1. Introduction
The interest of radiometals in Nuclear Medicine has increased dramatically over the last decade fostered by the successful clinical use of metal-based radiopharmaceuticals in combined targeted diagnosis and therapy (the so-called theragnostic concept) [
1,
2,
3,
4,
5]. To produce these radiometals, most hospitals would require the purchase of isotope generators, when available, or to make a substantial investment in a medical cyclotron with a solid target system. This is not a trivial option as most cyclotrons typically handle liquid and gas targets only and are used to produce non-metallic isotopes such as
18F,
11C and
13N. Therefore, the possibility to produce metal isotopes using a medical cyclotron without the investment in a solid-target system provides an easy and accessible way to produce these isotopes within a wide range of accelerator facilities [
6,
7,
8,
9,
10,
11]. Recent developments concerning the production of radiometals using liquid targets have been published by our group [
12,
13], paving the way for a new, safer and simplified procedure for automated loading and transfer of target solution to an automated chemistry module inside of a shielded hot-cell, and helping compliance with current Good Manufacturing Practices (GMP) regulations [
10].
The methods described allow the production of radioisotopes—such as
68Ga,
64Cu,
61Cu and others—through the irradiation of liquid targets, with a substantial reduction in processing time and cost when compared with the solid target approach. The process also eliminates the need for pre- and post-irradiation target preparation and simplifies the transfer of irradiated material from target to hotcell (
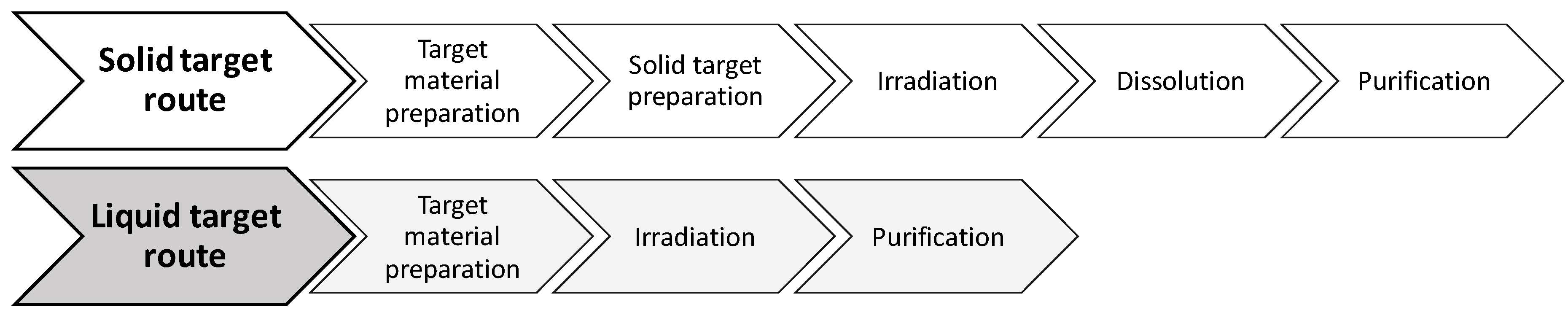
Figure 1).
Based on the potential of fast and cost-effective production of radiometals in medical cyclotrons, we present a fully automated process, using a commercially available module, for the purification of metal radioisotopes produced by cyclotron irradiation of liquid targets. This work describes the fully automated separation of
68Ga and
64Cu or
61Cu from target material and formulation in a solution for radiolabelling in compliance with European Pharmacopoeia (Ph. Eur.) requirements [
14]. The purified chloride solution can be used for labelling molecules using a conventional automated procedure in a reactor vial followed by post purification by a C18 cartridge [
15,
16,
17] or by means of a cold-kit based method [
18,
19,
20].
The process described can easily be extended to other metal radioisotopes. Irradiation of liquid targets in medical cyclotrons involves the previous preparation of a target solution containing the enriched (when needed) material in a process that benefits from the high yields provided by the same nuclear reactions used in the solid target while avoiding the inherent limitations of using such targets. In addition to the post-irradiation handling and transport of the solid target to a processing unit (shielded hotcell), such solid targets require a large amount of expensive enriched material (hundreds of mg are necessary) and such a long and complicated process is also associated with inevitable contamination with other metal ions due to the use of higher volumes of strong acids for the dissolution.
2. Materials and Methods
All steps required for the production and separation of a metal radioisotope from a liquid target are implemented in a fully integrated system. For each isotope, a dedicated IBA Nirta Conical® target system (IBA, Louvain-la-Neuve, Belgium) is used. To separate the metal isotopes from the target solution and reformulate them in a ready-to-use chloride solution, a commercially available IBA Synthera® Extension module (IBA, Louvain-la-Neuve, Belgium) is used with single-use kits. For the radiolabelling step, an IBA Synthera® Extension is used to label compounds with 64Cu/61Cu (e.g., bis(4-methyl-3-thiosemicarbazone), PTSM; diacetyl-2,3-bis(N4-methyl-3-thiosemicarbazone), ATSM) and an IBA Synthera@ for 68Ga-based compounds (e.g., 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) peptides, N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid (HBED) peptides. For metal trace analysis of samples, is used an inductively coupled plasma mass spectrometry (ICP-MS) equipment: Thermo Scientific iCAP Qc (Thermo Fisher Scientific, Waltham, MA, USA). To measure the activities of samples, an ISOMED 2010 (Nuklear-medizintechnik, Dresden, Germany) is used.
All chemicals and solvents used are trace-metal grade.
2.1. Targetry/Irradiation
As target material, the enriched isotopes are diluted in a 0.01 M nitric acid solution (
Table 1). Concentrations are adjusted to produce a maximum of required activity while avoiding precipitation and providing stability of the solution over time for storage and better behaviour under the cyclotron beam without corrosion of target support materials [
6,
7].
64Ni and 68Zn targets are typically irradiated with a beam current of about 70 µA and 45 µA, respectively, using an IBA 18/9 Cyclone cyclotron. The amount of enriched material on target varies from 10–100 mg of 64Ni and 100–400 mg of 68Zn, depending on the required activity. After irradiation, solutions are transferred to a processing hot-cell under nitrogen pressure.
2.2. Post-Processing
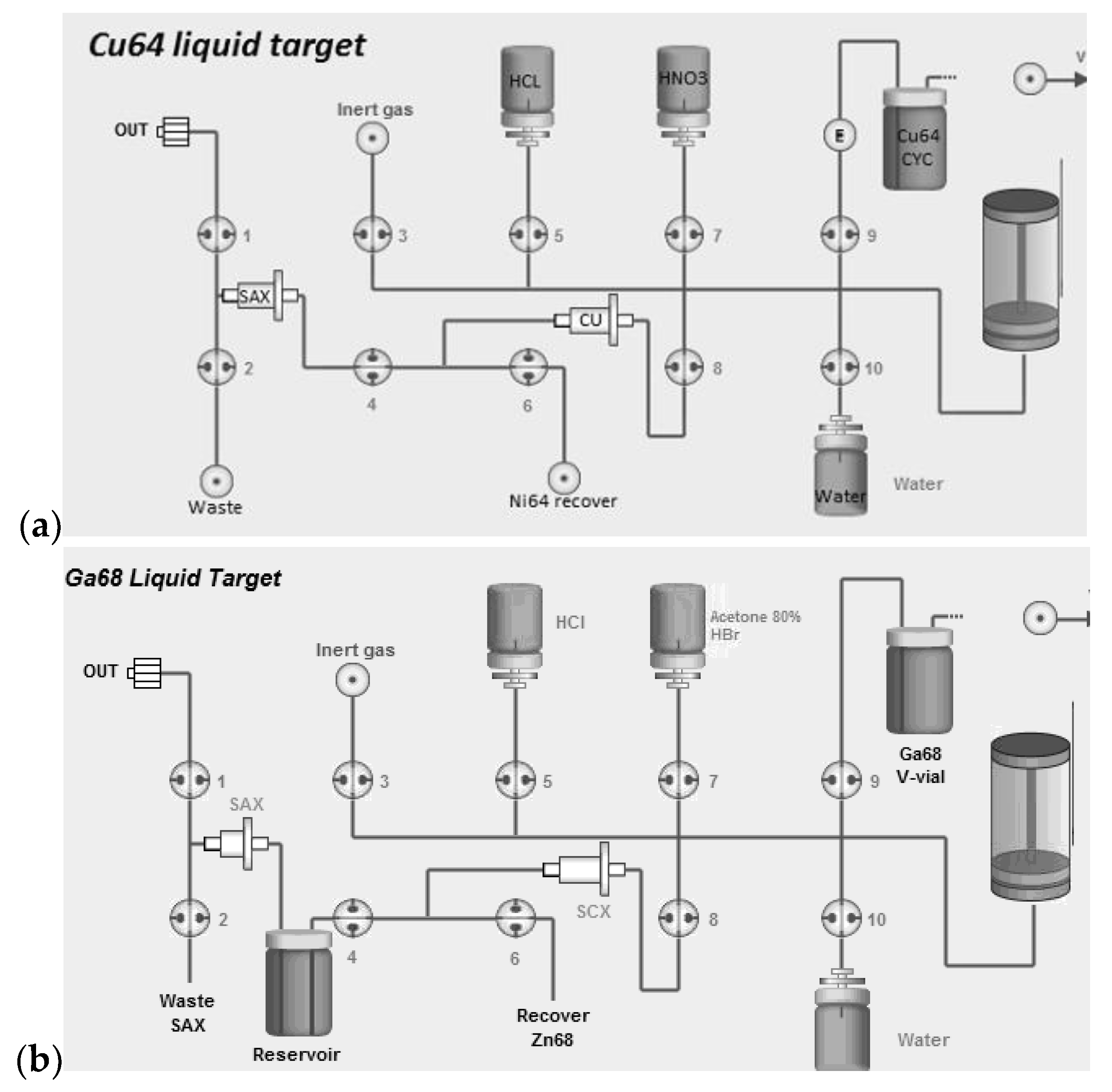
For the Gallium-68 production, the irradiated
68Zn target solution is dissolved multiple times in water and the solution is passed through a cation exchange resin (SCX; DOWEX 50W, 200–400 mesh, H+ form, treated with 10 mL of 3 M HCl followed by 10 mL of water) loaded on a 1 mL catridge. The cartridge is then washed with 30 mL of Acetone/HBr mixture to remove zinc ions as described by Strelow [
21,
22]. The adsorbed
68Ga cations are eluted from the SCX cartridge with 6 mL of HCl 3 M mixed with 10 mL of HCl 30% (to increase the molarity of HCl) to an intermediate reservoir (
Figure 2) and passed through an anion exchange resin (SAX; Biorad AG1 100 mesh, treated with 10 mL of water followed by 10 mL of HCl 8 M) loaded on 0.5 mL size-cartridge where the anionic complex [
68GaCl
4]
− remained strongly adsorbed [
23,
24]. A flow of inert gas is then applied to dry the column and remove any traces of HCl. Finally,
68Ga is eluted from the column with water into a final collection vial in the form of
68GaCl
3 solution in 0.1–0.25 M HCl. The
68Zn ions are collected on a separate vial and can be recycled to be reused as target material.
The entire purification process takes about 35 min from end of bombardment (EOB).
Conversely, for the production of copper radioisotopes (
61Cu and
64Cu) the irradiated
natZn or
64Ni liquid target solution is dissolved multiple times in water to bring the pH to a suitable range for the adsorption of the copper ions onto a highly selective Cu resin (TrisKem International, Bruz, France) loaded on 2 mL cartridge, as described by Dirks [
25]. The pH adjusted solution is then passed through the resin (pre-conditioned with 10 mL of water) that is then washed with 10 mL of HNO
3 1 mM to remove any traces of non-copper ions. The adsorbed
64Cu/
61Cu cations are eluted from the cartridge with 5 mL of HCl 3 M, directly to an anion exchange resin (SAX; TrisKem International, treated with 10 mL of water followed by 10 mL of HCl 8 M) (
Figure 2) loaded on 0.5 mL cartridge size where the anionic complex [
64CuCl
4]
−/[
61CuCl
4]
− remains strongly adsorbed. A flow of inert gas is then applied to dry the column and remove any traces of HCl. Finally, copper is eluted from the column with water into a final collection vial in the form of a copper chloride solution. In the case of
64Cu production,
64Ni ions are recovered on a separated vial and can be recovered to be recycled. As for
natZn, there is no need to recover, as natural zinc is quite inexpensive.
The entire purification process takes about 1 h from EOB.
2.3. Specific Activity and Trace Metal Analysis
Specific activities (TBq/μg) of 68Ga and 64Cu were calculated by measuring the total Ga and Cu present in the final chloride solution after purification using inductively coupled plasma mass spectrometry (ICP-MS). Other metal contaminants including Al, Co, Cu, Ga, Fe, Ni and Zn were also analysed by ICP-MS.
3. Results
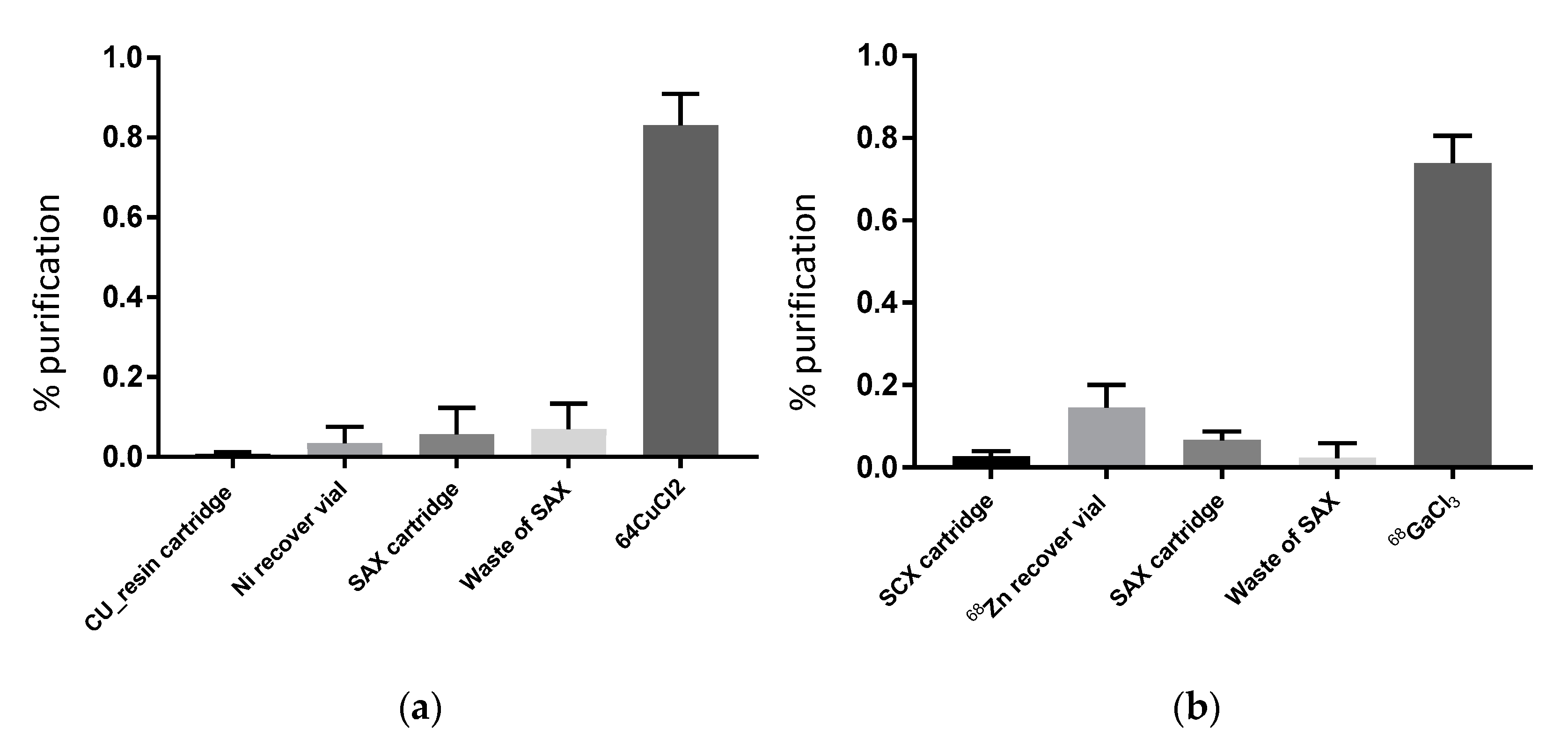
Figure 3 shows the successful separation of
68Ga,
61Cu and
64Cu from their target nuclides using the methods described. The presented procedure for processing radiometals is able to recover 81.2 ± 7.8% (
n = 10, average of 10 runs) of Copper-64 chloride solution in a small volume (4 mL) using the cartridge-based purification with a disposable kit on a commercial IBA Synthera
® extension module. Using an almost identical process, we recovered 73.9 ± 6.7% (
n = 33, average of 33 runs) of Gallium-68 chloride solution in 5–10 mL of volume using the ionic exchange principle applied on the same synthesizer module with a dedicated disposable tubing kit. The efficiency of our separation (
Figure 3) is consistent with the previously reported purification yields [
7,
9,
26].
Average production yields and respective specific molarities are summarized on
Table 2. In
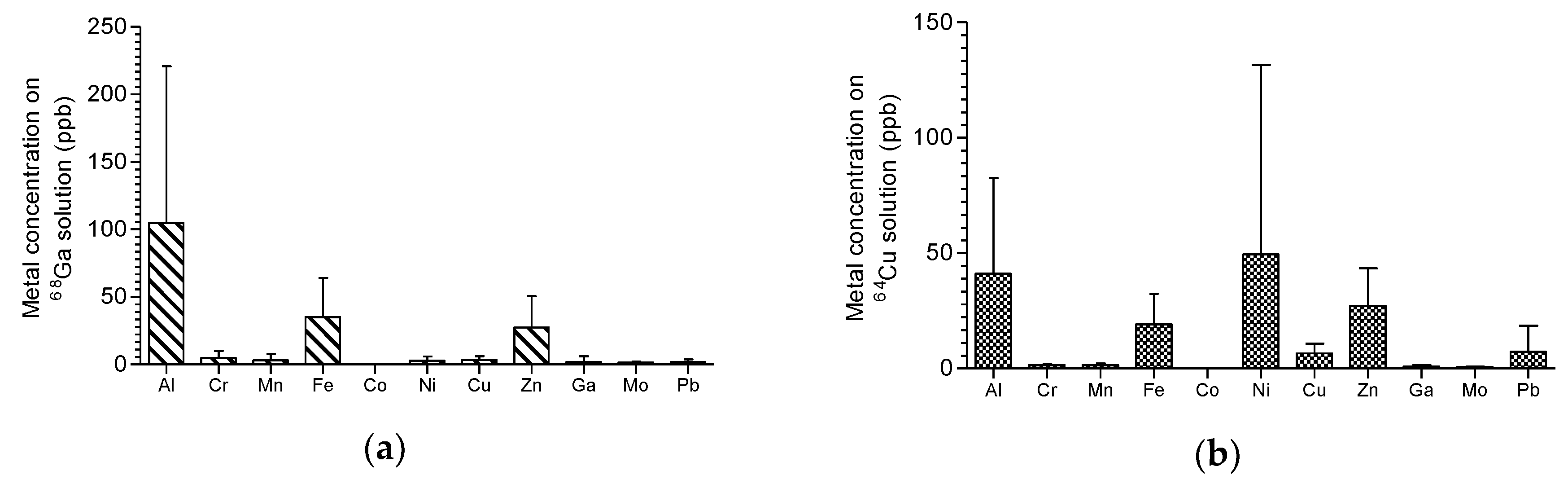
Figure 4, the results of ICP-MS analysis for determination of metal impurities in final solutions are presented.
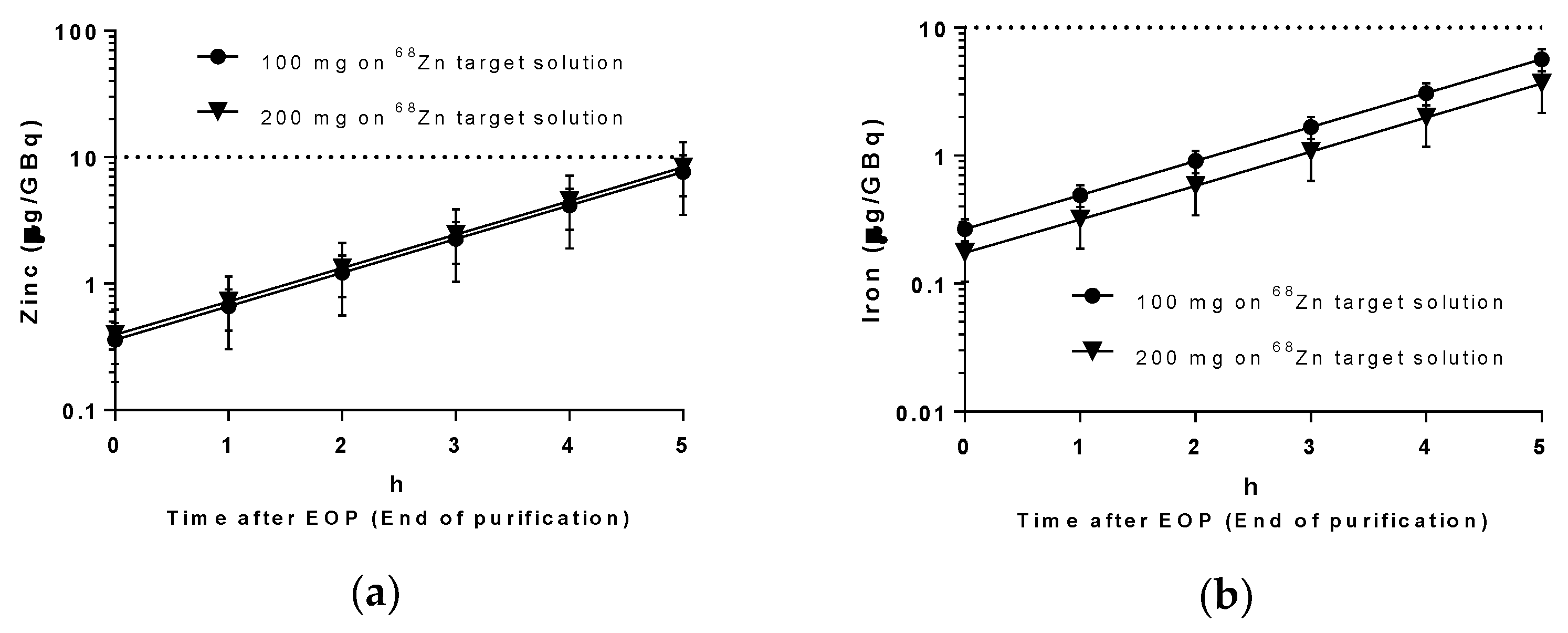
68Ga solutions produced were tested for the presence of iron and zinc using ICP-MS. Results are shown in
Figure 5 and are in accordance with the Ph. Eur. requirement of a maximum of 10 μg/GBq [
14] up to 4 h after the end of purification.
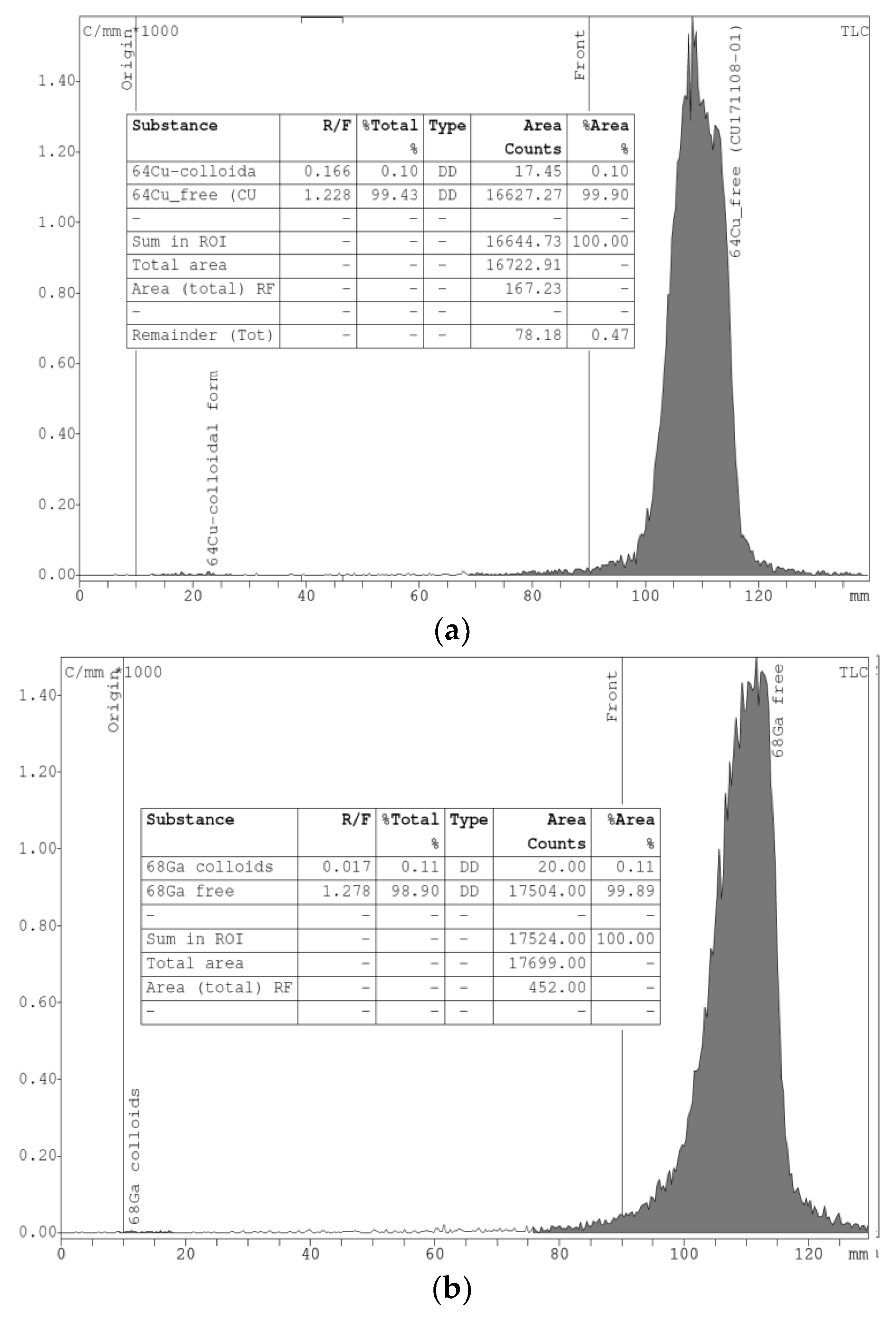
An iTLC analysis was made for all chloride solutions to confirm the presence of the ionic forms of the radiometal isotopes and the absence of colloidal complexes (
Figure 6).
4. Discussion
A complete setup for radiometal production and purification based on the irradiation of a liquid target was implemented using an IBA target and an IBA Synthera
® Extension module. Using commercially available disposable kits (Fluidomica, Coimbra, Portugal) the system is able to recover 81.2 ± 7.8% of copper-64 chloride solution for radiolabelling in less than one hour of processing time, and 73.9 ± 6.7% of gallium-68 chloride solution for radiolabelling in less than 35 min processing time. The activity of
68Ga lost to zinc-68 recover vial is explained with amount of SCX resin used on first purification step. It was decided to keep this to ensure the proper clean of zinc ions from resin without increasing the volume of washing solution and subsequent increasing of processing time. This is a significant improvement, with less processing time and considerably lower costs, when compared with a conventional solid target system [
27] or the published results from other liquid target systems [
11,
26]. The approach described here enables the production of purified radiometal solutions ready to be used for labelling radiopharmaceuticals for human use with final activities large enough for multiple doses and/or for distribution to other positron emission tomography (PET) facilities.
Specific activities were in the range of 0.3–24 TBq/µg for
68Ga and 5.0–122.8 TBq/µg for
64Cu. The presence of metal contaminants, especially iron and zinc, were very low and in compliance with the Ph. Eur. regarding the maximum amount permitted per GBq of activity in the final product vial. As
Figure 4 shows, for gallium and copper purification processes, it was found in the final chloride solutions, some traces (part per billion level) of aluminium that was explained by the glass storage container, and iron coming from all the reagents used by the “concentration” effect of process once iron has the same behavior of gallium and will be ‘carried’ together with to final purified solutions. Zinc presence on gallium-68 and copper-61 chloride solutions can be observed in small concentration at end once are the start target material. The same is said for nickel on copper-64 purification. The zinc present on copper-64 chloride solution was not explained and was assumed as possible contamination from used reagents.
Since the amount of expensive enriched material can be chosen and optimized to suit the requirements for each production, a very substantial cost reduction is achieved when compared to the solid target technique. The purified radiometal solution is ready to be used for radiolabelling in about 30 min for 68Ga and 1 h for 64Cu after EOB which is a significant improvement considering the inevitable time-consuming post-irradiation processing associated with the solid target technique.
This improvement is even more important for the case of 68Ga where the purity of the product is maintained for up to 5 h after EOB. When compared with generator obtained 68Ga, two major advantages emerged: (1) it is possible to make more consecutive runs as only 1 h 35 min is necessary to produce 68GaCl3, from the beginning of irradiation till the end of purification, compared with the generators’ 68Ga-grown waiting time and (2) no risk of contamination with long-lived impurities, as occurs with 68Ge/68Ga generators, where there is a significant risk of 68Ge breakthrough.
5. Conclusions
The described process makes feasible the production of metal radioisotopes, such as 68Ga, 64Cu and 61Cu, through the irradiation of a liquid target, using a medical cyclotron, with a considerable reduction in processing time and cost when compared with the traditional solid target approach. The process also eliminates the complex and time-consuming tasks associated with pre- and post-irradiation target preparation and simplifies the transfer of irradiated material from target to hot-cells.
Additionally, the automated process with disposable cassettes reduces radiation exposure to the operator, improves robustness of the production and provides documentation of the manufacturing process that can be used to fulfil GMP requirements.
Considering that virtually all medical cyclotrons installed worldwide are using liquid targets for routine production of PET radiopharmaceuticals, this approach provides an easier and accessible way to produce medical radioisotopes for human use in a wide range of accelerator facilities.
6. Patents
EP20150170854. Process for producing gallium-68 through the irradiation of a solution target. (Grant 2017-08-09; Publication 2017-08-09)
US15172905. Process for producing gallium-68 through the irradiation of a solution target. (Pending).
Author Contributions
Conceptualization, V.H.A.; Data curation, V.H.A.; Formal analysis, V.H.A.; Investigation, V.H.A.; Methodology, V.H.A.; Project administration, F.A. and A.J.A.; Resources, S.J.C.d.C., F.A. and A.J.A.; Supervision, F.A. and A.J.A.; Validation, F.A. and A.J.A.; Visualization, V.H.A.; Writing—original draft, V.H.A.; Writing—review & editing, F.A. and A.J.A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yordanova, A.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M.; Eppard, E.; Kürpig, S.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; et al. Theranostics in Nuclear Medicine Practice. Preprints 2017, 2017010094. [Google Scholar] [CrossRef]
- Velikyan, I. Prospective of 68Ga-radiopharmaceutical development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Continued rapid growth in 68Ga applications: Update 2013 to June 2014. J. Label. Compd. Radiopharm. 2015, 58, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Brasse, D.; Nonat, A. Radiometals: Towards a new success story in nuclear imaging? Dalt. Trans. 2015, 44, 4845–4858. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.J.C.; Alves, V.; Alves, F.; Abrunhosa, A.J. Fast and cost-effective cyclotron production of 61 Cu using a nat Zn liquid target: An opportunity for radiopharmaceutical production and R&D. Dalt. Trans. 2017, 46, 14556–14560. [Google Scholar] [CrossRef]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Hoehr, C.; Badesso, B.; Morley, T.; Trinczek, M.; Buckley, K.; Klug, J.; Zeisler, S.; Hanemaayer, V.; Ruth, T.R.; Benard, F.; et al. Producing radiometals in liquid targets: Proof of feasibility with 94mTc. AIP Conf. Proc. 2012, 1509, 56–60. [Google Scholar] [CrossRef]
- Hoehr, C.; Oehlke, E.; Hou, X.; Zeisler, S.; Adam, M.; Ruth, T.; Buckley, K.; Celler, A.; Benard, F.; Schaffer, P. Production of Radiometals in a Liquid Target. Available online: http://hzdr.qucosa.de/api/qucosa%3A22237/attachment/ATT-0/ (accessed on 13 September 2018).
- Alves, F.; Alves, V.H.; Neves, A.C.B.; do Carmo, S.J.C.; Nactergal, B.; Hellas, V.; Kral, E.; Gonçalves-Gameiro, C.; Abrunhosa, A.J.; Gonçalves-gameiro, C.; et al. Cyclotron production of Ga-68 for human use from liquid targets: From theory to practice. AIP Conf. Proc. 2017, 1845, 20001–20005. [Google Scholar] [CrossRef]
- Oehlke, E.; Hoehr, C.; Hou, X.; Hanemaayer, V.; Zeisler, S.; Adam, M.J.; Ruth, T.J.; Celler, A.; Buckley, K.; Benard, F.; et al. Production of Y-86 and other radiometals for research purposes using a solution target system. Nucl. Med. Biol. 2015, 42, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, A.; Alves, V.; Alves, F. Process for Producing Gallium-68 Through the Irradiation of a Solution Target 2016. U.S. Patent US15172905, 7 December 2016. [Google Scholar]
- Alves, V.H.; Abrunhosa, A.J.; Alves, F. Process for Producing Gallium-68 Through the Irradiation of a Solution Target 2015. U.S. Patent EP20150170854, 5 June 2015. [Google Scholar]
- European Pharmacopeia. Gallium (68Ga) Chloride Solution for Radiolabelling. Available online: https://www.edqm.eu/sites/default/files/content-list-90.pdf (accessed on 13 September 2018).
- Alves, V.H.; Prata, M.I.M.; Abrunhosa, A.J.; Castelo-Branco, M. GMP production of 68Ga-labelled DOTA-NOC on IBA Synthera. J. Radioanal. Nucl. Chem. 2015. [Google Scholar] [CrossRef]
- Ocak, M.; Antretter, M.; Knopp, R.; Kunkel, F.; Petrik, M.; Bergisadi, N.; Decristoforo, C. Full automation of 68Ga labelling of DOTA-peptides including cation exchange prepurification. Appl. Radiat. Isot. 2010, 68, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, M.; Bedeschi, P.; Scardaoni, R.; Sudati, F.; Savi, A.; Pepe, A.; Masiello, V.; Todde, S.; Gianolli, L.; Messa, C.; et al. Automated production of copper radioisotopes and preparation of high specific activity [64Cu]Cu-ATSM for PET studies. Appl. Radiat. Isot. 2010, 68, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Pandey, U.; Chakravarty, R.; Sarma, H.D.; Dash, A. Development of single vial kits for preparation of 68Ga-labelled peptides for PET imaging of neuroendocrine tumours. Mol. Imaging Biol. 2014, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Cullinane, C.; Waldeck, K.; Roselt, P.; Hicks, R.J.; Blower, P.J. Rapid kit-based (68)Ga-labelling and PET imaging with THP-Tyr(3)-octreotate: A preliminary comparison with DOTA-Tyr(3)-octreotate. EJNMMI Res. 2015, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Iori, M.; Capponi, P.C.; Rubagotti, S.; Fraternali, A.; Versari, A. Development of a simple kit-based method for preparation of pharmaceutical-grade 68Ga-DOTATOC. Nucl. Med. Commun. 2015, 36, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Strelow, F.W. Quantitative separation of gallium from zinc, copper, indium, iron(III) and other elements by cation-exchange chromatography in hydrobromic acid-acetone medium. Talanta 1980, 27, 231–236. [Google Scholar] [CrossRef]
- Van der Walt, T.N.; Strelow, F.W.E. Quantitative Separation of Gallium from Other Elements by Cation-Exchange Chromatography. Anal. Chem. 1983, 55, 212–216. [Google Scholar] [CrossRef]
- Velikyan, I. Synthesis, Characterization and Application of Ga-Labelled Peptides and Oligonucleotides; Licentiate Dissertation, Institute of Chemistry, Department of Organic Chemistry: Uppsala, Sweden, 2004. [Google Scholar]
- Meyer, G.J.; Macke, H.; Schuhmacher, J.; Knapp, W.H.; Hofmann, M. 68Ga-labelled DOTA-derivatised peptide ligands. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Dirks-Fandrei, C. Entwicklung von Methoden zur Selektiven Trennung von Scandium, Zirkonium und Zinn für Radiopharmazeutische Anwendungen; Philipps-Universität Marburg: Marburg, Germany, 2014; p. 20. [Google Scholar]
- Pandey, M.K.; Byrne, J.F.; Jiang, H.; Packard, A.B.; DeGrado, T.R. Cyclotron production of (68)Ga via the (68)Zn(p,n)(68)Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [Google Scholar] [PubMed]
- Malinconico, M.; Asp, J.; Lang, C.; Boschi, F.; Guidi, G.; Takhar, P. Radiometals Production by Only One Solid Target System. Available online: https://www.comecer.com/radiometals-production-by-only-one-solid-target-system/ (accessed on 10 July 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).