1. Introduction
Today, nanopowders are in great demand for the creation of new materials and technologies, fundamentally new devices. The process of obtaining nanopowders is therefore an important direction in nanotechnology.
The synthesis of nanocrystalline powders of metals and compounds using pyrolysis is associated with the use of complex and organometallic compounds. Numerous studies show that thermal decomposition is a complex process, depending on a variety of parameters. Therefore, the current task is to develop synthesis methods, that is, the simulation of deposition modes in which the most accurate particles can be obtained. By changing the conditions for thermal decomposition and input parameters, one can control the quality and morphology of the resulting metal nanoparticles.
The goal of this work was to analyze a model for obtaining nanopowders by thermal decomposition. The relevance of the pyrolysis process is that the rate of the formation and growth of metal nanoparticles is regulated by changes in the ratio of the number of reactants and the process temperature. Using this method, metal powders were obtained with a particle size in the range of 10–100 nm.
2. Thermal Decomposition Modeling Using COMSOL Multiphysics
The preparation of nanopowders of metals and compounds using pyrolysis is associated with the use of complex and organometallic compounds, such as polymers, hydroxides, carbonates, formates, nitrates, oxalates, and acetates. Heating precursors to a certain temperature is a consequence of their decomposition, resulting in the formation of the synthesizing substance and the release of the gas phase [
1].
Powders of highly dispersed metals are obtained by the pyrolysis of various salts. By carrying out the thermal decomposition of iron, nickel, and copper formats in vacuum or inert gas, it is possible to obtain metal powders with a particle size ranging from 100 to 300 nm [
2].
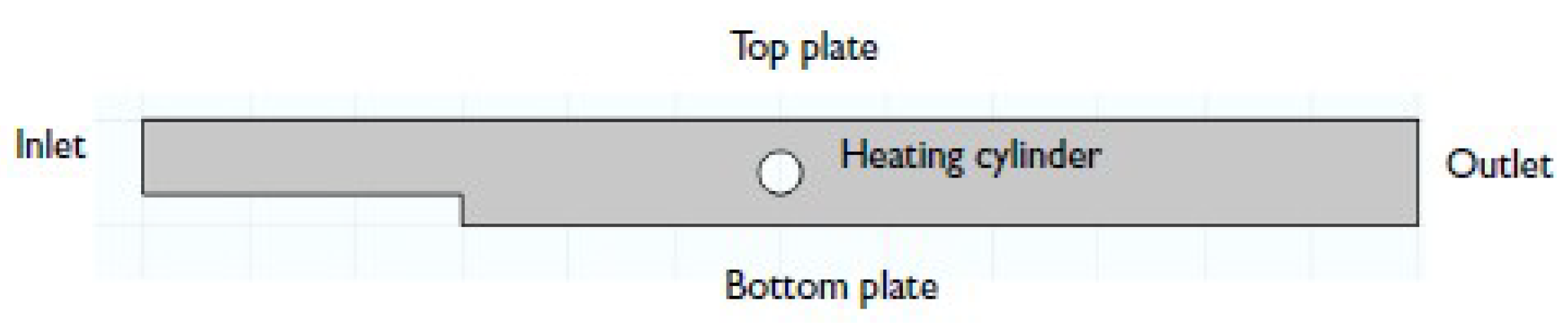
Thermal decomposition is an imperative process. Nanoparticles of various metals can be obtained by selecting and changing the parameters of the environment in which the process occurs. The model under review explored the single molecular decomposition of a chemical passing through a parallel plate reactor. Its scheme is shown in
Figure 1. After entering the reactor, the liquid first experienced expansion as it passed through the bottom plate, then through a heating cylinder [
3].
The analysis showed that, with such a geometry, the velocity of the laminar flow couldbe described by a parabolic equation
Two media were chosen to represent the pyrolysis process model: a water solution and ethanol. The following are the results of the work focusing on a method for choosing a more favorable environment for thermal decomposition.
The liquid solution enters the reactor at a temperature of 300 K and then it is heated when passing through the cylinder.
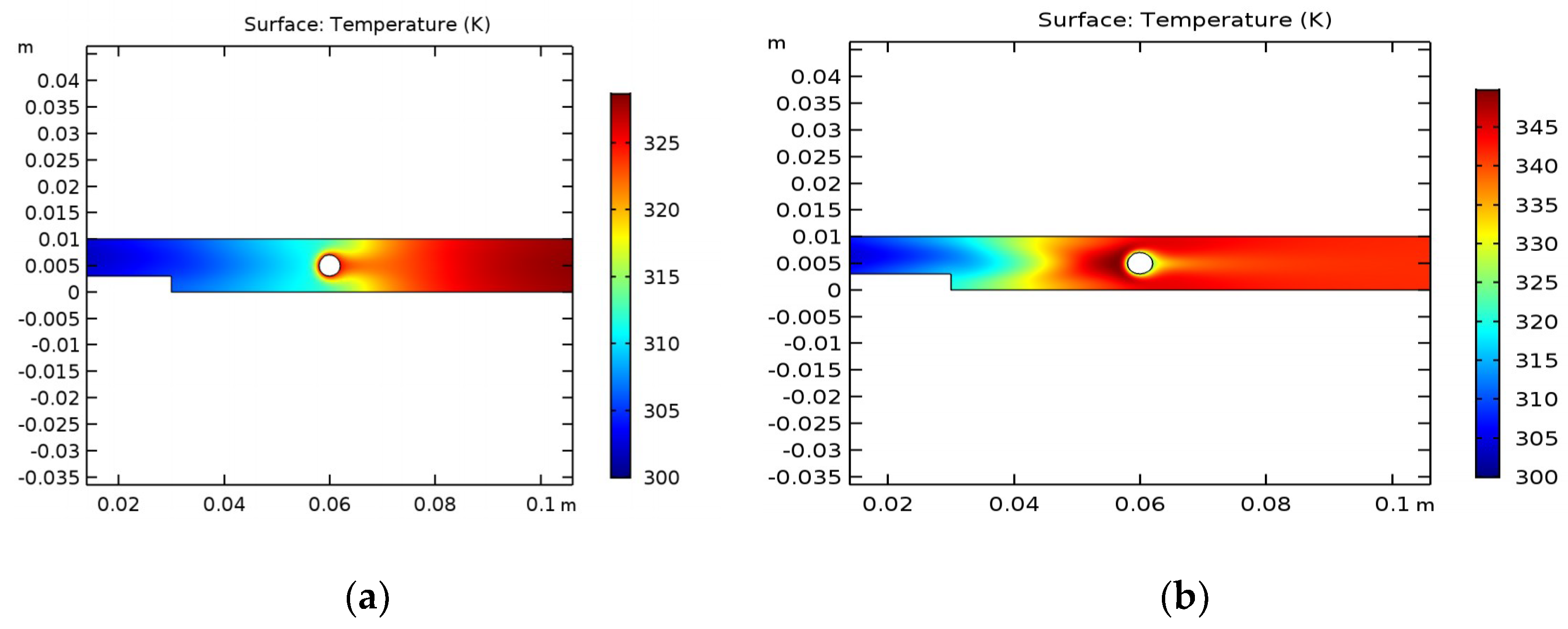
Figure 2 shows the temperature distribution in the reactor domain in the steady state.
It can be concluded that the water, passing through the cylinder, heats up and reaches high values only in the right side of the reactor. The maximum temperature is 330 K. In the case of ethanol, the high temperature covers almost the entire area of the reactor, including the cylinder, where the maximum temperature reaches 350 K.
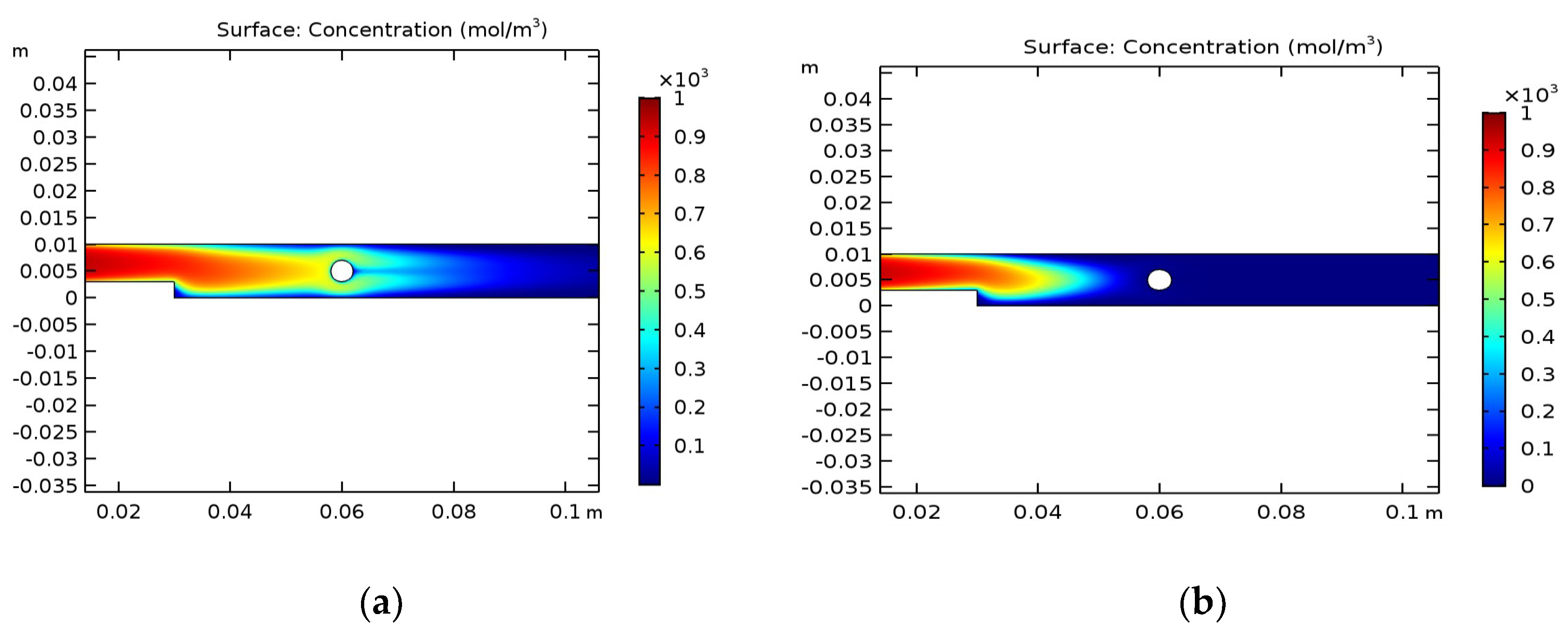
The distribution of the concentration of main carriers also depends on the choice of medium where pyrolysis will occur.
Figure 3 illustrates the concentration of a thermosensitive chemical (mol/m
3), depending on the position in the reactor.
These graphs reveal some differences. In
Figure 3a, it can be seen that decomposition occurs mainly after the liquid has been heated by the cylinder. In the first half of the reactor, where the temperature is relatively low, decomposition occurs near the walls. In the second part of the reactor, where heating occurs, areas with relatively low concentrations are visible. This has a physical meaning since the water velocity is relatively high. With regard to decomposition in ethanol,
Figure 3b proves that decomposition occurs only in the left part of reactor; in the right part, the concentration is close to zero.
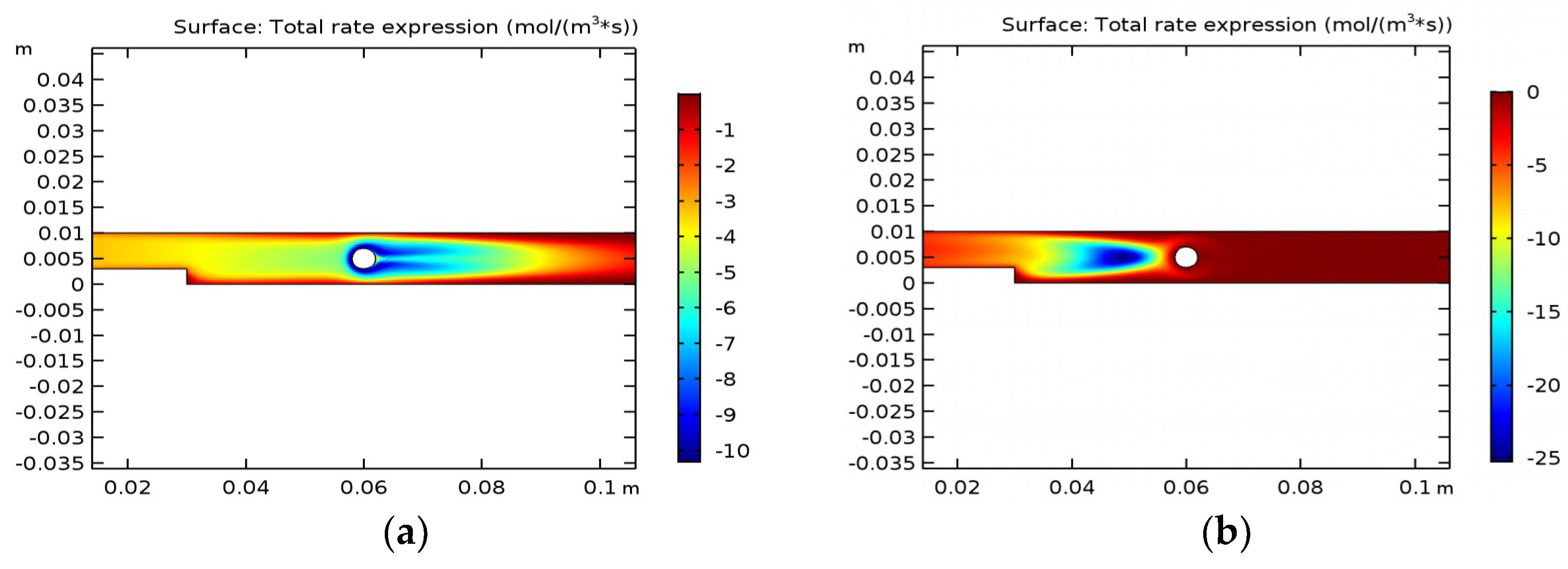
Figure 4 shows the reaction rate depending on the position in the reactor.
It can be seen from the figures that the maximum rate of thermal decomposition in water is observed only at the boundaries of the lower and upper plate of the reactor. During pyrolysis in ethanol, we observed the maximum temperature in almost all the reactor. Therefore, we can conclude that of the two environments presented above, ethanol is the most suitable one for the pyrolysis process.