Thiamine Deficiency M74 Developed in Salmon (Salmo salar) Stocks in Two Baltic Sea Areas after the Hatching of Large Year-Classes of Two Clupeid Species—Detected by Fatty Acid Signature Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmon and Sample Preparation
2.2. Chemical Analyses
2.3. Classification of Salmon into M74 and Non-M74 Females
2.4. Classification of the Salmon in Two Feeding Groups
2.5. Statistical Analyses
3. Results
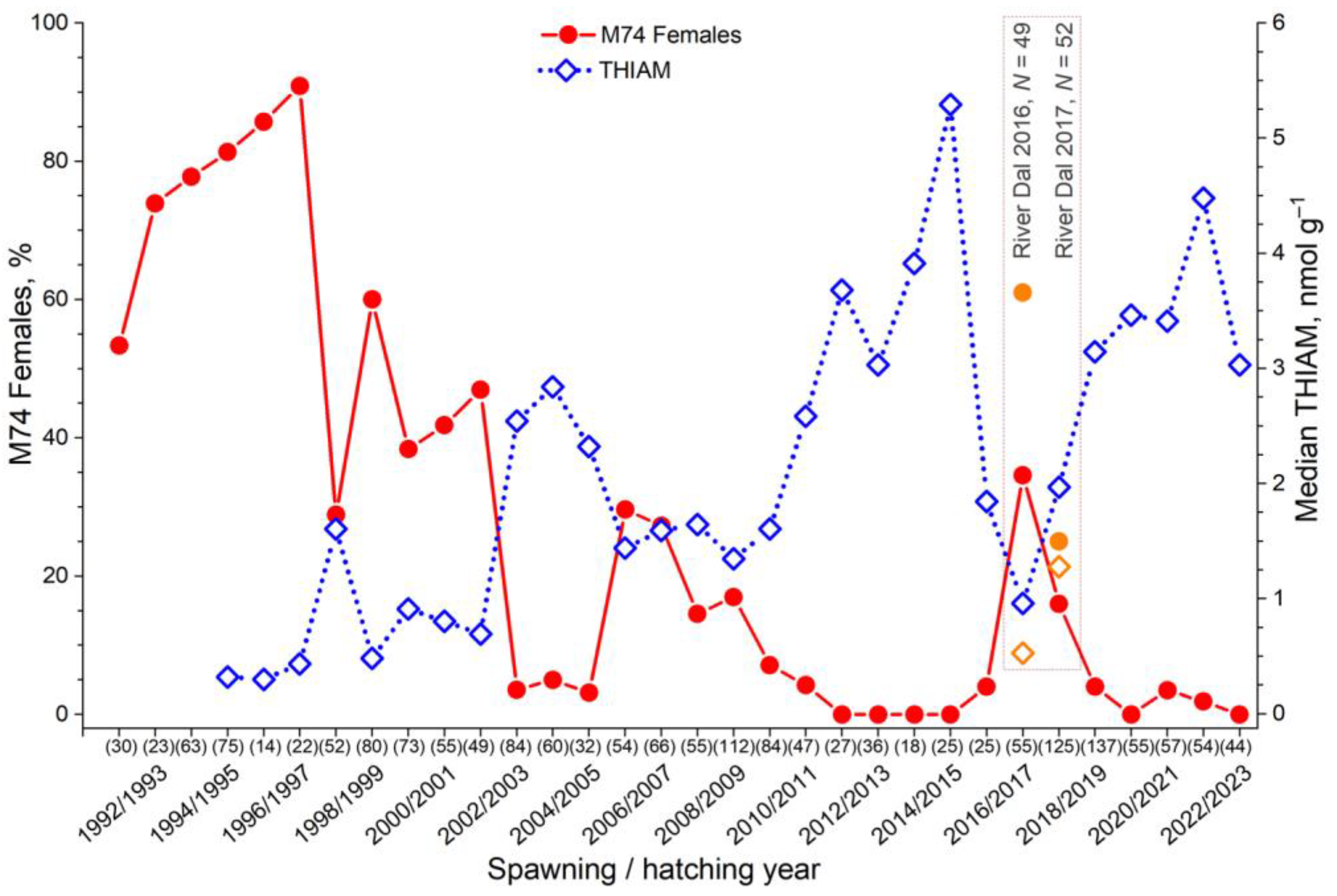
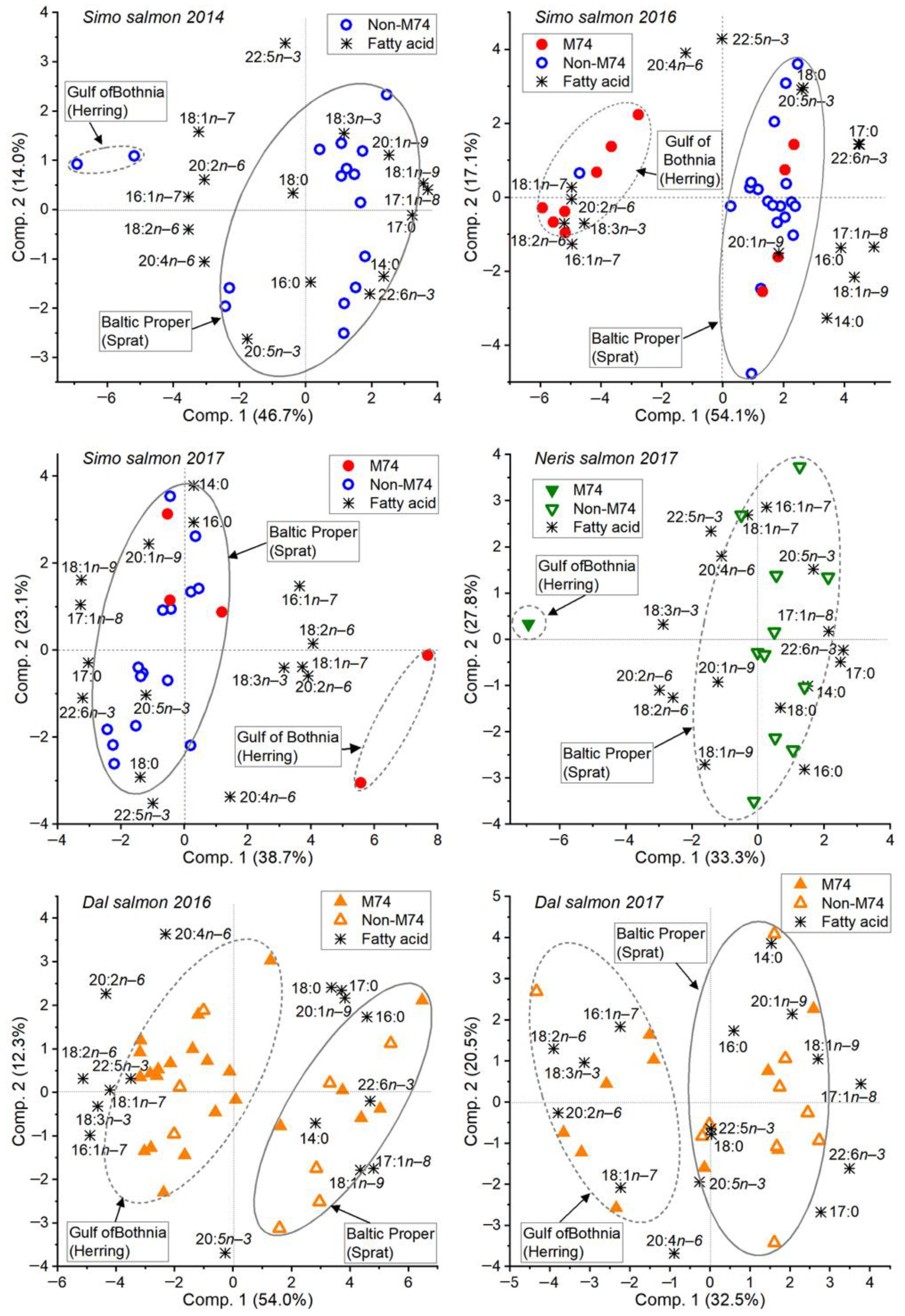
3.1. Variation in the Incidence of M74
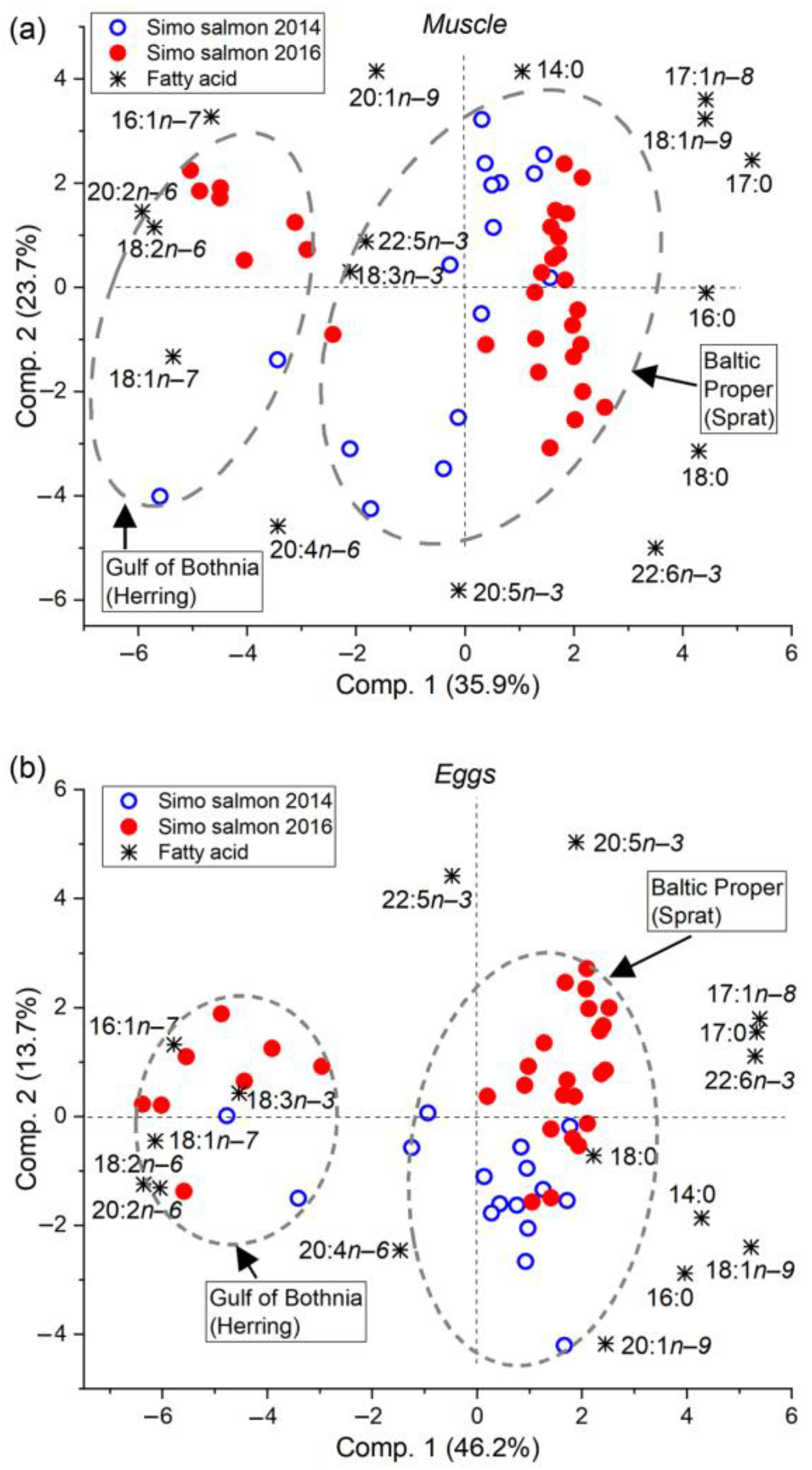
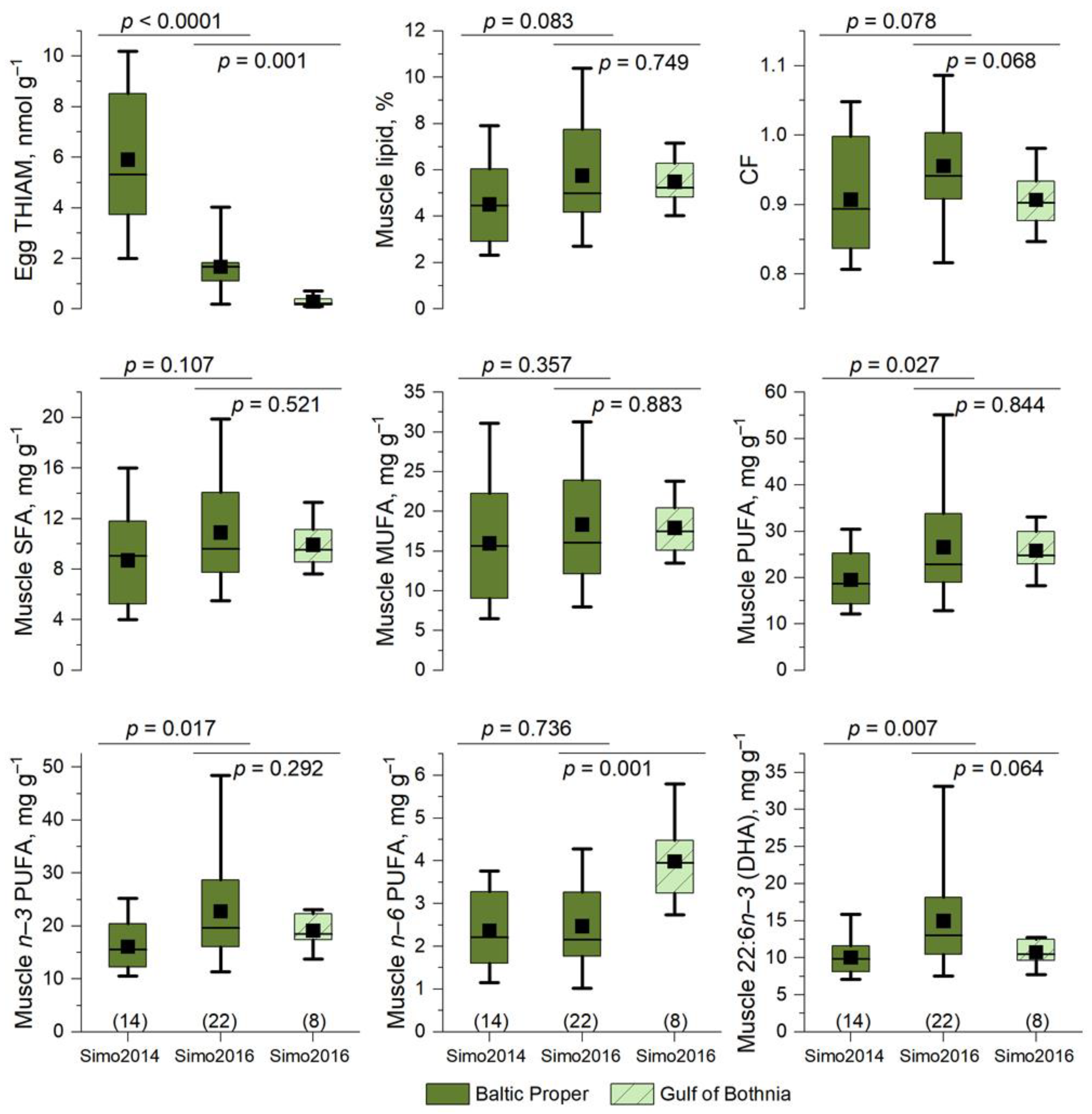
3.2. Feeding History Could Be Determined from the Fatty Acid Composition of Both Eggs and Muscle
3.3. Differences in Lipid Content and Fatty Acid Composition between Muscle and Eggs of the River Simojoki Salmon
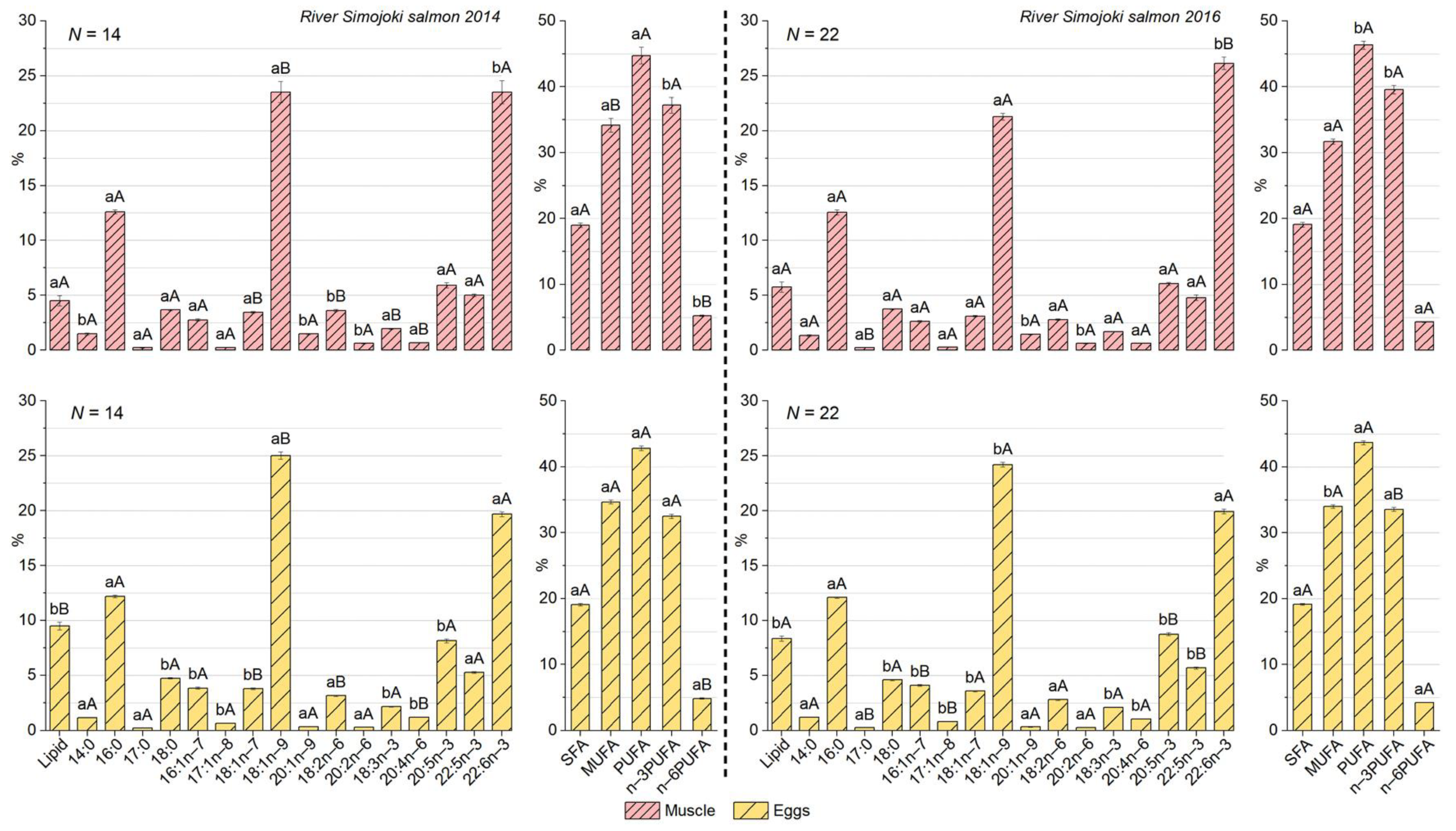
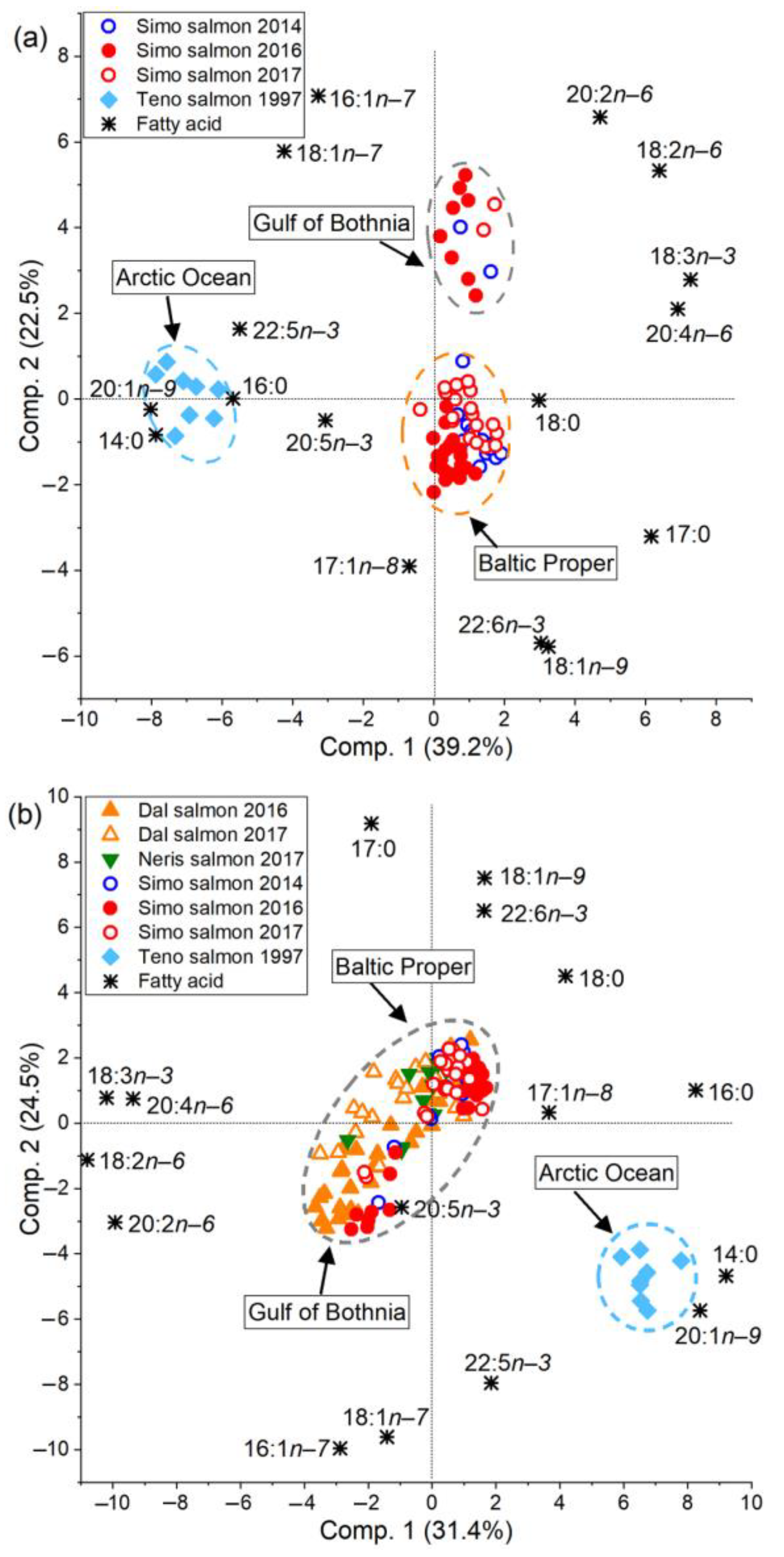
3.4. The Proportion of PUFAs in Eggs Was Higher for Baltic Salmon Than for Arctic Ocean Salmon
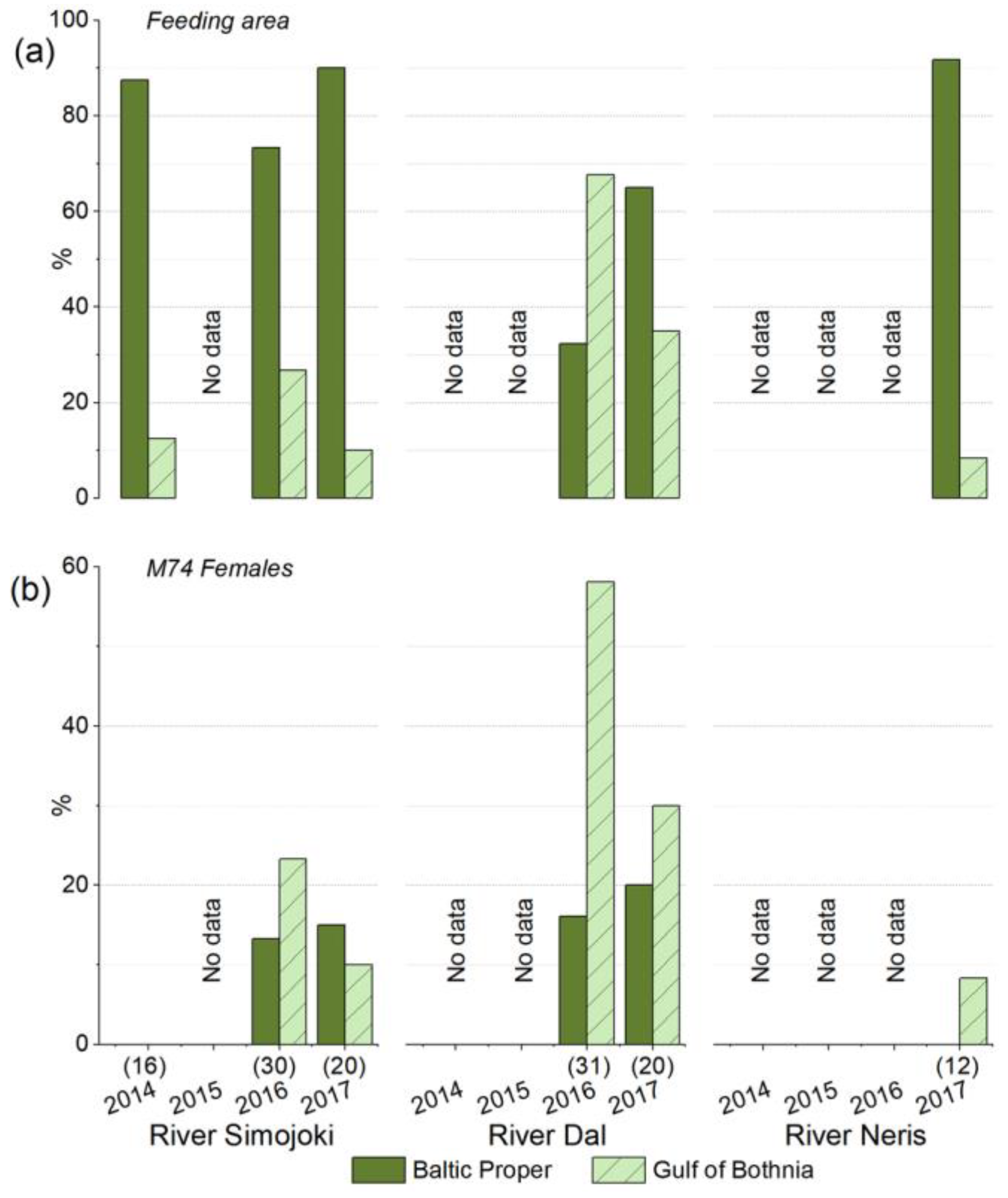
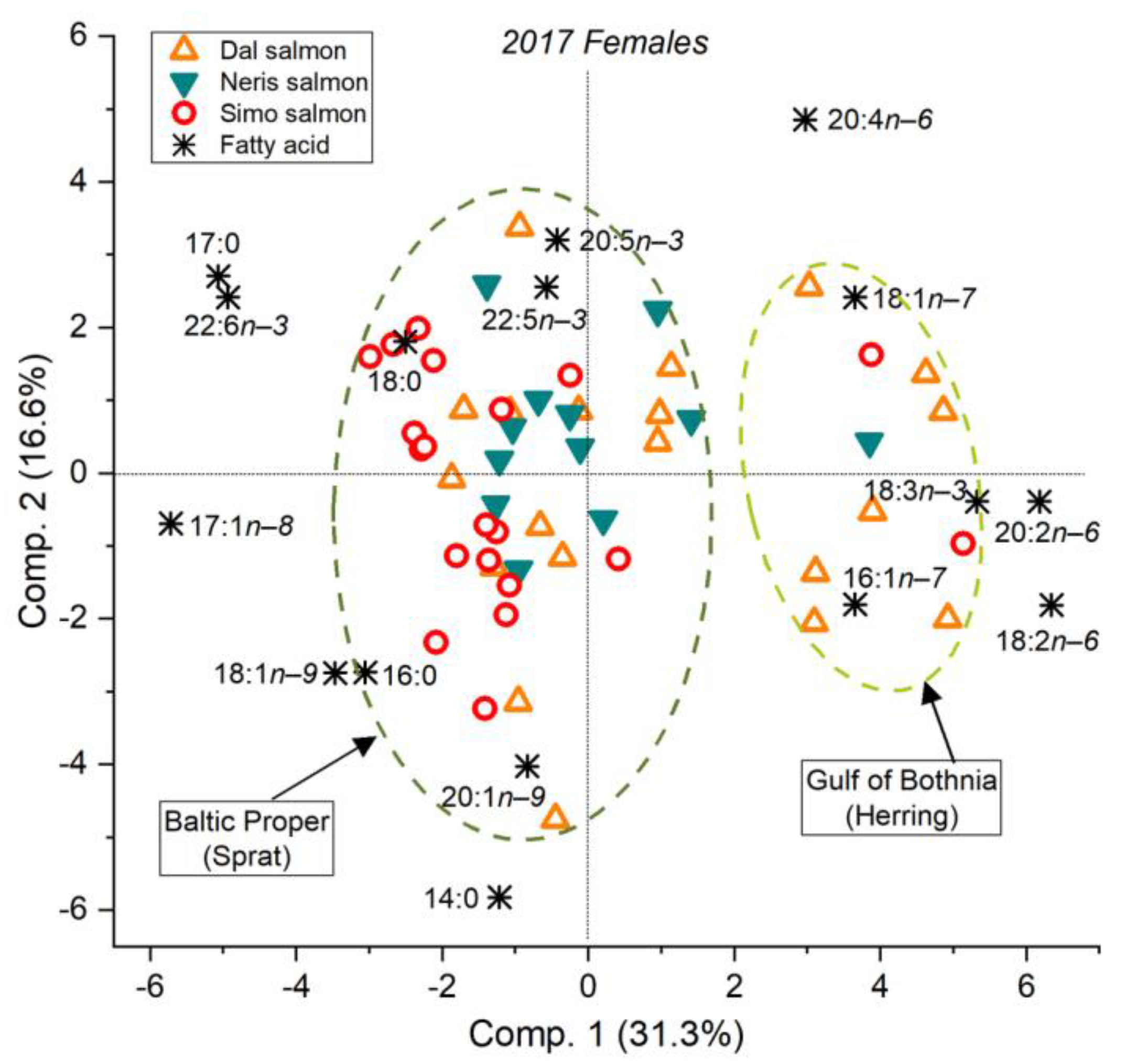
3.5. The River Dal Salmon Feed More in the Gulf of Bothnia Than the River Simojoki Salmon
3.6. More Salmon Fed in the Gulf of Bothnia when Young Herring Were Abundant
3.7. Salmon Grew Faster in the Baltic Proper Than in the Gulf of Bothnia

3.8. Relationships between Fish Size and Egg and Muscle Lipid and Egg THIAM
| Length | Mass | CF | Muscle Lipid | Egg Lipid | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2016 | 2014 | 2016 | 2014 | 2016 | 2014 | 2016 | 2014 | 2016 | |
| Muscle lipid | −0.610 | 0.317 | −0.715 | 0.312 | −0.572 | 0.181 | ||||
| 0.027 | 0.161 | 0.006 | 0.168 | 0.041 | 0.432 | |||||
| (13) | (21) | (13) | (21) | (13) | (21) | |||||
| Egg lipid | −0.376 | 0.063 | −0.339 | 0.213 | 0.011 | 0.336 | 0.009 | 0.157 | ||
| 0.205 | 0.785 | 0.258 | 0.354 | 0.971 | 0.136 | 0.975 | 0.486 | |||
| (13) | (21) | (13) | (21) | (13) | (21) | (14) | (22) | |||
| Egg THIAM | 0.555 | −0.233 | 0.671 | −0.123 | 0.460 | 0.157 | −0.633 | −0.393 | −0.563 | −0.124 |
| 0.049 | 0.323 | 0.012 | 0.605 | 0.114 | 0.508 | 0.015 | 0.078 | 0.036 | 0.592 | |
| (13) | (20) | (13) | (20) | (13) | (20) | (14) | (21) | (14) | (21) | |
3.9. Low Egg THIAM Concentrations Were Associated with High Muscle n−3 PUFA Concentrations
4. Discussion
4.1. The Fatty Acid Proportions of Muscle and Eggs Similarly Revealed the Prey Species and Feeding Area
4.2. Fatty Acid Composition of Baltic Salmon Differs from That of Arctic Ocean Salmon
4.3. Salmon Remain to Feed on Young Herring in the Gulf of Bothnia if Abundant
4.4. Different Salmon Stocks Have Different Feeding Patterns
4.5. Abundance of Young Clupeids Increased n−3 PUFA Content and Thiamine Deficiency in Salmon
4.6. Low Egg THIAM Concentration Was Associated with High Muscle, Not Egg, Lipid Content
4.7. Variation in the Growth, Lipid, and Egg THIAM Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keinänen, M.; Uddström, A.; Mikkonen, J.; Casini, M.; Pönni, J.; Myllylä, T.; Aro, E.; Vuorinen, P.J. The thiamine deficiency syndrome M74, a reproductive disorder of Atlantic salmon (Salmo salar) feeding in the Baltic Sea, is related to the fat and thiamine content of prey fish. ICES J. Mar. Sci. 2012, 69, 516–528. [Google Scholar] [CrossRef]
- Keinänen, M.; Käkelä, R.; Ritvanen, T.; Pönni, J.; Harjunpää, H.; Myllylä, T.; Vuorinen, P.J. Fatty acid signatures connect thiamine deficiency with the diet of the Atlantic salmon (Salmo salar) feeding in the Baltic Sea. Mar. Biol. 2018, 165, 161. [Google Scholar] [CrossRef]
- Koski, P.; Pakarinen, M.; Nakari, T.; Soivio, A.; Hartikainen, K. Treatment with thiamine hydrochloride and astaxanthine for the prevention of yolk-sac mortality in Baltic salmon fry (M74 syndrome). Dis. Aquat. Org. 1999, 37, 209–220. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Juntunen, E.-P.; Iivari, J.; Koski, P.; Nikonen, S.; Rokka, M.; Ritvanen, T.; Pakkala, J.; Heinimaa, P.; Keinänen, M. Lipid-related thiamine deficiency cause mortality of river lampreys (Lampetra fluviatilis) during pre-spawning fasting. Reg. Stud. Mar. Sci. 2023, 62, 14. [Google Scholar] [CrossRef]
- Crozier, L.G.; Siegel, J.E. A comprehensive review of the impacts of climate change on salmon: Strengths and weaknesses of the Literature by life stage. Fishes 2023, 8, 50. [Google Scholar] [CrossRef]
- Bylund, G.; Lerche, O. Thiamine therapy of M74 affected fry of Atlantic salmon Salmo salar. Bull. Eur. Assoc. Fish Pathol. 1995, 15, 93–97. [Google Scholar]
- Futia, M.H.; Connerton, M.J.; Weidel, B.C.; Rinchard, J. Diet predictions of Lake Ontario salmonines based on fatty acids and correlations between their fat content and thiamine concentrations. J. Great Lakes Res. 2019, 45, 934–948. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Rokka, M.; Nikonen, S.; Juntunen, E.-P.; Ritvanen, T.; Heinimaa, P.; Keinänen, M. Model for estimating thiamine deficiency-related mortality of Atlantic salmon (Salmo salar) offspring and variation in the Baltic salmon M74 syndrome. Mar. Freshw. Behav. Physiol. 2021, 54, 97–131. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Gibson, G.E.; Zhang, H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 2002, 40, 493–504. [Google Scholar] [CrossRef]
- Lonsdale, D.; Marrs, C. Thiamine Deficiency Disease, Dysautonomia, and High Calorie Malnutrition; Academic Press: London, UK; San Diego, CA, USA, 2019. [Google Scholar]
- Mommsen, T.P.; Walsh, P.J. Vitellogenesis and oocyte assembly. In Fish Physiology, Volume XI, The Physiology of Developing Fish, Part A Eggs and Larvae; Hoar, W.S., Randall, D.J., Eds.; Academic Press: London, UK, 1988; pp. 347–406. [Google Scholar]
- Keinänen, M.; Nikonen, S.; Käkelä, R.; Ritvanen, T.; Rokka, M.; Myllylä, T.; Pönni, J.; Vuorinen, P.J. High lipid content of prey fish and n-3 PUFA peroxidation impair the thiamine status of feeding-migrating Atlantic salmon (Salmo salar) and is reflected in hepatic biochemical indices. Biomolecules 2022, 12, 526. [Google Scholar] [CrossRef]
- Woodward, B. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture 1994, 124, 133–168. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. Thiamin. In The Vitamins, Fundamental Aspects in Nutrition and Health, 5th ed.; Academic Press: London/Cambridge/Oxford, UK; San Diego, CA, USA, 2017; pp. 297–314. [Google Scholar]
- Keinänen, M.; Käkelä, R.; Ritvanen, T.; Myllylä, T.; Pönni, J.; Vuorinen, P.J. Fatty acid composition of sprat (Sprattus sprattus) and herring (Clupea harengus) in the Baltic Sea as potential prey for salmon (Salmo salar). Helgol. Mar. Res. 2017, 71, 4. [Google Scholar] [CrossRef]
- Thomas, M.J. The role of free radicals and antioxidants: How do we know that they are working? Crit. Rev. Food Sci. 1995, 35, 21–39. [Google Scholar] [CrossRef]
- Spector, A.A. Lipid metabolism: Essential fatty acids. In Biochemical and Physiological Aspects of Human Nutrition; Stipanuk, M.H., Ed.; Saunders/Elsevier: Amsterdam, The Netherlands, 2000; pp. 365–383. [Google Scholar]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Lukienko, P.I.; Mel’nichenko, N.G.; Zverinskii, I.V.; Zabrodskaya, S.V. Antioxidant properties of thiamine. Bull. Exp. Biol. Med. 2000, 130, 874–876. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Rokka, M.; Ritvanen, T.; Käkelä, R.; Nikonen, S.; Pakarinen, T.; Keinänen, M. Changes in thiamine concentrations, fatty acid composition, and some other lipid-related biochemical indices in Baltic Sea Atlantic salmon (Salmo salar) during the spawning run and pre-spawning fasting. Helgol. Mar. Res. 2020, 74, 10. [Google Scholar] [CrossRef]
- Balon, E.K. Terminology of intervals in fish development. J. Fish. Board Can. 1975, 32, 1663–1670. [Google Scholar] [CrossRef]
- Brown, S.B.; Fitzsimons, J.D.; Palace, V.P.; Vandenbyllaardt, L. Thiamine and early mortality syndrome in lake trout. In Early Life Stage Mortality Syndrome in Fishes of the Great Lakes and the Baltic Sea; McDonald, G., Fitzsimons, J.D., Honeyfield, D.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 1998; pp. 18–25. [Google Scholar]
- Keinänen, M.; Tolonen, T.; Ikonen, E.; Parmanne, R.; Tigerstedt, C.; Rytilahti, J.; Soivio, A.; Vuorinen, P.J. Reproduction disorder of Baltic salmon—M74. In Kalatutkimuksia—Fiskundersökningar; Finnish Game and Fisheries Research Institute: Helsinki, Finland, 2000; Volume 165, 38p, (In Finnish with English Abstract). [Google Scholar]
- Brown, S.B.; Arts, M.T.; Brown, L.R.; Brown, M.; Moore, K.; Villella, M.; Fitzsimons, J.D.; Honeyfield, D.C.; Tillitt, D.E.; Zajicek, J.L.; et al. Can diet-dependent factors help explain fish-to-fish variation in thiamine-dependent early mortality syndrome? J. Aquat. Anim. Health 2005, 17, 36–47. [Google Scholar] [CrossRef]
- Amcoff, P.; Börjeson, H.; Landergren, P.; Vallin, L.; Norrgren, L. Thiamine (vitamin B1) concentrations in salmon (Salmo salar), brown trout (Salmo trutta) and cod (Gadus morhua) from the Baltic sea. Ambio 1999, 28, 48–54. [Google Scholar]
- Brown, S.B.; Honeyfield, D.C.; Hnath, J.G.; Wolgamood, M.; Marcquenski, S.V.; Fitzsimons, J.D.; Tillitt, D.E. Thiamine status in adult salmonines in the Great Lakes. J. Aquat. Anim. Health 2005, 17, 59–64. [Google Scholar] [CrossRef]
- Futia, M.H.; Hallenbeck, S.; Noyes, A.D.; Honeyfield, D.C.; Eckerlin, G.E.; Rinchard, J. Thiamine deficiency and the effectiveness of thiamine treatments through broodstock injections and egg immersion on Lake Ontario steelhead trout. J. Great Lakes Res. 2017, 43, 352–358. [Google Scholar] [CrossRef]
- Aro, E. A review of fish migration patterns in the Baltic. Rap. Proc.-Verb. Reun. Cons. Int. Explor. Mer. 1989, 190, 72–96. [Google Scholar]
- Bartel, R. Return of salmon back to Polish waters. Int. J. Ecohydr. Hydrobiol. 2001, 1, 377–392. [Google Scholar]
- Ikonen, E. The Role of the Feeding Migration and Diet of Atlantic Salmon (Salmo salar L.) in Yolk-Sac Fry Mortality (M74) in the Baltic Sea. Ph.D. Thesis, Department of Biological and Environmental Sciences, Faculty of Biosciences, University of Helsinki, Finland and Finnish Game and Fisheries Research Institute, Helsinki, Finland, 2006. [Google Scholar]
- Salminen, M.; Kuikka, S.; Erkamo, E. Divergence in feeding migration of Baltic salmon (Salmo salar L.); the significance of smolt size. Nord. J. Freshw. Res. 1994, 69, 32–42. [Google Scholar]
- Jutila, E.; Jokikokko, E.; Kallio-Nyberg, I.; Saloniemi, I.; Pasanen, P. Differences in sea migration between wild and reared Atlantic salmon (Salmo salar L.) in the Baltic Sea. Fish. Res. 2003, 60, 333–343. [Google Scholar] [CrossRef]
- Kallio-Nyberg, I.; Romakkaniemi, A.; Jokikokko, E.; Saloniemi, I.; Jutila, E. Differences between wild and reared Salmo salar stocks of two northern Baltic Sea rivers. Fish. Res. 2015, 165, 85–95. [Google Scholar] [CrossRef]
- Kallio-Nyberg, I.; Saloniemi, I.; Jutila, E.; Jokikokko, E. Effect of hatchery rearing and environmental factors on the survival, growth and migration of Atlantic salmon in the Baltic Sea. Fish. Res. 2011, 109, 285–294. [Google Scholar] [CrossRef]
- Karlsson, L.; Ikonen, E.; Mitans, A.; Hansson, S. The diet of salmon (Salmo salar) in the Baltic sea and connections with the M74 syndrome. Ambio 1999, 28, 37–42. [Google Scholar]
- Hansson, S.; Karlsson, L.; Ikonen, E.; Christensen, O.; Mitans, A.; Uzars, D.; Petersson, E.; Ragnarsson, B. Stomach analyses of Baltic salmon from 1959-1962 and 1994-1997: Possible relations between diet and yolk-sac-fry mortality (M74). J. Fish Biol. 2001, 58, 1730–1745. [Google Scholar] [CrossRef]
- Mikkonen, J.; Keinänen, M.; Casini, M.; Pönni, J.; Vuorinen, P.J. Relationships between fish stock changes in the Baltic Sea and the M74 syndrome, a reproductive disorder of Atlantic salmon (Salmo salar). ICES J. Mar. Sci. 2011, 68, 2134–2144. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Parmanne, R.; Vartiainen, T.; Keinänen, M.; Kiviranta, H.; Kotovuori, O.; Halling, F. PCDD, PCDF, PCB and thiamine in Baltic herring (Clupea harengus L.) and sprat [Sprattus sprattus (L.)] as a background to the M74 syndrome of Baltic salmon (Salmo salar L.). ICES J. Mar. Sci. 2002, 59, 480–496. [Google Scholar] [CrossRef]
- Røjbek, M.C.; Tomkiewicz, J.; Jacobsen, C.; Støttrup, J.G. Forage fish quality: Seasonal lipid dynamics of herring (Clupea harengus L.) and sprat (Sprattus sprattus L.) in the Baltic Sea. ICES J. Mar. Sci. 2014, 71, 56–71. [Google Scholar] [CrossRef]
- ICES. Baltic fisheries assessment working group (WGBFAS). ICES Sci. Rep. 2020, 2, 643. [Google Scholar] [CrossRef]
- Pönni, J. 3. Kilohaili. In Kalakantojen Tila Vuonna 2021 Sekä Ennuste Vuosille 2022 ja 2023; Luonnonvara- ja biotalouden tutkimus 72; Raitaniemi, J., Sairanen, S., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2022; pp. 24–28. (In Finnish) [Google Scholar]
- Pönni, J. 2. Silakka. In Kalakantojen Tila Vuonna 2021 Sekä Ennuste Vuosille 2022 ja 2023; Luonnonvara- ja biotalouden tutkimus 72; Raitaniemi, J., Sairanen, S., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2022; pp. 9–23. (In Finnish) [Google Scholar]
- Raitaniemi, J.; Natural Resources Institute Finland (Luke), Turku, Finland. Personal communication, 2016.
- Ackman, R.G. Characteristics of the fatty acid composition and biochemistry of some fresh-water fish oils and lipids in comparison with marine oils and lipids. Comp. Biochem. Physiol. 1967, 22, 907–922. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Bowen, W.D.; Ackman, R.G. Among- and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 2002, 59, 886–898. [Google Scholar] [CrossRef]
- Happel, A.; Pattridge, R.; Walsh, M.; Rinchard, J. Assessing diet compositions of Lake Ontario predators using fatty acid profiles of prey fishes. J. Great Lakes Res. 2017, 43, 838–845. [Google Scholar] [CrossRef]
- Happel, A.; Maier, C.; Farese, N.; Czesny, S.; Rinchard, J. Fatty acids differentiate consumers despite variation within prey fatty acid profiles. Freshw. Biol. 2019, 64, 1416–1426. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Keinänen, M.; Heinimaa, P.; Iivari, J.; Juntunen, E.-P.; Kannel, R.; Pakarinen, T.; Romakkaniemi, A. M74-oireyhtymän seuranta Itämeren lohikannoissa. In RKTL:n Työraportteja 41; Finnish Game and Fisheries Research Institute: Helsinki, Finland, 2014; 24p. (In Finnish) [Google Scholar]
- Hiilivirta, P.; Ikonen, E.; Lappalainen, J. Comparison of two methods for distinguishing wild from hatchery-reared salmon (Salmo salar Linnaeus, 1758) in the Baltic Sea. ICES J. Mar. Sci. 1998, 55, 981–986. [Google Scholar] [CrossRef]
- Lind, Y.; Huovila, T.; Käkelä, R. A retrospective study of fatty acid composition in Baltic herring (Clupea harengus membras) caught at three locations in the Baltic Sea (1973–2009). ICES J. Mar. Sci. 2018, 75, 330–339. [Google Scholar] [CrossRef]
- Happel, A.; Jonas, J.L.; McKenna, P.R.; Rinchard, J.; He, J.X.; Czesny, S.J. Spatial variability of lake trout diets in Lakes Huron and Michigan revealed by stomach content and fatty acid profiles. Can. J. Fish. Aquat. Sci. 2018, 75, 95–105. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Karstang, T.V. A general-purpose program for multivariate data- analysis. Chemometr. Intell. Lab. 1987, 2, 235–237. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M. SIMCA: A method for analyzing chemical data in terms of similarity and analogy. In Chemometrics: Theory and Application; Kowalski, B., Ed.; American Chemical Society: Washington, DC, USA, 1977; pp. 243–282. [Google Scholar]
- Corraze, G.; Kaushik, S. Lipids from marine and freshwater fish, Les lipides des poissons marins et d’eau douce. OCL 1999, 6, 111–115. [Google Scholar]
- Polvi, S.M.; Ackman, R.G. Atlantic salmon (Salmo salar) muscle lipids and their response to alternative dietary fatty acid sources. J. Agric. Food Chem. 1992, 40, 1001–1007. [Google Scholar] [CrossRef]
- Gélineau, A.; Corraze, G.; Boujard, T.; Larroquet, L.; Kaushik, S. Relation between dietary lipid level and voluntary feed intake, growth, nutrient gain, lipid deposition and hepatic lipogenesis in rainbow trout. Reprod. Nutr. Dev. 2001, 41, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Penney, Z.L.; Moffitt, C.M. Fatty-acid profiles of white muscle and liver in stream-maturing steelhead trout Oncorhynchus mykiss from early migration to kelt emigration. J. Fish Biol. 2015, 86, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.J.; Lopez-Bote, C.J.; Diez, A.; Corraze, G.; Arzel, J.; Dias, J.; Kaushik, S.J.; Bautista, J.M. Dietary fish oil and digestible protein modify susceptibility to lipid peroxidation in the muscle of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). Br. J. Nutr. 1998, 80, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kjær, M.; Todorcevic, M.; Torstensen, B.; Vegusdal, A.; Ruyter, B. Dietary n-3 HUFA affects mitochondrial fatty acid β-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon. Lipids 2008, 43, 813–827. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Paasivirta, J.; Piilola, T.; Surma-Aho, K.; Tarhanen, J. Organochlorine compounds in Baltic salmon and trout. I. Chlorinated hydrocarbons and chlorophenols 1982. Chemosphere 1985, 14, 1729–1740. [Google Scholar] [CrossRef]
- Jobling, M. Nutrient partitioning and the influence of feed composition on body composition. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 354–375. [Google Scholar]
- Jacobsen, J.A.; Hansen, L.P. Feeding habits of wild and escaped farmed Atlantic salmon, Salmo salar L., in the Northeast Atlantic. ICES J. Mar. Sci. 2001, 58, 916–933. [Google Scholar] [CrossRef]
- Hyvönen, L.; Koivistoinen, P. Fatty acid analysis, TAG equivalents as net fat value, and nutritional attributes of fish and fish products. J. Food Compos. Anal. 1994, 7, 44–58. [Google Scholar] [CrossRef]
- Thurow, F. On Food, Behaviour and Population Mechanism of Baltic Salmon; Report 4; Swedish Salmon Research Institute: Stockholm, Sweden, 1968; pp. 1–16. [Google Scholar]
- Saether, O.; Ellingsen, T.E.; Mohr, V. Lipids of North Atlantic krill. J. Lipid Res. 1987, 27, 274–285. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.-C. Krill for Human Consumption: Nutritional Value and Potential Health Benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Myllylä, T.; Ritvanen, T.; Keinänen, M. Lipid and fatty acid contents in three prey species of Baltic salmon (Salmo salar) from three areas of the Baltic Sea. Manuscript in preparation.
- Salminen, M.; Erkamo, E.; Salmi, J. Diet of post-smolt and one-sea-winter Atlantic salmon in the Bothnian Sea, northern Baltic. J. Fish Biol. 2001, 58, 16–35. [Google Scholar] [CrossRef]
- Budge, S.M.; Penney, S.N.; Lall, S.P. Response of tissue lipids to diet variation in Atlantic salmon (Salmo salar): Implications for estimating diets with fatty acid analysis. J. Exp. Mar. Biol. Ecol. 2011, 409, 267–274. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Keinänen, M.; Kiviranta, H.; Koistinen, J.; Kiljunen, M.; Myllylä, T.; Pönni, J.; Peltonen, H.; Verta, M.; Karjalainen, J. Biomagnification of organohalogens in Atlantic salmon (Salmo salar) from its main prey species in three areas of the Baltic Sea. Sci. Total Environ. 2012, 421–422, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Pickova, J.; Kiessling, A.; Pettersson, A.; Dutta, P.C. Comparison of fatty acid composition and astaxanthin content in healthy and by M74 affected salmon eggs from three Swedish river stocks. Comp. Biochem. Physiol. B 1998, 120, 265–271. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, N.; Zhao, Y.; Wang, X.; Wang, M. Comparison of the fatty acid and triglyceride profiles of big eye tuna (Thunnus obesus), Atlantic salmon (Salmo salar) and bighead carp (Aristichthysnobilis) heads. Molecules 2019, 24, 3983. [Google Scholar] [CrossRef]
- Kallio-Nyberg, I.; Peltonen, H.; Rita, H. Effects of stock-specific and environmental factors on the feeding migration of Atlantic salmon (Salmo salar) in the Baltic Sea. Can. J. Fish. Aquat. Sci. 1999, 56, 853–861. [Google Scholar] [CrossRef]
- Pakarinen, T.; Romakkaniemi, A.; Jokikokko, E.; Orell, P.; Erkinaro, J.; Koljonen, M.-L.; Keinänen, M.; Saura, A.; Jaala, E. 4. Lohi. In Kalakantojen Tila Vuonna 2016 Sekä Ennuste Vuosille 2017 ja 2018; Luonnonvara- ja biotalouden tutkimus 77; Raitaniemi, J., Manninen, K., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2017; pp. 27–49. (In Finnish) [Google Scholar]
- Jacobson, P.; Gådmark, A.; Huss, M. Population and size-specific distribution of Atlantic salmon Salmo salar in the Baltic Sea over five decades. J. Fish Biol. 2020, 96, 408–417. [Google Scholar] [CrossRef]
- Keinänen, M.; Iivari, J.; Juntunen, E.-P.; Kannel, R.; Heinimaa, P.; Nikonen, S.; Pakarinen, T.; Romakkaniemi, A.; Vuorinen, P.J. Thiamine Deficiency M74 of Salmon Can Be Prevented; Riista- ja kalatalous Selvityksiä 14/2014; Luke: Helsinki, Finland, 2014; 41p. (In Finnish) [Google Scholar]
- Holder, P.E.; Wood, C.M.; Lawrence, M.J.; Clark, T.D.; Suski, C.D.; Weber, J.-M.; Danylchuk, A.J.; Cooke, S.J. Are we any closer to understanding why fish can die after severe exercise? Fish Fish. 2022, 23, 1400–1417. [Google Scholar] [CrossRef]
- Pasternack, M.; Salminen, M.; Heinimaa, P. Physiological Condition and Migratory Readiness of Hatchery-Reared Neva Stock Salmon Smolts in 2007–2009; Riista- ja Kalatalous Selvityksiä 16/2010; Finnish Game and Fisheries Research Institute: Helsinki, Finland, 2010; 33p. (In Finnish) [Google Scholar]
- Pönni, J. 1. Silakka. In Kalakantojen Tila Vuonna 2016 Sekä Ennuste Vuosille 2017 ja 2018; Luonnonvara- ja biotalouden tutkimus 77; Raitaniemi, J., Manninen, K., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2017; pp. 6–17. (In Finnish) [Google Scholar]
- ICES. Report of the Baltic Fisheries Assessment Working Group (WGBFAS), 19–26 April 2017, Copenhagen, Denmark, Proceedings of the ICES CM 2017/ACOM:11; The International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2017; p. 810. [Google Scholar]
- Raitaniemi, J.; Manninen, K. (Eds.) Kalakantojen Tila Vuonna 2014 Sekä Ennuste Vuosille 2015 ja 2016; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2015; p. 90. (In Finnish) [Google Scholar]
- Raitaniemi, J.; Manninen, K. (Eds.) Kalakantojen Tila Vuonna 2015 Sekä Ennuste Vuosille 2016 ja 2017; Luonnonvara- ja biotalouden tutkimus 57; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2016; p. 85. (In Finnish) [Google Scholar]
- Pönni, J. Silakka. In Kalakantojen Tila Vuonna 2018 Sekä Ennuste Vuosille 2019 ja 2020; Sairanen, S., Raitaniemi, J., Eds.; Luonnonvara- ja biotalouden tutkimus 48; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2019; pp. 6–16. (In Finnish) [Google Scholar]
- Bagge, O.; Thurow, F.; Steffensen, E.; Bay, J. The Baltic cod. Dana 1994, 10, 1–28. [Google Scholar]
- Niiranen, S.; Orio, A.; Bartolino, V.; Bergström, U.; Kallasvuo, M.; Neuenfeldt, S.; Ustups, D.; Casini, M. Predator-prey body size relationships of cod in a low-diversity marine system. Mar. Ecol.-Progr. Ser. 2019, 627, 201–206. [Google Scholar] [CrossRef]
- Jacobson, P.; Gårdmark, A.; Östergren, J.; Casini, M.; Huss, M. Size-dependent prey availability affects diet and performance of predatory fish at sea: A case study of Atlantic salmon. Ecosphere 2018, 9, e02081. [Google Scholar] [CrossRef]
- ICES. Baltic fisheries assessment working group (WGBFAS). ICES Sci. Rep. 2021, 3, 732. [Google Scholar] [CrossRef]
- Möllmann, C.; Kornilovs, G.; Fetter, M.; Köster, F.W. Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J. Mar. Sci. 2005, 62, 1270–1280. [Google Scholar] [CrossRef]
- Casini, M.; Cardinale, M.; Hjelm, J. Inter-annual variation in herring, Clupea harengus, and sprat, Sprattus sprattus, condition in the central Baltic Sea: What gives the tune? Oikos 2006, 112, 638–650. [Google Scholar] [CrossRef]
- ICES. Baltic salmon and trout assessment working group (WGBAST). ICES Sci. Rep. 2020, 2, 261. [Google Scholar] [CrossRef]
- Axén, C.; Koski, P. Salmon Deaths in Torne River 2014–2016, Report of a Swedish-Finnish Survey, Dnr SVA 2017/59; Dnr Evira/2489/0165/2016; SVA: Uppsala, Sweden; Evira: Oulu, Finland, 2017; 92p, Available online: https://www.sva.se/media/lmkd2dbf/slutrapport_laxdoden-tornealv_2016.pdf (accessed on 21 April 2017)(In Swedish and Finnish).
- Salminen, M. Marine Survival of Atlantic Salmon in the Baltic Sea; Technical Report 4; North Pacific Anadromous Fish Commission: Vancouver, BC, Canada, 2002; pp. 27–29. [Google Scholar]
- Østbye, T.K.; Kjær, M.A.; Rørå, A.M.B.; Torstensen, B.; Ruyter, B. High n-3 HUFA levels in the diet of Atlantic salmon affect muscle and mitochondrial membrane lipids and their susceptibility to oxidative stress. Aquacult. Nutr. 2011, 17, 177–190. [Google Scholar] [CrossRef]
- Czesny, S.; Dettmers, J.M.; Rinchard, J.; Dabrowski, K. Linking egg thiamine and fatty acid concentrations of Lake Michigan lake trout with early life stage mortality. J. Aquat. Anim. Health 2009, 21, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation; Woodhead Publishing Limited: Cambridge, UK, 2012; p. 470. [Google Scholar]
- Parrish, C.C. Essential fatty acids in aquatic food webs. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009; pp. 309–326. [Google Scholar]
- Logue, J.A.; Howell, B.R.; Bell, J.G.; Cossins, A.R. Dietary n-3 long-chain polyunsaturated fatty acid deprivation, tissue lipid composition, ex vivo prostaglandin production, and stress tolerance in juvenile dover sole (Solea solea L.). Lipids 2000, 35, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, G.; Vrede, T.; Goedkoop, W. Fatty acid ratios in freshwater fish, zooplankton and zoobenthos—Are there spesific optima? In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009; pp. 147–178. [Google Scholar]
- Zheng, X.; Torstensen, B.E.; Tocher, D.R.; Dick, J.R.; Henderson, R.J.; Bell, J.G. Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar). BBA-Mol. Cell Biol. L. 2005, 1734, 13–24. [Google Scholar] [CrossRef]
- Morais, S.; Monroig, O.; Zheng, X.; Leaver, M.J.; Tocher, D.R. Highly unsaturated fatty acid synthesis in Atlantic salmon: Characterization of ELOVL5- and ELOVL2-like elongases. Mar. Biotechnol. 2009, 11, 627–639. [Google Scholar] [CrossRef]
- Jacobson, P. Size-Dependent Predator-Prey Interactions, Distribution and Mortality in Salmon: Effects on Individuals and Populations. Ph.D. Thesis, Swedish University of Agricultural Sciences, SLU, Öregrund, Sweden, 2020. [Google Scholar]
- Pönni, J. 2. Kilohaili. In Kalakantojen tila Vuonna 2016 Sekä Ennuste Vuosille 2017 ja 2018; Luonnonvara-ja biotalouden tutkimus 77; Raitaniemi, J., Manninen, K., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2017; pp. 18–21. (In Finnish) [Google Scholar]
- Grisdale-Helland, B.; Gatlin, D.M.; Helland, S.J. Optimization of dietary macronutrients for Atlantic salmon post-smolts using increasing ration levels. Aquaculture 2013, 408, 88–94. [Google Scholar] [CrossRef]
- Dessen, J.E.; Weihe, R.N.; Hatlen, B.; Thomassen, M.S.; Rorvik, K.A. Different growth performance, lipid deposition, and nutrient utilization in in-season (S1) Atlantic salmon post-smolt fed isoenergetic diets differing in protein-to-lipid ratio. Aquaculture 2017, 473, 345–354. [Google Scholar] [CrossRef]
- Hatlen, B.; Berge, K.; Nordrum, S.; Johnsen, K.; Kolstad, K.; Mørkøre, T. The effect of low inclusion levels of Antarctic krill (Euphausia superba) meal on growth performance, apparent digestibility and slaughter quality of Atlantic salmon (Salmo salar). Aquacult. Nutr. 2017, 23, 721–729. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Kiviranta, H.; Koistinen, J.; Pöyhönen, O.; Ikonen, E.; Keinänen, M. Organohalogen concentrations and feeding status in Atlantic salmon (Salmo salar L.) of the Baltic Sea during the spawning run. Sci. Total Environ. 2014, 468–469, 449–456. [Google Scholar] [CrossRef]
- Bell, J.G.; McEvoy, J.; Webster, J.L.; McGhee, F.; Millar, R.M.; Sargent, J.R. Flesh lipid and carotenoid composition of Scottish farmed Atlantic salmon (Salmo salar). J. Agric. Food Chem. 1998, 46, 119–127. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]

| Year | River | Mass | Length | CF | Sea Age | N | Samples for Analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Egg | |||||||||||||
| Lipid | FA | N | Th | N | Lipid | FA | N | |||||||
| 2014 | Simojoki | 7.3 ± 0.5 | 91 ± 2 | 0.94 ± 0.02 | n.a. | (26) | x | x | (16) | x | (26) | x | x | (16) |
| 2016 | Simojoki | 6.6 ± 0.3 | 88 ± 1 | 0.96 ± 0.01 | 2.1 ± 0.1 | (38) | x | x | (30) | x | (38) | x | x | (30) |
| 2016 | Dal | 6.2 ± 0.3 | 86 ± 1 | 0.91 ± 0.02 | 2.1 ± 0.0 | (49) | n.a. | n.a. | x | (49) | n.a. | x | (31) | |
| 2017 | Simojoki | 6.5 ± 3.4 | 86 ± 2 | 1.03 ± 0.07 | 2.2 ± 0.1 | (38) | n.a. | n.a. | x | (38) | n.a. | x | (20) | |
| 2017 | Dal | 6.3 ± 0.2 | 86 ± 1 | 0.96 ± 0.02 | 2.0 ± 0.0 | (52) | n.a. | n.a. | x | (52) | n.a. | x | (20) | |
| 2017 | Neris | 8.9 ± 0.5 | 98 ± 2 | 0.94 ± 0.02 | 2.1 ± 0.2 | (16) | n.a. | n.a. | x | (16) | n.a. | x | (12) | |
| 1997 | Tenojoki | 9.1 ± 1.1 | 98 ± 4 | 0.93 ± 0.03 | n.a. | (8) | n.a. | n.a. | x | (5) | x | x | (8) | |
| Non-M74 Females | M74 Females | Diff. % | |||
|---|---|---|---|---|---|
| Mass, kg | 6.55 ± 0.30 | (17) | 6.18 ± 0.27 | (4) | −5.6 |
| Length, cm | 87.6 ± 1.2 | (17) | 87.5 ± 1.7 | (4) | −0.1 |
| CF | 0.96 ± 0.02 | (17) | 0.92 ± 0.04 | (4) | −4.2 |
| THIAM, nmol g−1 | 1.98 ± 0.23 | (17) | 0.34 ± 0.06 | (4) | −82.7 |
| Muscle lipid, % | 5.47 ± 0.49 | (18) | 7.01 ± 1.31 | (4) | 28.2 |
| Egg lipid, % | 8.29 ± 0.27 | (18) | 8.71 ± 0.36 | (4) | 5.1 |
| 14:0, mg g−1 | 0.76 ± 0.11 | (18) | 0.93 ± 0.14 | (4) | 22.4 |
| 16:0, mg g−1 | 7.01 ± 0.66 | (18) | 7.82 ± 0.92 | (4) | 11.6 |
| 17:0, mg g−1 | 0.14 ± 0.01 | (18) | 0.17 ± 0.02 | (4) | 21.4 |
| 18:0, mg g−1 | 2.06 ± 0.18 | (18) | 2.39 ± 0.32 | (4) | 16.0 |
| 16:1n−7, mg g−1 | 1.48 ± 0.16 | (18) | 1.76 ± 0.27 | (4) | 18.9 |
| 17:1n−8, mg g−1 | 0.15 ± 0.02 | (18) | 0.18 ± 0.03 | (4) | 20.0 |
| 18:1n−7, mg g−1 | 1.73 ± 0.16 | (18) | 1.99 ± 0.26 | (4) | 15.0 |
| 18:1n−9, mg g−1 | 11.73 ± 1.13 | (18) | 14.94 ± 2.71 | (4) | 27.4 |
| 20:1n−9, mg g−1 | 0.81 ± 0.08 | (18) | 1.07 ± 0.23 | (4) | 32.1 |
| 18:2n−6, mg g−1 | 1.55 ± 0.15 | (18) | 1.87 ± 0.31 | (4) | 20.6 |
| 20:2n−6, mg g−1 | 0.34 ± 0.03 | (18) | 0.44 ± 0.09 | (4) | 29.4 |
| 18:3n−3, mg g−1 | 0.94 ± 0.09 | (18) | 1.17 ± 0.19 | (4) | 24.5 |
| 20:4n−6 (ARA), mg g−1 | 0.34 ± 0.02 | (18) | 0.43 ± 0.07 | (4) | 26.5 |
| 20:5n−3 (EPA), mg g−1 | 3.26 ± 0.28 | (18) | 4.27 ± 0.80 | (4) | 31.0 |
| 22:5n−3 (DPA), mg g−1 | 2.65 ± 0.30 | (18) | 3.72 ± 0.96 | (4) | 40.4 |
| 22:6n−3 (DHA), mg g−1 | 13.98 ± 1.15 | (18) | 19.32 ± 4.76 | (4) | 37.9 |
| n−3 PUFA, mg g−1 | 21.32 ± 1.84 | (18) | 29.12 ± 6.77 | (4) | 36.6 |
| n−6 PUFA, mg g−1 | 2.37 ± 0.21 | (18) | 2.92 ± 0.50 | (4) | 23.2 |
| SFA, mg g−1 | 10.7 ± 1.0 | (18) | 12.0 ± 1.4 | (4) | 12.1 |
| MUFA, mg g−1 | 17.47 ± 1.68 | (18) | 22.20 ± 4.00 | (4) | 26.9 |
| PUFA, mg g−1 | 25.00 ± 2.13 | (18) | 33.72 ± 7.58 | (4) | 34.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuorinen, P.J.; Käkelä, R.; Pakarinen, T.; Heinimaa, P.; Ritvanen, T.; Nikonen, S.; Rokka, M.; Keinänen, M. Thiamine Deficiency M74 Developed in Salmon (Salmo salar) Stocks in Two Baltic Sea Areas after the Hatching of Large Year-Classes of Two Clupeid Species—Detected by Fatty Acid Signature Analysis. Fishes 2024, 9, 58. https://doi.org/10.3390/fishes9020058
Vuorinen PJ, Käkelä R, Pakarinen T, Heinimaa P, Ritvanen T, Nikonen S, Rokka M, Keinänen M. Thiamine Deficiency M74 Developed in Salmon (Salmo salar) Stocks in Two Baltic Sea Areas after the Hatching of Large Year-Classes of Two Clupeid Species—Detected by Fatty Acid Signature Analysis. Fishes. 2024; 9(2):58. https://doi.org/10.3390/fishes9020058
Chicago/Turabian StyleVuorinen, Pekka J., Reijo Käkelä, Tapani Pakarinen, Petri Heinimaa, Tiina Ritvanen, Soili Nikonen, Mervi Rokka, and Marja Keinänen. 2024. "Thiamine Deficiency M74 Developed in Salmon (Salmo salar) Stocks in Two Baltic Sea Areas after the Hatching of Large Year-Classes of Two Clupeid Species—Detected by Fatty Acid Signature Analysis" Fishes 9, no. 2: 58. https://doi.org/10.3390/fishes9020058
APA StyleVuorinen, P. J., Käkelä, R., Pakarinen, T., Heinimaa, P., Ritvanen, T., Nikonen, S., Rokka, M., & Keinänen, M. (2024). Thiamine Deficiency M74 Developed in Salmon (Salmo salar) Stocks in Two Baltic Sea Areas after the Hatching of Large Year-Classes of Two Clupeid Species—Detected by Fatty Acid Signature Analysis. Fishes, 9(2), 58. https://doi.org/10.3390/fishes9020058






