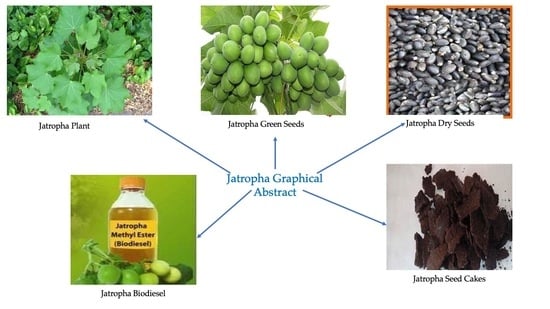
Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock
Abstract
1. Introduction
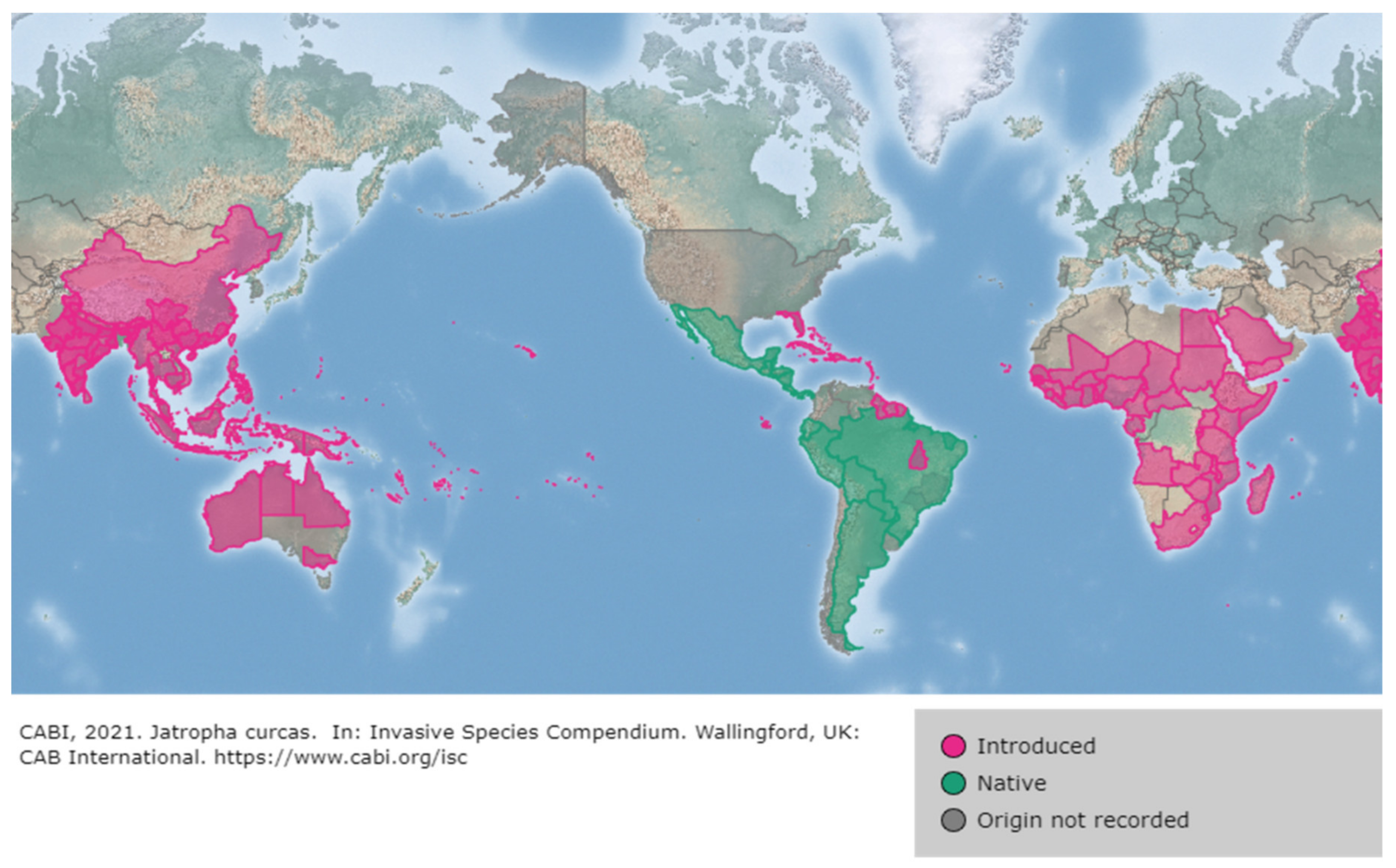
2. Origin, Distribution and Exploitation of J. curcas
3. Morphology and Phenology
4. Genetic Diversity
5. Cultivation Practices of Jatropha
5.1. Propagation
5.2. Soil Requirement
5.3. Irrigation and Fertilizer Management
5.4. Insect, Pest, and Disease Management
6. Biodiesel and Its Properties
6.1. Cetane Number
6.2. Cloud Point
6.3. Oxidative Stability
6.4. Viscosity
6.5. Lubricity
6.6. Density
6.7. Flashpoint
6.8. Pour Point
6.9. Cold Filter Plugging Point
6.10. Calorific Value
6.11. Acid Number
6.12. Iodine Number
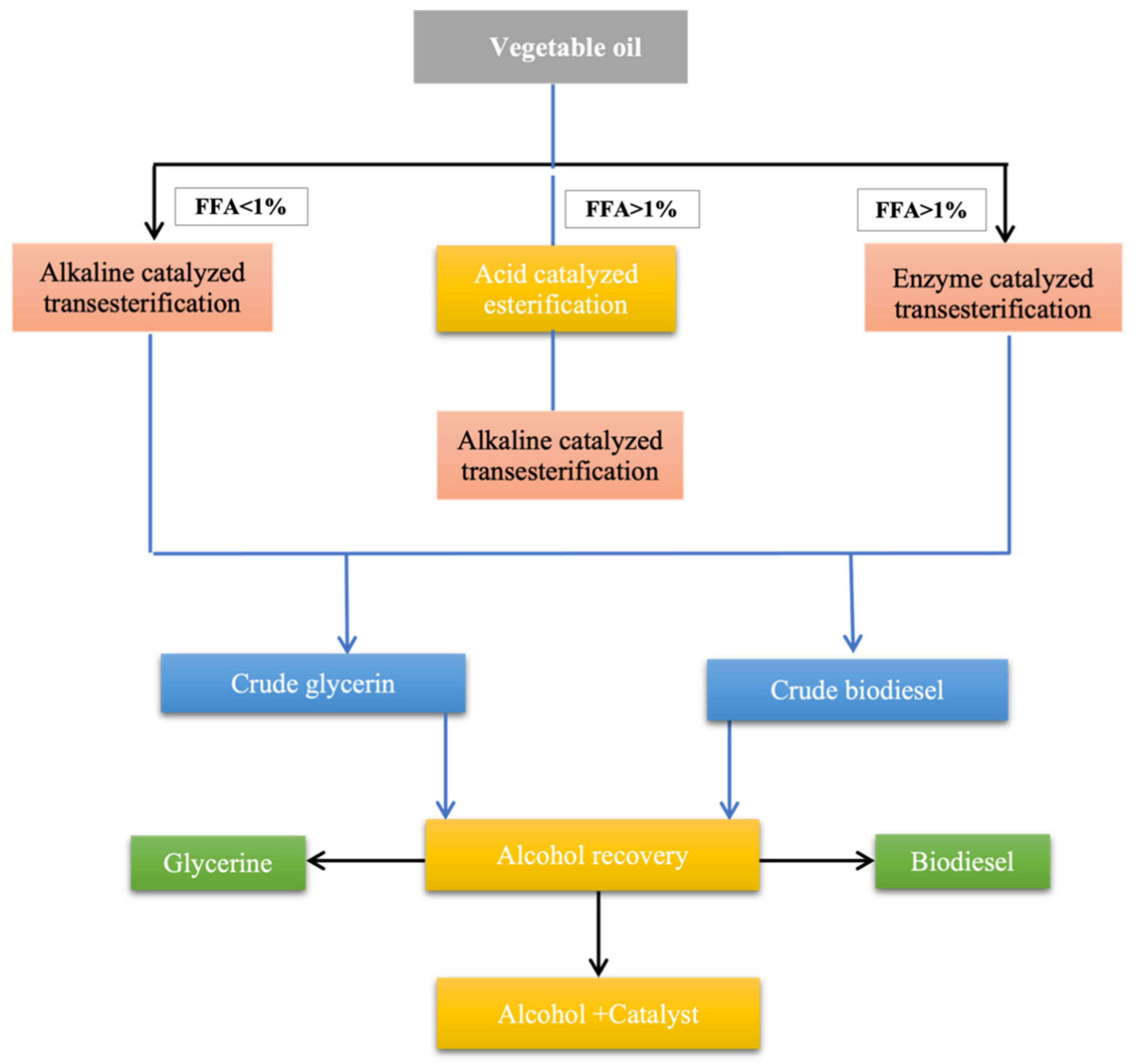
7. Methods of Biodiesel Production
7.1. Blending
7.2. Microemulsion Method
7.3. Pyrolysis or Thermal Cracking
7.4. Transesterification
7.4.1. Catalytic Transesterification
7.4.2. Base or Alkaline Catalyzed Transesterification
7.4.3. Acid-Catalyzed Transesterification
7.4.4. Biocatalytic or Enzymatic Transesterification
7.4.5. Supercritical Alcohol Transesterification
8. Jatropha as Second-Generation Biofuel Feedstocks from Non-Edible Source
8.1. Biodiesel Production Potential of J. curcas
8.2. Fatty Acid Composition
8.3. Composition of J. curcas Oil
9. Environmental Impacts and Economic Aspects of Jatropha Cultivation
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| J. curcas | Jatropha curcas L. |
| EN ISO | European Standards International Organization for Standardization |
| ASTM | American Society for Testing and Materials |
| FAME | fatty acid methyl ester |
References
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Plattner, G.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Climate change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 5–14. [Google Scholar]
- IEA. Sustainable Production of Second-Generation Biofuels: Potential and Perspectives in Major Economies and Developing Countries; International Energy Agency: Paris, France, 2010. [Google Scholar]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- UN Energy. Sustainable Bioenergy: A Framework for Decision Makers; UN Energy: New York, NY, USA, 2007. [Google Scholar]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Alherbawi, M.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Jatropha curcas for jet biofuel production: Current status and future prospects. Renew. Sustain. Energy Rev. 2020, 135, 110396. [Google Scholar] [CrossRef]
- Moeller, D.; Sieverding, H.L.; Stone, J.J. Comparative Farm-Gate Life Cycle Assessment of Oilseed Feedstocks in the Northern Great Plains. Biophys. Econ. Resour. Qual. 2017, 2, 13. [Google Scholar] [CrossRef]
- Lohaus, R.H.; Neupane, D.; Mengistu, M.A.; Solomon, J.K.; Cushman, J.C. Five-Year Field Trial of Eight Camelina sativa Cultivars for Biomass to be Used in Biofuel under Irrigated Conditions in a Semi-Arid Climate. Agronomy 2020, 10, 562. [Google Scholar] [CrossRef]
- Neupane, D.; Solomon, J.K.Q.; Davison, J.; Lawry, T. Nitrogen source and rate effects on grain and potential biodiesel production of camelina in the semiarid environment of northern Nevada. GCB Bioenergy 2018, 10, 861–876. [Google Scholar] [CrossRef]
- Neupane, D.; Solomon, J.K.Q.; Mclennon, E.; Davison, J.; Lawry, T. Sowing date and sowing method influence on camelina cultivars grain yield, oil concentration, and biodiesel production. Food Energy Secur. 2019, 8, 5. [Google Scholar] [CrossRef]
- Neupane, D.; Solomon, J.K.; Mclennon, E.; Davison, J.; Lawry, T. Camelina production parameters response to different irri-gation regimes. Ind. Crops Prod. 2020, 148, 112286. [Google Scholar] [CrossRef]
- Nleya, T.; Bhattarai, D.; Alberti, P. Agronomic Response of Camelina to Nitrogen and Seeding Rate on the Northern Great Plains. In Nitrogen in Agriculture-Physiological, Agricultural and Ecological Aspects; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Bhattarai, D.; Abagandura, G.O.; Nleya, T.; Kumar, S. Responses of soil surface greenhouse gas emissions to nitrogen and sulfur fertilizer rates to Brassica carinata grown as a bio-jet fuel. GCB Bioenergy 2020, 13, 627–639. [Google Scholar] [CrossRef]
- Jongschaap, R.; Corré, W.; Bindraban, P.; Brandenburg, W. Claims and Facts on Jatropha curcas L.: Global Jatropha curcas Evaluation. Breeding and Propagation Programme; Plant Research International: Wageningen, The Netherlands, 2007. [Google Scholar]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crop. Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, R. Physicochemical properties of Malaysian Jatropha curcas seed oil. Sains Malays. 2008, 37, 379–382. [Google Scholar]
- Sine, B.; Ouattara, B.; Sambakhé, D.; Ngom, A.W.; Ndiaye, A. Water Stress Limits Growth and Physiological Performance of Jatropha curcas L. Seedlings. J. Agric. Ecol. Res. Int. 2020, 21, 47–53. [Google Scholar]
- Berry, E.W. An eogene tropical forest in the Peruvian desert. Proc. Natl. Acad. Sci. USA 1929, 15, 345. [Google Scholar] [CrossRef][Green Version]
- Marshall, L.G. Land mammals and the great American interchange. Am. Sci. 1988, 76, 380–388. [Google Scholar]
- Heller, J. Physic Nut, Jatropha curcas L.; Bioversity International: Maccarese-Stazione, Spain, 1996; Volume 1. [Google Scholar]
- McVaugh, R. The Genus Jatropha in America: Principal Intrageneric Groups. Bull. Torrey Bot. Club 1945, 72, 271. [Google Scholar] [CrossRef]
- Wilbur, R.L. A synopsis of Jatropha, subsection Eucurcas, with the description of two new species from Mexico. J. Elisha Mitchell Sci. Soc. 1954, 70, 92–101. [Google Scholar]
- Basha, S.; Francis, G.; Makkar, H.; Becker, K.; Sujatha, M. A comparative study of biochemical traits and molecular markers for assessment of genetic relationships between Jatropha curcas L. germplasm from different countries. Plant. Sci. 2009, 176, 812–823. [Google Scholar] [CrossRef]
- Li, H.; Tsuchimoto, S.; Harada, K.; Yamasaki, M.; Sakai, H.; Wada, N.; Alipour, A.; Sasai, T.; Tsunekawa, A.; Tsujimoto, H.; et al. Genetic Tracing of Jatropha curcas L. from Its Mesoamerican Origin to the World. Front. Plant. Sci. 2017, 8, 1539. [Google Scholar] [CrossRef] [PubMed]
- Germplasm Resources Information Network (GRIN)-Global. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=20692 (accessed on 19 June 2021).
- Li, C.-Y.; Devappa, R.K.; Liu, J.-X.; Lv, J.-M.; Makkar, H.; Becker, K. Toxicity of Jatropha curcas phorbol esters in mice. Food Chem. Toxicol. 2010, 48, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Mahunnah, R.; Mshiu, E. Plants used in traditional medicine in Eastern Tanzania. III. Angiosperms (Euphorbiaceae to Menispermaceae). J. Ethnopharmacol. 1990, 28, 255–283. [Google Scholar] [CrossRef]
- Prasad, D.R.; Izam, A.; Khan, M.M.R. Jatropha curcas: Plant of medical benefits. J. Med. Plants Res. 2012, 6, 2691–2699. [Google Scholar]
- Staubmann, R.; Schubert-Zsilavecz, M.; Hiermann, A.; Kartnig, T. The anti-inflammatory effect of Jatropha curcas leaves. In Proceedings of the Symposium “Jatropha 97”, Managua, Nicaraqua, 23–27 February 1997. [Google Scholar]
- Singh, Y.N.; Ikahihifo, T.; Panuve, M.; Slatter, C. Folk medicine in tonga. A study on the use of herbal medicines for obstetric and gynaecological conditions and disorders. J. Ethnopharmacol. 1984, 12, 305–329. [Google Scholar] [CrossRef]
- Chitra, S.; Dhyani, S. Insect pests of Jatropha curcas L. and the potential for their management. Curr. Sci. 2006, 91, 162–163. [Google Scholar]
- Achten, W.; Verchot, L.; Franken, Y.; Mathijs, E.; Singh, V.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass-Bioenerg. 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Gübitz, G.M.; Mittelbach, M.; Trabi, M. Exploitation of the tropical oil seed plant Jatropha curcas L. Bioresour. Technol. 1999, 67, 73–82. [Google Scholar] [CrossRef]
- Openshaw, K. A review of Jatropha curcas: An oil plant of unfulfilled promise. Biomass-Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Osoniyi, O.; Onajobi, F. Coagulant and anticoagulant activities in Jatropha curcas latex. J. Ethnopharmacol. 2003, 89, 101–105. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Ezenwosu, I.V.; Ogidi, C.O.; Oyetayo, V.O. Antimicrobial potential of toothpaste formulated from extracts of Syzygium aromaticum, Dennettia tripetala and Jatropha curcas latex against some oral pathogenic microorganisms. AMB Express 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.N.; Masyitha, D.; Harris, A.; Balqis, U.; Iskandar, C.D.; Hambal, M. Darmawi Anti-inflammatory activity of Jatropha curcas Linn. latex in cream formulation on CD68 expression in mice skin wound. Veter World 2018, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Foidl, N.; Foidl, G.; Sanchez, M.; Mittelbach, M.; Hackel, S. Jatropha curcas L. as a source for the production of biofuel in Nicaragua. Bioresour. Technol. 1996, 58, 77–82. [Google Scholar] [CrossRef]
- Mujumdar, A.M.; Upadhye, A.S.; Misar, A.V. Studies on antidiarrhoeal activity of Jatropha curcus root extract in albino mice. J. Ethnopharmacol. 2000, 70, 183–187. [Google Scholar] [CrossRef]
- Shahinuzzaman, M.; Yaakob, Z.; Moniruzzaman, M. Medicinal and cosmetics soap production from Jatropha oil. J. Cosmet. Dermatol. 2016, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Olowoake, A.A.; Osunlola, O.S.; Ojo, J.A. Influence of compost supplemented with jatropha cake on soil fertility, growth, and yield of maize (Zea mays L.) in a degraded soil of Ilorin, Nigeria. Int. J. Recycl. Organ. Waste Agric. 2018, 7, 67–73. [Google Scholar] [CrossRef]
- Staubmann, R.; Foidl, G.; Foidl, N.; Gübitz, G.M.; Lafferty, R.M.; Arbizu, V.M.V.; Steiner, W. Biogas Production from Jatropha curcas Press-Cake. Appl. Biochem. Biotechnol. 1997, 457–467. [Google Scholar] [CrossRef]
- Aiyelaagbe, O. Antibacterial activity of Jatropha multifida roots. Fitoterapia 2001, 72, 544–546. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.; Adeniyi, B.; Fatunsin, O.; Arimah, B. In vitro Antimicrobial Activity and Phytochemical Analysis of Jatropha curcas Roots. Int. J. Pharmacol. 2006, 3, 106–110. [Google Scholar] [CrossRef]
- Carels, N. Jatropha curcas: A review. Bot. Res. 2009, 50, 39–86. [Google Scholar]
- Hartana, A. Flower characteristics and phenology of andromonoecious Jatropha curcas. Pakistan J. Bot. 2015, 47, 1501–1510. [Google Scholar]
- Zhang, F.-L.; Niu, B.; Wang, Y.-C.; Chen, F.; Wang, S.-H.; Xu, Y.; Jiang, L.-D.; Gao, S.; Wu, J.; Tang, L.; et al. A novel betaine aldehyde dehydrogenase gene from Jatropha curcas, encoding an enzyme implicated in adaptation to environmental stress. Plant Sci. 2008, 174, 510–518. [Google Scholar] [CrossRef]
- Sanou, H.; Angulo-Escalante, M.A.; Martínez-Herrera, J.; Koné, S.; Nikiema, A.; Kalinganire, A.; Hansen, J.K.; Kjær, E.D.; Graudal, L.; Nielsen, L.R. Loss of genetic diversity of Jatropha curcas L. through domestication: Implications for its genetic improvement. Crop. Sci. 2015, 55, 749–759. [Google Scholar] [CrossRef]
- Derero, A.; Gailing, O.; Finkeldey, R. Maintenance of genetic diversity in Cordia africana Lam., a declining forest tree species in Ethiopia. Tree Genet. Genomes 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Fuchs, E.J.; Lobo, J.A.; Quesada, M. Effects of Forest Fragmentation and Flowering Phenology on the Reproductive Success and Mating Patterns of the Tropical Dry Forest Tree Pachira quinata. Conserv. Biol. 2003, 17, 149–157. [Google Scholar] [CrossRef]
- Ginwal, H.S.; Phartyal, S.S.; Rawat, P.S.; Srivastava, R.L. Seed Source Variation in Morphology, Germination and Seedling Growth of Jatropha curcas Linn. in Central India. Silvae Genet. 2005, 54, 76–80. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R.P. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sustain. Energy Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Hegde, S.; Patil, C. Genetic divergence in rainfed rice. Karnataka J. Agric. Sci. 2000, 13, 549–553. [Google Scholar]
- Kaushik, N.; Kumar, K.; Kumar, S.; Roy, S. Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass-Bioenergy 2007, 31, 497–502. [Google Scholar] [CrossRef]
- Basha, S.D.; Sujatha, M. Inter and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population-specific SCAR markers. Euphytica 2007, 156, 375–386. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, D.; Wu, G.; Peng, J. ISSR-based genetic diversity of Jatropha curcas germplasm in China. Biomass- Bioenerg. 2010, 34, 1739–1750. [Google Scholar] [CrossRef]
- Grativol, C.; da Fonseca Lira-Medeiros, C.; Hemerly, A.S.; Ferreira, P.C.G. High efficiency and reliability of inter-simple se-quence repeats (ISSR) markers for evaluation of genetic diversity in Brazilian cultivated Jatropha curcas L. accessions. Mol. Biol. Rep. 2011, 38, 4245–4256. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.B.; Laviola, B.; Faria, D.A.; Pappas, M.; Bhering, L.L.; Quirino, B.; Grattapaglia, D. Molecular Markers Reveal Limited Genetic Diversity in a Large Germplasm Collection of the Biofuel Crop Jatropha curcas L. in Brazil. Crop. Sci. 2010, 50, 2372–2382. [Google Scholar] [CrossRef]
- Gautam Murty, S.; Patel, F.; Punwar, B.; Patel, M.; Singh, A.; Fougat, R. Comparison of RAPD, ISSR, and DAMD markers for genetic diversity assessment between accessions of Jatropha curcas L. and its related species. J. Agric. Sci. Technol. 2013, 15, 1007–1022. [Google Scholar]
- Mavuso, C.; Wu, Y.-P.; Chen, F.-C.; Huang, B.-H.; Lin, S.-J. Genetic diversity analysis of Jatropha curcas L. accessions cultivated in Taiwan using inter simple sequence repeats (ISSR) markers. Agrofor. Syst. 2015, 90, 417–431. [Google Scholar] [CrossRef]
- Vásquez-Mayorga, M.; Fuchs, E.J.; Hernández, E.J.; Herrera, F.; Hernández, J.; Moreira, I.; Arnáez, E.; Barboza, N.M. Molec-ular characterization and genetic diversity of Jatropha curcas L. in Costa Rica. PeerJ 2017, 5, e2931. [Google Scholar] [CrossRef]
- Maghuly, F.; Jankowicz-Cieslak, J.; Pabinger, S.; Till, B.J.; Laimer, M. Geographic origin is not supported by the genetic varia-bility found in a large living collection of Jatropha curcas with accessions from three continents. Biotechnol. J. 2015, 10, 536–551. [Google Scholar] [CrossRef]
- Trebbi, D.; Papazoglou, E.G.; Saadaoui, E.; Vischi, M.; Baldini, M.; Stevanato, P.; Cettul, E.; Sanzone, A.P.; Gualdi, L.; Fabbri, A. Assessment of genetic diversity in different accessions of Jatropha curcas. Ind. Crop. Prod. 2015, 75, 35–39. [Google Scholar] [CrossRef]
- Wahl, N.; Hildebrandt, T.; Moser, C.; Lüdeke-Freund, F.; Averdunk, K.; Bailis, R.; Barua, K.; Burritt, R.; Groeneveld, J.; Klein, A.-M. Insights into Jatropha Projects Worldwide-Key Facts & Figures from a Global Survey; Centre for Sustainability Management (CSM), Leuphana Universität Lüneburg: Lüneburg, Germany, 2012. [Google Scholar]
- Kochhar, S.; Singh, S.; Kochhar, V. Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant—Jatropha curcas. Biomass- Bioenergy 2008, 32, 1136–1143. [Google Scholar] [CrossRef]
- Nietsche, S.; Vendrame, W.A.; Crane, J.H.; Pereira, M.C. Assessment of reproductive characteristics of Jatropha curcas L. in south Florida. Gcb Bioenergy 2014, 6, 351–359. [Google Scholar] [CrossRef]
- El-Torky, M.; El-Naggar, H.; El-Shanhorey, N.; Yousef, A. In Vitro Propagation and Mutagenesis of Jatropha curcas. Alex. Sci. Exch. J. 2021, 42, 209–222. [Google Scholar] [CrossRef]
- Rodríguez, O.A.V.; Sánchez-Sánchez, O.; Pérez-Vázquez, A.; Caplan, J.; Danjon, F. Jatropha curcas L. Root Structure and Growth in Diverse Soils. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Tomar, N.S.; Ahanger, M.A.; Agarwal, R.M. Jatropha curcas: An. Overview; Springer: Berlin/Heidelberg, Germany, 2013; pp. 361–383. [Google Scholar] [CrossRef]
- van Eijck, J.; Romijn, H.; Balkema, A.; Faaij, A. Global experience with jatropha cultivation for bioenergy: An assessment of socio-economic and environmental aspects. Renew. Sustain. Energy Rev. 2014, 32, 869–889. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, N.; Kumar, R.; Mandal, D. Irrigation Scheduling and Fertilization Improves Production Potential of Jatropha (Jatropha curcas L.): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1703–1716. [Google Scholar] [CrossRef]
- Prasad, S.; Nirala, D.P.; Sinha, A.; Kumar, J.; Kumari, P. Oil content analysis of Jatropha curcas L. seeds under agronomical treatment with N, P, K and Gypsum. J. Pharmacogn. Phytochem. 2017, SP1, 1132–1134. [Google Scholar]
- Ramos-Robles, M.; Sánchez-Vega, M.; Aguirre-Uribe, L.A.; Méndez-López, A.; Martínez-Amador, S.Y.; Leal-Robles, A.I.; González-Méndez, L.M. Diversity and Species Turnover of Insects in a Jatropha curcas Agroecosystem. Southwest. Èntomol. 2021, 46, 115–128. [Google Scholar] [CrossRef]
- Lama, A.D.; Vuorisalo, T.; Niemela, P. Global patterns of arthropod herbivory on an invasive plant, the physic nut (Jatropha curcas L.). J. Appl. Èntomol. 2014, 139, 1–10. [Google Scholar] [CrossRef]
- Muniz, D.R.; Zaidan, I.R.; Dias, L.A.D.S.; Leite, J.P.V.; Diniz, J.A. Biocide Potential of Jatropha curcas L. Extracts. J. Biol. Life Sci. 2020, 11, 138–154. [Google Scholar] [CrossRef]
- Verma, M.; Pradhan, S.; Sharma, S.; Naik, S.; Prasad, R. Efficacy of karanjin and phorbol ester fraction against termites (Odontotermes obesus). Int. Biodeterior. Biodegrad. 2011, 65, 877–882. [Google Scholar] [CrossRef]
- Sharma, V.; Das, L.; Pradhan, R.; Naik, S.; Bhatnagar, N.; Kureel, R. Physical properties of tung seed: An industrial oil yielding crop. Ind. Crop. Prod. 2011, 33, 440–444. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different gener-ation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Fernández, J.R. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Mostafa, S.S.; El-Gendy, N.S. Evaluation of fuel properties for microalgae Spirulina platensis bio-diesel and its blends with Egyptian petro-diesel. Arab. J. Chem. 2017, 10, S2040–S2050. [Google Scholar] [CrossRef]
- Dixit, S.; Kanakraj, S.; Rehman, A. Linseed oil as a potential resource for bio-diesel: A review. Renew. Sustain. Energy Rev. 2012, 16, 4415–4421. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Atmanli, A. Comparative analyses of diesel–waste oil biodiesel and propanol, n-butanol or 1-pentanol blends in a diesel engine. Fuel 2016, 176, 209–215. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Abdulahad, W.S. Transesterification of mustard (Brassica nigra) seed oil with ethanol: Purification of the crude ethyl ester with activated carbon produced from de-oiled cake. Energy Convers. Manag. 2013, 77, 495–503. [Google Scholar] [CrossRef]
- Fattah, I.R.; Masjuki, H.; Kalam, A.; Wakil, M.; Rashedul, H.; Abedin, M. Performance and emission characteristics of a CI engine fueled with Cocos nucifera and Jatropha curcas B20 blends accompanying antioxidants. Ind. Crop. Prod. 2014, 57, 132–140. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, M. Performance and emission characteristics of a diesel engine fuelled with biodiesel blends. Int. J. Renew. Energy Res. (IJRER) 2016, 6, 658–662. [Google Scholar]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Bhuiya, M.; Rasul, M.; Khan, M.; Ashwath, N.; Azad, A.; Hazrat, M. Prospects of 2nd generation biodiesel as a sustainable fuel–Part 2: Properties, performance and emission characteristics. Renew. Sustain. Energy Rev. 2016, 55, 1129–1146. [Google Scholar] [CrossRef]
- Demirbas, A. A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008; ISBN 10:1846289947. [Google Scholar]
- Pratas, M.J.; Freitas, S.V.; Oliveira, M.B.; Monteiro, S.C.; Lima, Á.S.; Coutinho, J.A. Biodiesel density: Experimental measure-ments and prediction models. Energy Fuels 2011, 25, 2333–2340. [Google Scholar] [CrossRef]
- Alagu, R.; Sundaram, E.G. Preparation and characterization of pyrolytic oil through pyrolysis of neem seed and study of performance, combustion and emission characteristics in CI engine. J. Energy Inst. 2018, 91, 100–109. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Giménez, F.J.L.; De Castro, M.D.L.; Dorado, G.; Dorado, M.P. The Ideal Vegetable Oil-based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Sánchez, N.; Sánchez, R.; Encinar, J.M.; González, J.F.G.; Martínez, G. Complete analysis of castor oil methanolysis to obtain biodiesel. Fuel 2015, 147, 95–99. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2012, 18, 211–245. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef]
- López, I.; Quintana, C.; Ruiz, J.; Cruz-Peragón, F.; Dorado, M. Effect of the use of olive–pomace oil biodiesel/diesel fuel blends in a compression ignition engine: Preliminary exergy analysis. Energy Convers. Manag. 2014, 85, 227–233. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M. Prospects of biodiesel from Jatropha in India: A review. Renew. Sustain. Energy Rev. 2010, 14, 763–771. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M.K. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2020, 285, 119110. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative re-view. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Kartika, I.A.; Evon, P.; Cerny, M.; Suparno, O.; Hermawan, D.; Ariono, D.; Rigal, L. Simultaneous solvent extraction and transesterification of jatropha oil for biodiesel production, and potential application of the obtained cakes for binderless particleboard. Fuel 2016, 181, 870–877. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking Oil an Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Meher, L.C.; Kulkarni, M.G.; Dalai, A.K.; Naik, S.N. Transesterification of karanja (Pongamia pinnata) oil by solid basic catalysts. Eur. J. Lipid Sci. Technol. 2006, 108, 389–397. [Google Scholar] [CrossRef]
- Chitra, P.; Venkatachalam, P.; Sampathrajan, A. Optimisation of experimental conditions for biodiesel production from alka-li-catalysed transesterification of Jatropha curcus oil. Energy Sustain. Dev. 2005, 9, 13–18. [Google Scholar] [CrossRef]
- Lotero, E.; Goodwin, J.G., Jr.; Bruce, D.A.; Suwannakarn, K.; Liu, Y.; Lopez, D.E. The catalysis of biodiesel synthesis. Catalysis 2006, 19, 41–83. [Google Scholar]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Luque, S.; Cerveró, J.M.; Coca, J. Production of biodiesel from vegetable oils. Grasas Aceites 2008, 59, 494. [Google Scholar] [CrossRef]
- Goff, M.J.; Bauer, N.S.; Lopes, S.; Sutterlin, W.R.; Suppes, G.J. Acid-catalyzed alcoholysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 415–420. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Patil, P.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ling, F.W.; Jun, L.S. Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process. Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Hama, S.; Yamaji, H.; Kaieda, M.; Oda, M.; Kondo, A.; Fukuda, H. Effect of fatty acid membrane composition on whole-cell biocatalysts for biodiesel-fuel production. Biochem. Eng. J. 2004, 21, 155–160. [Google Scholar] [CrossRef]
- Al Basir, F.; Datta, S.; Roy, P.K. Studies on biodiesel production from Jatropha curcas oil using chemical and biochemical methods—A mathematical approach. Fuel 2015, 158, 503–511. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Production of FAME by palm oil transesterification via supercritical methanol technology. Biomass-Bioenergy 2009, 33, 1096–1099. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour. Technol. 2009, 100, 696–700. [Google Scholar] [CrossRef]
- Hamzah, N.H.C.; Khairuddin, N.; Siddique, B.M.; Hassan, M.A. Potential of Jatropha curcas L. as Biodiesel Feedstock in Malaysia: A Concise Review. Processes 2020, 8, 786. [Google Scholar] [CrossRef]
- Koh, M.Y.; Ghazi, T.I.M. A review of biodiesel production from Jatropha curcas L. oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Silitonga, A.; Atabani, A.; Mahlia, T.M.I.; Masjuki, H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Sekaran, U.; Singh, S.; Singh, J.; Ibrahim, M.A.; Subramanian, S.; Owens, V.N.; Kumar, S. Intercropping kura clover with prairie cordgrass mitigates soil greenhouse gas fluxes. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Gmünder, S.; Singh, R.; Pfister, S.; Adheloya, A.; Zah, R. Environmental Impacts of Jatropha curcas Biodiesel in India. J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Fuentes, A.; García, C.A.; Hennecke, A.; Masera, O. Life cycle assessment of Jatropha curcas biodiesel production: A case study in Mexico. Clean Technol. Environ. Policy 2018, 20, 1721–1733. [Google Scholar] [CrossRef]
- Ntaribi, T.; Paul, D.I. The economic feasibility of Jatropha cultivation for biodiesel production in Rwanda: A case study of Kirehe district. Energy Sustain. Dev. 2019, 50, 27–37. [Google Scholar] [CrossRef]
- van Eijck, J.; Romijn, H.; Smeets, E.; Bailis, R.; Rooijakkers, M.; Hooijkaas, N.; Verweij, P.; Faaij, A. Comparative analysis of key socio-economic and environmental impacts of smallholder and plantation based jatropha biofuel production systems in Tan-zania. Biomass Bioenergy 2014, 61, 25–45. [Google Scholar] [CrossRef]
- Goswami, K.; Saikia, J.; Choudhury, H.K. Economic benefits and costs of jatropha plantation in North-East India. Agric. Econ. Res. Rev. 2011, 24, 99–108. [Google Scholar]
- Timilsina, G.R.; Tiwari, U. The Economic Viability of Jatropha Biodiesel in Nepal. World Bank Policy Research Working Paper; World Bank Group Open Knowledge Repository: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]

| Plant Parts | Uses | References |
|---|---|---|
| Leaves | Medicinal uses | [23,31] |
| Anti-inflammatory | [32] | |
| Silk farming | [37] | |
| Latex | Coagulant and anticoagulant | [38] |
| Antimicrobial toothpaste | [39] | |
| Wound healing | [40] | |
| Seed oil | Biofuel | [41] |
| Medicinal uses | [42] | |
| Soap production | [43] | |
| Seed cake | Fertilizer | [44] |
| Biogas | [45] | |
| Fodder (non-toxic) | [23] | |
| Roots | Anti-microbial | [46] |
| Medicinal uses | [31,47] |
| N:P: K Treatment (kg ha−1 Year−1) | Other Additives (kg ha−1 Year−1) |
|---|---|
| 68:69:75 | Sulphur (12.5) |
| 46:46:46 | - |
| 46:48:24 | - |
| 60:30:0 | - |
| 37:37:37 | cow dung (25) |
| Feedstocks | Density at 15 °C (kg/m3) | Heating Value (MJ/kg) | Cloud Point (°C) | Flashpoint (°C) | Pour Point (°C) | Viscosity at 40 °C (mm2/s) | Cetane Number | Iodine Number | Sulfur Content (wt.%) | Add Value mg/g |
|---|---|---|---|---|---|---|---|---|---|---|
| Animal fat | 875 | 36.73 | - | - | - | 4.25 | 63.88 | 83.02 | - | 0.38 |
| Ankistrodesmus | 869 | 40.72 | 7 | 144 | −6 | 4.19 | - | - | - | - |
| Babassu | 872 | 31.8 | 4 | 117 | - | 4.2 | 63.25 | - | - | 0.425 |
| Beef tallow | 832 | 40.23 | - | 152–171 | 15 | 4.89 | 60.36 | 44.4 | - | 0.2 |
| Bitter almond | 884 | - | 4.5 | 169 | −6 | 4.6 | 45.18 | 117.29 | - | 0.27 |
| Camelina | 885 | 45.2 | 2.5 | 150 | −6.3 | 4.11 | 48.91 | 146.5 | 3 ppm | 0.2 |
| Camelus dromedaries | 871 | 39.52 | 12.7 | 158 | 15.5 | 3.39 | 58.7 | 65.3 | 0.031 | - |
| Canola | 878 | 35.74 L | −3.25 | 172.36 | −8 | 4.42 | 54 | 113.6 | 2 ppm | 0.49 |
| Castor | 922 | 38.09 | −11.16 | 178.56 | −20 | 17.14 | 37.55 | 85.53 | 1.3 | 0.148 |
| Chicken fat | 883 | 40.17 | −7 | 172 | - | 4.98 | 48 | - | 23.45 | 0.22 |
| Chlorella variabilis | 867 | 38.78 | - | 157 | - | 4.875 | 58.6 | - | 0 | 0 |
| Coconut | 867 | 35.2 L, 38.2 H | −1.6 | 113.83 | −8.3 | 3.2 | 64.65 | - | 3 ppm | 0.18 |
| Cottonseed | 887 | 39.75 | 1.7 | 210 | −12.5 | 4.19 | 48.1 | 120 | - | 0.5 |
| Crambe abyssinica | 872 | 39.56 | - | 136 | - | 6 | - | - | - | - |
| Fish oil | 881 | 40.54 | - | 177 | - | 4.45 | 47 | - | - | - |
| Fish oil | 885 | 40.05 | - | 114 | - | 4.74 | 52.6 | - | - | - |
| Fleshing oil | 907 | 39.61 | - | - | - | - | - | 52 | >990 ppm | - |
| Groundnut | 920 | 39.8 | 8 | 132 | 3 | 4.4 | 59.85 | 71.8 | 1.315 ppm | - |
| Hazelnut | 896 | 39.58 | −7.65 | 172.7 | −6 | 4.81 | 62.95 | 109 | 7 ppm | 0.351 |
| J. curcas L. | 865 | 40.79 | 5.66 | 175.5 | 6 | 4.25 | 55.43 | 95.75 | 0.008 | 0.24 |
| Jojoba | 866 | 44.77 | - | 80.5 | - | 2.2–19.2 | 63.5 | 48.97 | 0.3 | 0.8 |
| Karanja | 889 | 36.56 | 13.3 | 157.4 | 6.4 | 4.79 | 56.55 | 89 | 0.003 | - |
| Kusum | 875 | - | - | 152 | −2 | 5.34 | - | 37.59 | <0.005 | 0.435 |
| Lard | 877 | 36.5 | - | 143.5 | 7 | 4.84 | - | 66–77 | - | 0.12 |
| Linseed | 852 | 37.45 | 2.43 | 241 | −9.6 | 3.95 | 34.6 | 178 | 0.002 | 0.335 |
| Mahua | 895 | 36.9 L, 39.4 H | 4.33 | 161 | −6.8 | 4.77 | 55 | 74.2 | - | 0.41 |
| Michelia champaca | 870 | 39.51 | - | 158 | - | 5.11 | 50.28 | 104 | - | 0.44 |
| Mustard | 879 | 40.4 | 16 | 169.16 | −18 | 5.53 | 56 | 128 | <1 | 0.2 |
| Neem | 886 | 39.84 | 114.5 | 144.75 | 7 | 6.09 | 51.26 | 46.84 | 473 ppm | - |
| Neem seed pyrolysis oil | 982 | 20.8 | - | 55 | - | 9.38 | - | - | - | - |
| Olive pomace | 894 | 39.96 | 2 | 138 | 6 | 4.26 | 56.3 | 134.5 | - | 0.1 |
| Palm | 870 | 34.4 L, 40.13 H | 14.25 | 176.7 | 14.33 | 4.53 | 60.21 | 50.5 | 2 | 0.2 |
| Peanut | 878 | 35.33 | 12.6 | 176 | 11.5 | 4.69 | 58.24 | 67.45 | 6 ppm | - |
| Plastic pyrolysis oil | 981 | 38.3 | - | 13 | - | 1.91 | - | - | 0.155 | 41 |
| Pont water algae | 872 | 40.8 | - | - | −16 | 5.82 | - | - | - | 0.4 |
| Poultry fat | 877 | 38.58 | - | 172 | 3 | 6.86 | - | 78.8 | - | 0.55 |
| Rapeseed | 879 | 35.8 L, 41.1 H | −3.5 | 169.5 | −11 | 4.4 | 48.25 | 112 | 0.0024 | 0.26 |
| Rice bran | 889 | 38.17 | 0.55 | 157.4 | 6.4 | 5.15 | 64.95 | 106 | 6 | - |
| Rubber | 875 | 39.174 | 3.1 | 173.4 | −7 | 5.6 | 53 | 144 | - | 0.12 |
| Sesame | 867 | 40.25 | 0.5 | 176.67 | −4 | 4.23 | 58.97 | 83.52 | < 0.005 | 0.285 |
| Sludge pyrolysis oil | 980 | 37.04 | - | 68 | - | 12.3 | - | - | 0.55 | 26 |
| Sour plum | - | - | - | - | −6 | - | 61.39 | - | - | - |
| Soybean | 882 | 39.84 H | 0 | 140.1 | −3.2 | 4.15 | 44.7 | 117.7 | - | 0.18 |
| Spirulina | 860 | 41.36 | - | 130 | −18 | 5.66 | - | - | - | 0.45 |
| Spirulina platensis | 863 | 45.63 | −3 | 189 | −9 | 12.4 | 70 | 102 | 0 | - |
| Sunflower | 869 | 34.71 L, 40.6 H | 1.33 | 180.33 | −2 | 4.26 | 45.7 | 128.7 | - | 0.357 |
| Terminalia catappa | 876 | 37.33 | - | 90 | 6 | 4.3 | 57.1 | 83.2 | 13.3 | 0.5 |
| Tobacco | 865 | 42.22 | - | 165 | −12 | 3.56 | 51.5 | 136 | - | - |
| Trout oil | 885 | 37.8 | - | - | - | 4.25 | 51.3 | - | - | - |
| Waste cooking oil | 876 | 39.76 | - | 160 | - | 3.65 | 50.4 | 62 | - | - |
| Waste fry oil | 855 | 40.5 | −12 | 126 | - | 4.57 | 52.2 | - | - | - |
| Waste frying palm oil | 875 | 38.73 | - | 70.6 | - | 4.4 | 60.4 | - | - | 0.51 |
| Species | Seed Yield (×105 Mg ha−1 Year−1) | Oil Content (%) | Oil Yield (Mg ha−1 Year−1) |
|---|---|---|---|
| Jatropha | 2.0 | 40–60 | 2.0–3.0 |
| Mahua | 2.0 | 35–40 | 1.0–4.0 |
| Karanja | 0.6 | 30–40 | 2.0–4.0 |
| Caster | 2.5 | 45–60 | 0.5–1.0 |
| Linseed | 1.0 | 35–45 | 0.5–1.0 |
| Fatty Acid | Structure | Formula | Composition (%) | ||||
|---|---|---|---|---|---|---|---|
| Jatropha Seed Oil a | Karanja Oil | Sunflower Oil | Soybean Oil | Palm Kernel Oil | |||
| Myristic | (14:0) | C14H28O2 | 0–0.1 | - | - | 0.1 | 16.3 |
| Palmitic | (16:0) | C16H32O2 | 14.1–15.3 | 9.8 | - | 11.0 | 8.4 |
| Palmitoleic | (16:1) | C16H16O2 | 0–1.3 | - | - | - | - |
| Stearic | (18:0) | C18H36O2 | 3.7–9.8 | 6.2 | 4.5 | 4.0 | 2.4 |
| Oleic | (18:1) | C18H34O2 | 34.3–45.8 | 72.2 | 21.1 | 23.4 | 15.4 |
| Linoleic | (18:2) | C18H32O2 | 29.0–44.2 | 11.8 | 66.2 | 53.2 | 2.4 |
| Linolenic | (18:3) | C18H32O2 | 0–0.3 | - | - | 7.8 | - |
| Arachidic | (20:0) | C20H40O2 | 0–0.3 | - | 0.3 | - | 0.1 |
| Behenatic | (22:0) | C22H44O2 | 0–0.2 | - | - | - | - |
| Characteristics | Jatropha Biodiesel | Diesel Oil |
|---|---|---|
| Specific gravity 15 °C | 0.86–0.93 | 0.82–0.86 |
| Calorie value (MJ kg−1) | 38–42 | 42 |
| Pour point (°C) | −3 | −35 to 15 |
| Cloud point (°C) | 2 | −15 to 5 |
| Flashpoint (°C) | 210–240 | 50–98 |
| Cetane number | 38–51, up to 57 | 40–55 |
| Sulfur | 0.13 | 1.2 |
| Viscosity (cSt) at 30 °C | 37–55 | 1.3–4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.Q.; Qin, R.; Adhikari, P. Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions 2021, 6, 60. https://doi.org/10.3390/inventions6040060
Neupane D, Bhattarai D, Ahmed Z, Das B, Pandey S, Solomon JKQ, Qin R, Adhikari P. Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions. 2021; 6(4):60. https://doi.org/10.3390/inventions6040060
Chicago/Turabian StyleNeupane, Dhurba, Dwarika Bhattarai, Zeeshan Ahmed, Bhupendra Das, Sharad Pandey, Juan K. Q. Solomon, Ruijun Qin, and Pramila Adhikari. 2021. "Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock" Inventions 6, no. 4: 60. https://doi.org/10.3390/inventions6040060
APA StyleNeupane, D., Bhattarai, D., Ahmed, Z., Das, B., Pandey, S., Solomon, J. K. Q., Qin, R., & Adhikari, P. (2021). Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions, 6(4), 60. https://doi.org/10.3390/inventions6040060





_Qin.png)