Abstract
Recirculating aquaculture system (RAS) technology is seen worldwide as a solution for sustainable fish production. However, there are still deficiencies in the process technology imperiling consistent operation and thus economic results. Drawbacks are linked to essential processes of the water treatment systems such as denitrification. Nitrogenous waste needs to be removed from RAS process water to maintain an adequate production environment for fish and to mitigate the environmental impact of discharged process water. At present, denitrification lacks reliable process control, especially regarding the organic carbon feed to heterotrophic denitrification processes. An investigation into heterotrophic denitrification in an experimental RAS resulted in the discovery of a virtual sensor based on measurements of the oxidation reduction potential (ORP). The virtual sensor responds to an insufficient carbon feed to denitrification. It is based on the oxidation of nitrite in an ozone-enhanced foam flotation installed downstream of the denitrification. The sensor essentially delivers a binary signal denoting either a complete or an incomplete denitrification process. The virtual sensor can be used for reliably controlling heterotrophic denitrification. It requires an upgraded process chain employing ozone-enhanced foam flotation (protein skimmer) downstream of the denitrification. However, the virtual sensor does not require any additional instrumentation.
Keywords:
denitrification; foam flotation; nitrite; ozone; protein skimmer; RAS; recirculating aquaculture system Key Contribution:
Automated routines for nitrite detection based on a virtual sensor signal can avoid extended periods of malfunction of denitrification without additional analytical equipment. A well-controlled, stable denitrification process allows to reduce RAS water consumption, improves husbandry conditions in RASs and mitigates the environmental impact by minimizing the discharge of inorganic nitrogen and organic carbon.
1. Introduction
As aquaculture grows worldwide, the concept of sustainable industrial production has already been discussed for decades. A promising approach is aquaculture in largely closed technical environments, in so-called recirculating aquaculture systems (RASs). However, this applies only if by-products of the production, such as excreted organic matter, metabolic losses and residual feed, are removed from the water cycle [1].
RASs are intensive fish production systems in which the process water is continuously re-used. Descriptions of the current status of RAS technology and economic perspectives can be found in references [2,3,4]. A critical factor in a RAS water cycle is ammonia/ammonium excreted by fish [5]. To avoid accumulation of toxic ammonia species, total ammonium nitrogen is converted to nitrate by bacterial nitrification in an aerated biofilter. RASs operated intensively, with high feed loads and less than 10% exchange of system volume per day, are prone to nitrate accumulation. Nitrate is virtually absent in the natural environment of marine fish [6]. Although it was shown to be the least toxic dissolved nitrogen species in RASs, potentially harmful chronic effects should be considered [7,8,9,10]. Nitrate, as with any other nutrient discharged from an RAS, contributes to eutrophication in the receiving water body and is therefore subject to wastewater regulations [11].
Nitrate can be removed from RAS process water through heterotrophic denitrification. Several other biological nitrogen removal systems have been studied but have not yet been adopted in commercial fish farming [3,12]. Heterotrophic denitrification is carried out by bacteria that gain energy from the dissimilation of organic carbon substances while reducing nitrate to nitrogen gas. At low dissolved oxygen concentrations, these bacteria develop respirasomes, which can reduce nitrate stepwise via nitrite, nitric oxide and nitrous oxide to nitrogen gas [13]. In nature, denitrifying communities are consortia of various bacteria carrying out complete or partial denitrification reactions [14]. In an RAS biofilter, denitrifying consortia also develop spontaneously when appropriate conditions are provided, i.e., low dissolved oxygen concentration and sufficient organic carbon. If the carbon supply is too low, these consortia release intermediate products of the denitrification process into circulation [14]. Of particular interest here is nitrite. On the other side, excess carbon in process water increases bacterial activities and abundance [15,16], thereby fostering the spread of opportunistic bacteria and fish pathogens [17,18], and it hinders nitrification, an essential process in the water treatment of RASs [19,20,21,22]. All these effects can have a negative impact on fish growth [23].
The dissolved organic carbon available in the process water of a well-managed RAS is usually not sufficient to support a heterotrophic denitrification process. This is particularly true for marine clearwater RASs, which offer the best husbandry conditions for marine fish [24]. In these RASs, the well-controlled addition of an exogenous carbon source to the denitrification process is necessary. The stoichiometry of denitrification with an exogenous carbon source has been investigated in detail [25,26,27,28]. However, the adjustment of the carbon feed to the denitrification process is the critical task. Martins et al. (2010) [29] viewed denitrification technology as immature due to the lack of reliable process control. Cheng et al. (2012) [30] developed a thermodynamic model of denitrification indicating that the oxidation reduction potential (ORP) reflects the activity of denitrification. Stavrakidis-Zachou et al. (2019) [31] used the ORP as a manipulated variable of the denitrification to limit the feed of oxygen that enters the biofilter with the nitrate-rich RAS process water. The study showed that under these circumstances, ORP control was not sufficient to ensure stable denitrification.
RAS water treatment depends on microbial conversion processes, which lead to the formation of biomass in biofilms, flocs and free-floating bacteria. Detached biofilms and flocs can be removed by sedimentation and by drum filters. However, the latter may break up bacterial aggregates and resuspend bacterial cells [32,33]. Resuspended bacteria are microparticles that contribute to water turbidity. To avoid the accumulation of these fine particles, Waller et al. (2001) [1] and Orellana et al. (2014) [24] proposed the use of an ozone-enhanced foam flotation system, also called a protein skimmer. In foam flotation, macromolecules, microparticles and bacteria become trapped at the gas/liquid interphase of the rising bubbles and are skimmed off at the surface. Ozone is a powerful oxidant enhancing the efficiency of foam flotation. In addition to the oxidation of nitrite and yellow substance, an effective reduction in bacterial biomass was achieved by this technique [34,35].
The present paper describes coincidental observations in an experimental RAS where ozone-enhanced foam flotation was operated downstream of a denitrification biofilter. During the operation of the RAS, the ORP signal in the flotation occasionally showed a peculiar recurring pattern. The occurrence of the pattern appeared to be linked to an incomplete denitrification process that released nitrite. It was hypothesized and experimentally confirmed that the change in the ORP pattern is a virtual nitrite sensor, which can be used to control carbon dosing in the denitrification process.
2. Materials and Methods
2.1. RAS Operation
Experiments were carried out in an 8 m3 seawater RAS (Figure 1). The RAS was stocked with 1500 juvenile European sea bass (Dicentrarchus labrax). The animals came from the Écloserie de Gravelines (France). The feeding rate of the fish was based on growth and feed conversion rates recommended by Lupatsch’s (2001) growth model [36]. Fish were handfed from 9:00 to 13:00 h with pelleted feed (Emsland-Aller Aqua GmbH, Golssen, Germany). The stocking density was maintained at around 40 kg m−3 by regularly harvesting some fish.
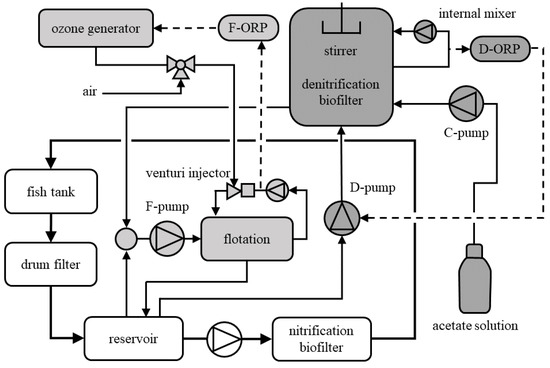
Figure 1.
Setup of experimental RAS. The main flow is shown by white boxes. Foam flotation and denitrification are shown in shades of grey and were operated in bypass-flows. Full lines represent process water and gas/air pipes. Dashed lines are control lines.
Fish were kept in a 6 m3 rectangular fish tank (4.85 × 1.90 × 0.65 m) in artificial seawater (ASM, Seequasal GmbH, Münster, Germany) at 21 psu salinity. The water temperature was maintained at 22.7 ± 0.9 °C. The pH levelled around 7.7 ± 0.2. The water flow rate through the fish tank was maintained at 18 m3 h−1. Additional airlifts were mounted at the tank wall to remove carbon dioxide and to oxygenate the water. The dissolved oxygen concentration was maintained around the saturation level (7.8 ± 0.6 mg L−1).
The water treatment system consisted of several treatment steps, shown in Figure 1. A drum filter (mesh size 60 μm) connected to the center drain of the fish tank passed the tank water into the reservoir. From here, the main circulation was maintained by a centrifugal pump delivering the process water to the nitrification biofilter. The moving bed biofilter was filled with Kaldness K3 biofilm carriers. From the nitrification biofilter, the water flowed back into the fish tank by gravity. The process water in the moving bed nitrification biofilter was aerated with a compressor (23 L min−1, Model-A025, Durr Technik, Bietigheim-Bissingen, Germany). Besides a stable nitrification process, this air supply contributed significantly to the gas exchange in the process water.
To remove fine particles and suspended solids that may arise during drum filtration [33], a foam flotation system (protein skimmer, Helgoland 500, Erwin Sander Elektroapparatebau, Uetze-Eltze, Germany) was installed in a bypass-flow (Figure 1). The foam flotation was continuously operated at a process water flow rate of 6 m3 h−1 (F-pump Ocean Runner, Aqua Medic Anlagenbau GmbH, Bissendorf, Germany; Figure 1). The flotation process was supported by feeding ozone gas (Multizon, Erwin Sander Elektroapparatebau, Uetze-Eltze, Germany) into the reaction space. Ozone was added as a mix of air and ozone through a venturi injector (Figure 1). The ozone generator was controlled by a two-point controller connected to an ORP probe (tec line ORP probe, Jumo GmbH, Fulda, Germany; F-ORP, Figure 1). Set points were between 350 and 450 mV, ensuring a minor ozone concentration (<0.05 mg L−1) in the foam flotation system.
The denitrification biofilter (volume 0.2 m3) was filled with 0.06 m3 polyethylene Kaldness K3 biofilm carriers. An electric stirrer operating at 40 rpm gently carried the biofilm carriers around. An internal circulation generated a steady up-flow (0.5 m3 h−1), mixing the process water in the biofilter (Figure 1). The biofilter was operated in a fed-batch mode, which was controlled by a two-point controller using the ORP measured in the biofilter (D-ORP, tec line ORP probe; Figure 1). D-ORP set points were −90 and −100 mV. At the lower set point (D-ORP = −100 mV), a centrifugal pump (D-pump, Ocean Runner; Figure 1) was actuated with an in-flow of 0.2 m3 h−1. This fed aerobic, nitrate-rich process water from the reservoir in the biofilter and increased the D-ORP. At the upper set point (D-ORP = −90 mV), the D-pump stopped. During the following batch phase, bacterial activity decreased the D-ORP until, at the lower set point, aerobic process water was fed again (for more details see [31]). Excess process water left the biofilter by gravitational flow and was mixed into the process water bypass-flow towards the foam flotation system (Figure 1). Due to the fed-batch operation, the denitrification effluent was mixed at intervals into the continuous process water flow for foam flotation. As the contribution of organic carbon in the process water was low (see Section 3), a metering pump (C-pump, Modelcraft, F3007; Figure 1) supplied acetate as an external carbon source (60 g L−1 C) for denitrification. The acetate supply was calculated from the nitrogen excretion of the fish based on the feed administered. The runtime of the C-pump was set manually according to the C/N ratio for denitrification with acetate [27]. The actual C/N ratio (expressed as g carbon per g nitrogen) was calculated retrospectively from concentrations and automatically recorded feed times of acetate-C and nitrate-N.
The RAS functioned largely automatically. The concentration of dissolved oxygen, salinity, temperature, pH, ORP and water level were continuously monitored. Process variables were automatically stabilized by appropriate actuators. The RAS automation was based on a supervisory control and data acquisition system (SCADA, Siemens, Munich, Germany). It involved a combination of PC-based visualization, an engineering station connected to an industrial S7410 5H Control CPU and ET 200S signal interface modules. Process control was based on the PCS7 V8.1 software (Siemens, Munich, Germany).
Concentrations of the nutrients nitrate (NO3-N) and nitrite (NO2-N) were determined manually using colorimetric assays (Hach Lange GmbH, Duesseldorf, Germany) calibrated for use in 20 psu seawater.
Biological oxygen demand (BOD) is a proxy for organic carbon available as a nutrient for microorganisms in aqueous environments. In the absence of oxygen, this carbon is available for denitrifying bacteria. Therefore, the biologically available organic carbon in the process water was estimated using standardized manometric measurement of the biological oxygen uptake over 5 days at 25 °C (BOD5) with the BOD Direct plus_GB_36_02 system of Hach Lange GmbH, Duesseldorf, Germany.
2.2. Validation Experiment
To validate the assumption that irregular ORP patterns in the foam flotation system were caused by nitrite formation in the denitrification process, the foam flotation system was disconnected from the RAS. The ozone regulation was inactivated, so that ozone was fed at a constant rate into the device upon activation only. An amperometric ozone probe (OPTISENS CL 1100, Krohne Messtechnik GmbH, Duisburg, Germany; ozone detection range 0.01 to 5 mg L−1) and a sampling valve were mounted in the measuring bypass of the foam flotation system in series with the F-ORP probe. While the F-ORP was recorded by the RAS automation system, the ozone values were recorded manually. Nitrite concentration was determined as specified above.
Validation experiments were carried out in batch mode. Before starting each experiment, sodium nitrite (0.05, 0.10 and 0.20 g nitrite-N) was added to the foam flotation system containing 0.35 ± 0.04 m3 of process water. After the nitrite was dissolved, the concentration was determined. The experiments began (t = 0) by starting the ozone generator. After a short lag time, ozone was fed at a constant rate of 0.04 g min−1 (manufacturer’s instruction). Subsequently, nitrite concentrations were determined in water samples until the detection limit of the test was reached. The ozone generator was switched off after the ozone concentration in the process water exceeded 0.05 mg L−1. For comparison of the mass conversions of nitrite and ozone, the oxidized nitrite mass (in moles) was calculated from the concentration using the volume of the process water in the foam flotation system. Ozone feed was calculated using the mass flow rate multiplied by the oxidation time, i.e., the time that was needed to oxidize all nitrite.
3. Results
3.1. Routine Operation of RAS
The performance of heterotrophic denitrification was investigated in an experimental RAS with European sea bass (Dicentrarchus labrax), maintained at a fish biomass of around 40 kg m−3. The operating conditions were kept in favorable limits during the experiment (Table 1). The water temperature (23 °C), salinity (21 psu) and pH (7.7) were in accordance with conditions in the natural distributional range of European sea bass. The mean dissolved oxygen concentration slightly exceeded air saturation, which can be attributed to multiple aeration systems. However, the concentration of nitrate-N, a nutrient rarely found in the natural habitat of sea bass, was higher than the anticipated maximum concentration of 100 mg L−1, indicating some irregularities in the denitrification process.

Table 1.
Operational conditions in the RAS based on some key variables. Mean: arithmetic mean; Stdev: standard deviation; Nobs: number of observations.
The pelagic marine habitat is characterized by few nutrients for bacteria and low turbidity. To achieve these conditions, the RAS was equipped with an ozone-enhanced foam flotation apparatus which can remove organic nutrients and fine particles, including bacteria. The efficiency of this treatment was examined by measuring bacterial oxygen demand in a 5-day standard assay (BOD5). The process water and the skimmed concentrate of the flotation showed average BOD5 values of 4 and 69 mg L−1, respectively. The BOD5 varied between 2 and 10 mg L−1 in the process water and between 17 and 136 mg L−1 in the skimmed foam (Figure 2). The average BOD5 was found to be different using the Kruskal–Wallis test (one-way ANOVA on ranks, p < 0.05). Apparently, organic material supporting oxygen uptake in the 5-day BOD5 tests was efficiently removed by foam flotation.
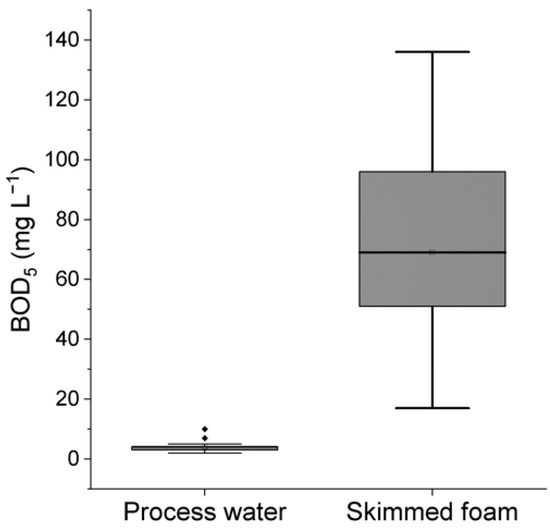
Figure 2.
Removal of BOD5 from process water by foam flotation. Box plot for BOD5 in RAS process water and in the concentrate skimmed off by foam flotation. The BOD5 determination was carried out in samples that were randomly taken from the experimental RAS. Each box encloses 50% of the data. The median value is displayed as a thin line. The top and bottom of the box mark the upper and lower quartiles. The lines extending from the top and bottom mark the minimum and maximum values. Dot markers mark outliers.
Ozone is a strong oxidant that rapidly oxidizes organic and inorganic molecules in water and enhances the efficiency of foam flotation. According to the Nernst equation, the ORP in a system with a stable pH and temperature is mainly determined by the concentration of ozone. Therefore, ORP was used to limit the generation of ozone and its addition to the foam flotation apparatus. The ozone generator was switched on and off by a two-point controller with a lower set point and an upper set point. These settings produced a sawtooth-like F-ORP pattern with lower and upper values corresponding to the set points (Figure 3A). In the following, this situation is referred to as the regular pattern of F-ORP. Figure 3B shows the nitrite concentration in the denitrification biofilter. Nitrite was transiently detected in the batch phase of denitrification (D-pump switching state = 0; Figure 3B,C) but was not present during the feed phase of the biofilter. The feed of process water into the biofilter (D-pump switching state = 1; Figure 3C) and the subsequent transfer to the foam flotation system had a barely detectable influence on the F-ORP.
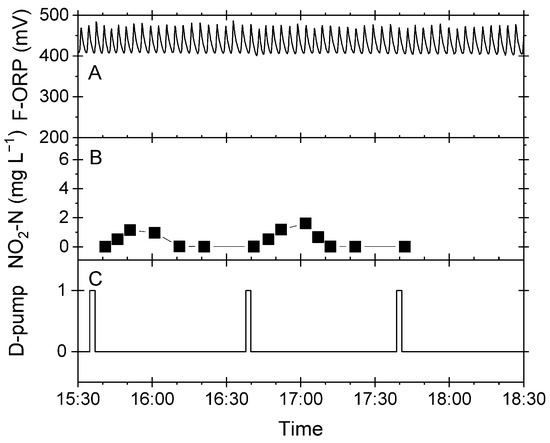
Figure 3.
Regular pattern of the F-ORP during a routine operation of the RAS. Excerpt from continuous data recording of a three-hour period. (A) F-ORP measured in the foam flotation system. (B) Nitrite concentration determined manually in the denitrification biofilter. (C) Switching state of the D-pump, which pumped process water into the biofilter and directed the overflow into the flotation system. The time of day is given as Central European Time (CET).
However, sometimes peculiar recurring patterns in the F-ORP were observed, which indicated a temporally disturbed ozonation process. During the time period shown in Figure 4, the regular sawtooth pattern was observed three times (09:45–10:15, 10:45–11:15 and 11:45–12:20 CET). The F-ORP varied between 355 and 474 mV (Figure 4A), reflecting the lower and upper set points of the two-point controller of the ozone generator. In between, periods were observed during which the ORP dropped down to values below 355 mV. This was lower than the set points of the two-point controller, indicating that the ozone feed was insufficient to keep the ORP within the expected range. This situation is referred to below as the irregular pattern of F-ORP.
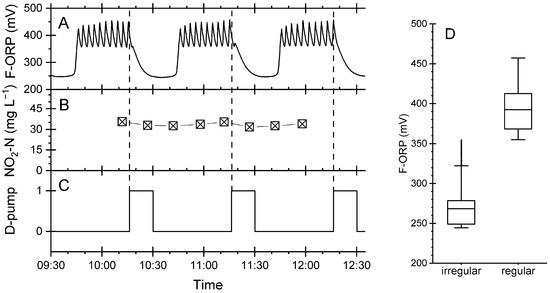
Figure 4.
Regular and irregular patterns of F-ORP in the foam flotation; excerpt from continuous data recording of a three-hour period. (A) F-ORP measured in the foam flotation. (B) Nitrite concentration determined in water samples taken from the denitrification biofilter. (C) Switching state of the D-Pump. (D) Box plot of the F-ORP data. See text for explanation.
Figure 4B shows the nitrite concentrations measured in the denitrification biofilter during an irregular pattern of F-ORP. The nitrite concentration remained high, above 30 mg L−1. This was clearly above the average process water concentration of 0.09 ± 0.04 mg L−1 (Table 1) and more than 20 times higher than during regular operation (Figure 3B). High nitrite concentrations point towards an incomplete denitrification process. Figure 4C shows the switching state of the D-pump. The irregular pattern of F-ORP always developed during the feed phase of the denitrification biofilter (switching state = 1). At the same time, the excess water left the device and flowed over into the adjacent foam flotation system. Some minutes after switching off the D-pump (Figure 4C), the irregular pattern was replaced by the regular one again (Figure 4A). The regular and irregular patterns describe distinct operational states of the F-ORP. A t-test for unequal variances (p < 0.05) proved that these two different operational states had two different statistical population means of 392 ± 27 mV and 268 ± 28 mV for the regular and irregular patterns, respectively.
The repeated occurrence of the irregular pattern of F-ORP led to the following hypothesis: if nitrite was formed during partial denitrification and transferred into the foam flotation system with the process water, it reacted with ozone to form nitrate. Although the feed of the flotation system from the reservoir in the main circulation of the RAS was much stronger than the feed from the biofilter, the nitrite in the latter was apparently sufficient to transiently consume all ozone. This kept the F-ORP at a low level, observed in the irregular pattern of the F-ORP. Thus, the F-ORP acted as a nitrite sensor.
3.2. Validation Experiments
The assumption formulated above was experimentally verified in the foam flotation system, which was decoupled from the RAS for this purpose.
Nitrite was added to the process water in the decoupled flotation system. The venturi injector was operated for a few minutes with air to dissolve the nitrite. Then, the ozone generator was switched on (t = 0). After a short lag time, ozone was injected at a constant rate of 0.04 g min−1 (manufacturer’s instruction). Irrespective of the initial nitrite concentration, nitrite started to decrease until it became non-detectable (Figure 5A). After the completion of nitrite oxidation, the ozone feed restored oxidative conditions. The ozone concentration rose to concentrations above 0.05 g m−3 (Figure 5B). Then, the generator was turned off manually and the ozone started to decay. The ozone accumulation also led to an increase in ORP, which reached upper levels higher than 640 mV (Figure 4C).
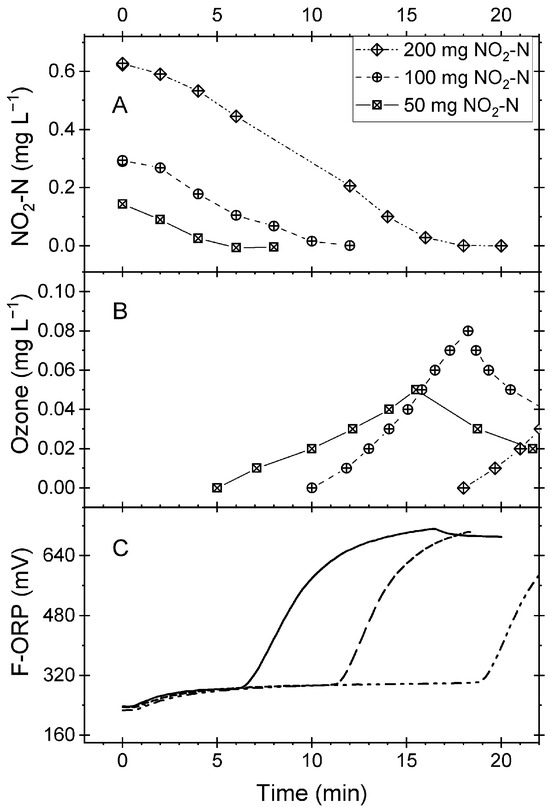
Figure 5.
Results from batch experiments in the foam flotation system. Variables measured in the measuring bypass of the reaction vessel. (A) Nitrite concentration measured manually at intervals. (B) Ozone concentration measured by probe. The values were read from the instrument and protocolled. (C) Automatically recorded F-ORP.
A cross-check confirmed that in the validation experiments, the oxidation of nitrite likely consumed all the ozone fed during the oxidation phase into the foam flotation system. In all three experiments, the ozone feed met the demand for the oxidation of the nitrite to a good approximation (Table 2). The average oxidation rate was 0.034 mg L−1 min−1. The oxidation rates calculated for all pairs of nitrite concentrations measured in the three experiments (Figure 5A) could be assigned to a single population (Kruskal–Wallis, p < 0.05).

Table 2.
Estimation of the ozone demand for the oxidation of nitrite and comparison with the ozone feed in the foam flotation based on the reaction O3 + NO2¯→ O2 + NO3¯.
Nitrite produced as an intermediate product in a partial denitrification process can be further reduced to nitrogen gas by increasing the carbon dosage for bacteria in the biofilter [14]. This relationship enabled validation of the hypothesis during routine operation of the experimental RAS. Figure 6 shows an excerpt from automatic monitoring starting at 07:00 CET. The D-pump, which was controlled by the D-ORP in the denitrification biofilter, fed process water from the reservoir at regular intervals (Figure 6A, right axis). The F-ORP in the flotation device showed a regular pattern (Figure 6A, left axis). The C-pump that fed acetate (Figure 6B, right axis) maintained a C/N ratio around 1.1 until 09:30. After 09:30 the actuation frequency of the C-pump was manually decreased. Subsequently, the C/N ratio dropped to 0.7. This low C/N ratio did not allow for a complete denitrification process. Around 12:00 CET, the sawtooth pattern of F-ORP (Figure 6A) showed first irregularities. At 15:00 CET, the irregular pattern developed whenever the D-pump delivered water via the biofilter into the foam flotation system. The virtual nitrite sensor flagged an insufficient C/N ratio for denitrification.
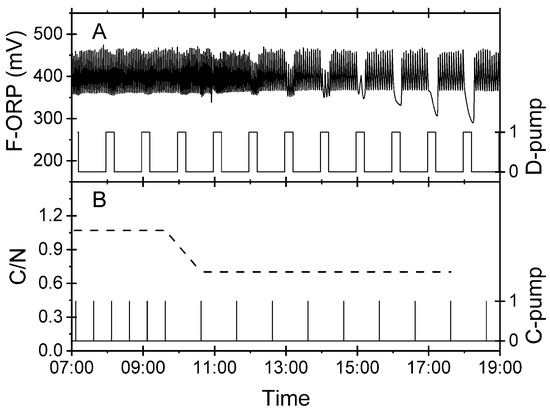
Figure 6.
Results of an experiment carried out in the experimental RAS to validate the capability of the virtual nitrite sensor to detect an upcoming carbon deficiency in denitrification. The C/N ratio was manually downregulated at 09:30 CET. (A) F-ORP signal in the foam flotation (left axis) and actuation pattern of D-pump (right axis). (B) C/N ratio (left axis, gC/gN) and actuation pattern of the C-pump (right axis) delivering acetate for denitrification.
Figure 7 shows a reverse experiment. Initially, insufficient carbon was supplied for denitrification. The C/N ratio in the feeds was calculated to be 0.9. The nitrite concentration in the biofilter had reached 20 mg L−1 N, indicating that the conversion of nitrate to nitrogen gas was incomplete. At this time, the nitrate-N concentration in the RAS process water was around 150 mg L−1. In the biofilter, only 37% of this nitrate was reduced. Whenever the D-pump delivered process water into the biofilter and passed the overflow to the foam flotation system, irregular F-ORP patterns were observed. At 17:30 CET, the frequency of the C-pump was upregulated. The C/N ratio increased to 1.4. The irregular patterns disappeared slowly, and normal routine operation was resumed at 22:00 CET.
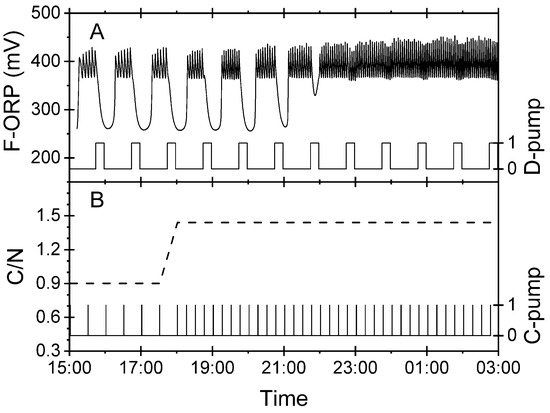
Figure 7.
Results of an experiment conducted in the experimental RAS to validate the ability of the virtual nitrite sensor to monitor the correction of a deficient carbon supply in the denitrification biofilter. The C-pump supplying acetate was manually upregulated at 18.00 CET. (A) F-ORP signal in the foam flotation system (left axis) and actuation pattern of the D-pump delivering nitrate-rich process water into the biofilter (right axis). (B) C/N ratio (left axis, gC/gN) and actuation pattern of the C-pump (right axis).
Figure 8A shows the disappearance of the irregular pattern of the F-ORP, which indicated an improving denitrification process. However, the D-ORP measured in the biofilter did not respond to this process improvement (Figure 8B). The steep increase in the D-ORP was caused by the feeding of oxygenated RAS process water (D-pump, switching state = 1; Figure 8B). During the batch phase (switching state = 0), the D-ORP decreased due to the metabolic demand of the facultatively aerobic heterotrophic microbiome in the biofilter. Thus, trends of increases and decreases can be adequately explained as fluctuations of the oxygen concentration in the fed-batch reactor. In such a system, the D-ORP is apparently not a reliable process variable to monitor and control the denitrification process. In contrast, the virtual sensor (Figure 8A) indicated a malfunction of denitrification through an irregular F-ORP pattern. The results show that the coupled operation of denitrification and foam flotation provided valuable additional information about the actual operation state of the denitrification process.
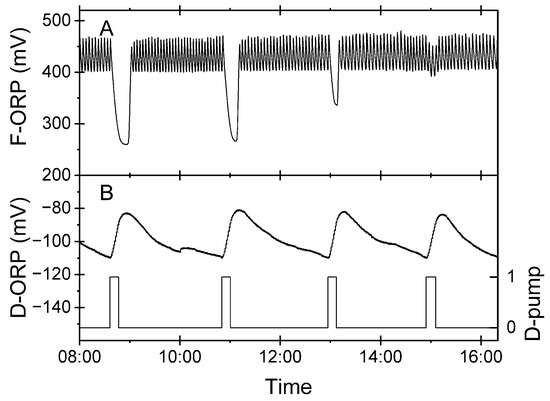
Figure 8.
Excerpt of a dataset showing the variation of the F-ORP and D-ORP during a period of improving denitrification. (A) F-ORP, acting as virtual sensor for nitrite, was measured in the foam flotation system. (B) D-ORP measured in the denitrification process (left axis) and actuation pattern of D-pump (right axis).
4. Discussion
Denitrification is a crucial process to make RASs a sustainable solution for future aquaculture production. Martins et al. (2010) [29] described denitrification as a spectacular development as it opens a theoretical perspective to close a RAS to nearly 100%. However, its application in commercial RASs is hampered by the fact that the implementation and process control have not yet been adequately solved. As already described by Badiola et al. (2012) [37], many commercial RASs still do not include sufficient denitrification technology.
Optimal husbandry conditions are a prerequisite for successful aquaculture production. Denitrification carried out as a continuous process in RAS water circulation appears necessary to ensure animal welfare in marine RASs. RASs without denitrification consume water at a rate of up to 10% of the system volume per day to keep the nitrate concentration within safe limits. Without integrated denitrification, all dissolved nutrients from feeding the fish end up in the environment. Although end-of-pipe treatment of the effluent can reduce this impact [38], RASs that are not ready to remove nutrients from the water circulation are not likely to comply with animal welfare issues and will contribute to eutrophication, as other aquaculture installations do.
Over the years, various nitrogen removal processes have been investigated for the treatment of RAS process water. Processes and stoichiometries of heterotrophic denitrification fed by an external carbon source have been well documented [25,26,27,28]. However, for nitrogen removal to work within an RAS, a reliable automatic control system that uses sensors to record and process key operating variables is required. To our knowledge, a key variable, namely the supply of dissolved organic carbon, has been described only for heterotrophic denitrification [39]. The virtual nitrite sensor described in this paper can identify an insufficient carbon feed for heterotrophic denitrification without additional analytical equipment [40]. The results shown in Figure 3, Figure 4, Figure 6 and Figure 7 demonstrate the functionality of the virtual sensor during RAS operation. The function was validated in additional experiments showing the correlation between ozone consumption by nitrite oxidation and a lowered F-ORP signal in the flotation apparatus (Figure 5). Stoichiometric nitrite oxidation by ozone, NO2¯ + O3 → NO3¯ + O2, was confirmed by the data obtained from these experiments (Table 2). The virtual sensor could be the basis for the reliable control of denitrification. However, it requires an upgraded process chain employing foam flotation downstream to the denitrification process (Figure 1).
In the RAS operated here, the biologically available organic carbon was maintained at low levels, as indicated by the low BOD5 in the process water (Figure 2). One reason was the thorough control of the addition of external carbon. By adding acetate at a ratio stoichiometric to the calculated nitrogen excretion by the fish, denitrification was always carried out close to carbon limitation. However, occasionally, this carbon feed became too low. A retrospective analysis of the automated F-ORP recordings showed that the virtual nitrite sensor had signaled carbon deficiency for denitrification eleven times during the 70-day observation period, either a few hours or even days before nitrite accumulation was detected by manual measurements. Over time, these periods of low nitrate reduction efficiency resulted in an increased nitrate concentration in the RAS (Table 1).
The experiments carried out during this study confirmed the results of [31] showing that the D-ORP recorded in the biofilter was not a reliable process variable of denitrification. The D-ORP varied because the feeding process water contained oxygen (Table 1). It reflected neither altered activity nor malfunction of the denitrification process. This was, however, visualized by the virtual nitrite sensor (Figure 8A). It can be envisaged that in future RASs, the carbon feed to a denitrification biofilter will be adjusted by stepwise upregulation of the feed after the virtual sensor has detected a carbon deficiency in the process. Subsequently, stepwise downregulation can reduce the carbon feed until the irregular patterns show up again. Using up- and downregulation, the carbon feed can be kept within safe limits while minimizing the costs of the external carbon source.
The virtual nitrite sensor was established in the ozone-enhanced foam flotation system (Figure 1). The foam flotation was installed to skim off fine materials not removed by mechanical filtration. The introduction of the discharge of the denitrification biofilter, a source of bacterial biomass [28], into the in-flow of the ozone-enhanced foam flotation system followed an obvious logic. This led to the discovery of a virtual nitrite sensor that signals carbon deficiency in the denitrification process based on a standard ORP sensor signal. However, the foam flotation is much more than a virtual detector for insufficient denitrification functioning. High BOD5 values in the skimmed material not only demonstrated an efficient concentration of organic waste in the foam (Figure 2) but also showed that this material is an important nutrient source for microorganisms. Microorganisms are part of a critical internal microbial loop in RASs, which is responsible not only for the growth rate reduction of fish [23] but also for the generation of the typical earthy-musty off-flavor of fish raised in aquaculture [41,42]. The formation of bacterial biomass in RAS process water and on the surfaces of these technical facilities must be seen as a major obstacle for an otherwise potentially sustainable and responsible aquaculture production technology. Regarding this study and the broad discussion in the literature, ozone-enhanced foam flotation must be seen as mandatory to establish safe RAS operation. As a reward, this virtual nitrite sensor for the control of the denitrification process comes without extra costs.
5. Conclusions
The enhancement of RAS technology is urgently needed to meet the goal of sustainable fish production in aquaculture. This applies not only to components of the process chain that already appear to be well defined; a much greater impact is to be expected from more sophisticated process automation.
Process automation and data processing played an important role in the discovery of the virtual nitrite sensor described in this paper. Automated routines for nitrite detection based on the virtual sensor signal can avoid extended periods of malfunction of denitrification without additional analytical equipment. A well-controlled, stable denitrification process allows to reduce RAS water consumption. In addition, it improves husbandry conditions in RASs and mitigates the environmental impact by minimizing the discharge of inorganic nitrogen and organic carbon.
The virtual sensor described here did not require additional investment. This raises the question of the extent to which similar virtual sensors could be found through continuous data analysis using machine learning routines in the frame of artificial intelligence (AI). Virtual sensors immediately respond to deviations from normal operation. In automated RAS operation, a much larger toolbox of virtual sensors is conceivable, which will improve RAS functionality in future.
Author Contributions
Conceptualization, A.E. and U.W.; methodology, C.S., K.W. and U.W.; software, K.W.; validation, A.E. and K.W.; investigation, A.E.; resources, U.W.; data curation, K.W.; writing—original draft, A.E.; writing—review and editing, U.W.; funding acquisition, U.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out at the Hochschule fuer Technik und Wirtschaft des Saarlandes, University of Applied Sciences, Saarbruecken, Germany. It was funded by The Federal Ministry of Education and Research (BMBF), Germany (AZ: 03FH004I3), and the COFASP ERA-NET partners, who received funding from the European Union’s Seventh Framework Program for research, technological development and demonstration under grant agreement no. 321553.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data are included in the paper.
Conflicts of Interest
Authors Christian Steinbach and Kai Wagner were employed by the company SEAWATER Cubes GmbH at the time of writing the paper. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Waller, U. Tank culture and recirculating systems. In Environmental Impacts of Aquaculture; Black, K.D., Ed.; Sheffield Academic Press: Sheffield, UK, 2001; pp. 99–127. [Google Scholar]
- Aich, N.; Nama, S.; Biswal, A.; Paul, T. A Review on Recirculating Aquaculture Systems: Challenges and Opportunities for Sustainable Aquaculture. Innov. Farming 2020, 5, 17–24. Available online: https://www.innovativefarming.in/index.php/IF/article/view/109 (accessed on 3 February 2024).
- Espinal, C.A.; Matulić, D. Recirculating Aquaculture Technologies. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–76. [Google Scholar]
- European Commission Directorate-General for Maritime Affairs and Fisheries. In Recirculation Aquaculture Systems; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2771/66025 (accessed on 3 February 2024).
- Kır, M.; Sunar, M.C.; Gök, M.G. Acute ammonia toxicity and the interactive effects of ammonia and salinity on the standard metabolism of European sea bass (Dicentrarchus labrax). Aquaculture 2019, 511, 734273. [Google Scholar] [CrossRef]
- Bristow, L.; Mohr, W.; Ahmerkamp, S.; Kuypers, M. Nutrients that limit growth in the ocean. Curr. Biol. 2017, 27, 474–478. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Williams, C.; Summerfelt, S.T. Evaluating the chronic effects of nitrate on the health and performance of post-smolt Atlantic salmon Salmo salar in freshwater recirculation aquaculture systems. Aquac. Eng. 2017, 79, 1–8. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Thermal plasticity of the cardiorespiratory system provides cross-tolerance protection to fish exposed to elevated nitrate. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108920. [Google Scholar] [CrossRef]
- Torno, J.; Einwächter, V.; Schroeder, J.P.; Schulz, C. Nitrate has a low impact on performance parameters and health status of on-growing European sea bass (Dicentrarchus labrax) reared in RAS. Aquaculture 2018, 489, 21–27. [Google Scholar] [CrossRef]
- van Bussel, C.G.J.; Schroeder, J.P.; Wuertz, S.; Schulz, C. The chronic effect of nitrate on production performance and health status of juvenile turbot (Psetta maxima). Aquaculture 2012, 326–329, 163–167. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Preena, P.G.; Rejish Kumar, V.J.; Singh, I.S.B. Nitrification and denitrification in recirculating aquaculture systems: The processes and players. Rev. Aquac. 2021, 13, 2053–2075. [Google Scholar] [CrossRef]
- Borrero-de Acuña, J.M.; Timmis, K.N.; Jahn, M.; Jahn, D. Protein complex formation during denitrification by Pseudomonas aeruginosa. Microb. Biotechnol. 2017, 10, 1523–1534. [Google Scholar] [CrossRef]
- Kraft, B.; Tegetmeyer, H.; Sharma, R.; Klotz, M.; Ferdelman, T.; Hettich, R.; Geelhoed, J.; Strous, M. The environmental controls that govern the end product of bacterial nitrate respiration. Science 2014, 345, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Tirado, P.; Pedersen, P.B.; Vadstein, O.; Pedersen, L.-F. Changes in microbial water quality in RAS following altered feed loading. Aquac. Eng. 2018, 81, 80–88. [Google Scholar] [CrossRef]
- Rojas-Tirado, P.; Pedersen, P.B.; Vadstein, O.; Pedersen, L.-F. Microbial dynamics in RAS water: Effects of adding acetate as a biodegradable carbon-source. Aquac. Eng. 2019, 84, 106–116. [Google Scholar] [CrossRef]
- Fossmark, R.O.; Vadstein, O.; Rosten, T.W.; Bakke, I.; Košeto, D.; Bugten, A.V.; Helberg, G.A.; Nesje, J.; Jørgensen, N.O.G.; Raspati, G.; et al. Effects of reduced organic matter loading through membrane filtration on the microbial community dynamics in recirculating aquaculture systems (RAS) with Atlantic salmon parr (Salmo salar). Aquaculture 2020, 524, 735268. [Google Scholar] [CrossRef]
- Mota, V.C.; Striberny, A.; Verstege, G.C.; Difford, G.F.; Lazado, C.C. Evaluation of a Recirculating Aquaculture System Research Facility Designed to Address Current Knowledge Needs in Atlantic Salmon Production. Front. Anim. Sci. 2022, 3, 876504. [Google Scholar] [CrossRef]
- Eding, E.; Kamstra, A.; Verreth, J.; Huisman, E.A.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Leonard, N.; Guiraud, J.P.; Gasset, E.; Cailleres, J.P.; Blancheton, J.P. Bacteria and nutrients—Nitrogen and carbon—In a recirculating system for sea bass production. Aquac. Eng. 2002, 26, 111–127. [Google Scholar] [CrossRef]
- Ling, J.; Chen, S. Impact of organic carbon on nitrification performance of different biofilters. Aquac. Eng. 2005, 33, 150–162. [Google Scholar] [CrossRef]
- Michaud, L.; Lo Giudice, A.; Interdonato, F.; Triplet, S.; Ying, L.; Blancheton, J.P. C/N ratio-induced structural shift of bacterial communities inside lab-scale aquaculture biofilters. Aquac. Eng. 2014, 58, 77–87. [Google Scholar] [CrossRef]
- de Jesus Gregersen, K.J.; Pedersen, L.-F. A case study comparing the addition of two different carbon sources in pilot scale RAS with trout with and without biofilters. Aquac. Eng. 2023, 103, 102370. [Google Scholar] [CrossRef]
- Orellana, J.; Waller, U.; Wecker, B. Culture of yellowtail kingfish (Seriola lalandi) in a marine recirculating aquaculture system (RAS) with artificial seawater. Aquac. Eng. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Michaels, J.T.; Beaulaton, C.M.; Graham, W.F.; Dutt, W.; Steinbach, P.; Losordo, T.M.; Schrader, K.K.; Main, K.L. Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquac. Eng. 2008, 38, 79–92. [Google Scholar] [CrossRef]
- Matějů, V.; Čižinská, S.; Krejčí, J.; Janoch, T. Biological water denitrification—A review. Enzym. Microb. Technol. 1992, 14, 170–183. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, Y.J. Biological nitrate removal in industrial wastewater treatment: Which electron donor we can choose. Appl. Microbiol. Biotechnol. 2009, 82, 415–429. [Google Scholar] [CrossRef]
- Strohm, T.; Griffin, B.; Zumft, W.; Schink, B. Growth Yields in Bacterial Denitrification and Nitrate Ammonification. Appl. Environ. Microbiol. 2007, 73, 1420–1424. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Cheng, H.-B.; Kumar, M.; Lin, J.-G. Interpretation of redox potential variation during biological denitrification using linear non-equilibrium thermodynamic model. Int. Biodeterior. Biodegrad. 2012, 67, 28–39. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Ernst, A.; Steinbach, C.; Wagner, K.; Waller, U. Development of denitrification in semi-automated moving bed biofilm reactors operated in a marine recirculating aquaculture system. Aquac. Int. 2019, 27, 1485–1501. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Pedersen, L.-F.; Pedersen, P.B. Daily micro particle distribution of an experimental recirculating aquaculture system—A case study. Aquac. Eng. 2014, 60, 28–34. [Google Scholar] [CrossRef]
- Langer, J.; Efthimiou, S.; Rosenthal, H.; Bronzi, P. Drum filter performance in a recirculating eel culture unit. J. Appl. Ichthyol. 2007, 12, 61–65. [Google Scholar] [CrossRef]
- Kovács, B.D.; de Jesus Gregersen, K.J.; Rüppel, F.; von Danwitz, A.; Pedersen, L.-F. Evaluating protein skimmer performance in a commercial seawater recirculating aquaculture system (RAS). Aquac. Eng. 2023, 103, 102369. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Croot, P.L.; Von Dewitz, B.; Waller, U.; Hanel, R. Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac. Eng. 2011, 45, 35–41. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D. Optimization of feeding regimes for European sea bass Dicentrarchus labrax: A factorial approach. Aquaculture 2001, 202, 289–302. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Almeida, P.; Dewasme, L.; Vande Wouwer, A. Denitrification Control in a Recirculating Aquaculture System—A Simulation Study. Processes 2020, 8, 1306. [Google Scholar] [CrossRef]
- Butinyac, M.G.; Montaño, V.A.; Downes, J.; Ruane, N.M.; Ryder, E.; Egan, F.; Staessen, T.; Paull, B.; Murray, E. Continuous nitrite and nitrate monitoring of recirculating aquaculture systems using a deployable ion chromatography-based analyser. Aquac. Int. 2024, 32, 1013–1026. [Google Scholar] [CrossRef]
- Azaria, S.; van Rijn, J. Off-flavor compounds in recirculating aquaculture systems (RAS): Production and removal processes. Aquac. Eng. 2018, 83, 57–64. [Google Scholar] [CrossRef]
- Zorzi, V.; Bertini, A.; Robertson, A.; Berardinelli, A.; Palmisano, L.; Parrino, F. The application of advanced oxidation processes including photocatalysis-based ones for the off-flavours removal (GSM and MIB) in recirculating aquaculture systems. Mol. Catal. 2023, 551, 113616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).