Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands
Abstract
1. Introduction
2. Materials and Methods
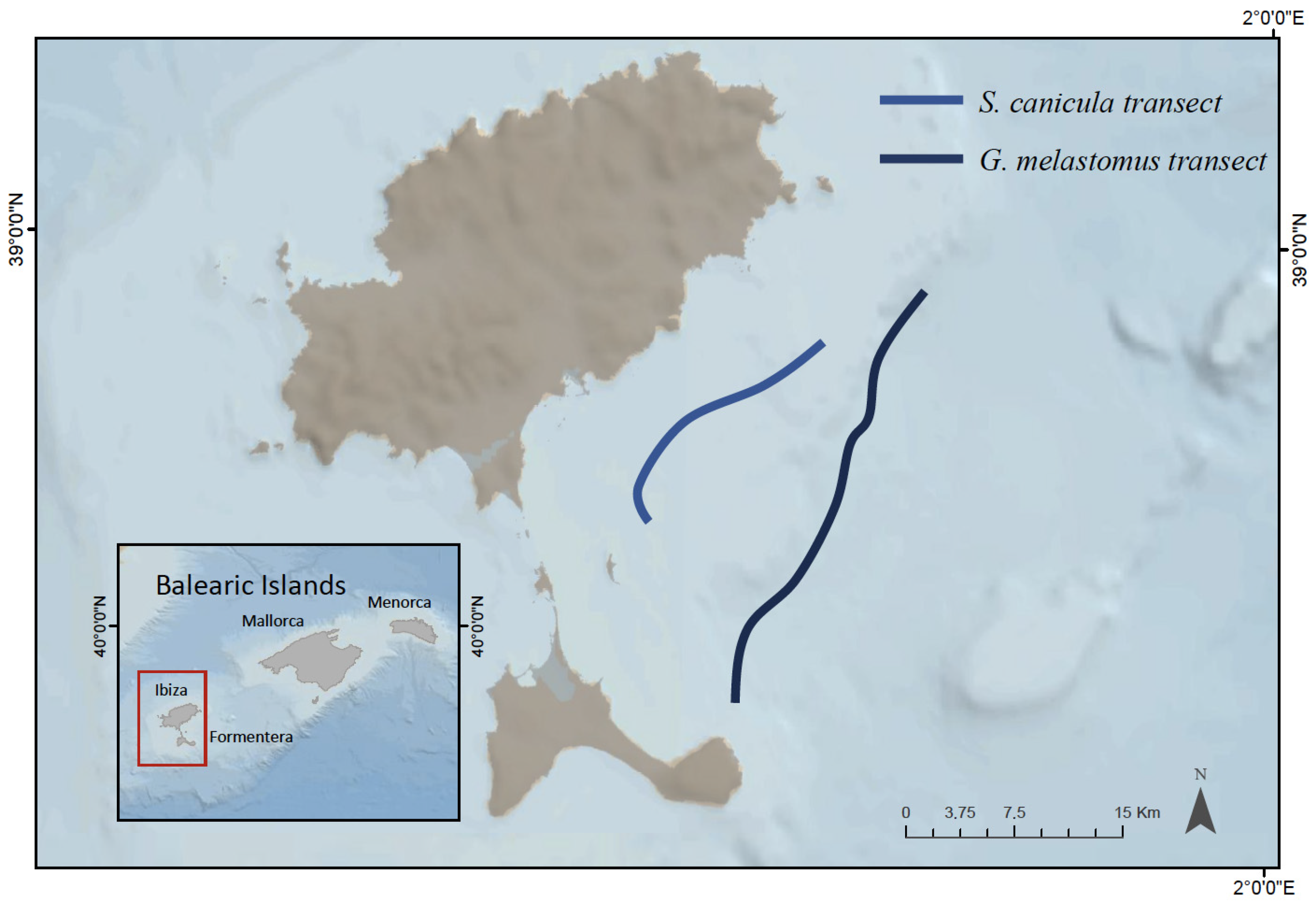
2.1. Fish Sampling and Processing
2.2. Microplastic Analysis
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Biometric Parameters
3.2. Microplastics in the Gastrointestinal Tract
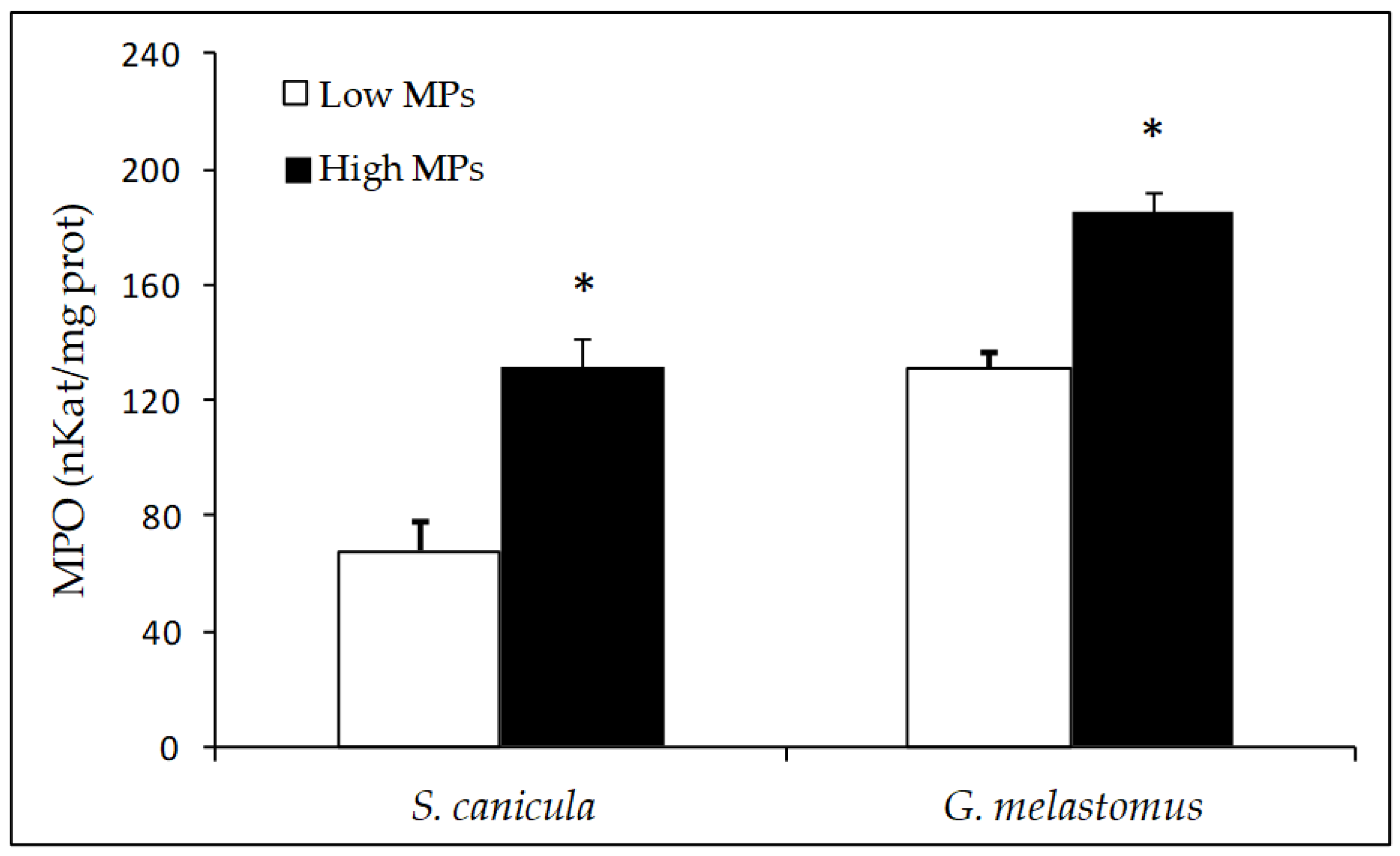
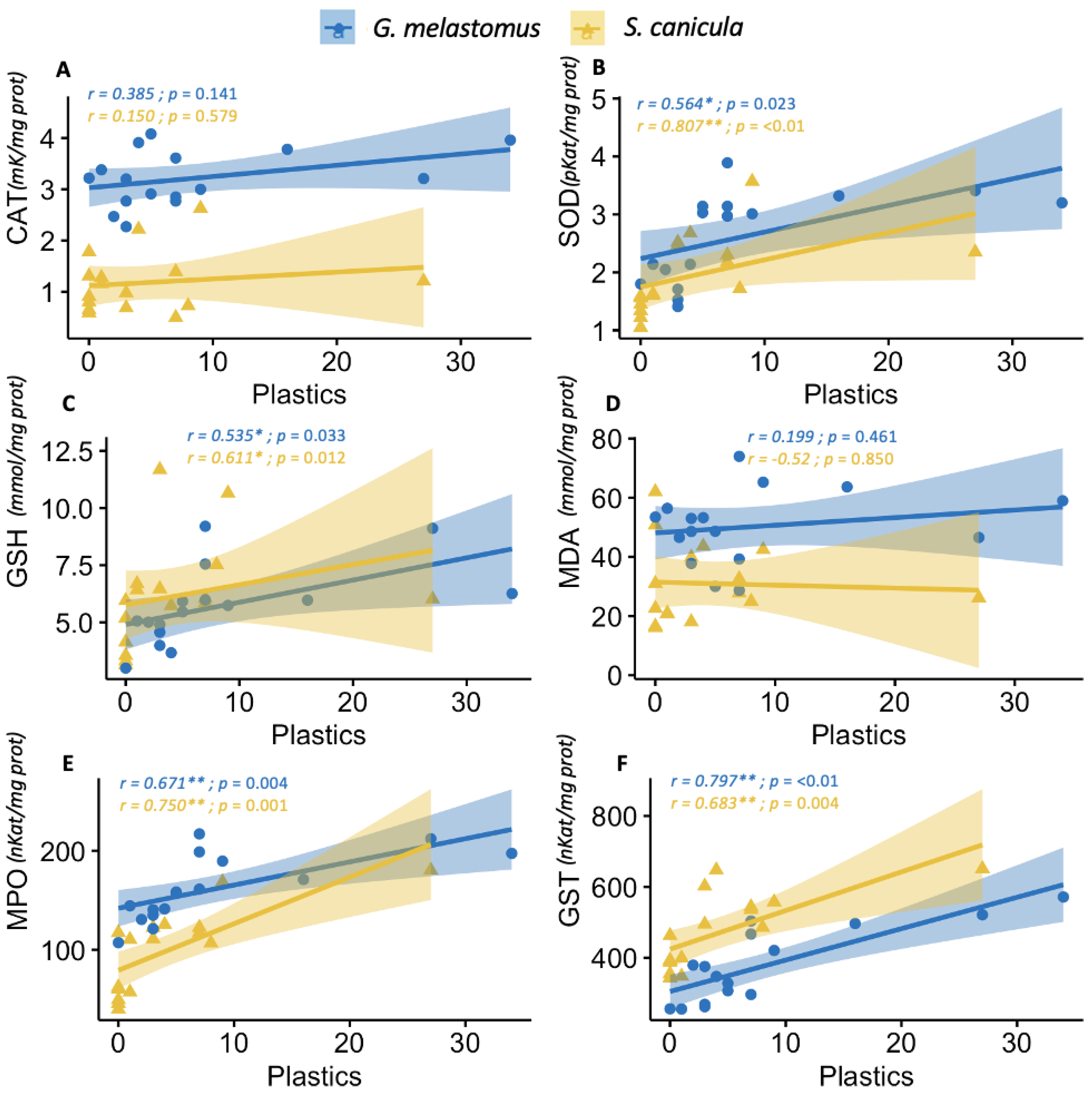
3.3. Biochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Europe Plastics. Plastics—The Facts 2020. An Analysis in European Plastic Production, Demand and Waste Data. Plastic Europe. 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 22 January 2023).
- Zhang, Y.; Gao, T.; Kang, S.; Shi, H.; Mai, L.; Allen, D.; Allen, S. Current Status and Future Perspectives of Microplastic Pollution in Typical Cryospheric Regions. Earth Sci. Rev. 2022, 226, 103924. [Google Scholar] [CrossRef]
- Solomando, A.; Pujol, F.; Sureda, A.; Pinya, S. Evaluating the Presence of Marine Litter in Cetaceans Stranded in the Balearic Islands (Western Mediterranean Sea). Biology 2022, 11, 1468. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Vandeperre, F.; Santos, M.R.; Herrera, L.; Parra, H.; Deshpande, A.; Bjorndal, K.A.; Pham, C.K. Litter Ingestion and Entanglement in Green Turtles: An Analysis of Two Decades of Stranding Events in the NE Atlantic. Environ. Pollut. 2022, 298, 118796. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal Alterations in European Sea Bass Dicentrarchus labrax (Linnaeus, 1758) Exposed to Microplastics: Preliminary Results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, R.; Wang, F.; Geng, Y.; Xu, T.; Zhu, M.; Lv, H.; Xu, S.; Guo, M.Y. Polyethylene Microplastics Trigger Cell Apoptosis and Inflammation via Inducing Oxidative Stress and Activation of the NLRP3 Inflammasome in Carp Gills. Fish Shellfish. Immunol. 2023, 132, 108470. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, Y.J.; Hong, N.H.; Hong, S.H.; Park, J.W. Toxicological Effects of Irregularly Shaped and Spherical Microplastics in a Marine Teleost, the Sheepshead Minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-Term Exposure to Virgin and Seawater Exposed Microplastic Enriched-Diet Causes Liver Oxidative Stress and Inflammation in Gilthead Seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef]
- Solomando, A.; Cohen-Sánchez, A.; Box, A.; Montero, I.; Pinya, S.; Sureda, A. Microplastic Presence in the Pelagic Fish, Seriola dumerili, from Balearic Islands (Western Mediterranean), and Assessment of Oxidative Stress and Detoxification Biomarkers in Liver. Environ. Res. 2022, 212, 113369. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A Meta-Analysis of the Effects of Exposure to Microplastics on Fish and Aquatic Invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Chen, H.; Ding, Y.; Wang, Y.; Liao, Q.; Wang, T.; Fan, Q.; Feng, Z.; Zhang, C.; Fu, G.; et al. Effects of Microplastics on the Toxicity of Co-Existing Pollutants to Fish: A Meta-Analysis. Water Res. 2023, 240, 120113. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of Microplastics by Demersal Fish from the Spanish Atlantic and Mediterranean Coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- López-López, L.; Preciado, I.; González-Irusta, J.M.; Arroyo, N.L.; Muñoz, I.; Punzón, A.; Serrano, A. Incidental Ingestion of Meso- and Macro-Plastic Debris by Benthic and Demersal Fish. Food Webs 2018, 14, 1–4. [Google Scholar] [CrossRef]
- Valente, T.; Sbrana, A.; Scacco, U.; Jacomini, C.; Bianchi, J.; Palazzo, L.; de Lucia, G.A.; Silvestri, C.; Matiddi, M. Exploring Microplastic Ingestion by Three Deep-Water Elasmobranch Species: A Case Study from the Tyrrhenian Sea. Environ. Pollut. 2019, 253, 342–350. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Morgan, E.; Hutchinson, D.; Gaion, A. Plastic Ingestion by the Small-Spotted Catshark (Scyliorhinus canicula) from the South West Coast of the United Kingdom. Bull. Environ. Contam. Toxicol. 2021, 106, 910–915. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S. Evidence of Microplastic Ingestion in the Shark Galeus melastomus Rafinesque, 1810 in the Continental Shelf off the Western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef]
- Pimiento, C.; Albouy, C.; Silvestro, D.; Mouton, T.L.; Velez, L.; Mouillot, D.; Judah, A.B. Functional Diversity of Sharks and Rays Is Highly Vulnerable and Supported by Unique Species and Locations Worldwide. Nat. Commun. 2023, 14, 7691. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a Century of Global Decline in Oceanic Sharks and Rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Walls, R.H.L.; Dulvy, N.K. Tracking the Rising Extinction Risk of Sharks and Rays in the Northeast Atlantic Ocean and Mediterranean Sea. Sci. Rep. 2021, 11, 15397. [Google Scholar] [CrossRef] [PubMed]
- Parton, K.J.; Godley, B.J.; Santillo, D.; Tausif, M.; Omeyer, L.C.M.; Galloway, T.S. Investigating the Presence of Microplastics in Demersal Sharks of the North–East Atlantic. Sci. Rep. 2020, 10, 12204. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, I.; Garibaldi, F.; Canesi, L.; Cristina, M.; Baini, M. First Data on Plastic Ingestion by Blue Sharks (Prionace glauca) from the Ligurian Sea (North-Western Mediterranean Sea). Mar. Pollut. Bull. 2018, 135, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, A.I.; Barría, C.; Broglio, E.; García-Barcelona, S. Plastic Debris Straps on Threatened Blue Shark Prionace glauca. Mar. Pollut. Bull. 2017, 115, 436–438. [Google Scholar] [CrossRef]
- Ordines, F.; Valls, M.; Jos, M.; Ramírez-Amaro, S.; García-Ruiz, C.; Massutí, E. Potential Factors Influencing the Condition of Demersal Sharks in the Mediterranean Deep Sea Ecosystems. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2021, 176, 103603. [Google Scholar] [CrossRef]
- Papadopoulo, K.; Villegas-Ríos, D.; Hillinger, A.; Alonso-Fernández, A.; Cabello, E.; Mucientes, G. Drivers of Behaviour and Spatial Ecology of the Small Spotted Catshark (Scyliorhinus canicula). Aquat. Conserv. 2023, 33, 443–457. [Google Scholar] [CrossRef]
- Massutí, E.; Moranta, J. Demersal Assemblages and Depth Distribution of Elasmobranchs from the Continental Shelf and Slope off the Balearic Islands (Western Mediterranean). ICES J. Mar. Sci. 2003, 60, 753–766. [Google Scholar] [CrossRef]
- Kousteni, V.; Karachle, P.K.; Megalofonou, P. Diet of the small-spotted catshark Scyliorhinus canicula in the Aegean Sea (Eastern Mediterranean). Mar. Biol. Res. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Sánchez, F.; Serrano, A.; Rodríguez-Cabello, C.; Cendrero, O. Trophic relations of lesser-spotted catshark (Scyliorhinus canicula) and blackmouth catshark (Galeus melastomus) in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 481–494. [Google Scholar] [CrossRef]
- Sbrana, A.; Cau, A.; Cicala, D.; Franceschini, S.; Giarrizzo, T.; Gravina, M.F.; Ligas, A.; Maiello, G.; Matiddi, M.; Parisi, A.; et al. Ask the shark: Blackmouth catshark (Galeus melastomus) as a sentinel of plastic waste on the seabed. Mar. Biol. 2022, 169, 98. [Google Scholar] [CrossRef]
- Ruiz-García, D.; Raga, J.A.; March, D.; Colmenero, A.I.; Quattrocchi, F.; Recasens, L.; Barría, C. Spatial distribution of the demersal chondrichthyan community from the western Mediterranean trawl bycatch. Front. Mar. Sci. 2023, 10, 1145176. [Google Scholar] [CrossRef]
- Mancuso, M.; Panarello, G.; Falco, F.; Di Paola, D.; Serena, S.; Capillo, G.; Romeo, T.; Presti, G.; Gullotta, E.; Spanò, N.; et al. Investigating the effects of microplastic ingestion in Scyliorhinus canicula from the South of Sicily. Sci. Total Environ. 2022, 850, 157875. [Google Scholar] [CrossRef]
- Porcino, N.; Bottari, T.; Mancuso, M. Is wild marine biota affected by microplastics? Animals 2022, 13, 147. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-Term Exposure to Microplastics Induces Oxidative Stress and a Pro-Inflammatory Response in the Gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic Ingestion by Mullus surmuletus Linnaeus, 1758 Fish and Its Potential for Causing Oxidative Stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Pedà, C.; Battaglia, P.; D’Alessandro, M.; Laface, F.; Malara, D.; Consoli, P.; Vicchio, T.M.; Longo, F.; Andaloro, F.; Baini, M.; et al. Coupling gastro-intestinal tract analysis with an airborne contamination control method to estimate litter ingestion in demersal elasmobranchs. Front. Environ. Sci. 2020, 8, 119. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Meena, B.; Valivittan, K.; Suresh, A. Glutathione-S-Transferase and Catalase Activity in Different Tissues of Marine Catfish Arius arius on Exposure to Cadmium. Int. J. Pharm. Pharm. Sci. 2014, 6, 326–332. [Google Scholar]
- Capeillère-Blandin, C. Oxidation of Guaiacol by Myeloperoxidase: A Two-Electron-Oxidized Guaiacol Transient Species as a Mediator of NADPH Oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef]
- Pinya, S.; Tejada, S.; Capó, X.; Sureda, A. Invasive Predator Snake Induces Oxidative Stress Responses in Insular Amphibian Species. Sci. Total Environ. 2016, 566–567, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Janardhanam, M.; Sivakumar, P.; Srinivasan, G.; Sivakumar, R.; Raman, T.; Singaram, G.; Harikrishnan, T. Microplastics in Demersal Sharks From the Southeast Indian Coastal Region. Front. Mar. Sci. 2022, 9, 914391. [Google Scholar] [CrossRef]
- Matupang, D.M.; Zulkifli, H.I.; Arnold, J.; Mat, A.; Abd, M.; Musa, S.M. Tropical sharks feasting on and swimming through microplastics: First evidence from Malaysia. Mar. Pollut. Bull. 2023, 189, 114762. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef] [PubMed]
- Koongolla, J.B.; Lin, L.; Pan, Y.F.; Yang, C.P.; Sun, D.R.; Liu, S.; Xu, X.R.; Maharana, D.; Huang, J.S.; Li, H.X. Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf, South China Sea. Environ. Pollut. 2020, 258, 113734. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Compa, M.; Alomar, C.; Fagiano, V.; Ventero, A.; Iglesias, M.; Deudero, S. Ubiquitous vertical distribution of microfibers within the upper epipelagic layer of the western Mediterranean Sea. Estuar. Coast. Shelf Sci. 2022, 266, 107741. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Perez-Rubín, C.; Gavara, R.; Handcock, R.N.; Rivas, M.L. Presence and implications of plastics in wild commercial fishes in the Alboran Sea (Mediterranean Sea). Sci. Total Environ. 2020, 850, 158025. [Google Scholar] [CrossRef] [PubMed]
- Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the deep: Comparing dietary and plastic ingestion data between two Mediterranean bathyal opportunistic feeder species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through stomach content analysis. J. Mar. Sci. Eng. 2022, 10, 624. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and Ecological Aspects of the Blackmouth Catshark (Galeus melastomus Rafinesque, 1810) in the Southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Mnasri, N.; El Kamel, O.; Boumaiza, M.; Reynaud, C.; Capape, C. Food and feeding habits of the small-spotted catshark, Scyliorhinus canicula (Chondrichthyes: Scyliorhinidae) from the northern coast of Tunisia (central Mediterranean). Cah. Biol. Mar. 2021, 53, 139. [Google Scholar]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Sánchez, A.; Solomando, A.; Pinya, S.; Tejada, S.; Valencia, J.M.; Box, A.; Sureda, A. Microplastic Presence in the Digestive Tract of Pearly Razorfish Xyrichtys novacula Causes Oxidative Stress in Liver Tissue. Toxics 2023, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Nie, J.; Qiu, D.; Li, S.; Sun, Y.; Wang, C. Toxic effect of chronic exposure to polyethylene nano/microplastics on oxidative stress, neurotoxicity and gut microbiota of adult zebrafish (Danio rerio). Chemosphere 2023, 339, 139774. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish. Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Ramanan, P.A.; Gorakh, S.S.; Pillai, D.; Vattiringal Jayadradhan, R.K. Sub-chronic exposure of Oreochromis niloticus to environmentally relevant concentrations of smaller microplastics: Accumulation and toxico-physiological responses. J. Hazard. Mater. 2023, 458, 131916. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Capó, X.; Alomar, C.; Compa, M.; Sole, M.; Sanahuja, I.; Soliz Rojas, D.L.; González, G.P.; Garcinuño Martínez, R.M.; Deudero, S. Quantification of differential tissue biomarker responses to microplastic ingestion and plasticizer bioaccumulation in aquaculture reared sea bream Sparus aurata. Environ. Res. 2022, 211, 113063. [Google Scholar] [CrossRef]
- Hossain, M.A.; Olden, J.D. Global meta-analysis reveals diverse effects of microplastics on freshwater and marine fishes. Fish Fish. 2022, 23, 1439–1454. [Google Scholar] [CrossRef]
- Jacob, H.; Besson, M.; Swarzenski, P.W.; Lecchini, D.; Metian, M. Effects of virgin micro- and nanoplastics on fish: Trends, meta-analysis, and perspectives. Environ. Sci. Technol. 2020, 54, 4733–4745. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Pelamatti, T.; Avio, C.G.; Camedda, A.; Costantini, M.L.; de Lucia, G.A.; Jacomini, C.; Piermarini, R.; Regoli, F.; Sbrana, A.; et al. One is not enough: Monitoring microplastic ingestion by fish needs a multispecies approach. Mar. Pollut. Bull. 2022, 184, 114133. [Google Scholar] [CrossRef] [PubMed]



| Scyliorhinus canicula | |||||||
| CAT | SOD | GSH | MDA | MPO | GST | ||
| Plastic items | r | 0.150 | 0.807 ** | 0.611 * | −0.52 | 0.750 ** | 0.683 ** |
| p | 0.579 | 0.000 | 0.012 | 0.850 | 0.001 | 0.004 | |
| Galeus melastomus | |||||||
| CAT | SOD | GSH | MDA | MPO | GST | ||
| Plastic items | r | 0.385 | 0.564 * | 0.535 * | 0.199 | 0.671 ** | 0.797 ** |
| p | 0.141 | 0.023 | 0.033 | 0.461 | 0.004 | 0.000 | |
| Study | Study Area | Species | Total Length (cm) | Wet Weight (g) | Sample Size | Nº MPs | MPs/Ind. (%) |
|---|---|---|---|---|---|---|---|
| Mancuso et al. 2022 [38] | Mediterranean Sea | Scyliorhinus canicula | 40.4 ± 5.1 | 212.7 ± 69.4 | 61 | 147 | 2.4 (80.3) |
| Sbrana et al. 2022 [36] | Tyrrhenian Sea | Galeus melastomus | - | - | 164 | 47 | 1.47 ± 0.28 |
| Alomar et al. 2018 [23] | Mediterranean Sea | Galeus melastomus | 29.94 ± 0.81 | 90.74 ± 6.65 | 125 | 21 | 0.34 ± 0.07 |
| Bellas et al. 2016 [18] | Atlantic Ocean (Cantabrian coast) | Scyliorhinus canicula | 33.3 ± 2.2 | - | 24 | - | 1.20 ± 0.45 (20.80) |
| Atlantic Ocean (Gulf of Cadiz) | Scyliorhinus canicula | 33.3 ± 2.2 | - | 24 | - | 1.20 ± 0.45 (20.80) | |
| Atlantic Ocean (Galician coast) | Scyliorhinus canicula | 33.3 ± 2.2 | - | 24 | - | 1.0 (4.20) | |
| Valente et al. 2019 [20] | Tyrrhenian Sea | Galeus melastomus | 37.7 ± 8.9 | 157.4 ± 92.0 | 143 | 14 | 4.47 ± 1.10 (40.6) |
| Scyliorhinus canicula | 34.4 ± 8.1 | 137.9 ± 100.1 | 75 | 8 | 2.50 ± 0.52 (26.7) | ||
| Mancia et al. 2020 [21] | Mediterranean Sea (Strait of Sicily) | Scyliorhinus canicula | 38.3 ± 3.7 | 189.1 ± 60.2 | 25 | 33 | 1.32 (71) |
| Mediterranean Sea (Gulf of Hammamet) | Scyliorhinus canicula | - | - | 25 | 26 | 1.04 (62) | |
| Mediterranean Sea (Strait of Sicily) | Scyliorhinus canicula | - | - | 25 | 6 | 0.24 (20) | |
| Mediterranean Sea (Gulf of Hammamet) | Scyliorhinus canicula | - | - | 25 | 5 | 0.2 (16) | |
| Morgan et al. 2021 [22] | South West Coast of the United Kingdom | Scyliorhinus canicula | - | - | 200 | 28 | NA (6.50%) |
| Lopez-Lopez et al. 2017 [19] | Global (review) | Galeus spp. | - | - | 2962 | - | 3 (0.10%) |
| Scyliorhinus canicula | - | - | 9981 | - | 7 (0.07%) | ||
| Scyliorhinus canicula | - | - | 9981 | - | 7 (0.07%) | ||
| Neves et al. 2015 [57] | Atlantic (Portuguese coast) | Scyliorhinus canicula | 43 (33–47) | 300 (127–433) | 17 | 3 | 0.12 ± 0.33 (12%) |
| Scyliorhinus canicula | - | - | 3 | 3 | 0.67 ± 0.58 (67%) | ||
| Pedà et al. 2020 [42] | Mediterranean Sea (South Tyrrhenian Sea) | Scyliorhinus canicula | 40.5 ± 6.3 | 187.4 ± 96.3 | 27 | 5 | 0.19 ± 0.48 (22.2%) |
| Galeus melastomus | 20.7 ± 3.0 | 24.9 ± 10.8 | 12 | 4 | 0.50 ± 0.80 (33%) | ||
| López Martinez et al. 2020 [58] | Mediterranean Sea (Alboran Sea) | Scyliorhinus canicula | 37.29 ± 4.86 | 145.22 ± 40.65 | 51 | 7 | 0.30 ± 0.45 (9.8%) |
| Scacco et al. 2022 [59] | Mediterranean Sea (Tyrrhenian Sea) | Galeus melastomus | 104–200 mm, 201–290 mm and 291–380 mm | - | 200 | 58 | 0.30 ± 0.45 (15%) |
| This Study | Mediterranean Sea | Scyliorhinus canicula | 38.8 ± 0.7 | 191.0 ± 9.7 | 16 | 70 | 4.38 ± 1.77 (63) |
| Galeus melastomus | 36.3 ± 3.1 | 197.8 ± 49.3 | 16 | 133 | 8.31 ± 2.46 (82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, S.; Compa, M.; Box, A.; Pinya, S.; Sureda, A. Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands. Fishes 2024, 9, 55. https://doi.org/10.3390/fishes9020055
Torres S, Compa M, Box A, Pinya S, Sureda A. Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands. Fishes. 2024; 9(2):55. https://doi.org/10.3390/fishes9020055
Chicago/Turabian StyleTorres, Susana, Montserrat Compa, Antonio Box, Samuel Pinya, and Antoni Sureda. 2024. "Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands" Fishes 9, no. 2: 55. https://doi.org/10.3390/fishes9020055
APA StyleTorres, S., Compa, M., Box, A., Pinya, S., & Sureda, A. (2024). Presence and Potential Effects of Microplastics in the Digestive Tract of Two Small Species of Shark from the Balearic Islands. Fishes, 9(2), 55. https://doi.org/10.3390/fishes9020055








