96 mg/g dw lycopene, and 2.71 mg/g dw β-carotene (Figure 2a). For E2, almost half of the amounts of E1 were quantified as follows: 0.56 mg/g dw astaxanthin, 4.74 mg/g dw zeaxanthin, 0.55 mg/g dw β-cryptoxanthin, 0.98 mg/g dw lycopene, and 1.31 mg/g dw β-carotene (Figure 2b). Our results are in agreement with Mihalcea et al. [15] and Ursache et al. [17], who also identified these compounds in sea buckthorn extracts. However, while Ursache et al. [17] reported zeaxanthin as the major compound from sea buckthorn extracts as we did, Mihalcea et al. [15] reported β-carotene and zeaxanthin palmitate as major compounds.
3.2. Evaluation of the Phytochemical Profile of the Powders
In this paper, the bioactive compounds from sea buckthorn were encapsulated by complex coacervation, using whey proteins and CMC. The choice of wall materials is justified by the fact that they are effective carriers for bioactive compounds, fats, oils, fatty acids, and flavors [19].
For both powders, the same amount of 0.5 g extract was used. For a better compound dispersion and taking into account their lipophilic profile, the extracts were mixed with sunflower oil. The characterization of the microencapsulated sea buckthorn extract was performed by quantifying the LC, βC, TC, TFC, TPC, and antioxidant activity. The results obtained from these experiments are shown in Table 3.

Table 3.
Phytochemical profile of the powders obtained by the microencapsulation of the sea buckthorn extracts.
The encapsulation efficiency is an important parameter that provides information about the delivery system. In our study, the extracts were encapsulated in CMC and WPI using the coacervation method, followed by freeze-drying to obtain stable powders. The encapsulation efficiency of P1 was significantly lower compared to P2 (p < 0.05). Our results are comparable to those reported by other researchers. Ursache et al. [17] reported an encapsulation efficiency of 56.16 ± 1.24% after the microencapsulation of sea buckthorn extract in WPI and acacia gum (1:1).
However, P1 presented the highest LC, TC, TFC, TPC, and DPPH antioxidant activity. The β-carotene content, on the other hand, was higher in P2, as the antioxidant activity against ABTS. Our results are in agreement with Ursache et al. [17] who reported values of 2.82 ± 0.17 mg/g dw for total carotenoids content after the microencapsulation of the sea buckthorn extract in a WPI and acacia gum matrix (1:1). Laos et al. [20] reported 11.12 mg/100 g of β-carotene content in sea buckthorn juice microspheres obtained by ionotropic gelation. Interesting results were also published by Neagu et al. [21]. They encapsulated the sea buckthorn extract obtained with supercritical fluids in WPI and casein using different encapsulation methods. They obtained values in the range of 302.98 ± 2.30 to 352.90 ± 1.02 mg/g dw for the total carotenoid contents. The results from our study demonstrate that the use of WPI and CMC as wall materials is suitable for the sea buckthorn phytochemical encapsulation.
The encapsulation efficiency over time can predict the stability of the compounds inside the encapsulation matrix. Table 4 shows the variation of encapsulation efficiency during 270 days of storage at 4 °C in the dark.

Table 4.
Initial encapsulation efficiency of the powders obtained by the microencapsulation of the sea buckthorn extracts and its stability during 270 days of storage.
A slight decrease over time in the encapsulation efficiencies can be observed for both powders (Table 4). Thus, the encapsulation efficiency of P1 is 7% lower after 270 days of storage than the initial encapsulation efficiency. P2 showed a 12% decrease in the encapsulation efficiency after 270 days of storage.
3.3. Colorimetric Analysis of the Powders and Its Stability
The results of colorimetric analysis conducted with the powders are reported in Table 5. The lightness (L*), the redness (a*), and the yellowness (b*) were the parameters analysed during 270 days of storage.

Table 5.
Colorimetric parameters of the powders obtained by the microencapsulation of the sea buckthorn extracts and its stability during 270 days of storage.
The b* values presented in Table 5 suggest a high initial yellowness for both powders due to the carotenoids and flavonoids from the extract, but higher for P2. Our results are in agreement with those of Ursache et al. [17], who also reported a high yellowness for the powders obtained by the microencapsulation of the sea buckthorn extract in WPI and acacia gum. The color measurements over time show a significantly decrease for the yellowness parameter (p < 0.05), which can be correlated with the encapsulation efficiency variation over the same time. This may be due to the release of the compounds from the matrices which led to degradation and lower color intensities.
3.4. The Confocal Laser Scanning Microscopy (CLSM)
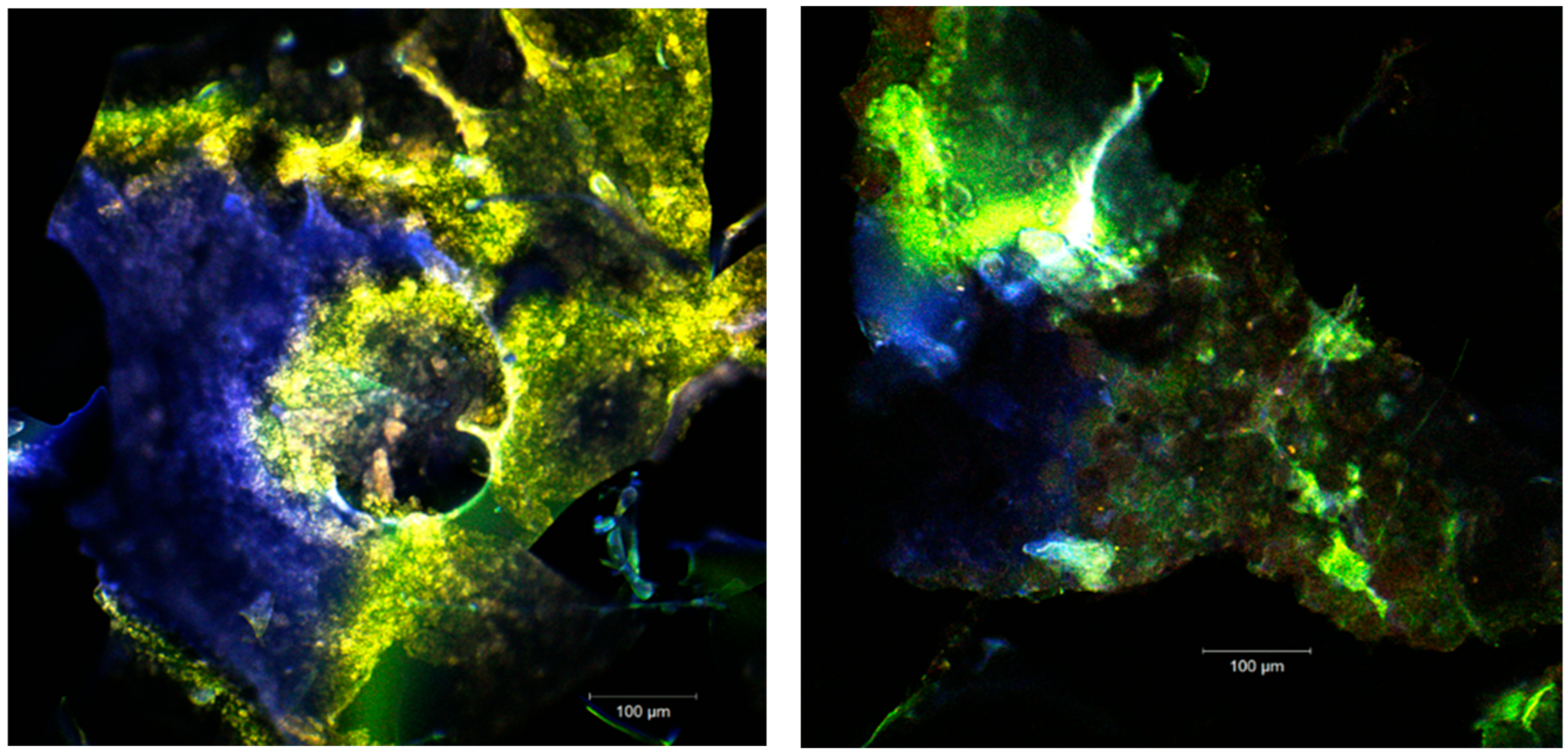
The biochemical complexity of the sea buckthorn fruits has been proven by numerous studies. The remarkable content of carotenoids (β-carotene, lycopene, lutein, zeaxanthin), vitamins (A, B, C, E, K), flavonoids (quercetin, kaempferol, catechin, isorhamnetin, etc.), and mono- and polyunsaturated fatty acids recommend this fruit as a food-medicine [17]. Depending on the variety, the climatic conditions, the time of harvest but also on the extraction method used, the proportion of biologically active compounds can differ considerably [22]. The confocal analysis of the two samples (Figure 3 and Figure 4) aimed at clarifying the morphological and structural aspect of the powders depending on the extraction method. For the excitation of the samples, the following lasers were used: Ar laser (458, 488, and 514 nm) and DPSS (561 nm pumped solid-state diodes) because the data from the literature indicate for the carotenoids’ absorption range the wavelengths of 448, 476, and 505 nm [23,24]. The studies by Llansola-Portoles et al. [25], conducted on an in vitro carotenoid assembly, recommend the excitation at 532 nm to obtain the full set of transient absorption signals. The complex aggregates of carotenoids and flavonoids microencapsulated in the polymer matrices showed an autofluorescence in a fairly wide range 530–630 nm, and sometimes it could be observed that the spectrum shifts to red (685–750 nm) upon the aggregation [23,25]. The carbohydrate biopolymers that have been introduced into the microencapsulating matrices displayed the maximum absorbance between 300 and 400 nm which have been excited by the diode laser (405 nm).
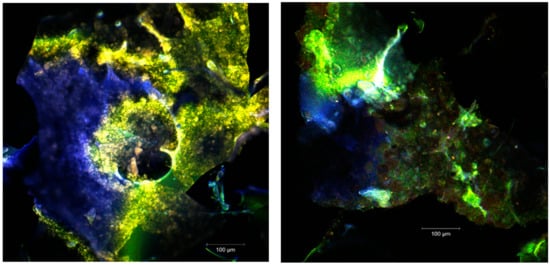
Figure 3.
The confocal laser scanning microscopy (CLSM) images of the unstained native powders.
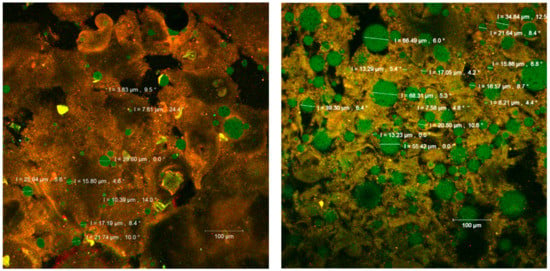
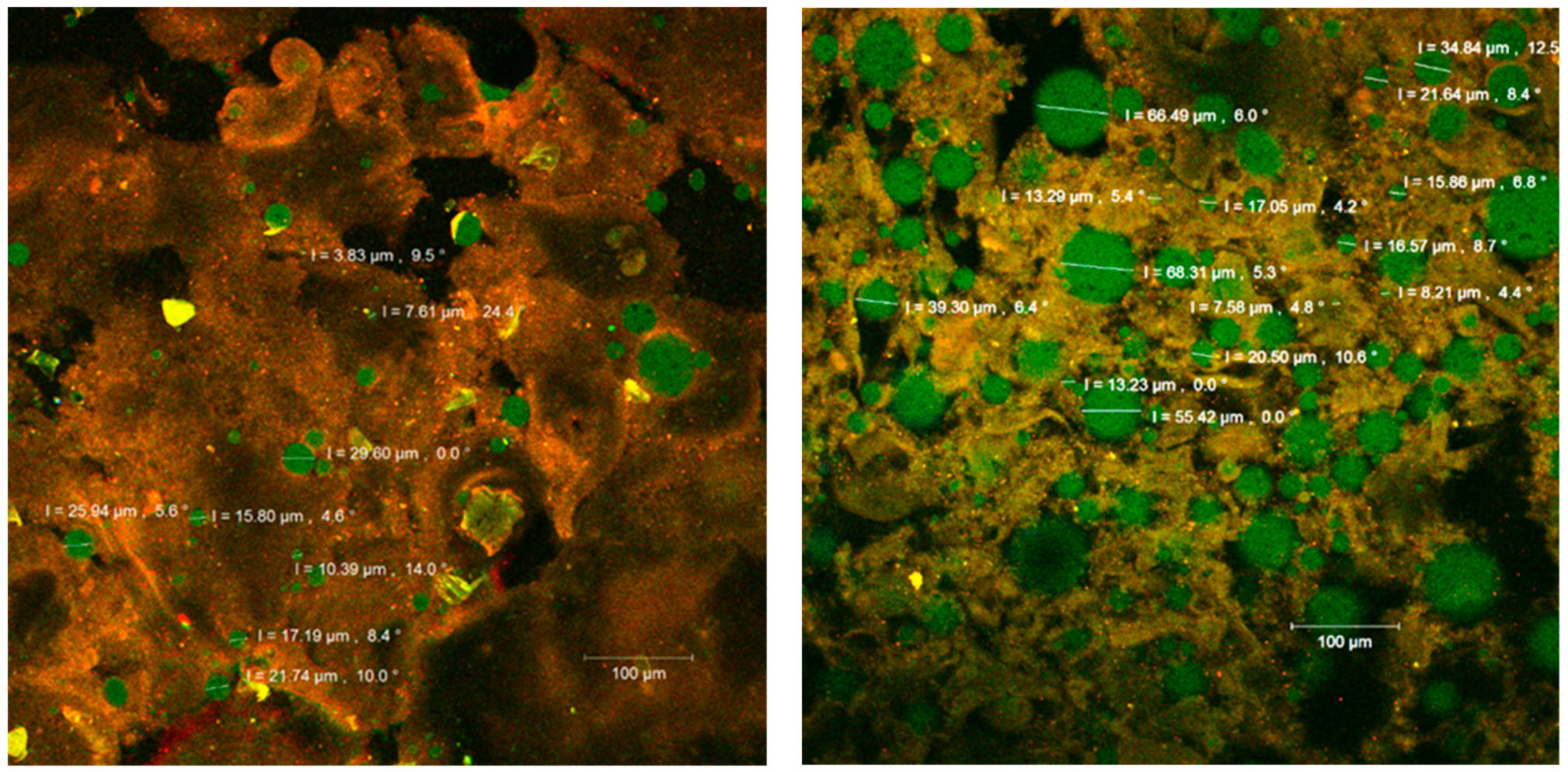
Figure 4.
The confocal laser scanning microscopy (CLSM) images of the fluorophore dyed powders.
The images acquired by point-by-point laser scanning (Figure 3) revealed a scalariform appearance of the microcapsules. The fine biofilms (in blue or green depending on the ratio between the WPI and the polysaccharide biopolymer) were formed. Within the biopolymer matrix, several plant pigments can be seen (in yellow-orange) microspherosomes (1–2µm) that are anchored. The interaction between the vegetal pigments from sea buckthorn fruits with the polysaccharide compounds from the microencapsules did not cause modifications on the absorbance pattern of the spectrum. The strong linkages are usually established between carotenoids and polyphenols, and these preserve the UV absorption capability of the pigments in time [26].
The confocal analysis of the samples after the fluorescent staining with Red Congo revealed the biologically active compounds from sea buckthorn fruit extracts in the form of spherosomes (in green) with different diameters between 3.83 and 215.78 µm. The Congo red dye binds the intact β-d-glucans from the biopolymer matrices [27] as well as the peptides from the WPI, marking them in orange (Figure 4). Small spherosomes (<25 µm) are visualized in P1, while in P2 they are of medium size. P2 displayed a large number of spherosomes of medium size, well individualized, with a tendency to group in clusters and well stabilized in the WPI and CMC matrix. Similar results were previously obtained by Mihalcea et al. [15], who use WPI and acacia gum as the encapsulation material, and by Neagu et al. [21], who compared the native sea buckthorn extract with the cross-linked one mediated by transglutaminase.
3.5. In Vitro Digestion
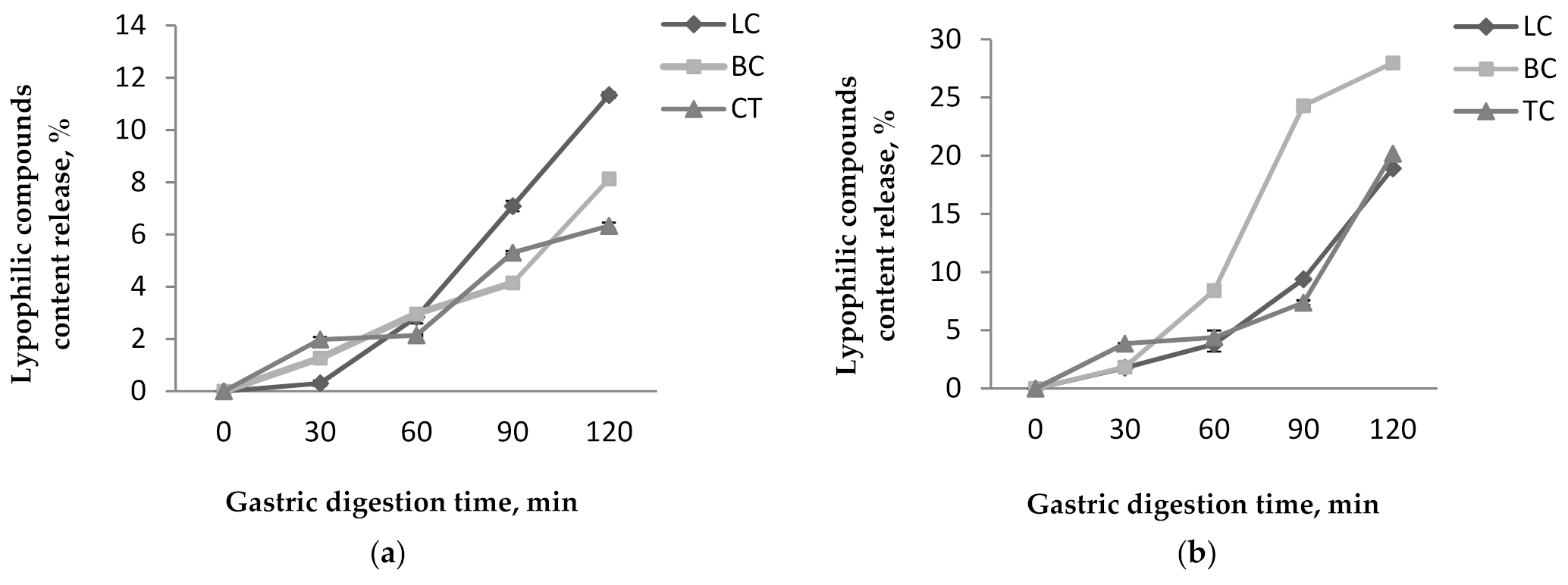
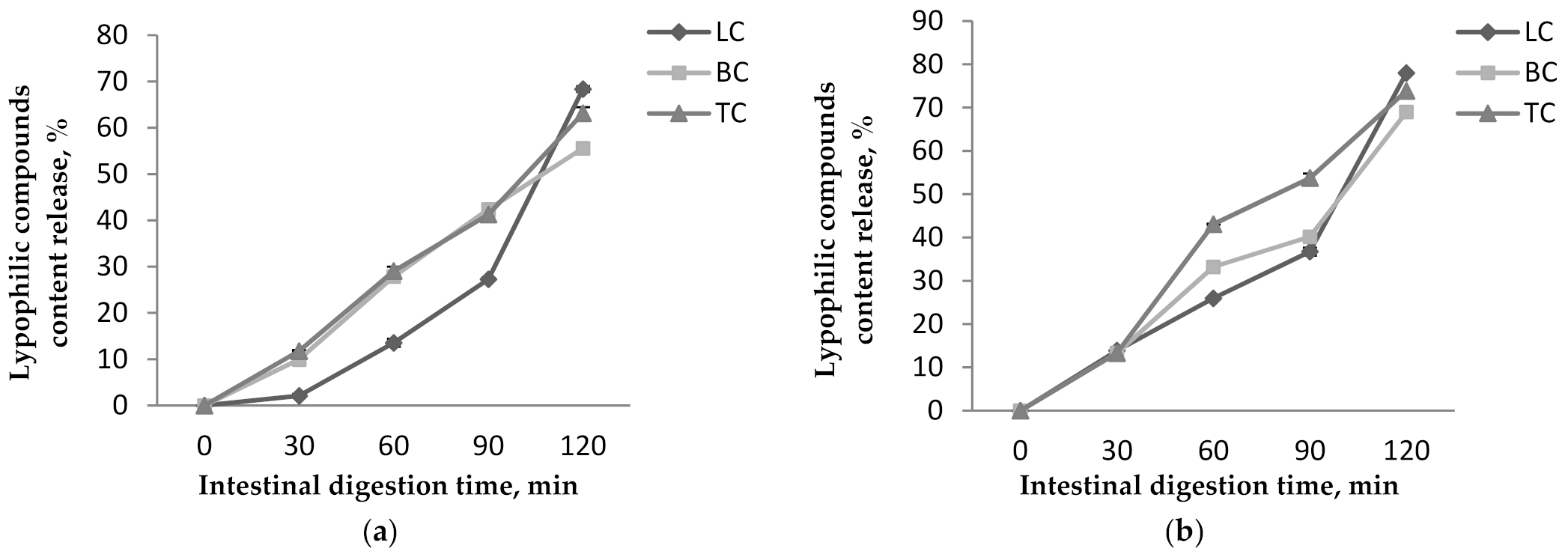
The in vitro digestibility of the encapsulated powders was also studied. Figure 5 and Figure 6 represent the release percentage of TC, βC, and LC from both powders at the gastric and intestinal level.
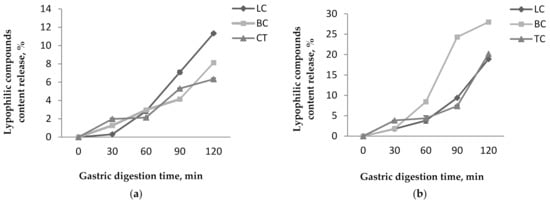
Figure 5.
Lypophilic compounds content release from P1 (a) and P2 (b) during the in vitro gastric digestion.
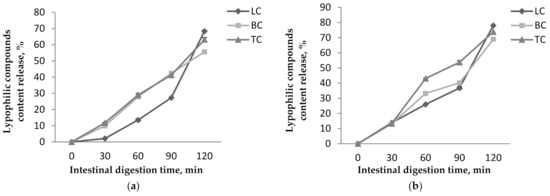
Figure 6.
Lypophilic compounds content release from P1 (a) and P2 (b) during the in vitro intestinal digestion.
The results obtained for the in vitro digestibility in simulated gastric conditions showed that the coating materials presented a protective effect on the lipophilic compounds, especially in P1. However, a slight release of the lipophilic compounds was observed for both powders. Among the investigated lipophilic compounds, in P1, the lycopene content presented the highest release from all compounds up to 11.33 ± 0.11% after 120 min of digestion (Figure 5a). The β-carotene and total carotenoids released up to 8.12 ± 0.1% and 6.33 ± 0.12%, respectively after 120 min. In P2, the β-carotene presented the highest release from all compounds up to 27.97 ± 0.26% after 120 min (Figure 5b). The lycopene and total carotenoids were released up to 18.90 ± 0.14% and 20.17 ± 0.20% after 120 min. For all that, P2 showed the highest release percentages for all lipophilic compounds.
In simulated intestinal conditions, the results show that the maximum amount of lipophilic compounds is released during 120 min of digestion and are recorded for P2 (Figure 6). Thus, in P1 release, values of 55.59 ± 0.99%, 63.14 ± 1.3%, and 68.36 ± 0.62% were achieved for βC, TC, and LC, respectively, after 120 min (Figure 6a). P2 presented higher release values, i.e., 68.98 ± 0.41 % of the βC, 73.86 ± 1.03% of the TC, and 78.01 ± 0.91% of the LC released after 120 min of digestion (Figure 6b). Our results are in agreement with other studies. In research conducted by Neagu et al. [21], a 10% release of the carotenoids in gastric juice was reported for the microencapsulated sea buckthorn extract in WPI and casein matrices. They also reported that, in simulated intestinal juice, the release increased significantly up to 65%.
4. Conclusions
This study aimed to increase the stability and bioavailability of sea buckthorn fruit’s bioactives. For this purpose, the biologically active compounds from Hippophae rhamnoides L. fruits have been extracted using the ultrasound-assisted extraction method. Different polar solvents have been used to extract as many bioactive compounds as possible. On one side, a combination between glacial acetic acid, acetone, and water was used (E1), with water only on another side (E2). By adding acetic acid and acetone into the water, the highest phytochemical content was obtained. The chromatographic profile of both extracts revealed the presence of five compounds, with zeaxanthin being the major one. Further, to increase the bioactive’s stability and bioavailability, both extracts were encapsulated in a matrix composed of CMC and WPI. The coacervation technique was used, followed by freeze-drying. Different analyses have been made to test the functionality of the ingredients. The phytochemicals characterization revealed that both powders presented high phytochemicals content. Although the powder with E2 presented the highest encapsulation efficiency, the one with E1 showed the highest phytochemical content and encapsulation efficiency stability. P2 highlighted the highest amount of yellowness, being correlated with the high content of phytochemicals. The CLSM images revealed that the coacervation led to the scalariform appearance of the microcapsules. The bioactives from sea buckthorn are observable as yellow-orange microspherosomes. Small spherosomes were visualized in P1, while in P2, they were of medium size. Both powders had a substantially higher release of the biologically active substances in the simulated intestinal juice, but the best transmission of the target substances has P2. This research suggests that, despite the differences between the powders, both could be used in food applications as targeted delivery vehicles for carotenoids and polyphenols as bioactive ingredients.
Author Contributions
Conceptualization, D.R., N.N.C. and G.R.; methodology, D.R.; software, E.E. and V.B.; validation, E.E, V.B. and I.A.; formal analysis, D.R.; investigation, D.R.; resources, G.E.B.; data curation G.R. and N.S.; writing—original draft preparation D.R. and N.N.C.; writing—review and editing, G.R. and N.N.C.; visualization, N.S.; supervision, G.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (G.R.) upon reasonable request.
Acknowledgments
The results of this work have been presented to the 9th edition of the Scientific Conference organized by the Doctoral Schools of “Dunărea de Jos” University of Galati (http://www.cssd-udjg.ugal.ro/ (accessed on 5 August 2021)), that was held on 10–11 June 2021 in Galati, Romania.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| AlCl3 | aluminum chloride |
| CE | Catechin Equivalents |
| CLSM | Confocal Laser Scanning Microscopy |
| CMC | Carboxymethil cellulose |
| DPPH | 2,2-diphenyl1-picrylhydrazyl |
| dw | dry weight |
| fr | fresh weight |
| GAE | Gallic Acid Equivalents |
| HCl | hydrochloric acid |
| HPLC | High Performance Liquid Chromatography |
| LC | Lycopene Content |
| Na2CO3 | sodium carbonate |
| NaCl | sodiul chloride |
| NaNO2 | sodium nitrite |
| NaOH | sodium hydroxide |
| nd | not detected |
| P | Powder |
| rpm | rotations per minute |
| SGF | Simulated Gastric Fluid |
| SIF | Simulated Intestinal Fluid |
| TC | Total Carotenoid Content |
| TFC | Total Flavonoid Content |
| TPC | Total Polyphenol Content |
| UAE | Ultrasounds Assisted Extraction |
| E | Extract |
| WPI | Whey Protein Isolate |
| βC | Beta carotene Content |
References
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Shutterstock. Available online: https://www.shutterstock.com/ro/image-photo/sea-buckthorn-fresh-ripe-berries-leaves-604664045 (accessed on 13 September 2021).
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, K.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Bioactive Encapsulated Powders for Functional Foods—A Review of Methods and Current Limitations. Food Bioproc. Tech. 2015, 8, 1825–1837. [Google Scholar] [CrossRef]
- Taneja, A.; Harjinder, S. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu. Rev. Food Sci. Technol. 2012, 3, 105–123. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, O.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, S.L.; Schmitt, C.; Sanchez, C. Protein-polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 63–70. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus: Washington, DC, USA, 2004; pp. 8–19. [Google Scholar]
- Turturică, M.; Râpeanu, G.; Stănciuc, N.; Bahrim, G. Fluorescence spectroscopy investigation on pH and heat changes of cherries anthocyanin extracts. J. Biotechnol. 2015, 208, S68. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturica, M.; Barbu, V.; Ionita, E.; Patrascu, L.; Cotarlet, M.; Dumitrascu, L.; Aprodu, I.; Rapeanu, G.; Stanciuc, N. Transglutaminase mediated microencapsulation of sea buckthorn supercritical CO2 extract in whey protein isolate and valorization in highly value added food products. Food Chem. 2018, 262, 30–38. [Google Scholar] [CrossRef]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Rapeanu, G.; Silvi, S.; Stanciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Ursache, F.M.; Ghinea, I.O.; Turturica, M.; Aprodu, I.; Rapeanu, G.; Stanciuc, N. Phytochemicals content and antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) as affected by heat treatment–Quantitative spectroscopic and kinetic approaches. Food Chem. 2017, 233, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Korekar, G.; Dolkar, P.; Singh, H.; Srivastava, R.B.; Stobdan, T. Variability and the genotypic effect on antioxidant activity, total phenolics, carotenoids and ascorbic acid content in seventeen natural population of Seabuckthorn (Hippophae rhamnoides L.) from trans-Himalaya. LWT 2014, 55, 157–162. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: A review. Carbohydr. Polym. 2020, 242, 219495666. [Google Scholar] [CrossRef] [PubMed]
- Laos, K.; Lõugas, T.; Mändmets, A.; Vokk, R. Encapsulation of β-carotene from sea buckthorn (Hippophaë rhamnoides L.) juice in furcellaran beads. Innov. Food Sci. Emerg. Technol. 2007, 3, 395–398. [Google Scholar] [CrossRef]
- Neagu, C.; Mihalcea, L.; Enachi, E.; Barbu, V.; Borda, D.; Bahrim, G.E.; Stanciunc, N. Cross-linked microencapsulation of CO2 supercritical extracted oleoresins from sea buckthorn: Evidence of targeted functionality and stability. Molecules 2020, 10, 2442. [Google Scholar] [CrossRef] [PubMed]
- Topală, C.M.; Mazilu, I.C.; Vulpe, M.; Vîjan, L.E. Quality study of fruits and extracts from six romanian sea buckthorn varieties. Curr. Trends Nat. Sci. 2020, 9, 273–283. [Google Scholar] [CrossRef]
- Katoh, T.; Nagashima, U.; Mimuro, M. Fluorescence properties of the allenic carotenoid fucoxanthin: Implication for energy transfer in photosynthetic pigment systems. Photosyn. Res. 1991, 27, 221–226. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F. HPLC determination of carotenoids in Bulgarian berries. J. Food Compost. Anal. 2007, 20, 370–374. [Google Scholar] [CrossRef]
- Llansola-Portoles, M.J.; Redeckas, K.; Streckaité, S.; Ilioaia, C.; Pascal, A.A.; Telfer, A.; Vengris, M.; Valkunasde, L.; Robert, B. Lycopene crystalloids exhibit singlet exciton fission in tomatoes. Phys. Chem. Chem. Phys. 2018, 20, 8640–8646. [Google Scholar] [CrossRef]
- Salgado, L.T.; Tomazetto, R.; Cinelli, L.P.; Farina, M.; Amado Filho, G.M. The influence of brown algae alginates on phenolic compounds capability of ultraviolet radiation absorption in vitro. Braz. J. Oceanogr. 2007, 55, 145–154. [Google Scholar] [CrossRef]
- McDonald, J.E.; Rooks, D.J.; McCarthy, A.J. Chapter nineteen-Methods for the Isolation of Cellulose-Degrading Microorganisms. In Methods in Enzymology; Gilbert, H.J., Ed.; Academic Press: London, UK, 2012; Volume 510, pp. 349–374. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).