Sea Bass (Dicentrarchus labrax) Tail-Beat Frequency Measurement Using Implanted Bioimpedance Sensing
Abstract
1. Introduction
2. Bioimpedance Measurement Principle and Applications
3. Materials and Methods
3.1. Ethical Approval
3.2. Materials
3.2.1. Animals
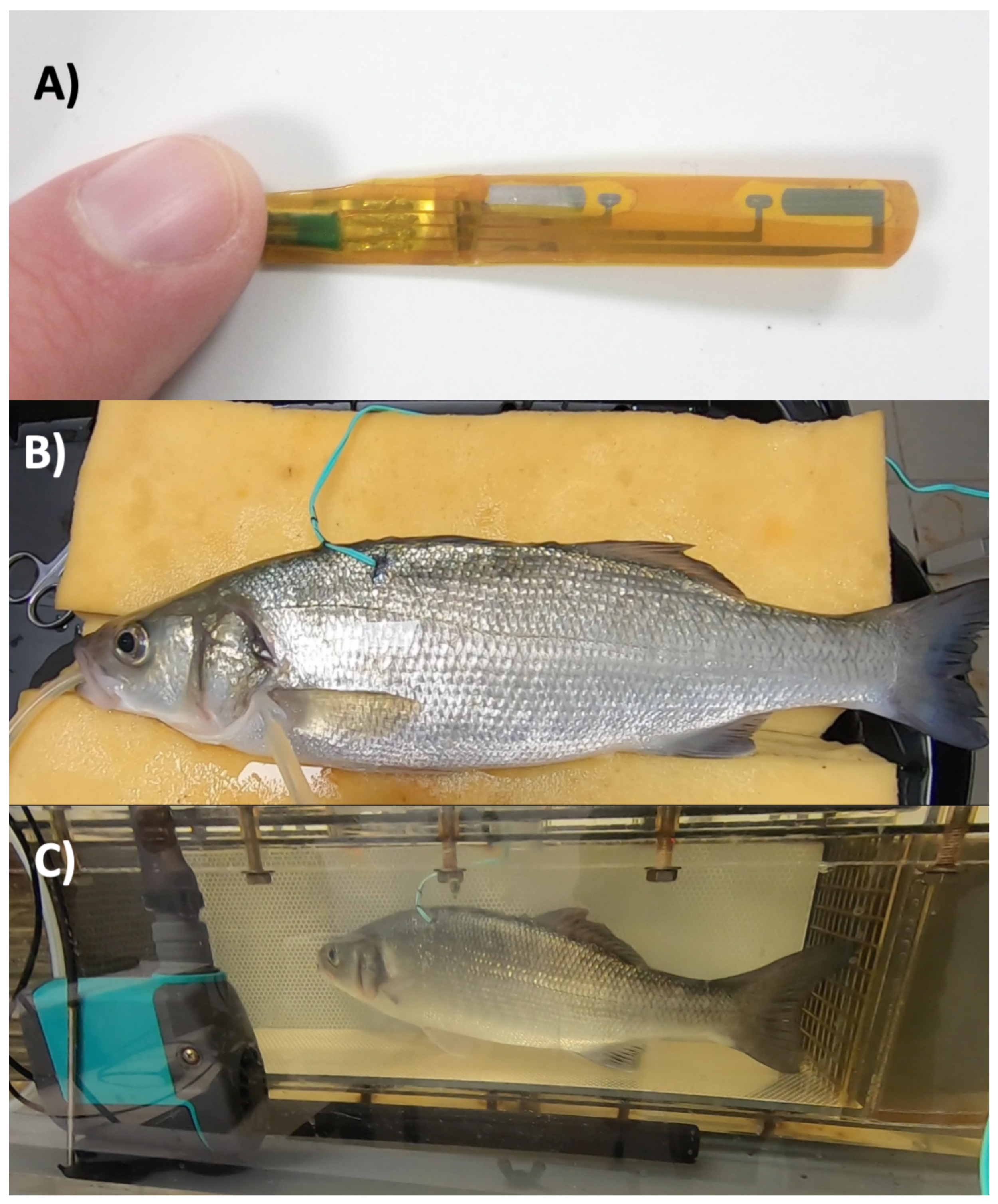
3.2.2. Bioimpedance Measurement
3.2.3. Respirometer
3.2.4. Video
3.3. Methods
3.3.1. Surgery
3.3.2. Respirometer
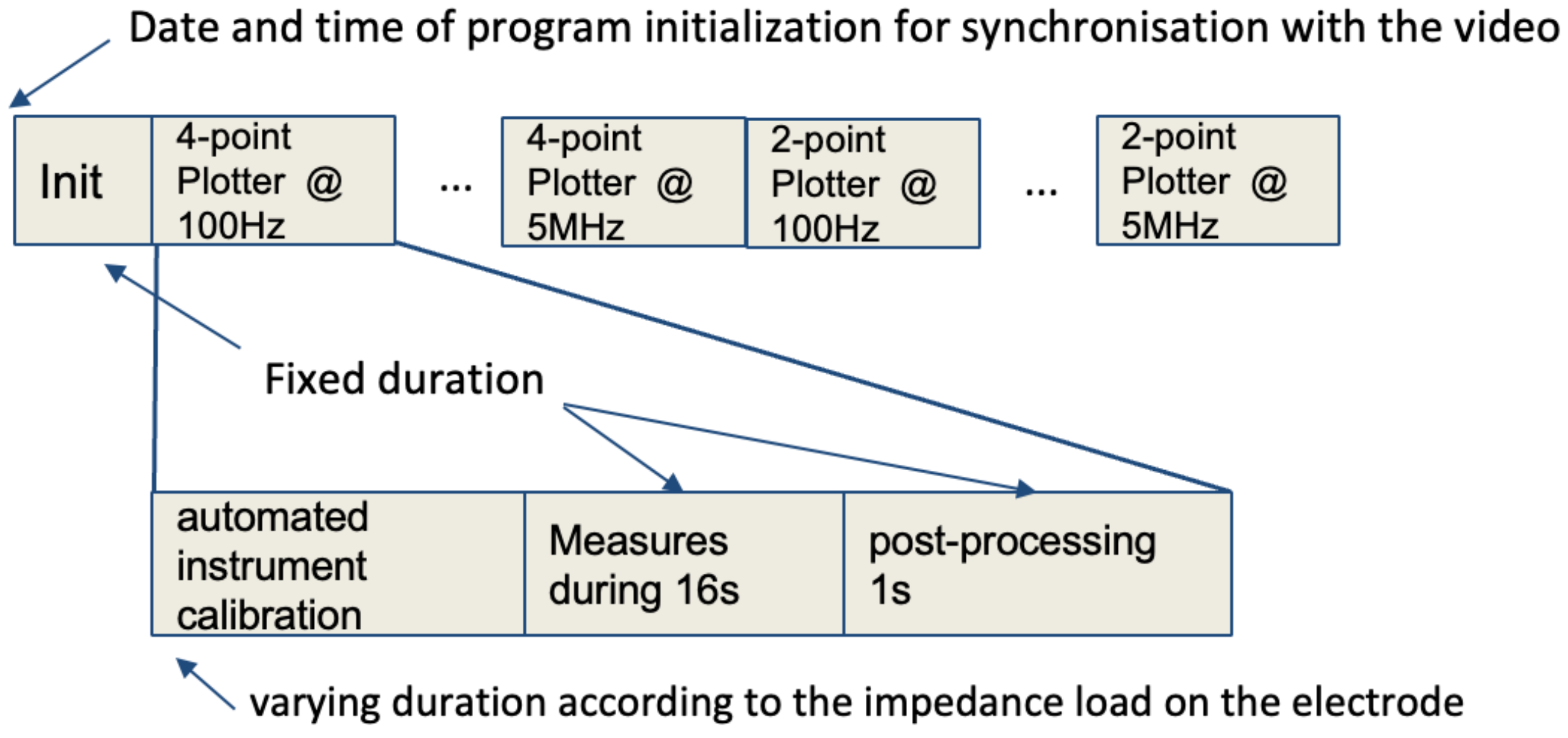
3.3.3. Bioimpedance Measurement
- Measurement setup: 4-point (4 pts) or 2-point (2 pts).
- Bioimpedance frequencies for each measurement: 100 , 1 , 10 , 100 , 1 , and 5 .
3.4. Data Analysis
3.4.1. Video
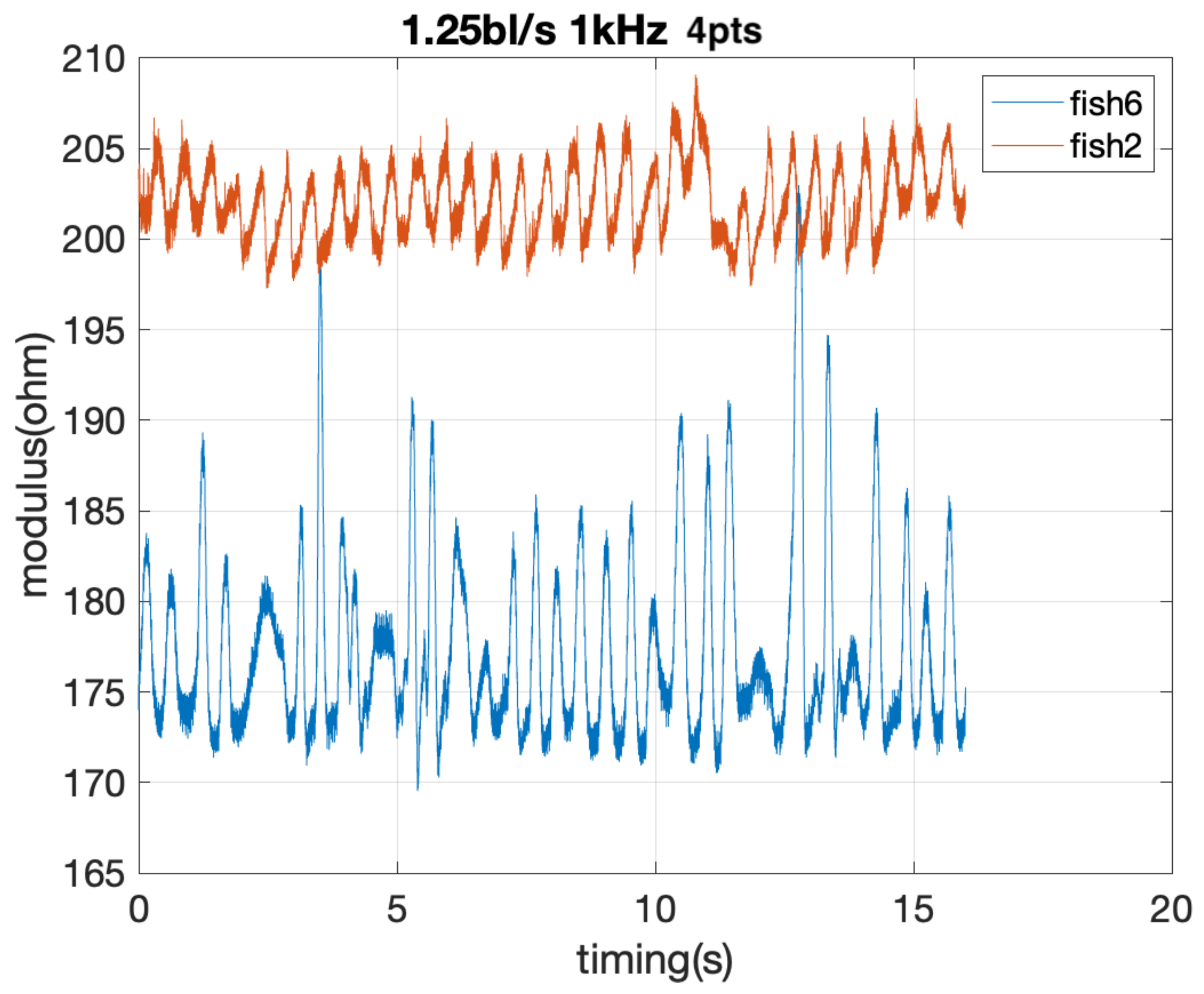
3.4.2. Bioimpedance
3.4.3. Statistical Analysis
4. Results
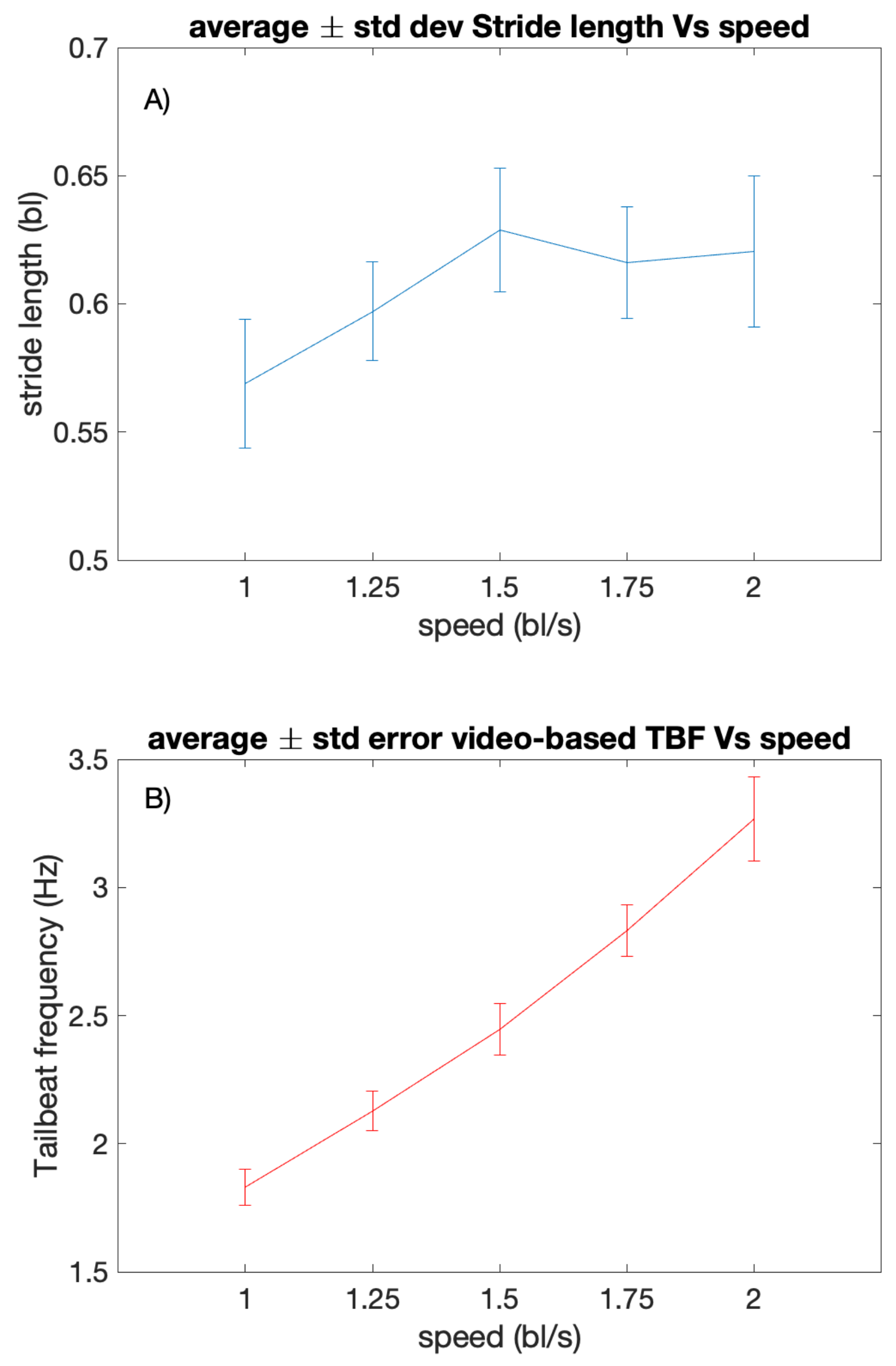
4.1. Stride Length and Video-Based TBF versus Water Speed
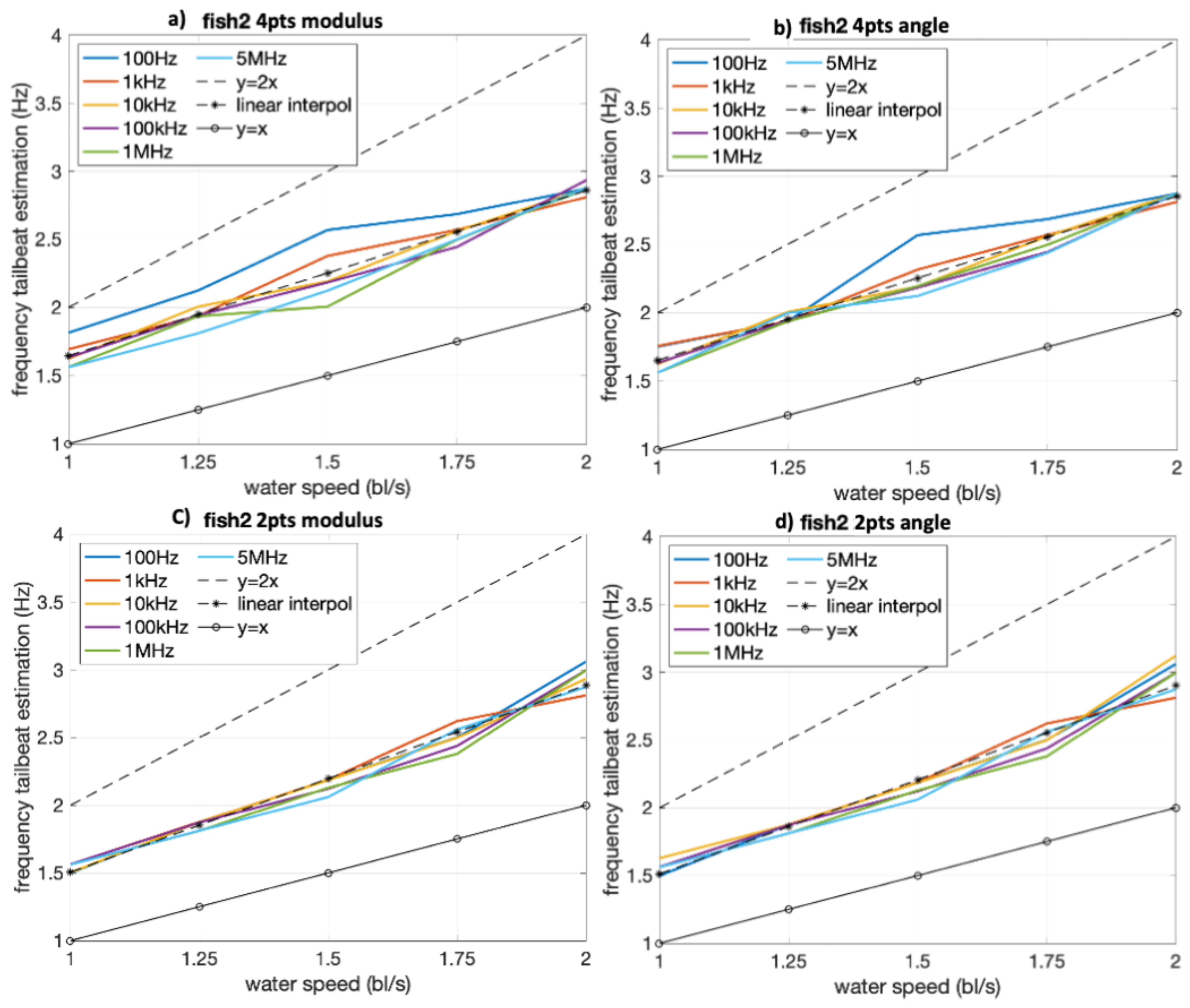
4.2. Bioimpedance-Based TBF Estimation in Relation to Swimming Speed
4.3. Fish TBF Measurement, Video versus Bioimpedance
4.3.1. Correlation and Agreement Considering Bioimpedance Setups and Resulting Electrical Parameters
- All t-tests for 4 pts setup measurements are 1 due to . For 2 pts setup measurements, the t-test is equal to 0 for real parts and imaginary parts, with p-values of and .
- The lowest CCC ( and ) are for 2 pts setup for modulus and angle electrical parameters. For 4 pts setup, CCC are over .
- r between and .
- 95% limits of agreements between and .
- CCC between and .
4.3.2. Correlation and Agreement Considering Different Frequencies of Bioimpedance Measurement
5. Discussion
- Accuracy using shorter measurement times and more advanced data processing techniques such as Short Time Fast Fourier Transform (STFFT).
- Response when the fish exhibits unsteady swimming with irregular tailbeats or turns.
- There is a need to instrument some fish with a biologger integrating a bioimpedance sensor in order to analyze the sensor response to some various behaviors resulting from free movements, such as burst swim and quick turn.
- Bioimpedance is known to be sensitive to temperature variations. Regarding TBF estimation using bioimpedance, we hypothesize that it would have only an impact on the signal amplitude, which shouldn’t affect the signal frequency estimation. Any way, a common solution is to integrate a temperature sensor in the electrode and calibrate the measurement according to temperature [44,45]
- One needed analysis regarding our newly proposed application is the effect of time. In order to be able to apply such an approach over a long time, there is a need for highly reliable electrodes that would resist repeated bendings in a harsh environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2 pts/4 pts | 2 points/4 points |
| CCC | Concordance Correlation Coefficient |
| CI1 | CCC lower limit of confidence interval |
| CI2 | CCC upper limit of confidence interval |
| FFT | Fast Fourier Transform |
| freq | frequency |
| ICC | Intraclass Correlation Coefficient |
| LLOA | Lower Limit of the Interval |
| mod | modulus |
| STFFT | Short Time Fast Fourier Transform |
| TBF | Tail-beat Frequency |
| ULOA | Upper Limit of the Interval |
References
- Videler, J.J. Fish Swimming; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Webb, P.W. Swimming. In The Physiologuy of Fishes; Evans, D., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 1–38. [Google Scholar]
- Bainbridge, R. The Speed of Swimming of Fish as Related to Size and to the Frequency and Amplitude of the Tail Beat. J. Exp. Biol. 1958, 35, 109–133. [Google Scholar] [CrossRef]
- Gleiss, A.C.; Schallert, R.J.; Dale, J.J.; Wilson, S.G.; Block, B.A. Direct measurement of swimming and diving kinematics of giant Atlantic bluefin tuna (Thunnus thynnus). R. Soc. Open Sci. 2019, 6, 190203. [Google Scholar] [CrossRef]
- Wardle, C.S. Limit of fish swimming speed. Nature 1975, 255, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.S.; Domenici, P.; Marras, S.; Krause, J.; Boswell, K.M.; Rodriguez-Pinto, I.; Wilson, A.D.M.; Kurvers, R.H.J.M.; Viblanc, P.E.; Finger, J.S.; et al. Maximum swimming speeds of sailfish and three other large marine predatory fish species based on muscle contraction time and stride length: A myth revisited. Biol. Open 2016, 5, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Handegard, N.O.; Pedersen, G.; Brix, O. Estimating tail-beat frequency using split-beam echosounders. ICES J. Mar. Sci. 2009, 66, 1252–1258. [Google Scholar] [CrossRef][Green Version]
- Carbonara, P.; Alfonso, S.; Dioguardi, M.; Zupa, W.; Vazzana, M.; Dara, M.; Spedicato, M.T.; Lembo, G.; Cammarata, M. Calibrating accelerometer data, as a promising tool for health and welfare monitoring in aquaculture: Case study in European sea bass (Dicentrarchus labrax) in conventional or organic aquaculture. Aquac. Rep. 2021, 21, 100817. [Google Scholar] [CrossRef]
- Cade, D.E.; Barr, K.R.; Calambokidis, J.; Friedlaender, A.S.; Goldbogen, J.A. Determining forward speed from accelerometer jiggle in aquatic environments. J. Exp. Biol. 2018, 221, jeb170449. [Google Scholar] [CrossRef]
- Warren-Myers, F.; Svendsen, E.; Føre, M.; Folkedal, O.; Oppedal, F.; Hvas, M. Novel tag-based method for measuring tailbeat frequency and variations in amplitude in fish. Anim. Biotelemetry 2023, 11, 12. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef]
- Clarke, T.M.; Whitmarsh, S.K.; Hounslow, J.L.; Gleiss, A.C.; Payne, N.L.; Huveneers, C. Using tri-axial accelerometer loggers to identify spawning behaviours of large pelagic fish. Mov. Ecol. 2021, 9, 26. [Google Scholar] [CrossRef]
- Kawabata, Y.; Noda, T.; Nakashima, Y.; Nanami, A.; Sato, T.; Takebe, T.; Mitamura, H.; Arai, N.; Yamaguchi, T.; Soyano, K. Use of a gyroscope/accelerometer data logger to identify alternative feeding behaviours in fish. J. Exp. Biol. 2014, 217, 3204–3208. [Google Scholar] [CrossRef]
- Broell, F.; Taylor, A.D.; Litvak, M.K.; Bezanson, A.; Taggart, C.T. Post-tagging behaviour and habitat use in shortnose sturgeon measured with high-frequency accelerometer and PSATs. Anim. Biotelemetry 2016, 4, 11. [Google Scholar] [CrossRef]
- Cruz-Font, L.; Shuter, B.J.; Blanchfield, P.J. Energetic costs of activity in wild lake trout: A calibration study using acceleration transmitters and positional telemetry. Can. J. Fish. Aquat. Sci. 2016, 73, 1237–1250. [Google Scholar] [CrossRef]
- Zupa, W.; Alfonso, S.; Gai, F.; Gasco, L.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Calibrating Accelerometer Tags with Oxygen Consumption Rate of Rainbow Trout (Oncorhynchus mykiss) and Their Use in Aquaculture Facility: A Case Study. Animals 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Bouyoucos, I.A.; Montgomery, D.W.; Brownscombe, J.W.; Cooke, S.J.; Suski, C.D.; Mandelman, J.W.; Brooks, E.J. Swimming speeds and metabolic rates of semi-captive juvenile lemon sharks (Negaprion brevirostris, Poey) estimated with acceleration biologgers. J. Exp. Mar. Biol. Ecol. 2017, 486, 245–254. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. (Eds.) Chapter 1—Introduction. In Bioimpedance and Bioelectricity Basics, 3rd ed.; Academic Press: Oxford, UK, 2015; pp. 1–7. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Willis, J.; Hobday, A.J. Application of bioelectrical impedance analysis as a method for estimating composition and metabolic condition of southern bluefin tuna (Thunnus maccoyii) during conventional tagging. Fish. Res. 2008, 93, 64–71. [Google Scholar] [CrossRef]
- Zaniboni-Filho, E.; Hermes-Silva, S.; Weingartner, M.; Jimenez, J.E.; Borba, M.R.; Fracalossi, D.M. Bioimpedance as a tool for evaluating the body composition of suruvi (Steindachneridion scriptum). Braz. J. Biol.-Rev. Bras. Biol. 2015, 75. [Google Scholar] [CrossRef][Green Version]
- Mesa, M.G.; Rose, B.P. An assessment of morphometric indices, blood chemistry variables and an energy meter as indicators of the whole body lipid content in Micropterus dolomieu, Sander vitreus and Ictalurus punctatus. J. Fish Biol. 2015, 86, 755–764. [Google Scholar] [CrossRef]
- Hafs, A.W.; Hartman, K.J. Developing bioelectrical impedance analysis methods for age-0 brook trout. Fish. Manag. Ecol. 2014, 21, 366–373. [Google Scholar] [CrossRef]
- Khramtsova, N.I.; Plaksin, S.A. Two-electrode bioelectrical impedance measurement in body composition analysis before and after liposuction. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Robert, M.; Dagorn, L.; Bodin, N.; Pernet, F.; Arsenault-Pernet, E.J.; Deneubourg, J.L. Comparison of condition factors of skipjack tuna (Katsuwonus pelamis) associated or not with floating objects in an area known to be naturally enriched with logs. Can. J. Fish. Aquat. Sci. 2014, 71, 472–478. [Google Scholar] [CrossRef]
- Blanco-Almazán, D.; Groenendaal, W.; Catthoor, F.; Jané, R. Chest Movement and Respiratory Volume both Contribute to Thoracic Bioimpedance during Loaded Breathing. Sci. Rep. 2019, 9, 20232. [Google Scholar] [CrossRef] [PubMed]
- Detrez, E.; Kerzérho, V.; Belhaj, M.M.; Vergnet, A.; de Verdal, H.; Rouyer, T.; Bonhommeau, S.; Lamlih, A.; Julien, M.; Ben Ali, F.; et al. Study differentiating fish oocyte developmental stages using bioimpedance spectroscopy. Aquaculture 2022, 547, 737396. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Measures of agreement. Perspect. Clin. Res. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Martin Bland, J.; Altman, D. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Rik. BlandAltmanPlot. 2021. Available online: https://fr.mathworks.com/matlabcentral/fileexchange/71052-blandaltmanplot/ (accessed on 19 September 2023).
- Lin, L.I.K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Matthew, R. f_CCC. 2018. Available online: https://fr.mathworks.com/matlabcentral/fileexchange/66896-f_ccc (accessed on 19 September 2023).
- Georgopoulou, D.G.; Stavrakidis-Zachou, O.; Mitrizakis, N.; Papandroulakis, N. Tracking and Analysis of the Movement Behavior of European Seabass (Dicentrarchus labrax) in Aquaculture Systems. Front. Anim. Sci. 2021, 2, 754520. [Google Scholar] [CrossRef]
- Marras, S.; Killen, S.S.; Domenici, P.; Claireaux, G.; McKenzie, D.J. Relationships among Traits of Aerobic and Anaerobic Swimming Performance in Individual European Sea Bass Dicentrarchus labrax. PLoS ONE 2013, 8, e72815. [Google Scholar] [CrossRef]
- Tyler, R.H.; Boyer, T.P.; Minami, T.; Zweng, M.M.; Reagan, J.R. Electrical conductivity of the global ocean. Earth Planets Space 2017, 69, 156. [Google Scholar] [CrossRef]
- Kolz, A.L. Electrical Conductivity as Applied to Electrofishing. Trans. Am. Fish. Soc. 2006, 135, 509–518. [Google Scholar] [CrossRef]
- Lamlih, A.; Freitas, P.; Belhaj, M.M.; Salles, J.; Kerzérho, V.; Soulier, F.; Bernard, S.; Rouyer, T.; Bonhommeau, S. A Hybrid Bioimpedance Spectroscopy Architecture for a Wide Frequency Exploration of Tissue Electrical Properties. In Proceedings of the 2018 IFIP/IEEE International Conference on Very Large Scale Integration (VLSI-SoC), Verona, Italy, 8–10 October 2018; pp. 168–171. [Google Scholar] [CrossRef]
- Analog Devices Impedance Measurement Products List. Available online: https://www.analog.com/en/solutions/instrumentation-and-measurement/electronic-test-and-measurement/impedance-measurement-and-analysis.html (accessed on 29 September 2024).
- Kerzérho, V.; Azaïs, F.; Bernard, S.; Bonhommeau, S.; Brisset, B.; De Knyff, L.; Julien, M.; Renovell, M.; Rouyer, T.; Saraux, C.; et al. Multilinear Regression Analysis between Local Bioimpedance Spectroscopy and Fish Morphological Parameters. Fishes 2023, 8, 88. [Google Scholar] [CrossRef]
- Hartman, K.J.; Margraf, F.J.; Hafs, A.W.; Cox, M.K. Bioelectrical Impedance Analysis: A New Tool for Assessing Fish Condition. Fisheries 2015, 40, 590–600. [Google Scholar] [CrossRef]
- Pothoven, S.A.; Ludsin, S.A.; Höök, T.O.; Fanslow, D.L.; Mason, D.M.; Collingsworth, P.D.; Van Tassell, J.J. Reliability of Bioelectrical Impedance Analysis for Estimating Whole-Fish Energy Density and Percent Lipids. Trans. Am. Fish. Soc. 2008, 137, 1519–1529. [Google Scholar] [CrossRef]
- Duncan, M.; Craig, S.R.; Lunger, A.N.; Kuhn, D.D.; Salze, G.; McLean, E. Bioimpedance assessment of body composition in cobia Rachycentron canadum (L. 1766). Aquaculture 2007, 271, 432–438. [Google Scholar] [CrossRef]
- Ruiz-Vargas, A.; Ivorra, A.; Arkwright, J.W. Design, Construction and Validation of an Electrical Impedance Probe with Contact Force and Temperature Sensors Suitable for in-vivo Measurements. Sci. Rep. 2018, 8, 14818. [Google Scholar] [CrossRef]
- Leung, T.K.W.; Ji, X.; Peng, B.; Chik, G.K.K.; Dai, D.S.H.S.; Fang, G.; Zhang, T.; Cheng, X.; Kwok, K.W.; Tsang, A.C.O.; et al. Micro-electrodes for in situ temperature and bio-impedance measurement. Nano Select 2021, 2, 1986–1996. [Google Scholar] [CrossRef]



| Bioimpedance Setup and Parameter | r | ULOA | LLOA | t-Test | t-Test p | CCC | CI1 | CI2 | |
|---|---|---|---|---|---|---|---|---|---|
| 4 pts mod | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0 | 0.97 | 0.96 | 0.98 |
| 4 pts angle | 0.97 | −0.03 | 0.26 | −0.32 | 1 | 0.01 | 0.96 | 0.95 | 0.97 |
| 2 pts mod | 0.95 | −0.06 | 0.29 | −0.39 | 1 | 0 | 0.94 | 0.92 | 0.96 |
| 2 pts angle | 0.93 | −0.05 | 0.37 | −0.46 | 1 | 0.01 | 0.92 | 0.89 | 0.94 |
| 4 pts real | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0.01 | 0.97 | 0.96 | 0.98 |
| 4 pts imag | 0.97 | −0.03 | 0.26 | −0.32 | 1 | 0.01 | 0.96 | 0.95 | 0.97 |
| 2 pts real | 0.97 | −0.01 | 0.26 | −0.29 | 0 | 0.32 | 0.97 | 0.95 | 0.97 |
| 2 pts imag | 0.96 | −0.02 | 0.29 | −0.33 | 0 | 0.21 | 0.96 | 0.94 | 0.97 |
| Bioimpedance Setup and Mean of Parameters | r | ULOA | LLOA | t-Test | t-Test p | CCC | CI1 | CI2 | |
|---|---|---|---|---|---|---|---|---|---|
| 4 pts mean (mod, angle) | 0.98 | −0.03 | 0.24 | −0.30 | 1 | 0.01 | 0.97 | 0.96 | 0.98 |
| 4 pts mean (real, imag) | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0.01 | 0.97 | 0.96 | 0.98 |
| 4 pts mean (mod, real) | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0.01 | 0.97 | 0.96 | 0.98 |
| 4 pts mean (mod, angle, real, imag) | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0.01 | 0.97 | 0.96 | 0.98 |
| 2 pts mean (mod, angle) | 0.95 | −0.05 | 0.31 | −0.41 | 1 | 0 | 0.94 | 0.91 | 0.95 |
| 2 pts mean (real, imag) | 0.97 | −0.01 | 0.27 | −0.30 | 0 | 0.24 | 0.96 | 0.95 | 0.97 |
| 2 pts mean (mod, real) | 0.97 | −0.03 | 0.24 | −0.30 | 1 | 0 | 0.96 | 0.95 | 0.97 |
| 2 pts mean (mod, angle, real, imag) | 0.97 | −0.03 | 0.25 | −0.31 | 1 | 0.01 | 0.96 | 0.95 | 0.97 |
| Bioimpedance Setup, Parameter and Frequency Number | r | ULOA | LLOA | t-Test | t-Test p | CCC | CI1 | CI2 | |
|---|---|---|---|---|---|---|---|---|---|
| 4 pts mod freq1 | 0.93 | 0.01 | 0.45 | −0.42 | 0 | 0.79 | 0.92 | 0.84 | 0.96 |
| 4 pts mod freq2 | 0.98 | −0.02 | 0.27 | −0.31 | 0 | 0.44 | 0.97 | 0.94 | 0.98 |
| 4 pts mod freq3 | 0.99 | −0.05 | 0.13 | −0.23 | 1 | 0.01 | 0.98 | 0.96 | 0.99 |
| 4 pts mod freq4 | 0.98 | −0.05 | 0.19 | −0.29 | 0 | 0.06 | 0.97 | 0.94 | 0.99 |
| 4 pts mod freq5 | 0.99 | −0.04 | 0.12 | −0.20 | 1 | 0.02 | 0.99 | 0.97 | 0.99 |
| 4 pts mod freq6 | 0.99 | −0.05 | 0.15 | −0.24 | 1 | 0.03 | 0.98 | 0.96 | 0.99 |
| 2 pts mod freq1 | 0.93 | 0.01 | 0.45 | −0.42 | 0 | 0.78 | 0.92 | 0.84 | 0.96 |
| 2 pts mod freq2 | 0.98 | −0.02 | 0.27 | −0.31 | 0 | 0.44 | 0.97 | 0.94 | 0.98 |
| 2 pts mod freq3 | 0.98 | −0.05 | 0.13 | −0.23 | 1 | 0.01 | 0.98 | 0.96 | 0.99 |
| 2 pts mod freq4 | 0.98 | −0.05 | 0.19 | −0.29 | 0 | 0.06 | 0.97 | 0.94 | 0.99 |
| 2 pts mod freq5 | 0.99 | −0.04 | 0.12 | −0.20 | 1 | 0.02 | 0.99 | 0.97 | 0.99 |
| 2 pts mod freq6 | 0.99 | −0.05 | 0.15 | −0.24 | 1 | 0.03 | 0.98 | 0.96 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerzerho, V.; Belhaj, M.-M.; Bernard, S.; Bonhommeau, S.; Rouyer, T.; Soulier, F.; McKenzie, D.J. Sea Bass (Dicentrarchus labrax) Tail-Beat Frequency Measurement Using Implanted Bioimpedance Sensing. Fishes 2024, 9, 399. https://doi.org/10.3390/fishes9100399
Kerzerho V, Belhaj M-M, Bernard S, Bonhommeau S, Rouyer T, Soulier F, McKenzie DJ. Sea Bass (Dicentrarchus labrax) Tail-Beat Frequency Measurement Using Implanted Bioimpedance Sensing. Fishes. 2024; 9(10):399. https://doi.org/10.3390/fishes9100399
Chicago/Turabian StyleKerzerho, Vincent, Mohamed-Moez Belhaj, Serge Bernard, Sylvain Bonhommeau, Tristan Rouyer, Fabien Soulier, and David J. McKenzie. 2024. "Sea Bass (Dicentrarchus labrax) Tail-Beat Frequency Measurement Using Implanted Bioimpedance Sensing" Fishes 9, no. 10: 399. https://doi.org/10.3390/fishes9100399
APA StyleKerzerho, V., Belhaj, M.-M., Bernard, S., Bonhommeau, S., Rouyer, T., Soulier, F., & McKenzie, D. J. (2024). Sea Bass (Dicentrarchus labrax) Tail-Beat Frequency Measurement Using Implanted Bioimpedance Sensing. Fishes, 9(10), 399. https://doi.org/10.3390/fishes9100399






