Selenium Protects Yellow Catfish (Tachysurus fulvidraco) from Low-Temperature Damage via the Perspective Analysis of Metabolomics and Intestinal Microbes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish and Feeding Management
2.3. Sample Collection and Preparation
2.4. Growth Analysis
2.5. Antioxidant Ability Analysis
2.6. Metabolomics Analysis
2.7. Analysis of Intestinal Microbe Composition
2.8. Correlation Analysis of the Metabolomics and the Intestinal Microbes’ Composition
2.9. Data and Statistical Analysis
3. Results
3.1. Effect of Selenium on the Growth Performance and Antioxidant Ability under Low-Temperature Stress of T. fulvidraco
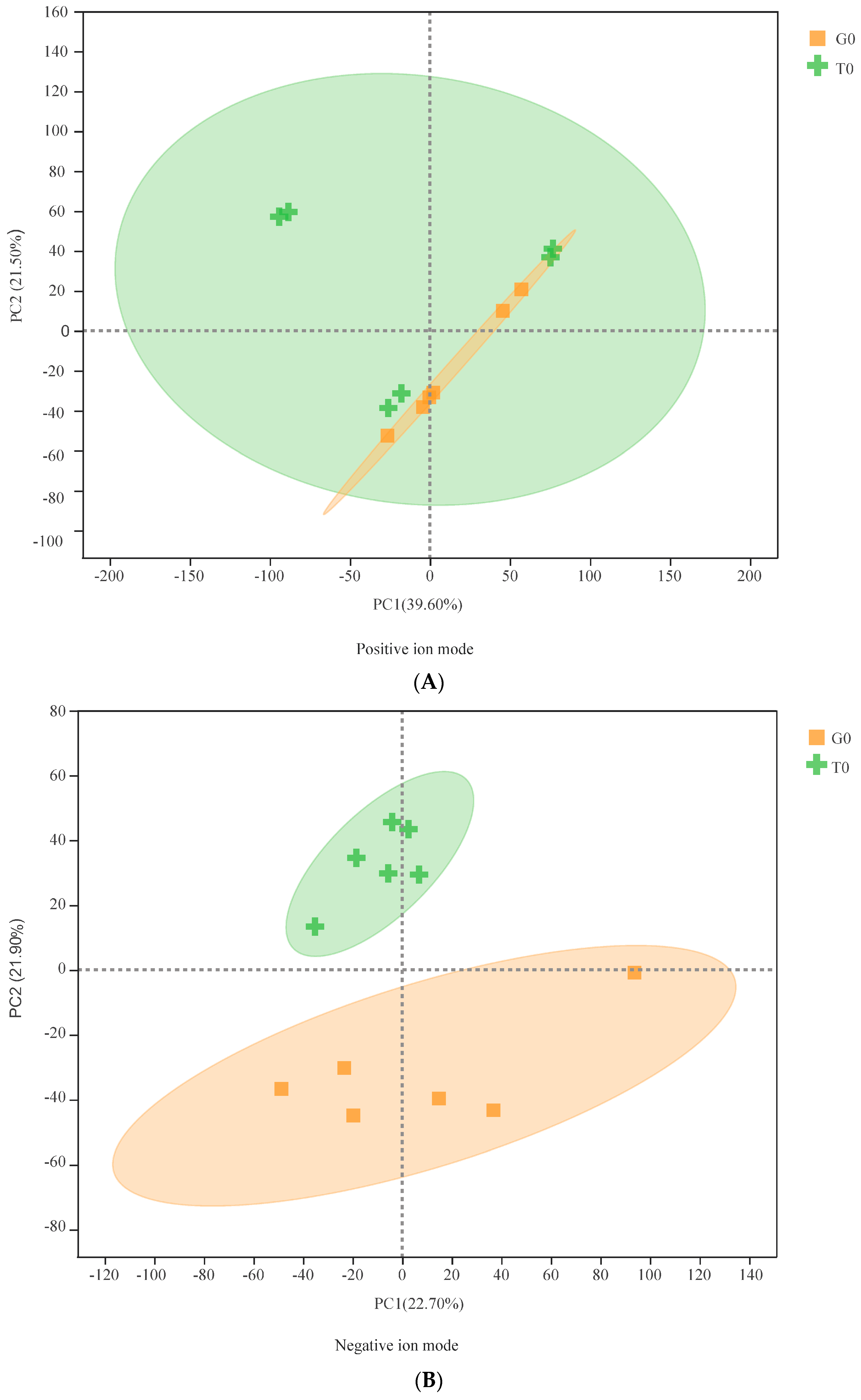
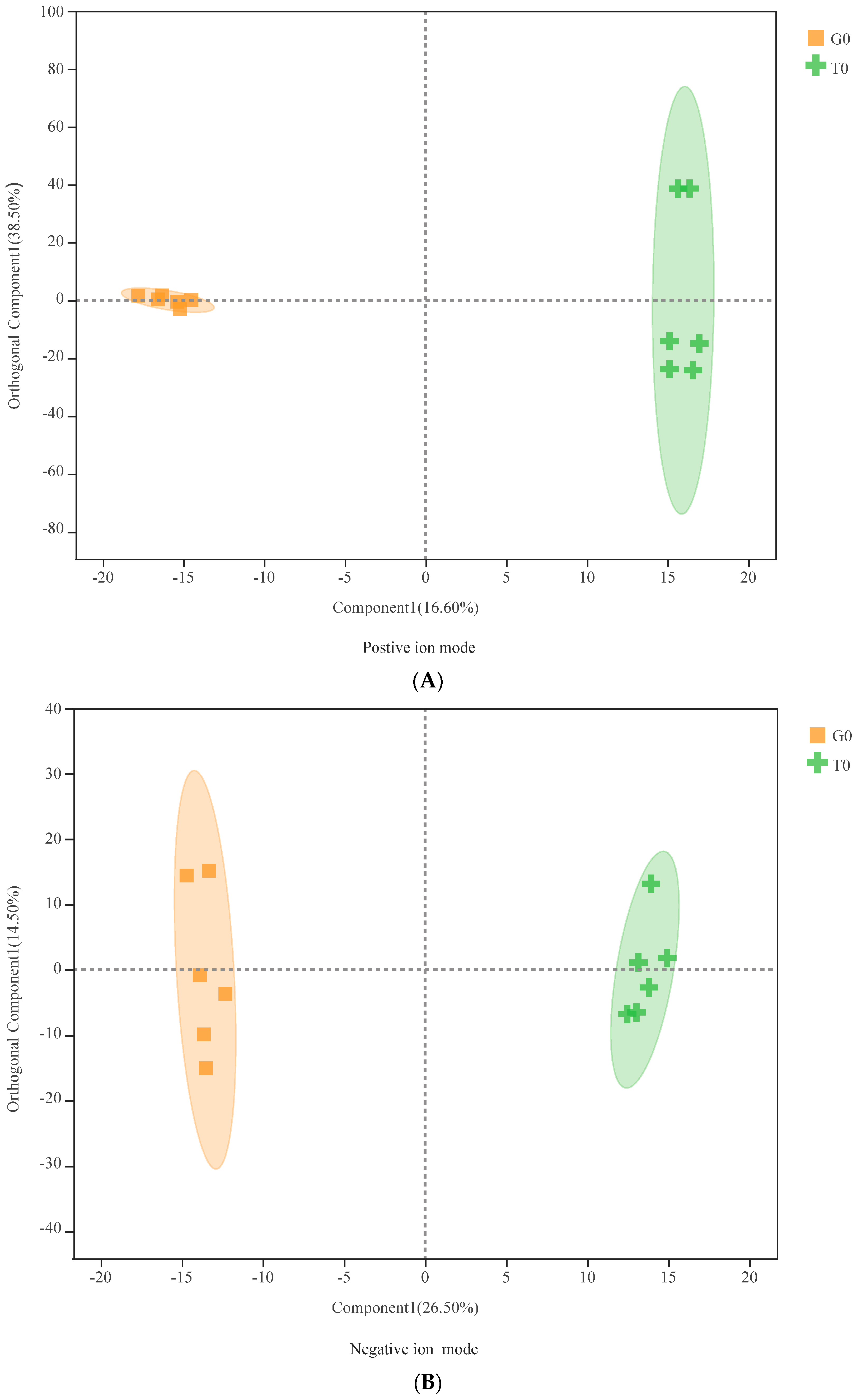
3.2. Multivariate Statistical Analysis
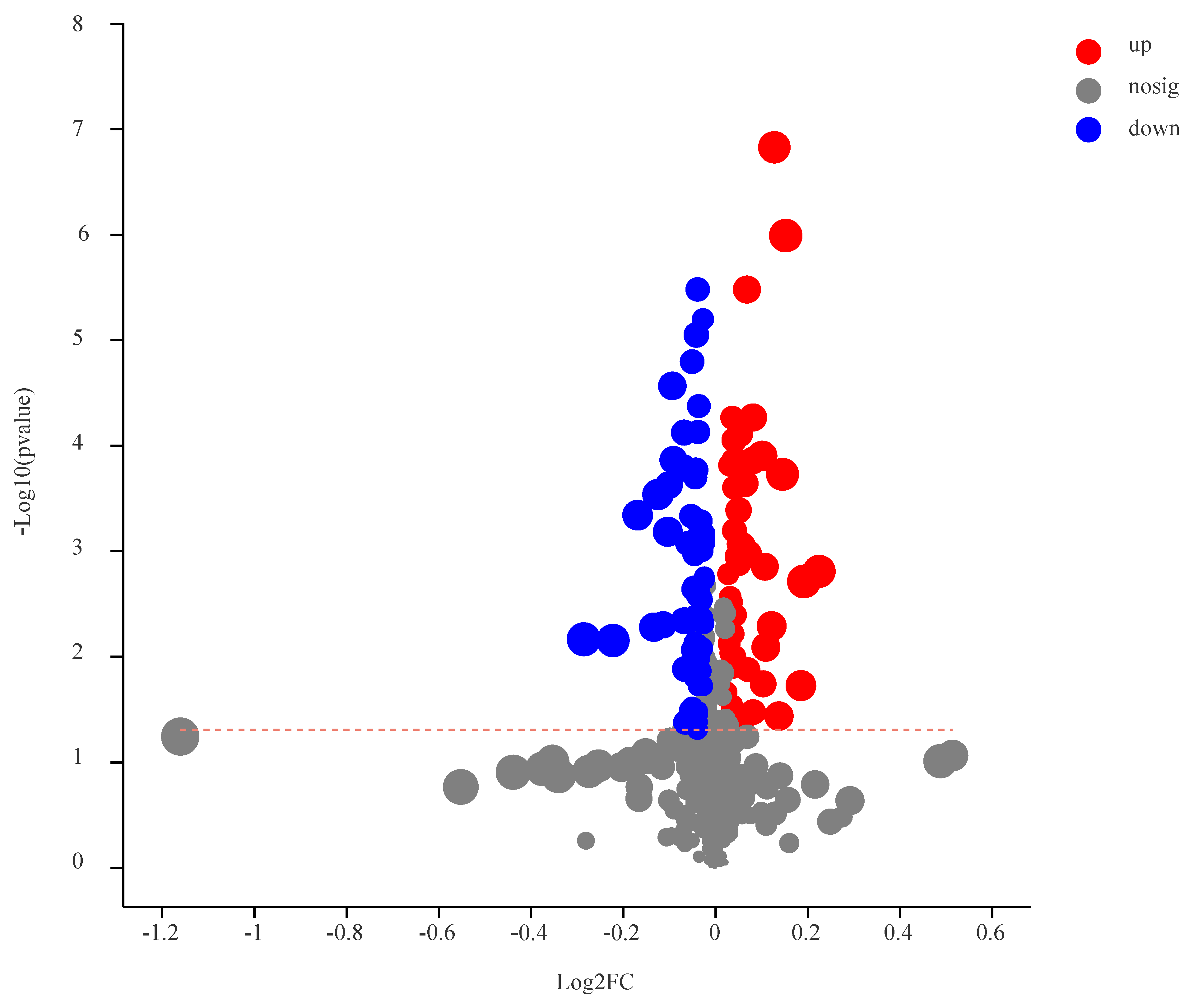
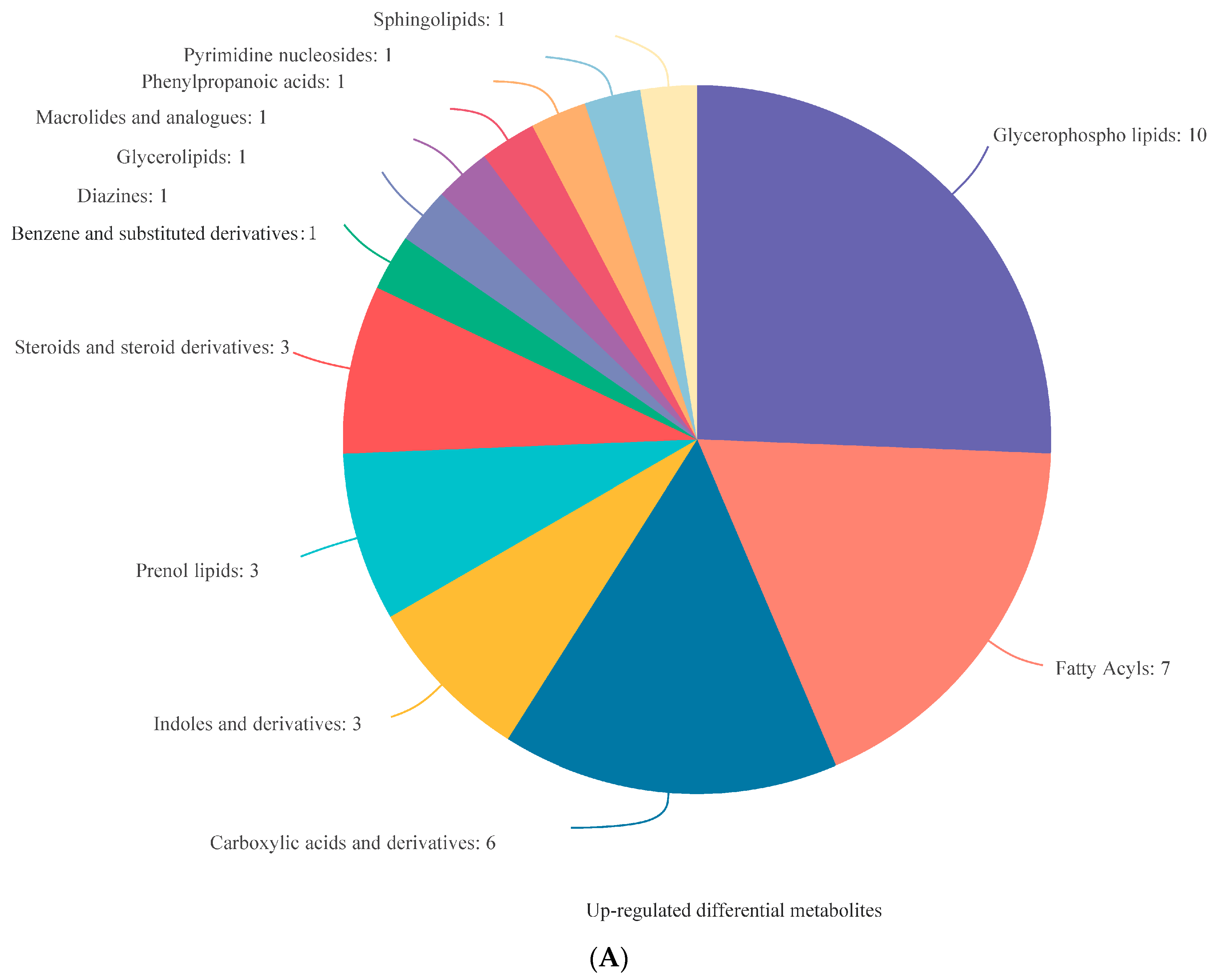
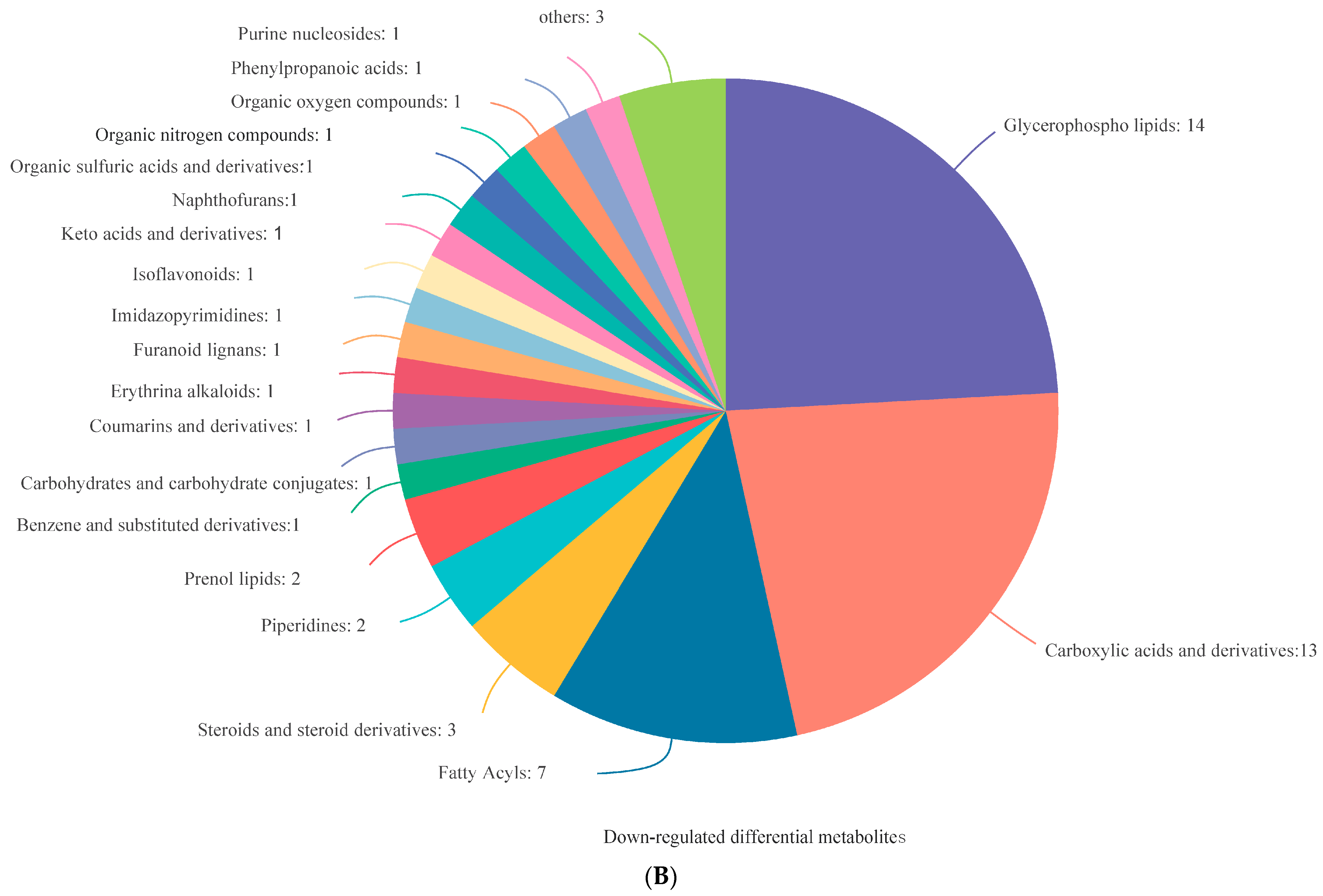
3.3. Identification of DEMs
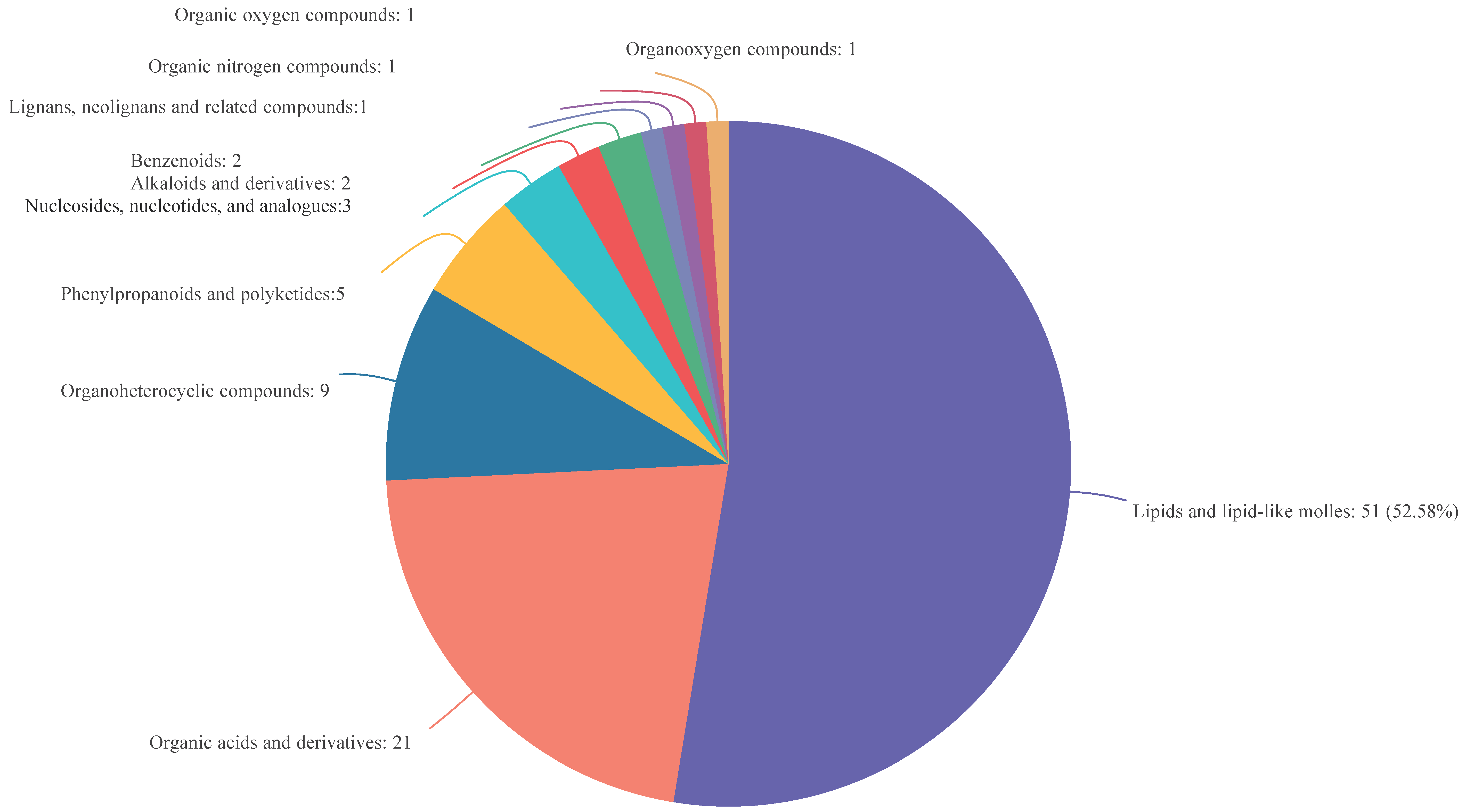
3.4. Analysis of DEMs
3.5. Metabolic Pathway Analysis
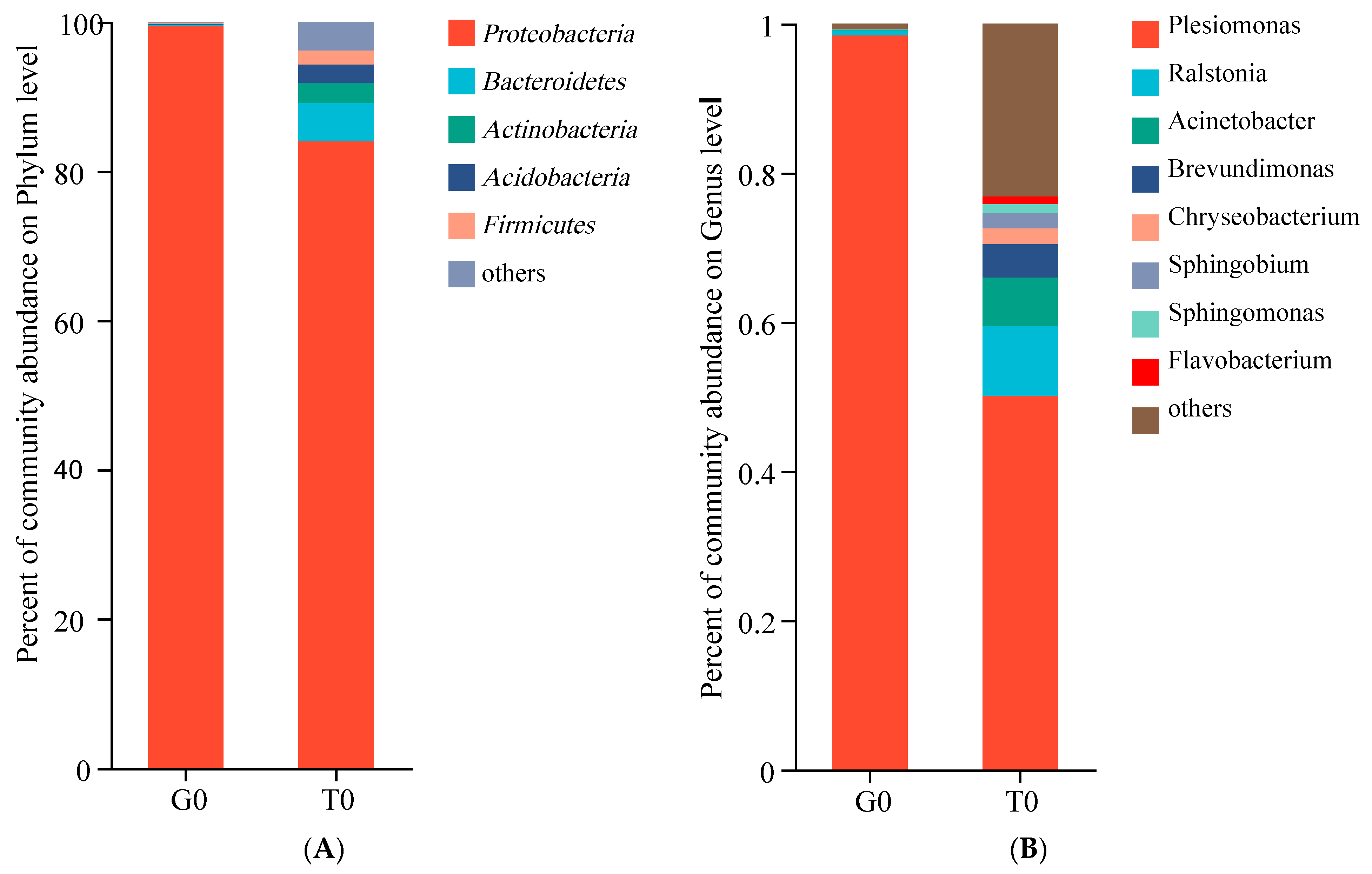
3.6. Intestinal Microbes Composition
3.7. Correlation between Metabolites and Intestinal Microbes
4. Discussion
4.1. Growth Performance
4.2. Lipid Metabolism
4.3. Carbohydrate Metabolism
4.4. Amino Acid Metabolism
4.5. Nucleotide Metabolism
4.6. Oxidation Products and Other Metabolites
4.7. Intestinal Microbes Diversity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.S.; Jia, X.Q.; Yu, X.D.; Wang, P.P.; Yin, S.W.; Zhao, C. Effect of water temperature on sex ratio and growth rate of juvenile Pelteobagrus fulvidraco, P. vachelli and hybrids [P. fulvidraco (♀) × P. vachelli (♂)]. Aquac. Rep. 2016, 3, 115–119. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, C.S.; Chen, J.C. Salinity and temperature tolerance of brown marbled grouper Epinephelus fuscoguttatus. Fish Physiol. Biochem. 2013, 39, 277–286. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold shock and fish. J. Fish. Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Physiol. 2010, 72, 127–145. [Google Scholar] [CrossRef]
- Farzad, R.; Kuhn, D.D.; Smith, S.A.; O’Keefe, S.F.; Hines, I.S.; Bushman, T.J.; Galagarza, O.A.; Stevens, A.M. Effects of selenium-enriched prebiotic on the growth performance, innate immune response, oxidative enzyme activity and microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 531, 735980. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Ott, K.H.; Aranibar, N.; Singh, B.; Stockton, G.W. Metabonomics classifies pathways affected by bioactive compounds. Artuficial neural network classification of NMR spectra of plant extracts. Phytochemistry 2003, 62, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Anderstanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yuan, R.M.; Liu, Y.J.; Yang, H.J.; Liang, G.Y.; Tian, L.X. Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2015, 435, 431–436. [Google Scholar] [CrossRef]
- Neissi, A.; Rafiee, G.; Farahmand, H.; Rahimi, S.; Mijakovic, I. Cold-Resistant heterotrophic ammonium and nitrite-removing bacteria improve aquaculture conditions of rainbow trout (Oncorhynchus mykiss). Microb. Ecol. 2020, 80, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.M.U.; Consuegra, S.; de Leaniz, C.G. Early life stress causes persistent impacts on the microbiome of Atlantic salmon. Comp. Biochem. Phys. D 2021, 40, 100888. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, Z.; Quan, J.Q.; Lu, J.H.; Zhao, G.Y.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Wang, G.X.; Sun, Y.P.; Zhao, H.X.; Huang, Y.H.; Cao, J.M. Effects of dietary sodium selenite and selenoyeast on growth performance, antioxidant responses and low-temperature stress resistance of juvenile yellow catfish (Pelteobagrus fulvidraco). J. Fish. China 2019, 43, 2394–2404. (In Chinese) [Google Scholar]
- Hu, J.R.; Yuan, X.C.; Huang, W.; Li, G.L.; Zhang, Y.F.; Wang, G.X.; Zhao, H.X.; Huang, Y.H. Transcriptome analysis of different Se sources on regulation signal pathways and key genes of Pelteobrus fulvidraco under cold stress. Chin. J. Anim. Nutr. 2021, 33, 4662–4674. (In Chinese) [Google Scholar]
- Hu, J.R.; Huang, Y.H.; Wang, G.X.; Wu, Y.X.; Xian, J.A.; Wang, A.L.; Cao, J.M. Defificient and excess dietary selenium levels affect growth performance, blood cells apoptosis and liver HSP70 expression in juvenile yellow catfifish Pelteobagrus fulvidraco. Fish. Physiol. Biochem. 2016, 42, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.L.; Chen, Y.N.; Kuo, C.M. Physiological responses, desaturase activity, and fatty acid composition in milkfish (Chanos chanos) under cold acclimation. Aquaculture 2003, 220, 903–918. [Google Scholar] [CrossRef]
- Dietricha, M.A.; Hliwab, P.; Adamekc, M.; Steinhagen, D.; Karol, H.; Ciereszko, A. Acclimation to cold and warm temperatures is associated with differential expression of male carp blood proteins involved in acute phase and stress responses, and lipid metabolism. Fish Shellfish Immunol. 2018, 76, 305–315. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef]
- Ibarz, A.; Blasco, J.; Beltrán, M.; Gallardo, M.A.; Sanchez, J.; Sala, R.; Fernandez-Borras, J. Cold-induced alterations on proximate composition and fatty acid profiles of several tissues in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 477–486. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Kuo, C.M. Stearoyl-CoA desaturase expression and fatty acid composition in milkfish (Chanos chanos) and grass carp (Ctenopharyngodon idella) during cold acclimation. Comp. Biochem. Phys. A 2005, 141, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tan, X.; Jiao, S.; Wang, Q.; Tan, X.G.; Jiao, S.; You, F.; Zhang, P.J. Analyzing cold tolerance mechanism in transgenic zebrafish (Danio rerio). PLoS ONE 2014, 9, e102492. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; You, F.; Wang, Q.; Weng, S.D.; Liu, H.; Wang, L.J.; Zhang, P.J.; Tan, X.G. Transcriptional responses of olive flounder (Paralichthys olivaceus) to low temperature. PLoS ONE 2014, 9, e108582. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Dong, L.; Shi, X.; Li, X.P.; Sui, J.; Luo, K.; Luan, S.; Chen, B.L.; Cao, B.X.; Cao, J.W.; et al. Screening of the candidate genes related to low-temperature tolerance of Fenneropenaeus chinensis based on high-throughput transcriptome sequencing. PLoS ONE 2019, 14, e0211182. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Hu, Y.N.; Fu, H.T.; Sun, S.M.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Analysis of testis metabolome and transcriptome from the oriental river prawn (Macrobrachium nipponense) in response to different temperatures and illumination times. Comp. Biochem. Phys. D 2020, 34, 100662. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Yu, Z.X.; Xu, Y.; Zhang, Y.B.; Mu, C.M.; Liu, P.; Li, J. Integrated transcriptomic and metabolomic responses in the hepatopancreas of kuruma shrimp (Marsupenaeus japonicus) under cold stress. Ecotoxicol. Environ. Saf. 2020, 206, 111360. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhang, D.G.; Ling, S.C.; Chen, G.H.; Tan, X.Y. Effects of dietary selenium on lipid metabolism and mirnas expression in mesenteric adipose tissue of yellow catfish Pelteobagrus fulvidraco. Acta Hydrobiol. Sin. 2020, 44, 685–692. (In Chinese) [Google Scholar]
- Callejon-Leblic, B.; Selma-Royo, M.; Collado, M.C.; Gomez-Ariza, J.L.; Abril, N.; Garcia-Barrera, T. Untargeted gut metabolomics to delve the interplay between selenium supplementation and gut microbiota. J. Proteome Res. 2022, 21, 758–767. [Google Scholar] [CrossRef]
- Henderson, R.J.; Tocher, D.R. The lipid composition and biochemistry of freshwater fish. Prog. Lipid Res. 1987, 26, 281–347. [Google Scholar] [CrossRef]
- Haug, A.; Eich-Greatorex, S.; Bernhoft, A.; Wold, J.P.; Hetland, H.; Christophersen, O.A.; Sogn, T. Effect of dietary selenium and omega-3 fatty acids on muscle composition and quality in broilers. Lipids Health Dis. 2007, 6, 29. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhu, S.G.; Xu, C.F. Biochemistry 3rd Edition II; Higher Education Press: Beijing, China, 2002; p. 111. (In Chinese) [Google Scholar]
- Sauer, U.; Lasko, D.R.; Fiaux, J.; Hochuli, M.; Glaser, R.; Szyperski, T.; Wüthrich, K.; Bailey, J.E. Metabolic flux Ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. J. Bacteriol. 1999, 181, 6679–6688. [Google Scholar] [CrossRef]
- Bennett, B.D.; Kimball, E.H.; Gao, M.; Osterhout, R.; Van Dien, S.J.; Rabinowitz, J.D. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 2009, 5, 593–599. [Google Scholar] [CrossRef]
- Karanova, M.V.; Andreev, A.A. Free amino acids and reducing sugars in the freshwater shrimp gammarus lacustris (Crustacea, Amphipoda) at the initial stage of preparation to winter season. J. Evol. Biochem. Physiol. 2010, 46, 279–283. [Google Scholar] [CrossRef]
- Karanova, M.V. Change in free amino acid content in tissue fluids of the freshwater mollusc lymnaea stagnalis in the process of seasonal adaptation to low positive temperatures. Izv. Akad. Nauk. SSSR Biol. 2006, 6, 719–724. [Google Scholar]
- Daniels, L.A. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 1996, 54, 185–199. [Google Scholar] [CrossRef]
- Wischhusen, P.; Heraud, C.; Skjærven, K.; Kaushik, S.J.; Fauconneau, B.; Prabhu, P.A.J.; Fontagné-Dicharry, S. Long-term effect of parental selenium supplementation on the one-carbon metabolism in rainbow trout (Oncorhynchus mykiss) fry exposed to hypoxic stress–Corrigendum. Brit. J. Nutr. 2022, 127, 148–149. [Google Scholar] [CrossRef]
- Ge, X.G.; Wang, D.H.; Wei, G.Y.; Nie, M.; Shao, N. Improvement of physiological characteristic of selenium enriched candida utilis with amino acids addition. Biomed. Res. Int. 2011, 9225, 238456. [Google Scholar]
- Melis, R.; Sanna, R.; Braca, A.; Bonaglini, E.; Cappuccinelli, R.; Slawski, H.; Roggio, T.; Uzzau, S.; Anedda, R. Molecular details on gilthead sea bream (Sparus aurata) sensitivity to low water temperatures from 1HNMR metabolomics. Comp. Biochem. Phys. A 2017, 204, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; You, Q.C.; Chi, C.F.; Luo, S.Y.; Song, H.B.; Lou, B.; Takeuchi, Y. Transcriptional response to low temperature in the yellow drum (Nibea albiflflora) and identifification of genes related to cold stress. Comp. Biochem. Phys. D 2018, 28, 80–89. [Google Scholar]
- Fan, L.F.; Wang, L.; Wang, Z.L. Proteomic characterization of the hepatopancreas in the Pacifific white shrimp Litopenaeus vannamei under cold stress: Revealing the organism homeostasis mechanism. Fish Shellfish Immun. 2019, 92, 438–449. [Google Scholar] [CrossRef]
- Shi, L.G.; Xun, W.J.; Yue, W.B.; Zhang, C.X.; Ren, Y.S.; Qian, Q.L.; Shi, W.L. Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Anim. Feed Sci. Technol. 2011, 163, 136–142. [Google Scholar] [CrossRef]
- Ferreira, A.V.D.; Cominotte, A.; Ladeira, M.M.; Casagrande, D.R.; Teixeira, P.D.; van Cleef, E.; Ezequiel, J.; Castagnino, P.; Neto, O.R.M. Feedlot diets with soybean oil, selenium and vitamin E alters rumen metabolism and fatty acids content in steers. Anim. Feed. Sci. Technol. 2020, 260, 114362. [Google Scholar] [CrossRef]
- Fournier, V.; Gouillou-Coustans, M.F.; Métailler, R.; Vachot, C.; Moriceau, J.; Le Delliou, H.; Huelvan, C.; Desbruyeres, E.; Kaushik, S.J. Excess dietary arginine affects urea excretion but does not improve N utilisation in rainbow trout Oncorhynchus mykiss and turbot Psetta maxima. Aquaculture 2003, 217, 559–576. [Google Scholar] [CrossRef]
- Levander, O.A. Considerations in the design of selenium bioavailability studies. Fed. Proc. 1983, 42, 1721–1725. [Google Scholar] [PubMed]
- Li, L.L.; Liu, Z.; Quan, J.Q.; Lu, J.H.; Zhao, G.Y.; Sun, J. Dietary nanoselenium supplementation for heat-stressed rainbow trout: Effects on organizational structure, lipid changes, and biochemical parameters as well as heat-shock protein and selenoprotein-related gene expression. Fish Physiol. Biochem. 2022, 48, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Al-Masqari, Z.A.; Guo, H.P.; Wang, R.Y.; Yan, H.Z.; Dong, P.S.; Wang, G.S.; Zhang, D.M. Effects of high temperature on water quality, growth performance, enzyme activity and the gut bacterial community of shrimp (Litopenaeus vannamei). Aquac. Res. 2022, 53, 3283–3296. [Google Scholar] [CrossRef]
- Cruz-Flores, R.; Rodríguez, M.H.; Flores, J.S.O.G.; Dhar, A.K. Formalin-fixed paraffin-embedded tissues for microbiome analysis in rainbow trout (Oncorhynchus mykiss). J. Microbiol. Meth. 2022, 192, 106389. [Google Scholar]
- Soriano, E.L.; Ramírez, D.T.; Araujo, D.R.; Gómez-Gil, B.; Castro, L.I.; Sánchez, C.G. Effect of temperature and dietary lipid proportion on gut microbiota in yellowtail kingfifish Seriola lalandi juveniles. Aquaculture 2018, 497, 269–277. [Google Scholar] [CrossRef]
- Rong, X. Impacts of Environmental Factors on the Intestinal Micromicrobiota of Apositchopus japonicus and Its Association with Bacterial Diseases. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2012. (In Chinese). [Google Scholar]
- Hu, J.R.; Zhao, H.X.; Wang, G.X.; Sun, Y.P.; Wang, L. Energy consumption and intestinal microbiome disorders of yellow catfish (Pelteobagrus fulvidraco) under cold stress. Front. Physiol. 2022, 13, 1935. [Google Scholar] [CrossRef]
- Baia, Z.A.; Ren, T.J.; Han, Y.Z.; Rahman, M.M.; Hu, Y.A.; Li, Z.Q.; Jiang, Z.Q. Influences of dietary selenomethionine exposure on tissue accumulation, blood biochemical profifiles, gene expression and intestinal microbiota of Carassius auratus. Comp. Biochem. Phys. C 2018, 218, 21–29. [Google Scholar]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fifish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [PubMed]
- Wu, S.G.; Gao, T.H.; Zheng, Y.Z.; Wang, W.W.; Cheng, Y.Y.; Wang, G.T. Microbial diversity of intestinal contents and mucus in yellow catfifish (Pelteobagrus fulvidraco). Aquaculture 2010, 303, 1–7. [Google Scholar] [CrossRef]
- Kim, D.H.; Brunt, J.; Austin, B. Microbial diversity of intestinal contents and mucus in rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2007, 102, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S. Effects of dietary blackberry syrup supplement on growth performance, antioxidant, and immunological responses, and resistance of Nile tilapia, Oreochromis niloticus to Plesiomonas shigelloides. Fish Shellfish Immun. 2019, 84, 1125–1133. [Google Scholar] [CrossRef]
- Burenjargal, M.; Lee, Y.S.; Yoo, J.M.; Kim, Y.C.; Lee, Y.M.; Oh, S.; Yun, Y.P.; Hong, J.T.; Chung, Y.B.; Moon, D.C.; et al. Endogenous sphingolipid metabolites related to the growth in Sphingomonas chungbukensis. Arch. Pharm. Res. 2007, 30, 317–322. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Shen, Y.; Niu, J.; Ding, F.; Ren, Y.; Chen, X.X.; Han, B.Z. Bacteria-induced amino acid metabolism involved in appearance characteristics of high-temperature Daqu. J. Sci. Food Agric. 2023, 103, 243–254. [Google Scholar] [CrossRef]


| Items | Content |
|---|---|
| Ingredients | |
| Casein | 46.00 |
| Corn starch | 26.50 |
| Fish oil | 8.00 |
| Lecithin oil | 2.00 |
| Vitamin premix (1) | 0.50 |
| Mineral premix (2) | 1.50 |
| Choline chloride | 0.50 |
| Vitamin C phosphate | 0.10 |
| NaCl | 0.40 |
| Ca(H2PO4)2 | 2.00 |
| Betaine | 0.50 |
| Carboxymethyl cellulose | 3.00 |
| Microcrystalline cellulose | 9.00 |
| Total | 100.00 |
| Nutrient levels (3) | |
| Crude protein | 41.29 |
| Crude lipid | 9.78 |
| Ash | 4.73 |
| Moisture | 8.42 |
| Groups | Weight Gain Rate (%) | Specific Growth Rate (%/d) | Survival Rate (%) | Feed Conversion Rate | GSH-Px (U/mL) | SOD (U/mL) | MDA (nmol/mL) |
|---|---|---|---|---|---|---|---|
| G0 group | 116.48 ± 7.12 | 1.84 ± 0.08 | 95.00 ± 2.95 | 1.45 ± 0.08 | 196.81 ± 3.72 | 0.93 ± 0.01 | 6.71 ± 0.08 |
| T0 group | 133.31 ± 13.43 | 2.01 ± 0.14 | 97.86 ± 2.14 | 1.42 ± 0.09 | 228.98 ± 25.77 | 0.81 ± 0.06 | 5.85 ± 0.15 |
| Metab ID | Metabolite | Formula | Upregulated/Downregulated |
|---|---|---|---|
| metab_16358 | LysoPA (22:5(4Z,7Z,10Z,13Z,16Z)/0:0) | C25H41O7P | Upregulated |
| metab_11598 | LysoPC (20:5(5Z,8Z,11Z,14Z,17Z)) | C28H48NO7P | Upregulated |
| metab_1757 | Ornithine | C5H12N2O2 | Upregulated |
| metab_10830 | Valine | C5H11NO2 | Upregulated |
| metab_292 | Cytidine | C9H13N3O5 | Upregulated |
| metab_15415 | PC (22:6(4Z,7Z,10Z,13Z,16Z,19Z) | C48H84NO7P | Downregulated |
| metab_1538 | Lotaustralin | C11H19NO6 | Downregulated |
| metab_10678 | L-isoleucine | C6H13NO2 | Downregulated |
| metab_1066 | Deoxyinosine | C10H12N4O4 | Downregulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, L.; Wang, G.; Zhao, H.; Lu, H.; Peng, K.; Huang, W.; Liu, Z.; Liu, D.; Sun, Y. Selenium Protects Yellow Catfish (Tachysurus fulvidraco) from Low-Temperature Damage via the Perspective Analysis of Metabolomics and Intestinal Microbes. Fishes 2024, 9, 56. https://doi.org/10.3390/fishes9020056
Hu J, Wang L, Wang G, Zhao H, Lu H, Peng K, Huang W, Liu Z, Liu D, Sun Y. Selenium Protects Yellow Catfish (Tachysurus fulvidraco) from Low-Temperature Damage via the Perspective Analysis of Metabolomics and Intestinal Microbes. Fishes. 2024; 9(2):56. https://doi.org/10.3390/fishes9020056
Chicago/Turabian StyleHu, Junru, Lei Wang, Guoxia Wang, Hongxia Zhao, Huijie Lu, Kai Peng, Wen Huang, Zhenxing Liu, Ding Liu, and Yuping Sun. 2024. "Selenium Protects Yellow Catfish (Tachysurus fulvidraco) from Low-Temperature Damage via the Perspective Analysis of Metabolomics and Intestinal Microbes" Fishes 9, no. 2: 56. https://doi.org/10.3390/fishes9020056
APA StyleHu, J., Wang, L., Wang, G., Zhao, H., Lu, H., Peng, K., Huang, W., Liu, Z., Liu, D., & Sun, Y. (2024). Selenium Protects Yellow Catfish (Tachysurus fulvidraco) from Low-Temperature Damage via the Perspective Analysis of Metabolomics and Intestinal Microbes. Fishes, 9(2), 56. https://doi.org/10.3390/fishes9020056






