Feeding Behavior and Bait Selection Characteristics for the Portunidae Crabs Portunus sanguinolentus and Charybdis natator
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Crab Species and Experimental Data Collection
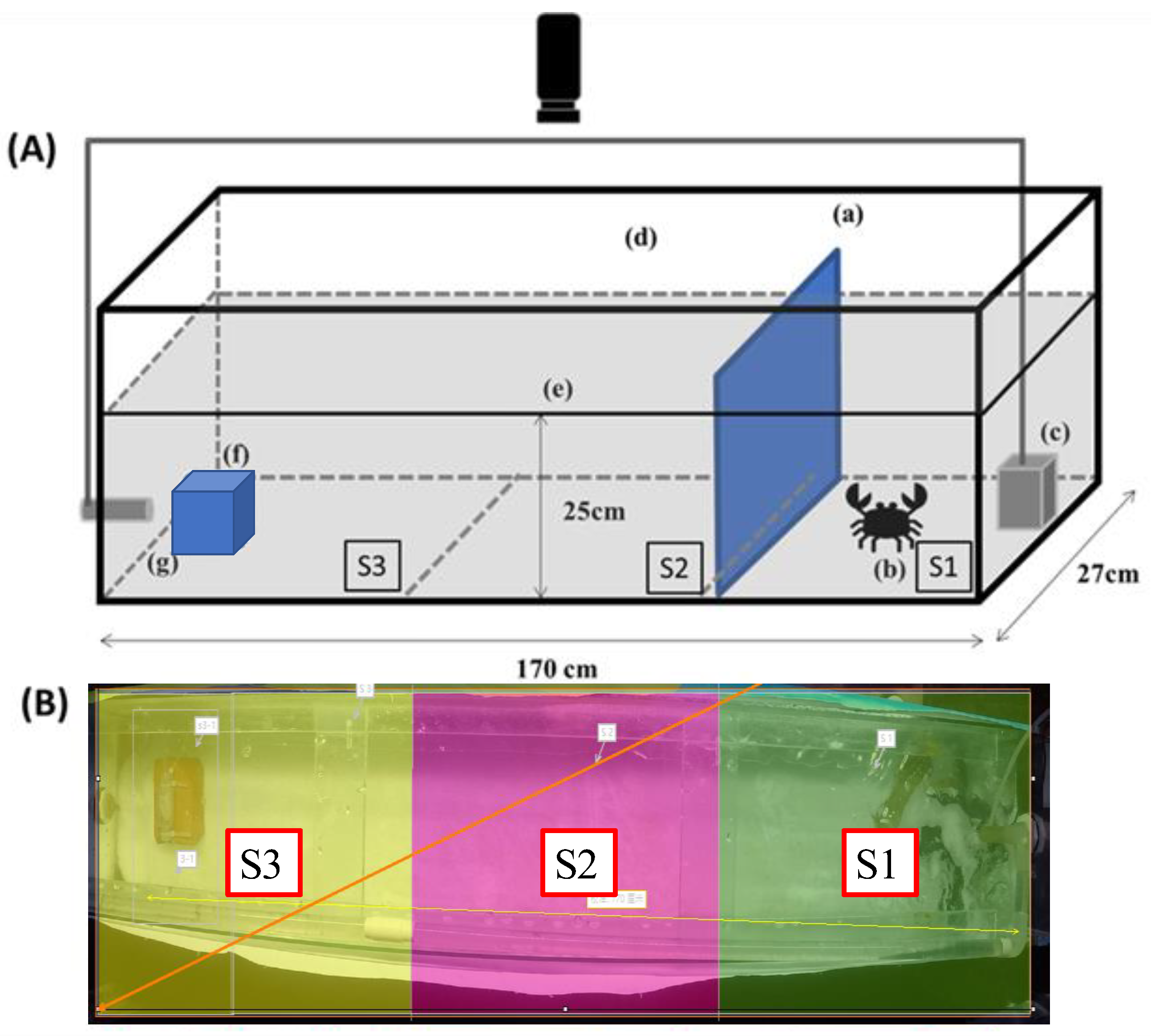
2.2. Captivity Behavior Experimental Design
2.3. Image Processing and Analysis
- I.
- Duration time (DT): The parameter measures crabs’ duration time in each area (S1–S3).
- II.
- Movement speed (MS): The speed in cm/s of crab moving during the trial.
2.4. Statistical Analysis
3. Results
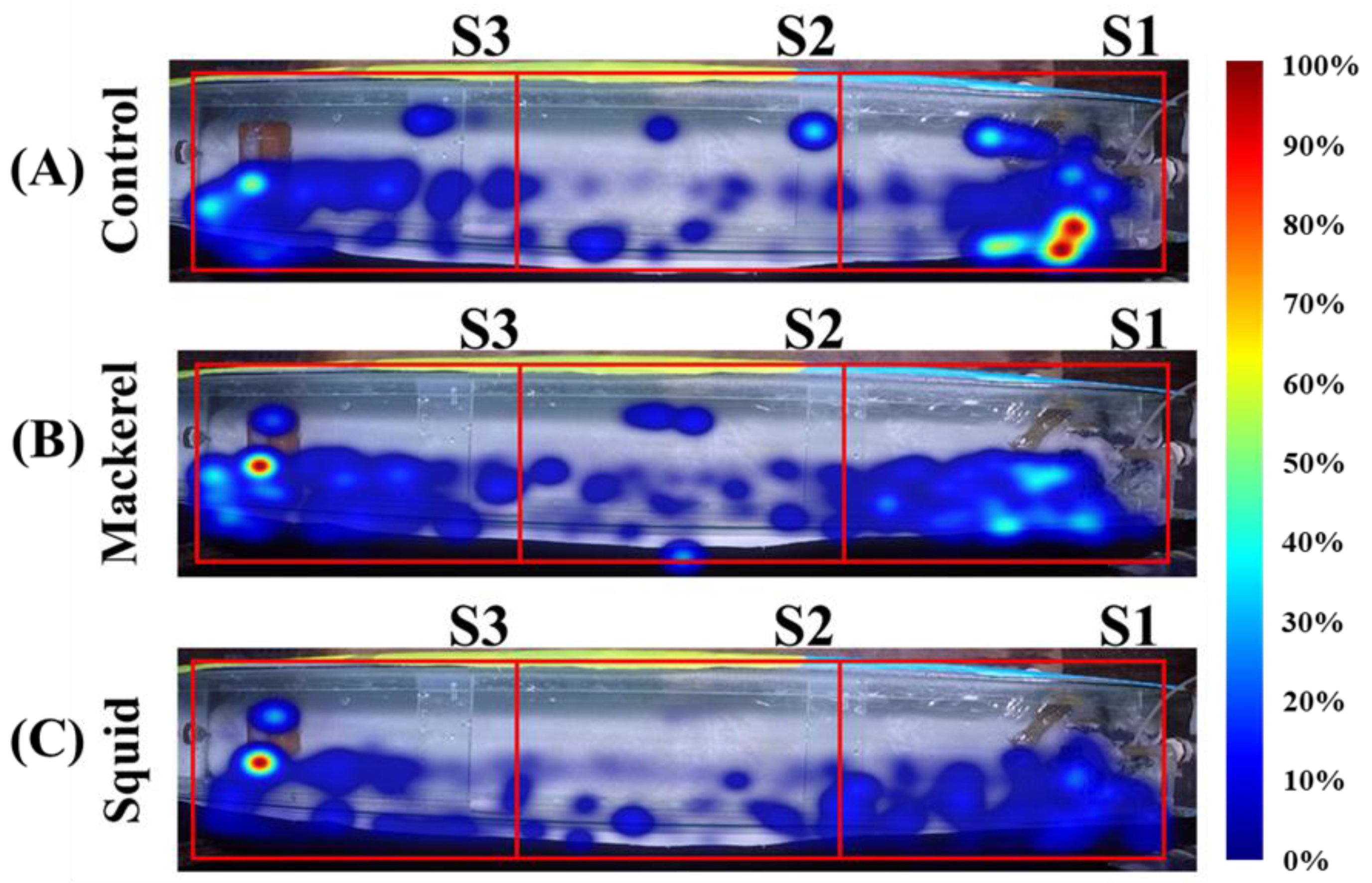
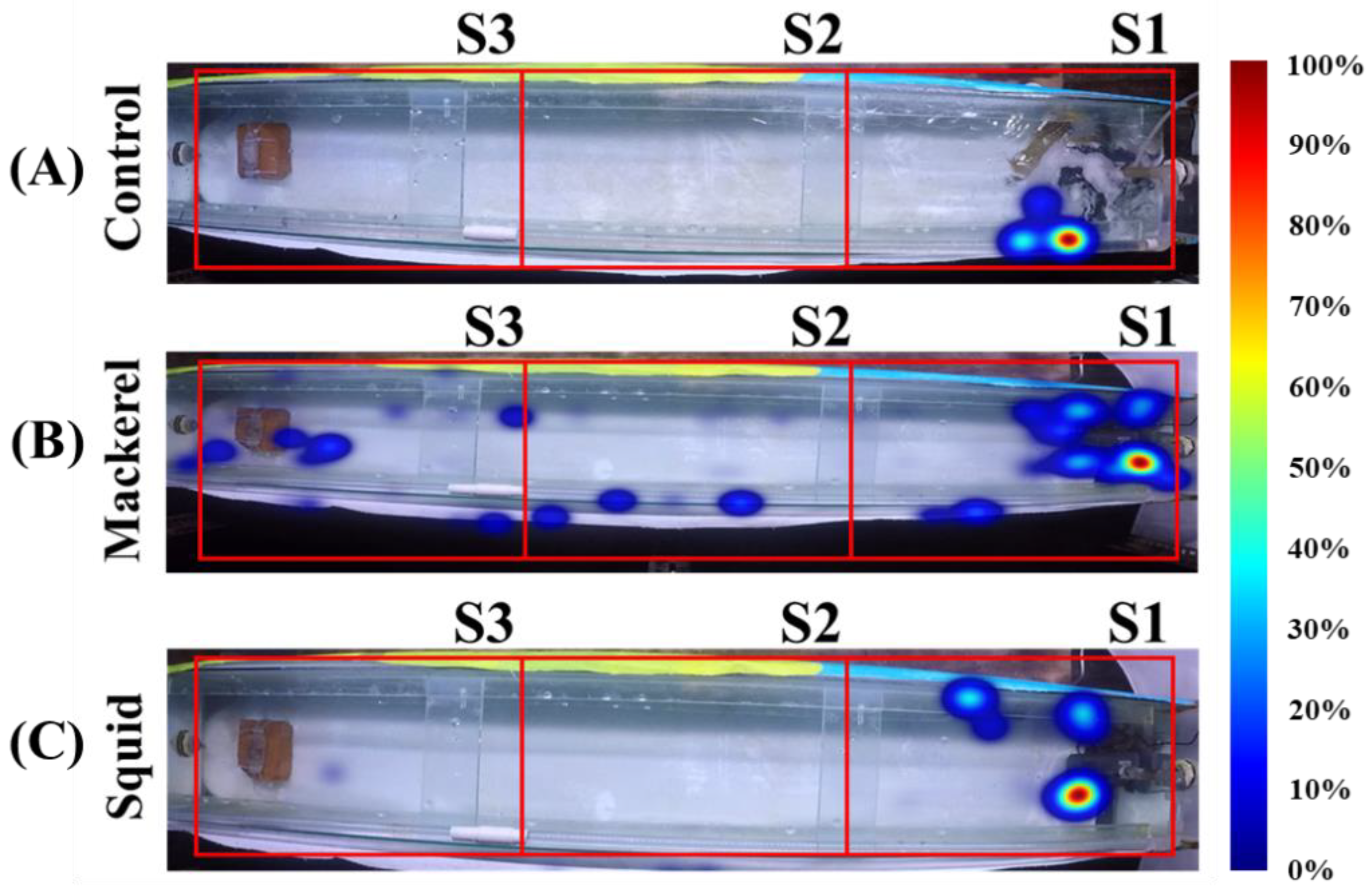
3.1. Spatial Distribution
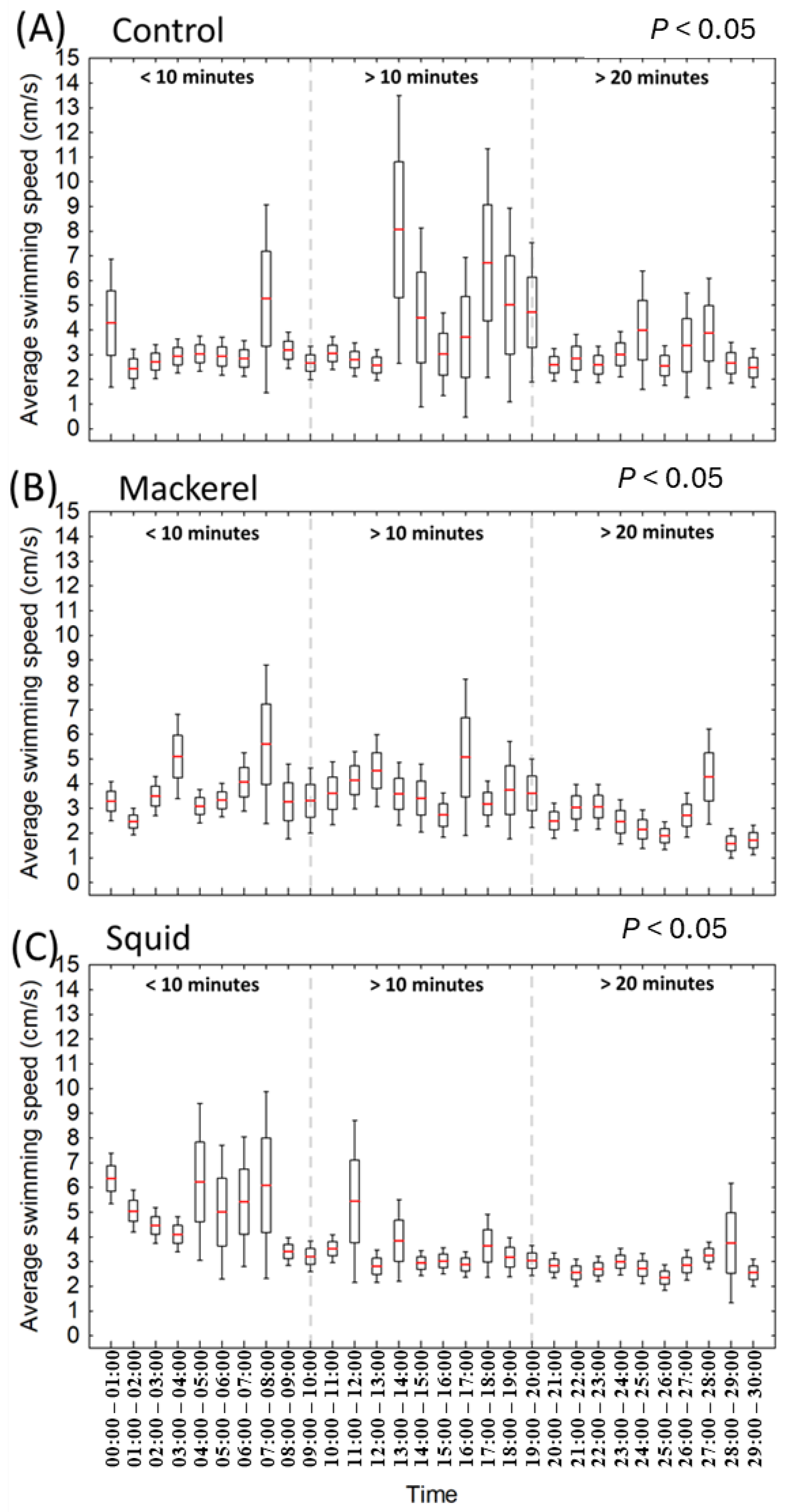
3.2. Swimming Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, W.; Rees, M. The endeavour and other Australian Museum collections of portunid crabs (Crustacea, Decapoda, Portunidae). Rec. Aust. Mus. 1968, 27, 285–298. [Google Scholar] [CrossRef][Green Version]
- Spiridonov, V.A. Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 8. Swimming crabs of Ambon (Crustacea: Decapoda: Portunidae). Zool. Meded. 1999, 73, 63–97. [Google Scholar]
- Sumpton, W.D.; Smith, G.S.; Potter, M.A. Notes on the biology of the portunid crab, Portunus sanguinolentus (Herbst), in subtropical Queensland waters. Mar. Freshw. Res. 1989, 40, 711–717. [Google Scholar] [CrossRef]
- Ng, P.K.; Wang, C.H.; Ho, P.H.; Shih, H.T. An Annotated Checklist of Brachyuran Crabs from Taiwan (Crustacea: Decapoda); National Taiwan Museum: Taipei, Taiwan, 2001; pp. 1–86. [Google Scholar] [CrossRef]
- Hsueh, P.W.; Hung, H.T. Temporal and spatial reproductive patterns of subtidal brachyuran crabs in coastal waters of Taiwan. Crustaceana 2009, 82, 449–465. [Google Scholar] [CrossRef]
- Naimullah, M.; Lan, K.W.; Liao, C.H.; Hsiao, P.Y.; Liang, Y.R.; Chiu, T.C. Association of environmental factors in the Taiwan strait with distributions and habitat characteristics of three swimming crabs. Remote Sens. 2020, 12, 2231. [Google Scholar] [CrossRef]
- Naimullah, M.; Lee, W.Y.; Wu, Y.L.; Chen, Y.K.; Huang, Y.C.; Liao, C.H.; Lan, K.W. Effect of soaking time on targets and bycatch species catch rates in fish and crab trap fishery in the southern East China Sea. Fish. Res. 2022, 250, 106258. [Google Scholar] [CrossRef]
- Naimullah, M.; Wu, Y.L.; Lee, M.A.; Lan, K.W. Effect of the El Niño–Southern Oscillation (ENSO) cycle on the catches and habitat patterns of three swimming crabs in the Taiwan Strait. Front. Mar. Sci. 2021, 8, 763543. [Google Scholar] [CrossRef]
- Naimullah, M.; Lan, K.W.; Liao, C.H.; Yang, Y.J.; Chen, C.C.; Liew, H.J.; Ikhwanuddin, M. Effects of spatial–temporal conditions and fishing-vessel capacity on the capture of swimming crabs by using different fishing gear around the waters of Taiwan. Mar. Freshw. Res. 2023, 74, 1244–1261. [Google Scholar] [CrossRef]
- Huner, J.V.; Pfister, V.A.; Romaire, R.P.; Baum, T.J. Effectiveness of commercially formulated and fish baits in trapping cambarid crawfish 1. J. World Aquac. Soc. 1990, 21, 288–294. [Google Scholar] [CrossRef]
- Billock, W.L.; Dunbar, S.G. Shell and food acquisition behaviors: Evidence for contextual decision hierarchies in hermit crabs. J. Exp. Mar. Biol. Ecol. 2011, 398, 26–32. [Google Scholar] [CrossRef]
- Naimullah, M.; Lan, K.W.; Mammel, M.; Chen, L.C.; Wu, Y.L.; Hsiao, P.Y.; WaiHo, K. Effect of climate change on habitat suitability and recruitment dynamics of swimming crabs in the Taiwan Strait. Mar. Freshw. Res. 2024, 75, 1244–1261. [Google Scholar] [CrossRef]
- Council of Agriculture. Control Measures for Crab Fishing along Offshorefishing Vessels. 2022. Available online: https://law.moa.gov.tw/LawContent.aspx?id=GL000522 (accessed on 15 March 2024).
- Lee, W.Y.; Lan, K.W.; Chang, H.H.; Naimullah, M. Residency and swimming behavior of Acanthopagrus schlegelii, Trachinotus blochii, and Acanthopagrus latus in relation to artificial reef models in a captivity experiment. Appl. Anim. Behav. Sci. 2022, 257, 105778. [Google Scholar] [CrossRef]
- Lim, C.J.; Platt, B.; Janhunen, S.K.; Riedel, G. Comparison of automated video tracking systems in the open field test: ANY-Maze versus EthoVision XT. J. Neurosci. Methods 2023, 397, 109940. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. GLMM and GAMM. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 323–341. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Bolker, B.M. Glmm TMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Magnusson, K.; Nilsson, A.; Andersson, G.; Hellner, C.; Carlbring, P. Level of agreement between problem gamblers’ and collaterals’ reports: A bayesian random-effects two-part model. J. Gambl. Stud. 2019, 35, 1127–1145. [Google Scholar] [CrossRef]
- Favaro, B.; Butt, M.A.; Bergshoeff, J.A. Comparison of catch per unit effort of invasive European green crab (Carcinus maenas) across four bait types. Fish. Res. 2020, 225, 105484. [Google Scholar] [CrossRef]
- McLay, C.L.; Osborne, T.A. Burrowing behaviour of the paddle crab Ovalipes catharus (White, 1843) (Brachyura: Portunidae). N. Z. J. Mar. Freshw. Res. 1985, 19, 125–130. [Google Scholar] [CrossRef]
- Bellwood, O. The occurrence, mechanics and significance of burying behaviour in crabs (Crustacea: Brachyura). J. Nat. Hist. 2002, 36, 1223–1238. [Google Scholar] [CrossRef]
- Su, X.; Liu, J.; Wang, F.; Zhang, D.; Zhu, B.; Liu, D. Effect of temperature on agonistic behavior and energy metabolism of the swimming crab (Portunus trituberculatus). Aquaculture 2020, 516, 734573. [Google Scholar] [CrossRef]
- Su, X.; Zhu, B.; Wang, F. Feeding strategy changes boldness and agonistic behaviour in the swimming crab (Portunus trituberculatus). Aquac. Res. 2022, 53, 419–430. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, H.; Liu, D.; Lu, Y.; Wang, F. Importance of substrate on welfare in swimming crab (Portunus trituberculatus) culture: A territorial behavior perspective. Aquac. Rep. 2022, 24, 101113. [Google Scholar] [CrossRef]
- Lee, H.H.; Hsu, C.C. Population biology of the swimming crab Portunus sanguinolentus in the waters off northern Taiwan. J. Crustac. Biol. 2003, 23, 691–699. [Google Scholar] [CrossRef]
- Yang, C.P.; Li, H.X.; Li, L.; Xu, J.; Yan, Y. Population structure, morphometric analysis and reproductive biology of Portunus sanguinolentus (Decapoda: Brachyura: Portunidae) in honghai bay, South China Sea. J. Crustac. Biol. 2014, 34, 722–730. [Google Scholar] [CrossRef]
- Lin, B.A.; Jiang, Y.; Liu, M. Population structure and reproductive dynamics of the ridged swimming crab Charybdis natator in the southern Taiwan Strait of China: Significant changes within 25 years. Front. Mar. Sci. 2023, 10, 1056640. [Google Scholar] [CrossRef]
- Ye, Q.T. Biological characteristic and resource status of Charybdis natator in Minnan-Taiwan shallow shoal fishing ground. Mar. Fish. 1999, 10, 107–111. [Google Scholar]
- Westerberg, H.; Westerberg, K. Properties of odour plumes from natural baits. Fish. Res. 2011, 110, 459–464. [Google Scholar] [CrossRef]
- Vabø, R.; Huse, G.; Fernö, A.; Jørgensen, T.; Løkkeborg, S.; Skaret, G. Simulating search behaviour of fish towards bait. ICES J. Mar. Sci. 2004, 61, 1224–1232. [Google Scholar] [CrossRef]
- Collins, M.A.; Yau, C.; Guilfoyle, F.; Bagley, P.; Everson, I.; Priede, I.G.; Agnew, D. Assessment of stone crab (Lithodidae) density on the South Georgia slope using baited video cameras. ICES J. Mar. Sci. 2002, 59, 370–379. [Google Scholar] [CrossRef][Green Version]
- Lees, K.J.; Mill, A.C.; Skerritt, D.J.; Robertson, P.A.; Fitzsimmons, C. Movement patterns of a commercially important, free-ranging marine invertebrate in the vicinity of a bait source. Anim. Biotelemetry 2018, 6, 8. [Google Scholar] [CrossRef]
- Stiansen, S.; Fernö, A.; Furevik, D.; Jørgensen, T.; Løkkeborg, S. Horizontal and vertical odor plume trapping of red king crabs explains the different efficiency of top-and side-entrance pot designs. Trans. Am. Fish. Soc. 2010, 139, 483–490. [Google Scholar] [CrossRef]
- Stevens, B.G. The ups and downs of traps: Environmental impacts, entanglement, mitigation, and the future of trap fishing for crustaceans and fish. ICES J. Mar. Sci. 2021, 78, 584–596. [Google Scholar] [CrossRef]
- Krouse, J.S. 14 Performance and selectivity of trap fisheries for crustaceans. In Marine Invertebrate Fisheries: Their Assessment and Management; Wiley: Hoboken, NJ, USA, 1989; p. 307. [Google Scholar]
- Stoner, A.W. Effects of environmental variables on fish feeding ecology: Implications for the performance of baited fishing gear and stock assessment. J. Fish Biol. 2004, 65, 1445–1471. [Google Scholar] [CrossRef]
- Hewitt, D.E.; Taylor, M.D.; Suthers, I.M.; Johnson, D.D. Environmental drivers of variation in southeast Australian Giant Mud Crab (Scylla serrata) harvest rates. Fish. Res. 2023, 268, 106850. [Google Scholar] [CrossRef]
- Leitao, F.; Monteiro, J.N.; Cabral, P.; Teodósio, M.A.; Roa-Ureta, R.H. Revealing the role of crab as bait in octopus fishery: An ecological and fishing approach to support management decisions. Mar. Policy 2023, 158, 105878. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A.; Incatasciato, J.M.; Taylor, M.L. Seasonal catch, size, color, and assessment of trapping variables for the European green crab Carcinus maenas (Brachyura: Portunoidea: Carcinidae), a nonindigenous species in Massachusetts, USA. J. Crustac. Biol. 2017, 37, 556–570. [Google Scholar] [CrossRef]
- Robertson, W.D. Factors affecting catches of the crab Scylla serrata (Forskål) (Decapoda: Portunidae) in baited traps: Soak time, time of day and accessibility of the bait. Estuar. Coast. Shelf Sci. 1989, 29, 161–170. [Google Scholar] [CrossRef]
- Zainal, K.A. Natural food and feeding of the commercial blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) along the coastal waters of the Kingdom of Bahrain. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 13, 1–7. [Google Scholar] [CrossRef][Green Version]
- Rasheed, S.; Mustaquim, J. Natural diet of two commercial crab species, Portunus segnis (Forskål, 1775) and P. sanguinolentus (Herbst, 1783), in the Coastal Waters of Karachi. Anim. Vet. Sci. 2018, 6, 35–42. [Google Scholar] [CrossRef]
- Westneat, M.W.; Wainwright, S.A. 7. Mechanical design for swimming: Muscle, tendon, and bone. Fish Physiol. 2001, 19, 271–311. [Google Scholar]
- Kier, W.M. Squid cross-striated muscle: The evolution of a specialized muscle fiber type. Bull. Mar. Sci. 1991, 49, 389–403. [Google Scholar]
- Bone, Q.; Pulsford, A.; Chubb, A.D. Squid mantle muscle. J. Mar. Biol. Assoc. United Kingd. 1981, 61, 327–342. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Ballantyne, J.; MacDonald, D.; Gosline, J.; Hochachka, P.W. Analogues of red and white muscle in squid mantle. Proc. Natl. Acad. Sci. USA 1981, 78, 3274–3278. [Google Scholar] [CrossRef] [PubMed]
- Baird, H.P.; Patullo, B.W.; Macmillan, D.L. Reducing aggression between freshwater crayfish (Cherax destructor Clark: Decapoda, Parastacidae) by increasing habitat complexity. Aquac. Res. 2006, 37, 1419–1428. [Google Scholar] [CrossRef]
- Marshall, S.; Warburton, K.; Paterson, B.; Mann, D. Cannibalism in juvenile blue-swimmer crabs Portunus pelagicus (Linnaeus, 1766): Effects of body size, moult stage and refuge availability. Appl. Anim. Behav. Sci. 2005, 90, 65–82. [Google Scholar] [CrossRef]
- Mirera, O.D.; Moksnes, P.O. Cannibalistic interactions of juvenile mud crabs Scylla serrata: The effect of shelter and crab size. Afr. J. Mar. Sci. 2013, 35, 545–553. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B.; Lu, Y.; Yu, L.; Wang, F.; Liu, D. An evaluation of the preferences of the juvenile swimming crab, Portunus trituberculatus, for different structural properties of shelters. Aquaculture 2022, 557, 738316. [Google Scholar] [CrossRef]
- Naimullah, M.; Lan, K.W.; Ikhwanuddin, M.; Amin-Safwan, A.; Lee, W.Y. Unbaited light-emitting diode traps performance for catching orange mud crabs. J. Mar. Sci. Technol. 2022, 30, 5. [Google Scholar] [CrossRef]
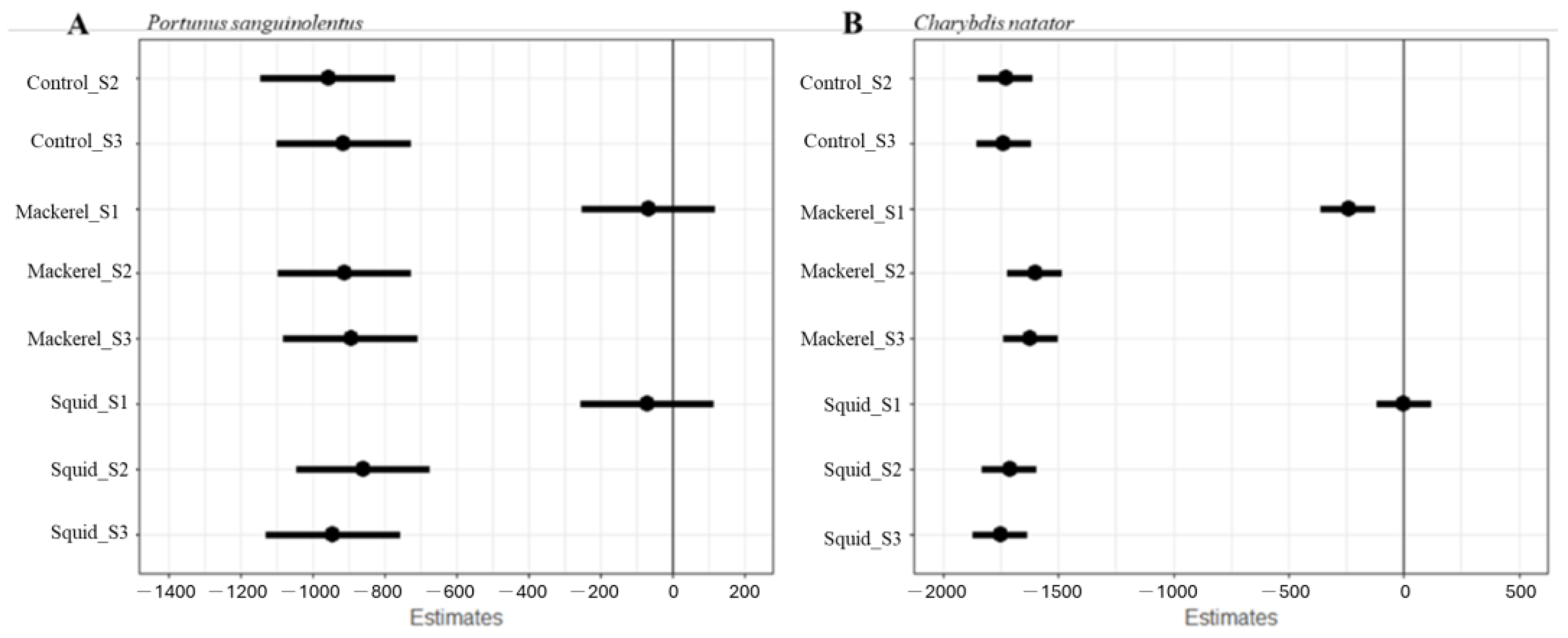




| (a) Portunus sanguinolentus | (b) Charybdis natator | |||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | z-Value | p | Estimate | z-Value | p |
| (Intercept) | 1223.11 | 18.19 | <0.05 | 1755.12 | 40.97 | <0.05 |
| ControlS2 | −956.65 | −10.06 | <0.05 | −1727.92 | −28.52 | <0.05 |
| ControlS3 | −912.69 | −9.59 | <0.05 | −1737.45 | −28.68 | <0.05 |
| MackerelS1 | −66.19 | −0.69 | >0.05 | −241.24 | −3.98 | <0.05 |
| MackerelS2 | −910.12 | −9.57 | <0.05 | −1602.29 | −26.45 | <0.05 |
| MackerelS3 | −893.03 | −9.39 | <0.05 | −1621.84 | −26.77 | <0.05 |
| SquidS1 | −68.49 | −0.72 | >0.05 | 0.34 | 0.01 | >0.05 |
| SquidS2 | −858.58 | −9.03 | <0.05 | 1712.64 | −28.27 | <0.05 |
| SquidS3 | −942.27 | −9.91 | <0.05 | 1753.09 | −28.93 | <0.05 |
| Portunus sanguinolentus | ||||||
|---|---|---|---|---|---|---|
| Control | Mackerel | Squid | ||||
| Male | Female | Male | Female | Male | Female | |
| S1 starting area | 65% | 71% | 64% | 64% | 67% | 61% |
| S2 middle area | 17% | 12% | 19% | 16% | 21% | 20% |
| S3 bait area | 17% | 17% | 17% | 20% | 12% | 19% |
| S1–S3 area Average moving speed in seconds (cm/s) | 0.24 | 0.26 | 0.43 | 0.62 | 0.80 | 1.33 |
| S1–S3 area average moving time (s) | 458.89 | 423.10 | 260.39 | 180.52 | 139.69 | 84.11 |
| Charybdis natator | ||||||
| Control | Mackerel | Squid | ||||
| Male | Female | Male | Female | Male | Female | |
| AS1 starting area | 100% | 100% | 82% | 86% | 97% | 98% |
| S2 middle area | 0% | 0% | 10% | 7% | 3% | 2% |
| S3 bait area | 0% | 0% | 8% | 6% | 0% | 0% |
| S1–S3 area Average moving speed in seconds (cm/s) | - | - | 0.43 | 0.38 | - | - |
| S1–S3 area average moving time (s) | - | - | 257.51 | 291.56 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-Y.; Wu, Y.-L.; Naimullah, M.; Liang, T.-Y.; Lan, K.-W. Feeding Behavior and Bait Selection Characteristics for the Portunidae Crabs Portunus sanguinolentus and Charybdis natator. Fishes 2024, 9, 400. https://doi.org/10.3390/fishes9100400
Lee W-Y, Wu Y-L, Naimullah M, Liang T-Y, Lan K-W. Feeding Behavior and Bait Selection Characteristics for the Portunidae Crabs Portunus sanguinolentus and Charybdis natator. Fishes. 2024; 9(10):400. https://doi.org/10.3390/fishes9100400
Chicago/Turabian StyleLee, Wei-Yu, Yan-Lun Wu, Muhamad Naimullah, Ting-Yu Liang, and Kuo-Wei Lan. 2024. "Feeding Behavior and Bait Selection Characteristics for the Portunidae Crabs Portunus sanguinolentus and Charybdis natator" Fishes 9, no. 10: 400. https://doi.org/10.3390/fishes9100400
APA StyleLee, W.-Y., Wu, Y.-L., Naimullah, M., Liang, T.-Y., & Lan, K.-W. (2024). Feeding Behavior and Bait Selection Characteristics for the Portunidae Crabs Portunus sanguinolentus and Charybdis natator. Fishes, 9(10), 400. https://doi.org/10.3390/fishes9100400






