Characterization and Functional Evaluation of NK-lysin from Clownfish (Amphiprion ocellaris)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish, Cell Lines, Virus, and Bacteria
2.3. Cloning the Open Reading Frame (ORF) of AoNK-lysin
2.4. Sequence Analysis of the AoNK-lysin ORF Gene
2.5. Distribution Expression of AoNK-lysin
2.6. Clownfish Challenged after SGIV Infection
2.7. Plasmid Construction
2.8. Yeast Expression and Purification of the AoNK-lysin Protein
2.9. Antibacterial Function of AoNK-lysin
2.10. Effect of the AoNK-lysin Protein on Viral Gene Transcription
2.11. Statistical Analysis
3. Results
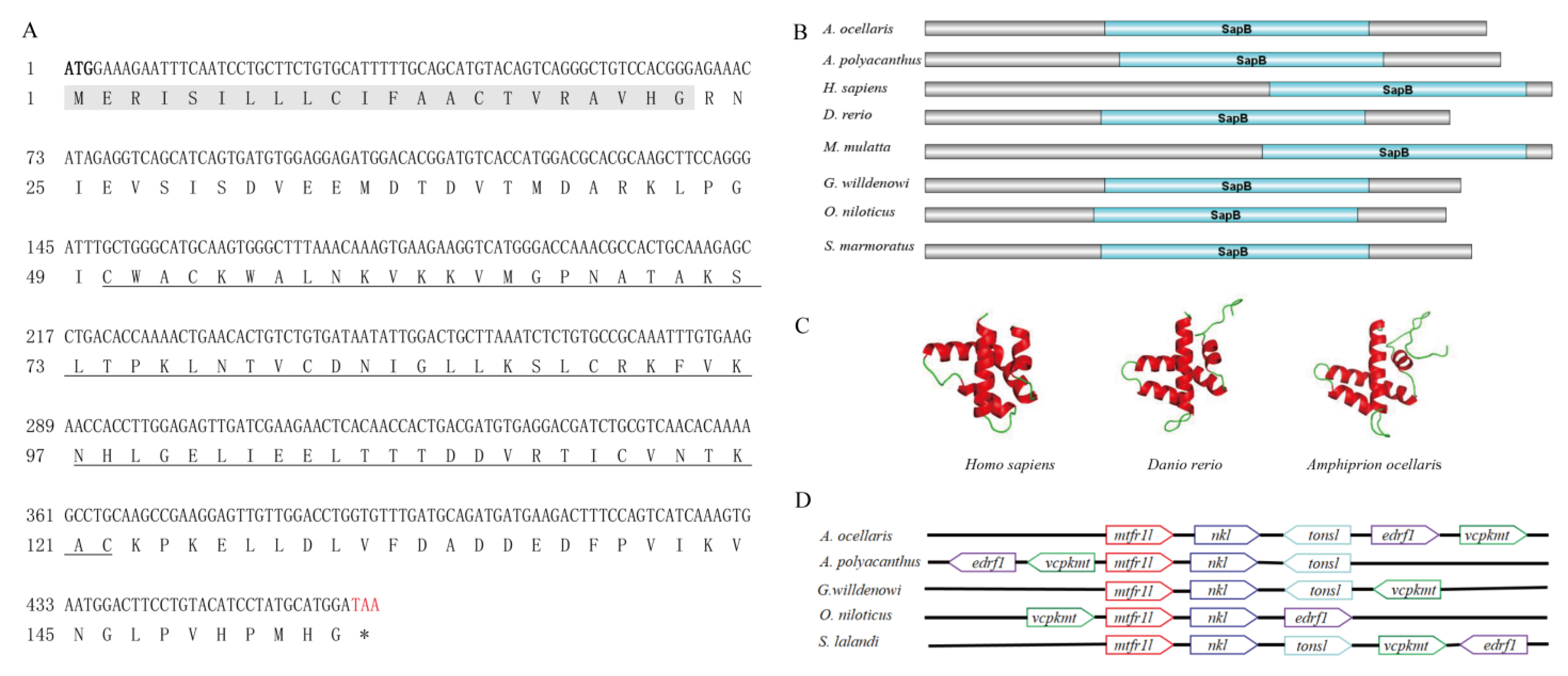
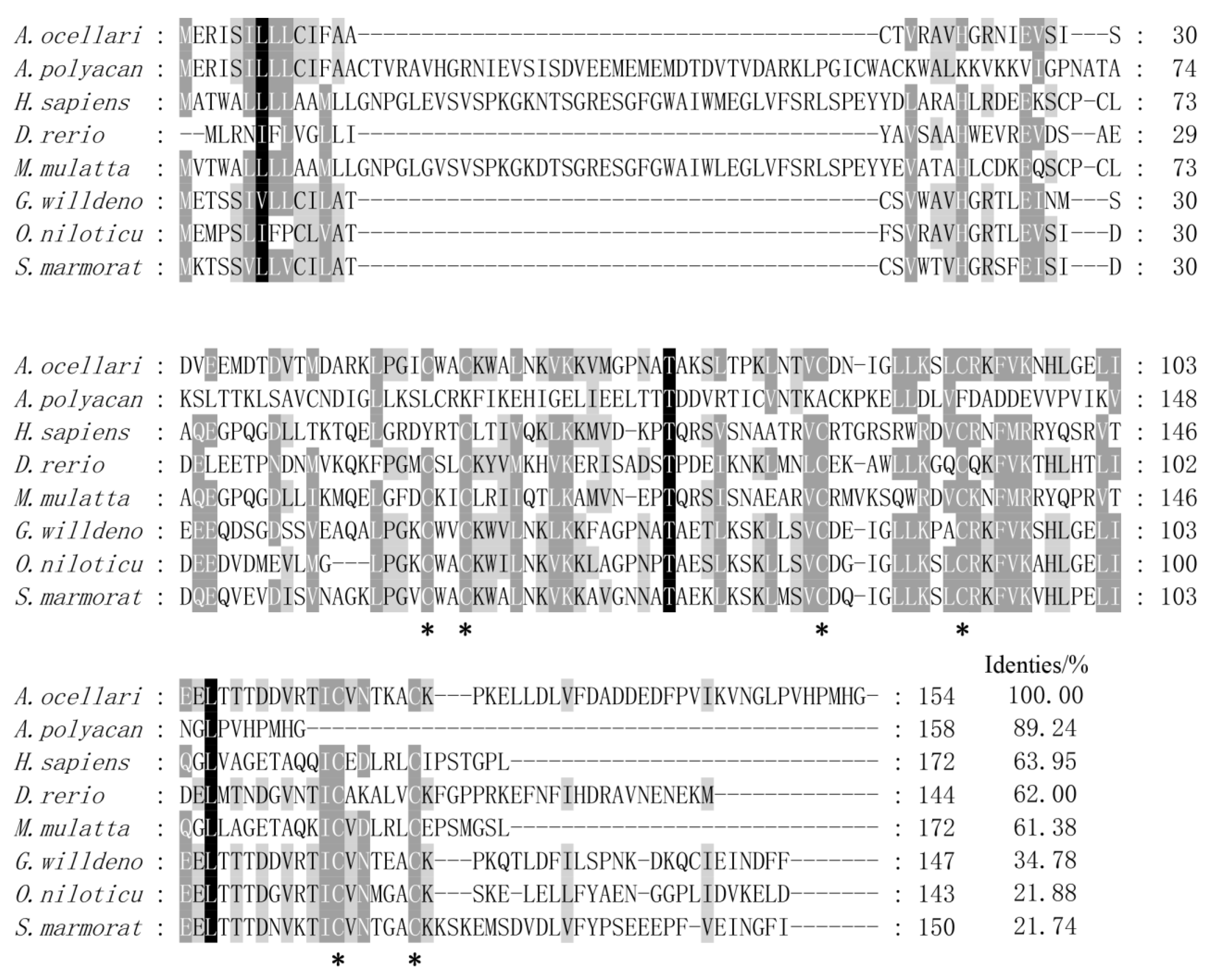
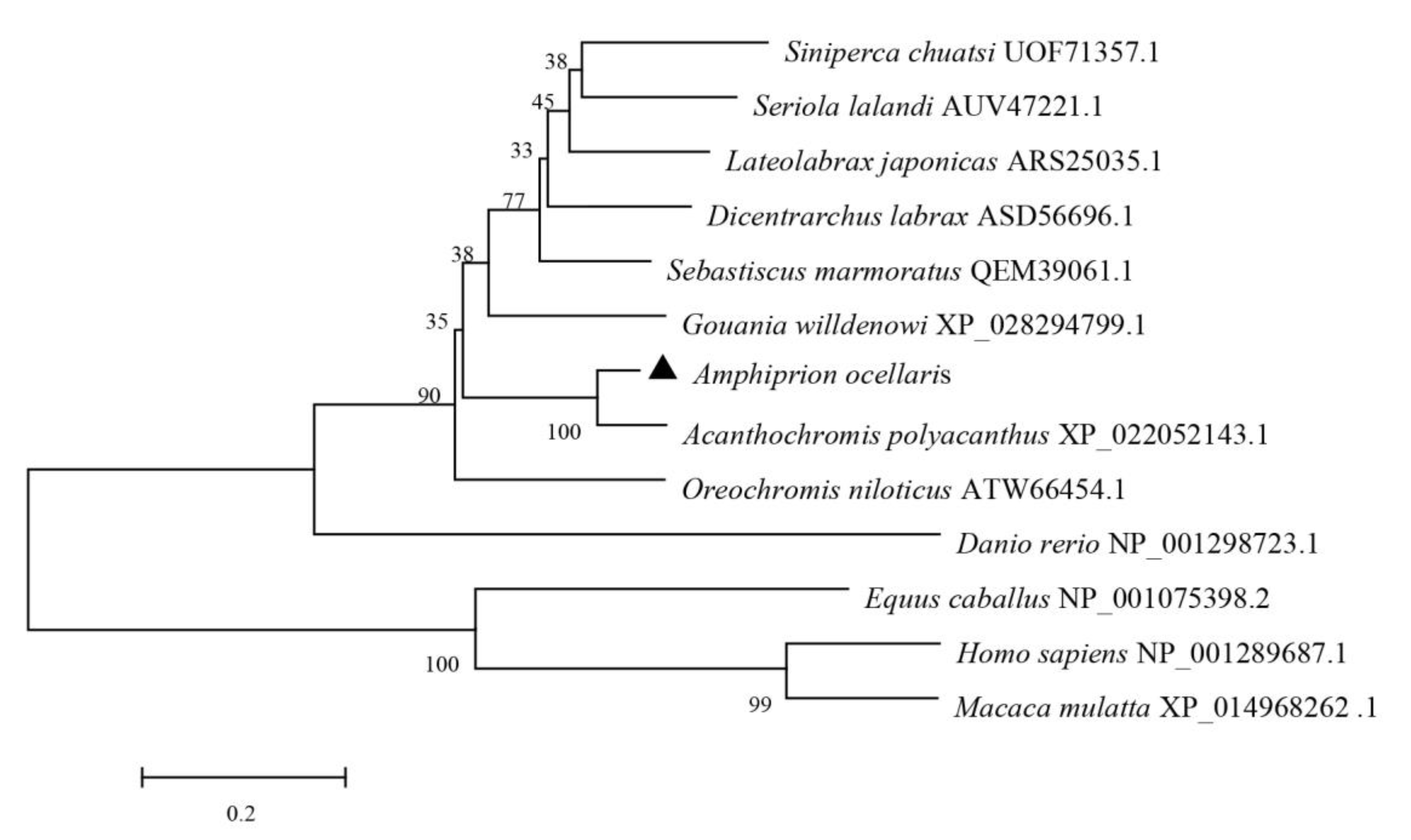
3.1. Sequence Analysis of AoNK-lysin
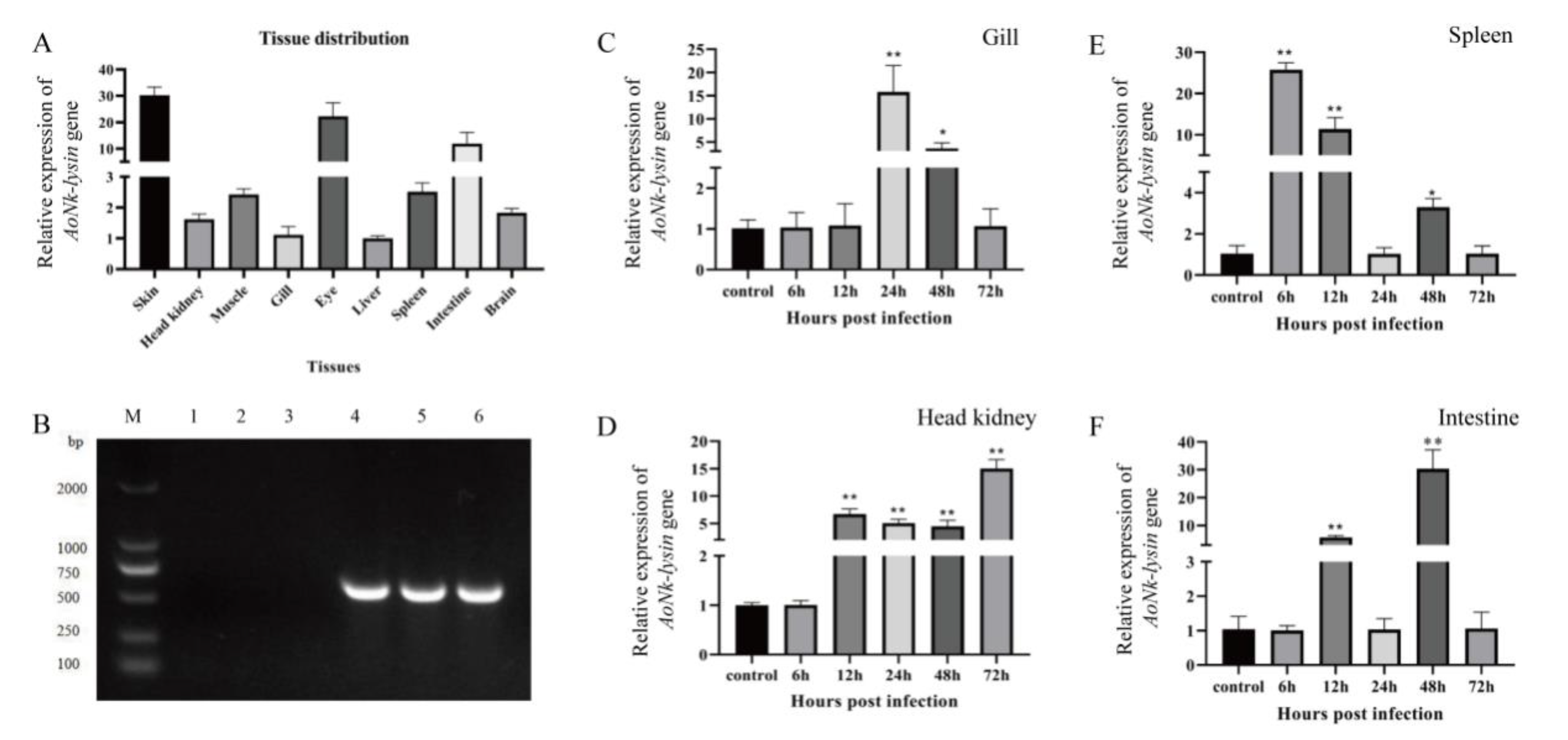
3.2. The Expressions of AoNK-lysin in Clownfish
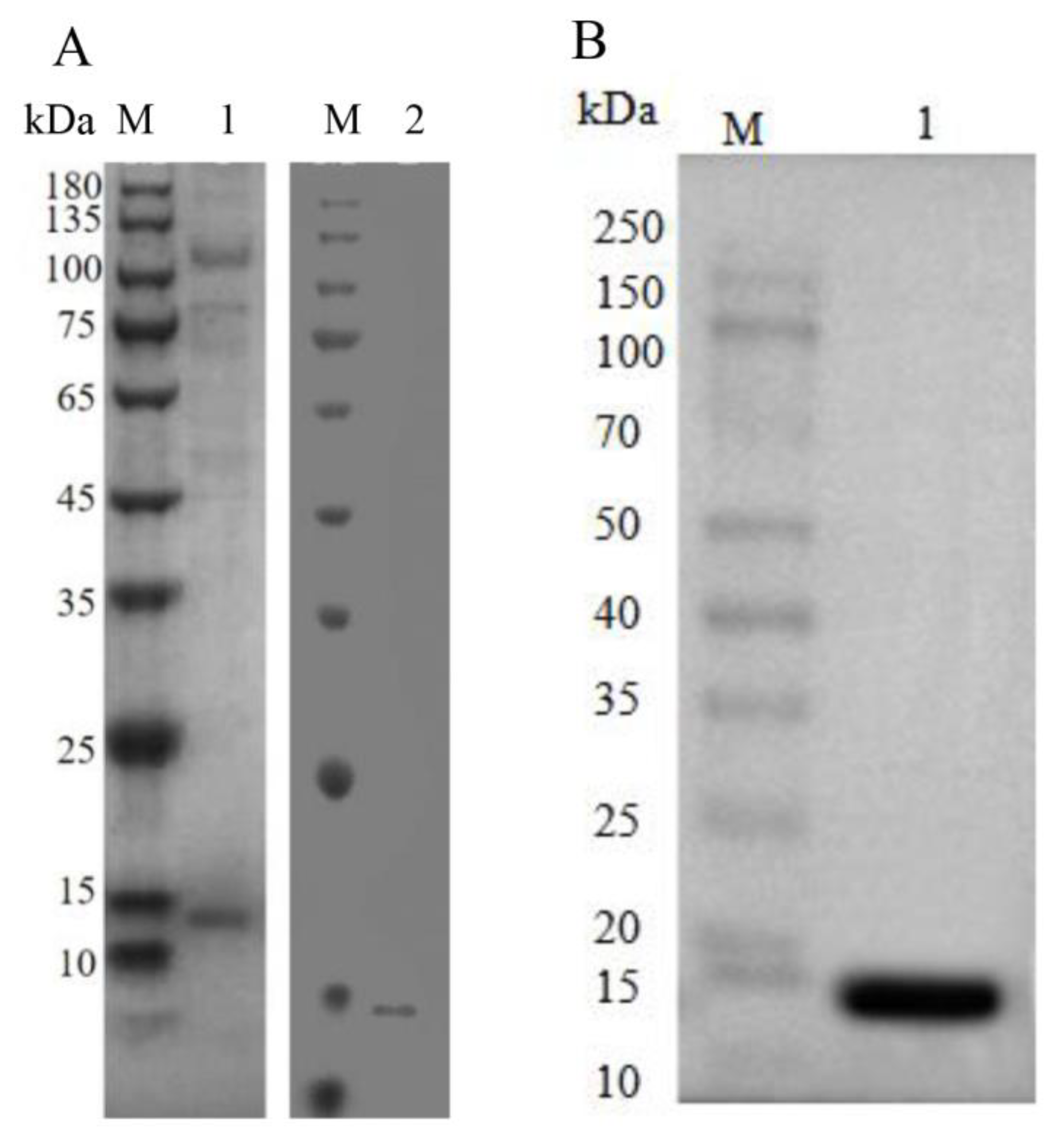
3.3. Recombinant AoNK-lysin Protein Expression and Antibacterial Activity
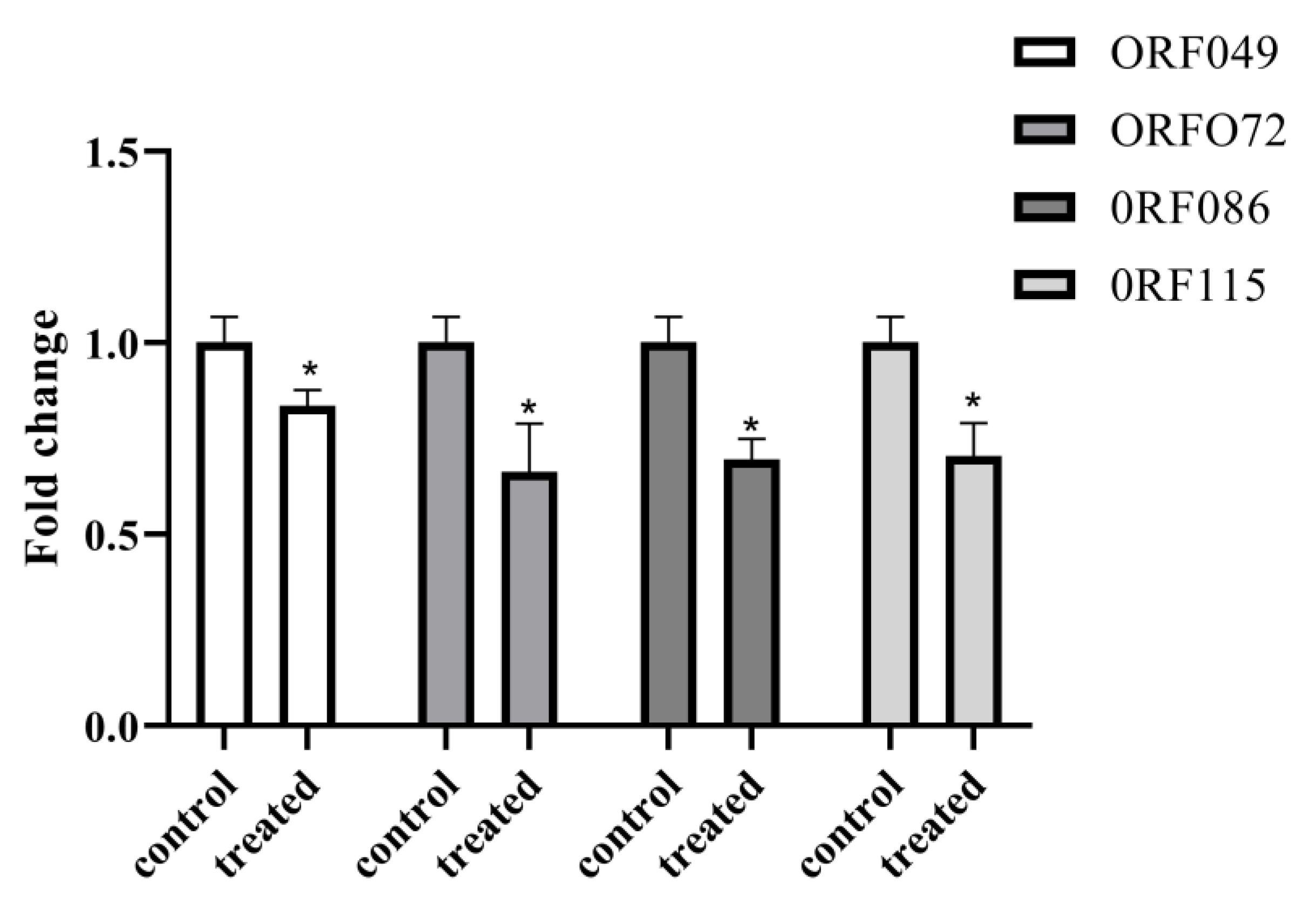
3.4. Bioactivity Analysis of AoNK-lysin Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roux, N.; Salis, P.; Lee, S.-H.; Besseau, L.; Laudet, V. Anemonefish, a Model for Eco-Evo-Devo. Evodevo 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, D.S.; Omeka, W.K.M.; Yang, H.; Lim, C.; Kwon, H.; Choi, C.Y.; Lee, J. Expression Profiling, Immune Functions, and Molecular Characteristics of the Tetraspanin Molecule CD63 from Amphiprion Clarkii. Dev. Comp. Immunol. 2021, 123, 104168. [Google Scholar] [CrossRef] [PubMed]
- Izumida, M.; Nagai, M.; Ohta, A.; Hashimoto, S.; Kawado, M.; Murakami, Y.; Tada, Y.; Shigematsu, M.; Yasui, Y.; Taniguchi, K. Epidemics of Drug-Resistant Bacterial Infections Observed in Infectious Disease Surveillance in Japan, 2001–2005. J. Epidemiol. 2007, 17, S42–S47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Li, M.; Sun, L. NKLP27: A Teleost NK-lysin Peptide That Modulates Immune Response, Induces Degradation of Bacterial DNA, and Inhibits Bacterial and Viral Infection. PLoS ONE 2014, 9, e106543. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engstrm, A.; Bennich, H.; Boman, H.G. Sequence and Specificity of Two Antibacterial Proteins Involved in Insect Immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial CDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Rao, A.G. Antimicrobial Peptides. Mol. Plant Microbe Interact. 1995, 8, 6–13. [Google Scholar] [CrossRef]
- Ernst, W.A.; Thoma-Uszynski, S.; Teitelbaum, R.; Ko, C.; Hanson, D.A.; Clayberger, C.; Krensky, A.M.; Leippe, M.; Bloom, B.R.; Ganz, T.; et al. Granulysin, a T Cell Product, Kills Bacteria by Altering Membrane Permeability. J. Immunol. 2000, 165, 7102–7108. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Li, Y.; Xia, H.; Xia, L.; Lei, Y.; Dong, Z.; Ye, J.; Lu, Y. Study on the Expression of NK-lysin from Nile Tilapia (Oreochromis Niloticus) in Pichia Pastoris and Its Biological Function. Aquaculture 2022, 557, 738321. [Google Scholar] [CrossRef]
- Bruhn, H.; Leippe, M. Comparative Modeling of Amoebapores and Granulysin Based on the NK-lysin Structure-Structural and Functional Implications. Biol. Chem. 1999, 380, 1001–1007. [Google Scholar] [CrossRef]
- Yu, D.; Weng, T.; Chen, J.; Lu, Y. Functional Characterization of a Grouper Nklysin with Antibacterial and Antiviral Activity. Fish Shellfish Immunol. 2022, 131, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Long, H.; Sun, L. A NK-lysin from Cynoglossus Semilaevis Enhances Antimicrobial Defense against Bacterial and Viral Pathogens. Dev. Comp. Immunol. 2013, 40, 258–265. [Google Scholar] [CrossRef]
- Acosta, J.; Roa, F.; González-Chavarría, I.; Astuya, A.; Maura, R.; Montesino, R.; Muñoz, C.; Camacho, F.; Saavedra, P.; Valenzuela, A.; et al. In Vitro Immunomodulatory Activities of Peptides Derived from Salmo Salar NK-lysin and Cathelicidin in Fish Cells. Fish Shellfish Immunol. 2019, 88, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Chen, J.; Liu, Z.-M.; Lin, Z.-H.; Guo, Z.-P. Barbel Steed (Hemibarbus Labeo) NK-lysin Protects against Aeromonas Hydrophila Infection via Immunomodulatory Activity. Dev. Comp. Immunol. 2021, 122, 104114. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; González-Fernández, C.; Cárdenas, C.; Guzmán, F.; León, R.; Cuesta, A. NK-lysin Peptides Ameliorate Viral Encephalopathy and Retinopathy Disease Signs and Provide Partial Protection against Nodavirus Infection in European Sea Bass. Antivir. Res. 2021, 192, 105104. [Google Scholar] [CrossRef] [PubMed]
- Lama, R.; Pereiro, P.; Costa, M.M.; Encinar, J.A.; Medina-Gali, R.M.; Pérez, L.; Lamas, J.; Leiro, J.; Figueras, A.; Novoa, B. Turbot (Scophthalmus Maximus) NK-lysin Induces Protection against the Pathogenic Parasite Philasterides Dicentrarchi via Membrane Disruption. Fish Shellfish Immunol. 2018, 82, 190–199. [Google Scholar] [CrossRef]
- Meng, D.-M.; Li, W.-J.; Shi, L.-Y.; Lv, Y.-J.; Sun, X.-Q.; Hu, J.-C.; Fan, Z.-C. Expression, Purification and Characterization of a Recombinant Antimicrobial Peptide Hispidalin in Pichia Pastoris. Protein Expr. Purif. 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.-P.; Chen, B.; Hao, H.; Wang, K.-J. Optimized Production of Scygonadin in Pichia Pastoris and Analysis of Its Antimicrobial and Antiviral Activities. Protein Expr. Purif. 2012, 82, 37–44. [Google Scholar] [CrossRef]
- Cai, J.; Yu, D.; Wei, S.; Tang, J.; Lu, Y.; Wu, Z.; Qin, Q.; Jian, J. Identification of the Bcl-2 Family Protein Gene BOK from Orange-Spotted Grouper (Epinephelus Coioides) Involved in SGIV Infection. Fish Shellfish Immunol. 2016, 52, 9–15. [Google Scholar] [CrossRef]
- Decuypere, E.; Hassanzadeh, M.; Buys, N.; Buyse, J. Further Insights into the Susceptibility of Broilers to Ascites. Vet. J. 2005, 169, 319–320. [Google Scholar] [CrossRef]
- Endsley, J.; Furrer, J.L.; Endsley, M.; Mcintosh, M.; Maue, A.; Waters, W.; Lee, D.R.; Estes, D. Characterization of Bovine Homologues of Granulysin and NK-lysin. J. Immunol. 2004, 173, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Q.; Niu, J.; Tang, J.; Wang, B.; Abarike, E.D.; Lu, Y.; Cai, J.; Jian, J. NK-lysin from Oreochromis Niloticus Improves Antimicrobial Defence against Bacterial Pathogens. Fish Shellfish Immunol. 2018, 72, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, Z.; Mao, L.; Chang, J. Isolation and Identification of Vibrio Rotiferianus from Diseased Half-Smooth Tongue Sole (Cynoglossus Semilaevis Günther). In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; pp. 2267–2272. [Google Scholar] [CrossRef]
- Pereiro, P.; Varela, M.; Diaz-Rosales, P.; Romero, A.; Dios, S.; Figueras, A.; Novoa, B. Zebrafish NK-lysins: First Insights about Their Cellular and Functional Diversification. Dev. Comp. Immunol. 2015, 51, 148–159. [Google Scholar] [CrossRef]
- Liu, B.; Liu, G.-D.; Guo, H.-Y.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Jiang, S.-G.; Zhang, D.-C. Functional Characterization of NK-lysin in Golden Pompano Trachinotus Ovatus (Linnaeus 1758). Dev. Comp. Immunol. 2020, 107, 103658. [Google Scholar] [CrossRef]
- Ding, F.F.; Li, C.H.; Chen, J. Molecular Characterization of the NK-lysin in a Teleost Fish, Boleophthalmus Pectinirostris: Antimicrobial Activity and Immunomodulatory Activity on Monocytes/Macrophages. Fish Shellfish Immunol. 2019, 92, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.; Ming, C.; Ying, L.; Qian, Z.B.; Cfl, A.; Pan, T.; Ezl, A.; Pnb, C.; Hai, X. Identification, Expression Analysis, and Antibacterial Activity of NK-lysin from Common Carp Cyprinus Carpio. Fish Shellfish Immunol. 2018, 73, 11–21. [Google Scholar] [CrossRef]
- Valero, Y.; Cortés, J.; Mercado, L. NK-lysin from Skin-Secreted Mucus of Atlantic Salmon and Its Potential Role in Bacteriostatic Activity. Fish Shellfish Immunol. 2019, 87, 410–413. [Google Scholar] [CrossRef]
- Qin, Q.W.; Lam, T.J.; Sin, Y.M.; Shen, H.; Chang, S.F.; Ngoh, G.H.; Chen, C.L. Electron Microscopic Observations of a Marine Fish Iridovirus Isolated from Brown-Spotted Grouper, Epinephelus Tauvina. J. Virol. Methods 2001, 98, 17–24. [Google Scholar] [CrossRef]
- Ou-Yang, Z.; Wang, P.; Huang, Y.; Huang, X.; Wan, Q.; Sheng, Z.; Wei, J.; Zhou, Y.; Qin, Q. Selection and Identification of Singapore Grouper Iridovirus Vaccine Candidate Antigens Using Bioinformatics and DNA Vaccination. Vet. Immunol. Immunopathol. 2012, 149, 38–45. [Google Scholar] [CrossRef]
- Sun, Z.P.; Xiang, X.U.; Qiang, L.I.; Shi-Gen, Y.E.; Hua, L.I. Cloning, and Expression of Red Sea Bream Iridovirus Liaoning Strain (ORF049L) Gene. J. Dalian Ocean Univ. 2013, 28, 148–153. [Google Scholar]
- Xia, L.; Zhang, H.; Liang, H.; Liu, S. Prokaryotic Expression, Purification and Antibody Preparation of SGIV ORF086 Protein. J. Shanghai Ocean Univ. 2010, 19, 1–6. [Google Scholar]
| Primer Name | Sequence (5′−3′) | Purpose |
|---|---|---|
| AoNK-OF | ATGGAAAGAATTTCAATCCTG | Amplification of ORF of AoNK-lysin |
| AoNK-OR | TCCATGCATAGGATGTACAG | |
| AoNK-MF | CCGGAATTCAGAAACATAGAGGTCAGCATCAGTGA | Amplification of sequence of mature AoNK-lysin |
| AoNK-MR | AAGGAAAAAAGCGGCCGCTTACATCATCACCATCACCATTCCATGCATAGGATGTACAGGA | |
| 5′ AOX1 | GACTGGTTCCAATTGACAAGC | Identification of integration in pPic9K |
| 3′ AOX1 | GCAAATGGCATTCTGACATCC | |
| SGIV-F | AGAGTTTTCGGTCGGGGTTC | Amplification of sequence of SGIV MCP |
| SGIV-R | GAAACGAGACCCACGGTCAT | |
| β-actin-F | GGGCCAAAAGGACAGCTAC | qRT-PCR in AoNK-lysin |
| β-actin-R | CAGGGTCAGGATACCCCTCT | |
| qNKlysin-F | TGGAGGAGATGGACACGGAT | |
| qNKlysin-R | TGGAGGAGATGGACACGGAT | |
| qORF115-F | CGGAAAGAACACAGATAACGG | qRT-PCR in SGIV |
| qORF115-R | AAAAAACACATGGCTTGCAAA | |
| qORF072-F | GCACGCTTCTCTCACCTTCA | |
| qORF072-R | AACGGCAACGGGAGCACTA | |
| qORF049-F | ATGTACGTATACCCCGCAAT | |
| qORF049-R | TCATTTTTTTTGCCTAA | |
| qORF086-F | ATCGGATCTACGTGGTTGG | |
| qORF086-R | CCGTCGTCGGTGTCTATTC | |
| qActin-F | TACGAGCTGCCTGACGGACA | |
| qActin-R | GGCTGTGATCTCCTTCTGCA |
| Bacterial Strains | Minimal Inhibitory Concentration (MIC; μg/mL) | ||
|---|---|---|---|
| AoNK-lysin | Kana+ | ||
| Gram-positive bacteria | Staphylococcus aureus | 125 | + |
| Bacillus subtilis | 15.63 | + | |
| Streptococcus agalactiae | 7.81 | + | |
| Streptococcus iniae | 7.81 | + | |
| Gram-negative bacteria | Edwardsiella tarda | 31.25 | + |
| Escherichia coli | >250 | + | |
| Vibrio cholerae | >250 | + | |
| Klebsiella pnenmoniae | 31.25 | + | |
| Salmonella typhi | 7.81 | + | |
| Aeromonas hydrophila | 31.25 | + | |
| Shigella sonnei | 7.81 | + | |
| Pseudomonas aeruginosa | 7.81 | + | |
| Aeromonas caviae | 7.81 | + | |
| Proteus vulgaris | >250 | + | |
| Salmonella typhimurium | 125 | + | |
| Proteus mirabilis | 15.63 | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Zhao, H.; Wen, Y.; Li, T.; Xia, H.; Wang, Z.; Gan, Z.; Xia, L.; Chen, J.; Lu, Y. Characterization and Functional Evaluation of NK-lysin from Clownfish (Amphiprion ocellaris). Fishes 2023, 8, 533. https://doi.org/10.3390/fishes8110533
Yu D, Zhao H, Wen Y, Li T, Xia H, Wang Z, Gan Z, Xia L, Chen J, Lu Y. Characterization and Functional Evaluation of NK-lysin from Clownfish (Amphiprion ocellaris). Fishes. 2023; 8(11):533. https://doi.org/10.3390/fishes8110533
Chicago/Turabian StyleYu, Dapeng, Haohang Zhao, Yiming Wen, Tao Li, Hongli Xia, Zhiwen Wang, Zhen Gan, Liqun Xia, Jianlin Chen, and Yishan Lu. 2023. "Characterization and Functional Evaluation of NK-lysin from Clownfish (Amphiprion ocellaris)" Fishes 8, no. 11: 533. https://doi.org/10.3390/fishes8110533
APA StyleYu, D., Zhao, H., Wen, Y., Li, T., Xia, H., Wang, Z., Gan, Z., Xia, L., Chen, J., & Lu, Y. (2023). Characterization and Functional Evaluation of NK-lysin from Clownfish (Amphiprion ocellaris). Fishes, 8(11), 533. https://doi.org/10.3390/fishes8110533






