The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Acclimation
2.2. Trial Design
2.3. Sampling Procedures
2.4. Experimental Analysis
2.4.1. Calculation of Condition, Weight Growth and Feed Conversion Ratio
2.4.2. RNA Extraction and cDNA Synthesis
2.4.3. Gene Expression Analysis
2.4.4. Activity of Metabolic Enzymes Measurement
2.4.5. Statistical Analysis
3. Results
3.1. Growth Performance, Survival Rate and Condition Factor
3.2. Gene Transcription Profiles in Liver
3.2.1. Metabolic Gene Expression
3.2.2. hsp70 Gene Expression
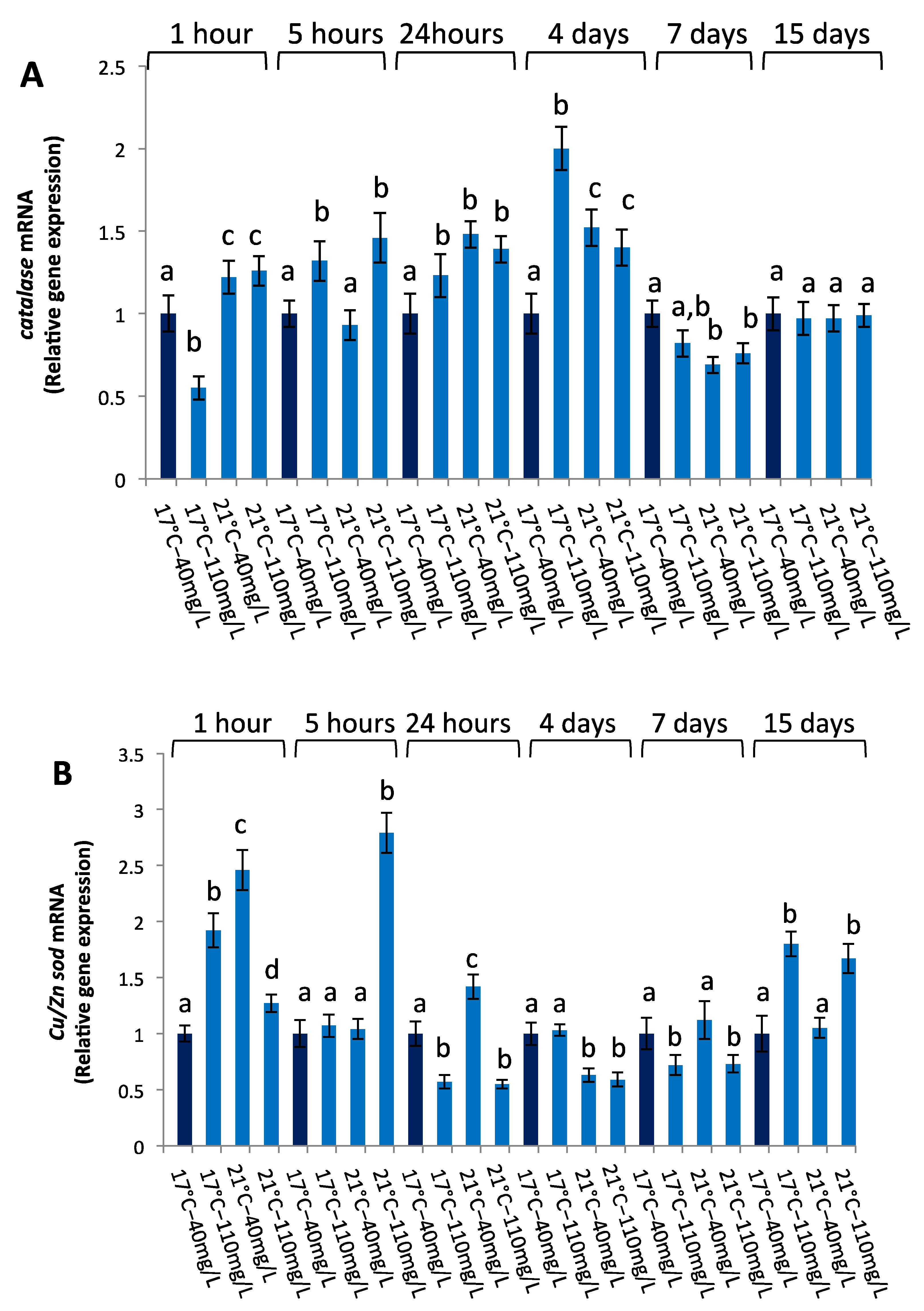
3.2.3. Antioxidant Gene Expression
3.2.4. tnf-a Gene Expression
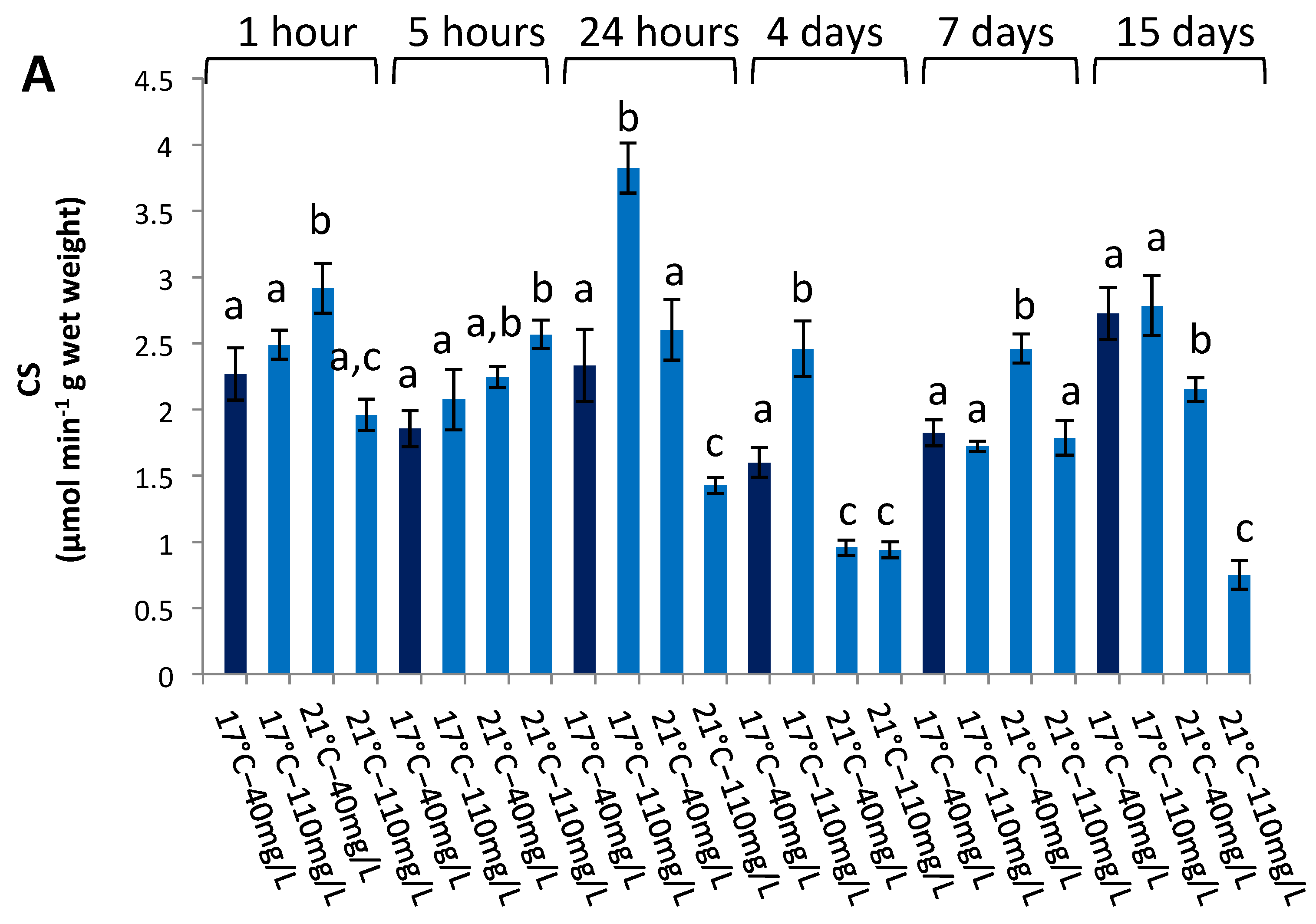
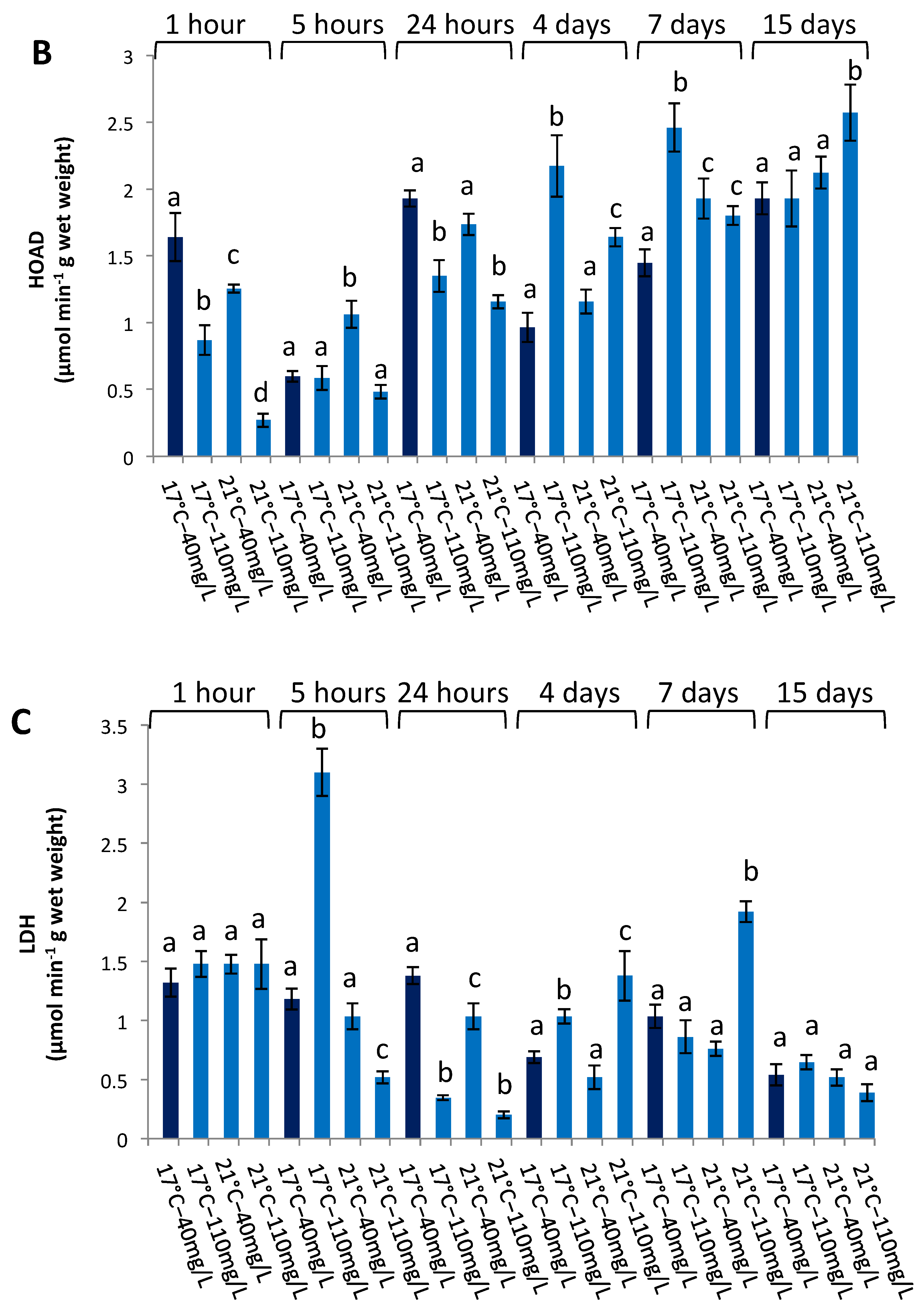
3.3. Enzymatic Activities in the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willer, D.F.; Aldridge, D.C. Microencapsulated diets to improve bivalve shellfish aquaculture for global food security. Glob. Food Secur. 2019, 23, 64–73. [Google Scholar] [CrossRef]
- Lennard, W.; Goddek, S. Aquaponics: The basics. In Aquaponics Food Production Systems, 1st ed.; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 113–143. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; HaїssamJijakli, M.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Vasdravanidis, C.; Alvanou, M.V.; Lattos, A.; Papadopoulos, D.K.; Chatzigeorgiou, I.; Ravani, M.; Ntinas, G.K.; Giantsis, I.A. Aquaponics as a promising strategy to mitigate impacts of climate change on rainbow trout culture. Animals 2022, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, L.; Archer, G.; Panades-Estruch, L.; Vrscaj, D. Ten Technologies Which Could Change Our Lives: Potential Impacts and Policy Implications. European Parliamentary Research Service Scientific Foresight Unit. 2019. Available online: http://www.europarl.europa.eu/EPRS/EPRS_IDAN_527417_ten_trends_to_change_your_life.pdf (accessed on 21 July 2022).
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Kiuru, T.; Aalto, S.L.; Koskela, J.; Vielma, J. Startup and effects of relative water renewal rate on water quality and growth of rainbow trout (Oncorhynchus mykiss) in a unique RAS research platform. Aquac. Eng. 2018, 82, 38–45. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Nagel, F.; Meyer, S.; Schroeder, J.P.; Schulz, C. Comparative life cycle assessment (LCA) of raising rainbow trout (Oncorhynchus mykiss) in different production systems. Aquac. Eng. 2013, 54, 85–92. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; Available online: https://www.fao.org/3/i4021e/i4021e.pdf (accessed on 21 July 2022).
- Birolo, M.; Bordignon, F.; Trocino, A.; Fasolato, L.; Pascual, A.; Godoy, S.; Nicoletto, C.; Maucieri, C.; Xiccato, G. Effects of stocking density on the growth and flesh quality of rainbow trout (Oncorhynchus mykiss) reared in a low-tech aquaponic system. Aquaculture 2020, 529, 735653. [Google Scholar] [CrossRef]
- Deviller, G.; Palluel, O.; Aliaume, C.; Asanthi, H.; Sanchez, W.; Franco Nava, M.A.; Blancheton, J.P.; Casellas, C. Impact assessment of various rearing systems on fish health using multibiomarker response and metal accumulation. Ecotoxicol. Environ. Saf. 2015, 61, 89–97. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. Comparing the effects of high vs. low nitrate on the health, performance, and welfare of juvenile rainbow trout Oncorhynchus mykiss within water recirculating aquaculture systems. Aquac. Eng. 2014, 59, 30–40. [Google Scholar] [CrossRef]
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled systems on trial: Eliminating bottlenecks to improve aquaponic processes. PLoS ONE 2017, 12, e0183056. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Tsai, S.J.; Chen, J.C. Accumulation of nitrate in the tissues of Penaeus monodon following elevated ambient nitrate exposure after different time periods. Aquat. Toxicol. 2002, 56, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Simultaneous exposure to nitrate and low pH reduces the blood oxygen-carrying capacity and functional performance of a freshwater fish. Conserv. Physiol. 2020, 8, coz092. [Google Scholar] [CrossRef]
- Schram, E.; Roques, J.A.; van Kuijk, T.; Abbink, W.; van de Heul, J.; van de Vries, P.; Bierman, S.; van de Vis, H.; Flik, G. The impact of elevated water ammonia and nitrate concentrations on physiology, growth and feed intake of pikeperch (Sander lucioperca). Aquaculture 2014, 420, 95–104. [Google Scholar] [CrossRef]
- van Bussel, C.G.; Schroeder, J.P.; Wuertz, S.; Schulz, C. The chronic effect of nitrate on production performance and health status of juvenile turbot (Psetta maxima). Aquaculture 2012, 326, 163–167. [Google Scholar] [CrossRef]
- Luo, S.; Wu, B.; Xiong, X.; Wang, J. Short-term toxicity of ammonia, nitrite, and nitrate to early life stages of the rare minnow (Gobiocyprisrarus). Environ. Toxicol. Chem. 2016, 35, 1422–1427. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture, 3rd ed.; Ithaca Publishing Company LLC: New York, NY, USA, 2013. [Google Scholar]
- Jobling, M. Bioenergetics: Feed Intake and Energy Partitioning, in Fish Ecophysiology; Rankin, J.C., Jense, F.B., Eds.; Chapman & Hall: London, UK, 1993. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, S.; Liu, C.; Zhou, Y. Temperature tolerance of juvenile rainbow trout and steelhead trout (Oncorhynchus mykiss). J. Ocean. Univ. China 2019, 49, 57–62. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Pal, A.K.; Sahu, N.P.; Ciji, A.; Mahanta, P.C. Thermal tolerance, oxygen consumption and haemato-biochemical variables of Tor putitora juveniles acclimated to five temperatures. Fish Physiol. Biochem. 2013, 39, 1387–1398. [Google Scholar] [CrossRef]
- Kaufman, R.C.; Coalter, R.; Nordman, N.L.; Cocherell, D.; Cech, J.J.; Thompson, L.C.; Fangue, N.A. Effects of temperature on hardhead minnow (Mylopharodonconocephalus) blood-oxygen equilibria. Environ. Biol. Fishes 2013, 96, 1389–1397. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Bock, C.; Mark, F.C. Oxygen-and capacity-limited thermal tolerance: Bridging ecology and physiology. J. Exp. Biol. 2017, 220, 2685–2696. [Google Scholar] [CrossRef]
- Wang, T.; Lefevre, S.; Iversen, N.K.; Findorf, I.; Buchanan, R.; McKenzie, D.J. Anaemia only causes a small reduction in the upper critical temperature of sea bass: Is oxygen delivery the limiting factor for tolerance of acute warming in fishes? J. Exp. Biol. 2014, 217, 4275–4278. [Google Scholar] [CrossRef]
- Muñoz, N.J.; Farrell, A.P.; Heath, J.W.; Neff, B.D. Hematocrit is associated with thermal tolerance and modulated by developmental temperature in juvenile Chinook salmon. Physiol. Biochem. Zool. 2018, 91, 757–762. [Google Scholar] [CrossRef]
- Rodgers, E.M.; Opinion, A.G.R.; Gomez Isaza, D.F.; Rašković, B.; Poleksić, V.; De Boeck, G. Double whammy: Nitrate pollution heightens susceptibility to both hypoxia and heat in a freshwater salmonid. Sci. Total Environ. 2021, 765, 142777. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Little, A.G.; Seebacher, F. Temperature determines toxicity: Bisphenol A reduces thermal tolerance in fish. Environ. Pollut. 2015, 197, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.W.; Chapman, J.C.; Lim, R.P.; Gehrke, P.C.; Sunderam, R.M. Interactions between water temperature and contaminant toxicity to freshwater fish. Environ. Toxicol. Chem. 2015, 34, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Egea-Serrano, A.; Van Buskirk, J. Responses to nitrate pollution, warming and density in common frog tadpoles (Rana temporaria). Amphib. Reptil. 2016, 37, 45–54. [Google Scholar] [CrossRef]
- Opinion, A.G.R.; De Boeck, G.; Rodgers, E.M. Synergism between elevated temperature and nitrate: Impact on aerobic capacity of European grayling, Thymallusthymallus in warm, eutrophic waters. Aquat. Toxicol. 2020, 226, 105563. [Google Scholar] [CrossRef] [PubMed]
- Opinion, A.G.R.; Çakir, R.; De Boeck, G. Better together: Cross-tolerance induced by warm acclimation and nitrate exposure improved the aerobic capacity and stress tolerance of common carp Cyprinus carpio. Ecotoxicol. Environ. Saf. 2021, 225, 112777. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalination Water Treat. 2011, 32, 422–430. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef]
- Westin, D.T. Nitrate and nitrite toxicity to salmonoid fishes. Progress. Fish Cult. 1974, 36, 86–89. [Google Scholar] [CrossRef]
- McGurk, M.D.; Landry, F.; Tang, A.; Hanks, C.C. Acute and chronic toxicity of nitrate to early life stages of lake trout (Salvelinus namaycush) and lake whitefish (Coregonus clupeaformis). Environ. Toxicol. Chem. 2006, 25, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Colt, J.; Tchobanoglous, G. Chronic exposure of channel catfish, Ictalurus punctatus, to ammonia: Effects on growth and survival. Aquaculture 1978, 15, 353–372. [Google Scholar] [CrossRef]
- Bolger, T.; Connolly, P.L. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 1989, 34, 171–182. [Google Scholar] [CrossRef]
- Horiba Scientific. Laqua. Waterproof Pocket Water Quality Meters; Brochure PBT-02-2017A. 2017. Available online: https://static.horiba.com/fileadmin/Horiba/Water_Quality/04_Support/Brochures/Pocket_Meters/Brochure_-_PBT-02-2017A_LAQUAtwin_Pocket_Water_Quality_Meter_-_LOWRES.pdf (accessed on 25 October 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Teimouri, M.; Yeganeh, S.; Mianji, G.R.; Najafi, M.; Mahjoub, S. The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45, 977–986. [Google Scholar] [CrossRef]
- Blair, S.D.; Glover, C.N. Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia 2019, 836, 155–167. [Google Scholar] [CrossRef]
- Moltesen, M.; Laursen, D.C.; Thörnqvist, P.O.; Andersson, M.Å.; Winberg, S.; Höglund, E. Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2016, 219, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Thibault, M.; Blier, P.U.; Guderley, H. Seasonal variation of muscle metabolic organization in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 1997, 16, 139–155. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.-D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish. Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Ineno, T.; Tamaki, K.; Yamada, K.; Kodama, R.; Tan, E.; Kinoshita, S.; Muto, K.; Yada, T.; Kitamura, S.; Asakawa, S.; et al. Evaluation of the thermal tolerances of different strains of rainbow trout Oncorhynchus mykiss by measuring the effective time required for loss of equilibrium at an approximate upper lethal temperature. Fish. Sci. 2019, 85, 839–845. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, S.; Liu, R.; Huang, M.; Dong, K.; Ge, J.; Gao, Q.; Zhou, Y. Effects of temperature, dissolved oxygen, and their interaction on the growth performance and condition of rainbow trout (Oncorhynchus mykiss). J. Therm. Biol. 2021, 98, 102928. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. The effects of ozone and water exchange rates on water quality and rainbow trout Oncorhynchus mykiss performance in replicated water recirculating systems. Aquac. Eng. 2011, 44, 80–96. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S.T. Abnormal swimming behavior and increased deformities in rainbow trout Oncorhynchus mykiss cultured in low exchange water recirculating aquaculture systems. Aquac. Eng. 2011, 45, 109–117. [Google Scholar] [CrossRef]
- Pedersen, L.F.; Suhr, K.I.; Dalsgaard, J.; Pedersen, P.B.; Arvin, E. Effects of feed loading on nitrogen balances and fish performance in replicated recirculating aquaculture systems. Aquaculture 2012, 338, 237–245. [Google Scholar] [CrossRef]
- Gagnon, M.M.; Holdway, D.A. Metabolic enzyme activities in fish gills as biomarkers of exposure to petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 1999, 44, 92–99. [Google Scholar] [CrossRef]
- Somero, G.N.; Childress, J.J. A violation of the metabolism-size scaling paradigm: Activities of glycolytic enzymes in muscle increase in larger-size fish. Physiol. Zool. 1980, 53, 322–337. [Google Scholar] [CrossRef]
- Guerreiro, I.; Magalhães, R.; Coutinho, F.; Couto, A.; Sousa, S.; Delerue-Matos, C.; Domingues, V.F.; Oliva-Teles, A.; Peres, H. Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J. Appl. Phycol. 2019, 31, 2115–2124. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Stanley, C.; Merkt, J.; Sumar-Kalinowski, J. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: An interpretive hypothesis. Respir. Physiol. 1983, 52, 303–313. [Google Scholar] [CrossRef]
- Dewes, L.J.; Sandrini, J.Z.; Monserrat, J.M.; Yunes, J.S. Biochemical and physiological responses after exposure to microcystins in the crab Chasmagnathusgranulatus (Decapoda, Brachyura). Ecotoxicol. Environ. Saf. 2006, 65, 201–208. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Sundh, H.; Gräns, A.; Hinchcliffe, J.; Ekström, A.; Sundell, K.; Olsson, C.; Axelsson, M.; Pichaud, N. Increased mitochondrial coupling and anaerobic capacity minimizes aerobic costs of trout in the sea. Sci. Rep. 2017, 7, 45778. [Google Scholar] [CrossRef]
- McClelland, G.B.; Craig, P.M.; Dhekney, K.; Dipardo, S. Temperature-and exercise-induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J. Physiol. 2006, 577, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Resende, A.C.; Pereira, D.M.C.; Schleger, I.C.; de Souza, M.R.D.P.; Neundorf, A.K.A.; Romão, S.; Herrerias, T.; Donatti, L. Effects of heat shock on energy metabolism and antioxidant defence in a tropical fish species Psalidodon bifasciatus. J. Fish Biol. 2002, 100, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Pichaud, N.; Ekström, A.; Breton, S.; Sundström, F.; Rowinski, P.; Blier, P.U.; Sandblom, E. Cardiac mitochondrial plasticity and thermal sensitivity in a fish inhabiting an artificially heated ecosystem. Sci. Rep. 2019, 9, 17832. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Machado, J.; Pousão-Ferreira, P.; Rosa, R. Seabream larval physiology under ocean warming and acidification. Fishes 2019, 5, 1. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Negative impacts of elevated nitrate on physiological performance are not exacerbated by low pH. Aquat. Toxicol. 2018, 200, 217–225. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Thermal acclimation offsets the negative effects of nitrate on aerobic scope and performance. J. Exp. Biol. 2020, 223, jeb224444. [Google Scholar] [CrossRef]
- Panserat, S.; Plagnes-Juan, E.; Kaushik, S. Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2001, 204, 2351–2360. [Google Scholar] [CrossRef]
- Soengas, J.L.; Polakof, S.; Chen, X.; Sangiao-Alvarellos, S.; Moon, T.W. Glucokinase and hexokinase expression and activities in rainbow trout tissues: Changes with food deprivation and refeeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R810–R821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panserat, S.; Capilla, E.; Gutierrez, J.; Frappart, P.O.; Vachot, C.; Plagnes-Juan, E.; Aguirre, P.; Brèque, J.; Kaushik, S. Glucokinase is highly induced and glucose-6-phosphatase poorly repressed in liver of rainbow trout (Oncorhynchus mykiss) by a single meal with glucose. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 128, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Plagnes-Juan, E.; Lansard, M.; Seiliez, I.; Médale, F.; Corraze, G.; Kaushik, S.; Panserat, S.; Skiba-Cassy, S. Insulin regulates the expression of several metabolism-related genes in the liver and primary hepatocytes of rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2008, 211, 2510–2518. [Google Scholar] [CrossRef]
- Caseras, A.; Metón, I.; Fernández, F.; Baanante, I.V. Glucokinase gene expression is nutritionally regulated in liver of gilthead sea bream (Sparus aurata). Biochim. Biophys. Acta 2000, 1493, 135–141. [Google Scholar] [CrossRef]
- Martinez-Cayuela, M. Oxygen free radicals and human disease. Biochimie 1995, 77, 147–161. [Google Scholar] [CrossRef]
- Ekström, A.; Brijs, J.; Clark, T.D.; Gräns, A.; Jutfelt, F.; Sandblom, E. Cardiac oxygen limitation during an acute thermal challenge in the European perch: Effects of chronic environmental warming and experimental hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R440–R449. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Thermal plasticity of the cardiorespiratory system provides cross-tolerance protection to fish exposed to elevated nitrate. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108920. [Google Scholar] [CrossRef]
- Klaiman, J.M.; Fenna, A.J.; Shiels, H.A.; Macri, J.; Gillis, T.E. Cardiac remodeling in fish: Strategies to maintain heart function during temperature change. PLoS ONE 2011, 6, e24464. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Dhillon, R.S.; Boulding, E.G.; Farrell, A.P.; Glebe, B.D.; Elliott, J.A.K.; Wolters, W.R.; Schulte, P.M. Variation in temperature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. J. Exp. Biol. 2013, 216, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Xiao, Y.; Li, X.; Xu, X.; Zhao, H.; Wu, L.; Li, J. Effects of chronic nitrate exposure on the intestinal morphology, immune status, barrier function, and microbiota of juvenile turbot (Scophthalmus maximus). Ecotoxicol. Environ. Saf. 2021, 207, 111287. [Google Scholar] [CrossRef]
- Adams, O.A.; Zhang, Y.; Gilbert, M.H.; Lawrence, C.S.; Snow, M.; Farrell, A.P. An unusually high upper thermal acclimation potential for rainbow trout. Conserv. Physiol. 2022, 10, coab101. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, H.; Zhang, X.; Huang, W.; Zhang, C.; Wang, C.; Gao, Q.; Dong, S. Temperature acclimation improves high temperature tolerance of rainbow trout (Oncorhynchus mykiss) by improving mitochondrial quality and inhibiting apoptosis in liver. Sci. Total Environ. 2023, 912, 169452. [Google Scholar] [CrossRef] [PubMed]




| Target Gene | Forward Primer (5′–3′) Reverse Primer (5′–3′) | Amplicon (bp) | Primer Efficiency | GenBank Accession No. | Reference |
|---|---|---|---|---|---|
| catalase | GAGGGCAACTGGGACCTTACT GGACGAAGGACGGGAACAG | 79 | 1.95 | BE669040.1 | [46] |
| Cu/Zn sod | AGGACCCACTGATGCTGTT GTTGCCTCCTTTTCCCAGA | 178 | 1.94 | AF469663.1 | This study |
| hsp70 | AGGACATCAGCCAGAACAAG TGGTGATGGAGGTGTAGAAG | 144 | 1.95 | AB062281.1 | This study |
| glucokinase | CTACTGAACTGGACCAAAGG CCATGTAGCAAGCGTTACAC | 217 | 1.98 | AF053331.2 | This study |
| citrate synthase | GATAACTTCCCTACCAACCT CGGTAGATCTTAGCAGCAAC | 188 | 1.92 | XM_021616545.2 | This study |
| tnf-a | CTACAAGGGAACCAAATCCT GCCAAATAACGTGACTCAGA | 124 | 1.94 | AJ401377.1 | This study |
| EF-1α | CTGTTGCCTTTGTGCCCATC CATCCCTTGAACCAGCCCAT | 82 | 1.97 | AF498320.1 | [47] |
| b-actin | AGAGCTACGAGCTGCCTGAC GTGTTGGCGTACAGGTCCTT | 179 | 1.98 | NM_001124235.1 | [48] |
| Treatment | Final Weight (g) | Condition Factor | SGRw (%) | FCR | Survival (%) |
|---|---|---|---|---|---|
| 17 °C and 40 mg/L (17L) | 14.37 ± 0.41 a | 1.28 ± 0.13 a | 1.91 ± 0.08 a | 0.87 ± 0.05 a | 100 |
| 17 °C and 110 mg/L (17H) | 14.23 ± 0.31 a | 1.17 ± 0.14 b | 1.89 ± 0.08 a | 0.88 ± 0.06 a | 100 |
| 21 °C and 40 mg/L (21L) | 12.84 ± 0.29 b | 1.11 ± 0.12 b | 1.36 ± 0.16 b | 1.25 ± 0.08 b | 100 |
| 21 °C and 110 mg/L (21H) | 12.89 ± 0.32 b | 1.13 ± 0.12 b | 1.38 ± 0.19 b | 1.25 ± 0.12 b | 100 |
| Condition Factor | FCR | SGRw | |
|---|---|---|---|
| temperature | 0.00002 | 0.00001 | 0.00003 |
| NO3− | 0.019 | 0.9402 | 0.9612 |
| temperature × NO3− | 0.0008 | 0.8333 | 0.577 |
| citrate synthase | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.0002 | 0.0018 | 0.0002 | 0.0002 | 0.0002 | 0.0016 |
| NO3− | 0.0013 | 0.0021 | 0.0019 | 0.4134 | 0.0079 | 0.003 |
| temperature × NO3− | 0.0003 | 0.0054 | 0.0003 | 0.0001 | 0.0196 | 0.0092 |
| glucokinase | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.4182 | 0.00006 | 0.00005 | 0.00008 | 0.00005 | 0.00007 |
| NO3− | 0.4088 | 0.0025 | 0.0001 | 0.00005 | 0.4814 | 0.00007 |
| temperature × NO3− | 0.4221 | 0.7216 | 0.0024 | 0.00005 | 0.0131 | 0.00002 |
| hsp70 | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.00005 | 0.00005 | 0.00005 | 0.4198 | 0.00004 | 0.00004 |
| NO3− | 0.202 | 0.0207 | 0.00004 | 0.00005 | 0.00006 | 0.0797 |
| temperature × NO3− | 0.1247 | 0.5985 | 0.00005 | 0.00007 | 0.00004 | 0.5677 |
| catalase | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.0001 | 0.4211 | 0.0002 | 0.206 | 0.0002 | 0.8399 |
| NO3− | 0.0022 | 0.0002 | 0.0005 | 0.0003 | 0.0288 | 0.8350 |
| temperature × NO3− | 0.0006 | 0.3521 | 0.0003 | 0.0001 | 0.0004 | 0.2455 |
| Cu/Zn sod | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.00005 | 0.00004 | 0.00009 | 0.00004 | 0.2474 | 0.1383 |
| NO3− | 0.0013 | 0.00003 | 0.00005 | 0.7794 | 0.00005 | 0.00006 |
| temperature × NO3− | 0.00004 | 0.00003 | 0.00003 | 0.0992 | 0.0124 | 0.0191 |
| tnf-a | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.00005 | 0.0164 | 0.0202 | 0.0663 | 0.00005 | 0.00004 |
| NO3− | 0.00005 | 0.00005 | 0.1013 | 0.00008 | 0.00005 | 0.00006 |
| temperature × NO3− | 0.0002 | 0.00007 | 0.0199 | 0.0001 | 0.00003 | 0.00005 |
| Citrate synthase | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.5176 | 0.0006 | 0.0001 | 0.0001 | 0.0015 | 0.0001 |
| NO3− | 0.0014 | 0.0069 | 0.0958 | 0.0001 | 0.0009 | 0.0001 |
| temperature × NO3− | 0.0001 | 0.534 | 0.0001 | 0.0001 | 0.0042 | 0.0001 |
| HOAD | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.0005 | 0.0057 | 0.05501 | 0.0429 | 0.2104 | 0.0013 |
| NO3− | 0.0002 | 0.0003 | 0.0003 | 0.0003 | 0.0004 | 0.0286 |
| temperature × NO3− | 0.2092 | 0.0004 | 0.0002 | 0.0011 | 0.0003 | 0.0284 |
| LDH | ||||||
| 1 h | 5 h | 24 h | 4 d | 7 d | 15 d | |
| Temperature | 0.395 | 0.0004 | 0.0013 | 0.203 | 0.0022 | 0.0415 |
| NO3− | 0.3485 | 0.0003 | 0.0002 | 0.0003 | 0.0005 | 0.8459 |
| temperature × NO3− | 0.3703 | 0.0001 | 0.0714 | 0.0034 | 0.0004 | 0.0712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, D.K.; Lattos, A.; Chatzigeorgiou, I.; Tsaballa, A.; Ntinas, G.K.; Giantsis, I.A. The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup. Fishes 2024, 9, 74. https://doi.org/10.3390/fishes9020074
Papadopoulos DK, Lattos A, Chatzigeorgiou I, Tsaballa A, Ntinas GK, Giantsis IA. The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup. Fishes. 2024; 9(2):74. https://doi.org/10.3390/fishes9020074
Chicago/Turabian StylePapadopoulos, Dimitrios K., Athanasios Lattos, Ioanna Chatzigeorgiou, Aphrodite Tsaballa, Georgios K. Ntinas, and Ioannis A. Giantsis. 2024. "The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup" Fishes 9, no. 2: 74. https://doi.org/10.3390/fishes9020074
APA StylePapadopoulos, D. K., Lattos, A., Chatzigeorgiou, I., Tsaballa, A., Ntinas, G. K., & Giantsis, I. A. (2024). The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup. Fishes, 9(2), 74. https://doi.org/10.3390/fishes9020074











