A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Sample Collection
2.3. qRT–PCR Analysis
2.4. Total RNA Extraction, cDNA Library Construction, and Sequencing
2.5. MeRIP-seq Data Analysis
2.6. RNA-seq Data Analysis
2.7. MeRIP-seq and RNA-seq Association Analysis
2.8. Statistical Analysis
3. Results
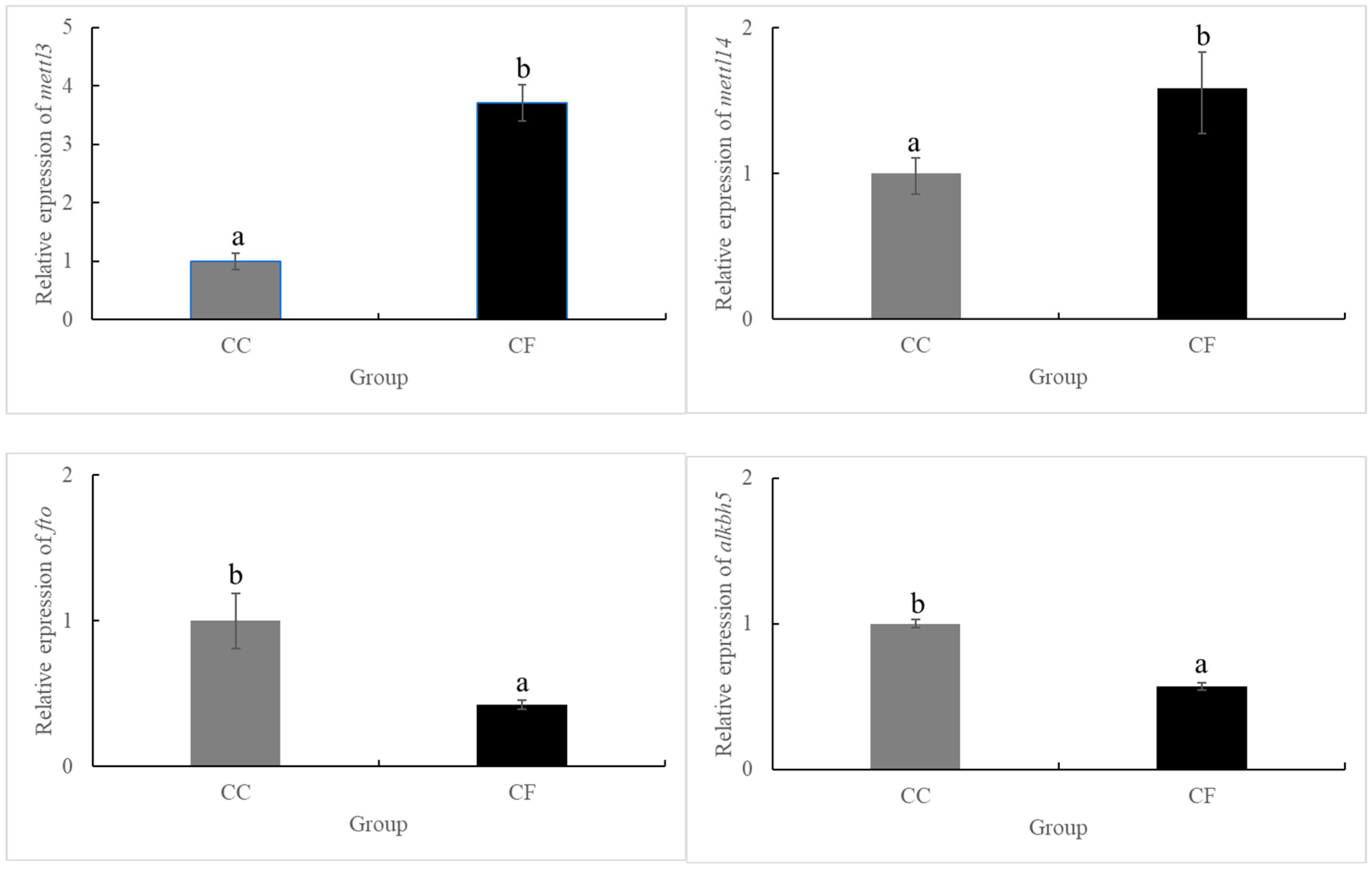
3.1. Starvation at Low Temperatures Enhanced the Expression of Genes Related to the Modification of m6A RNA Methylation
3.2. Statistics and Quality Assessment of MeRIP-seq
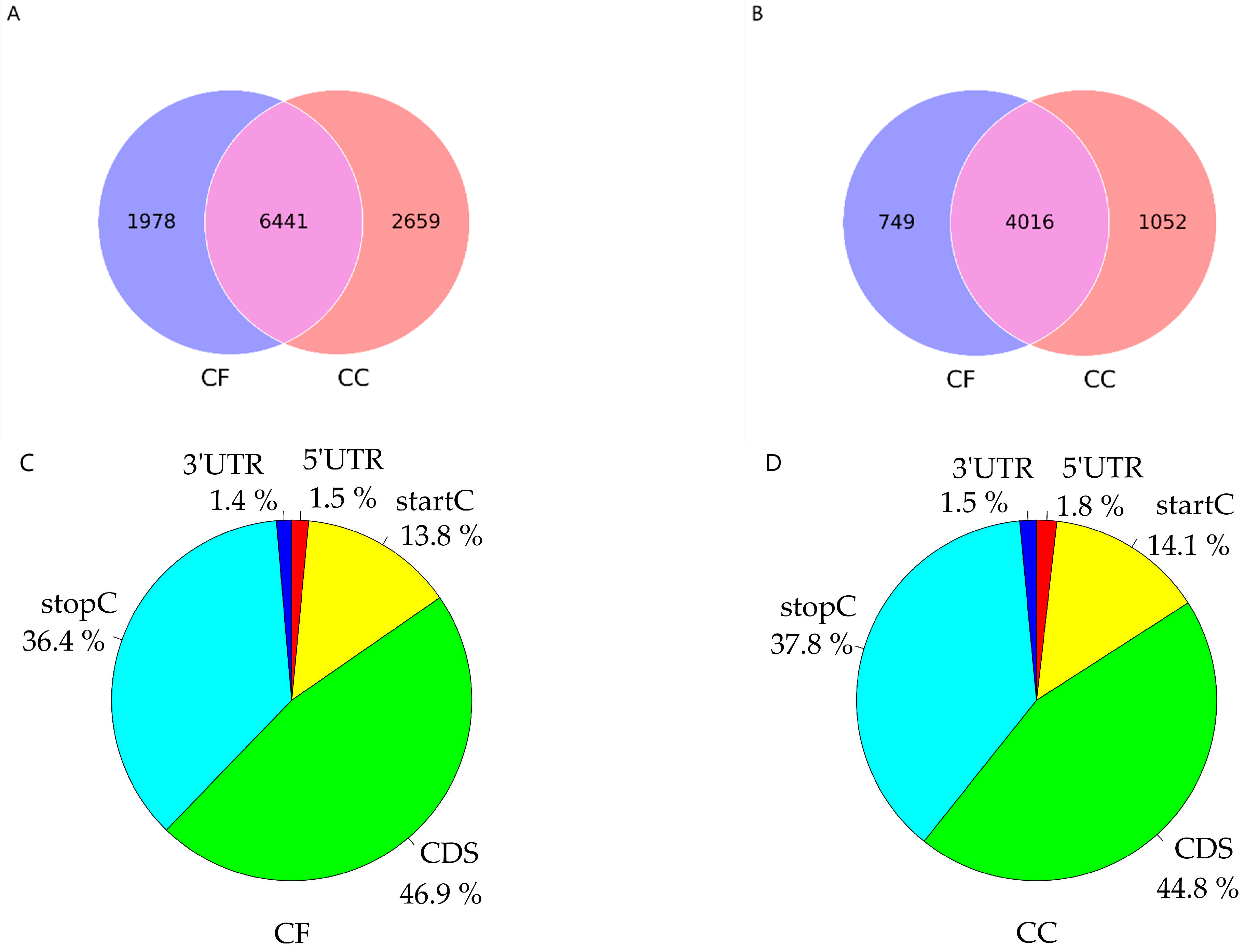
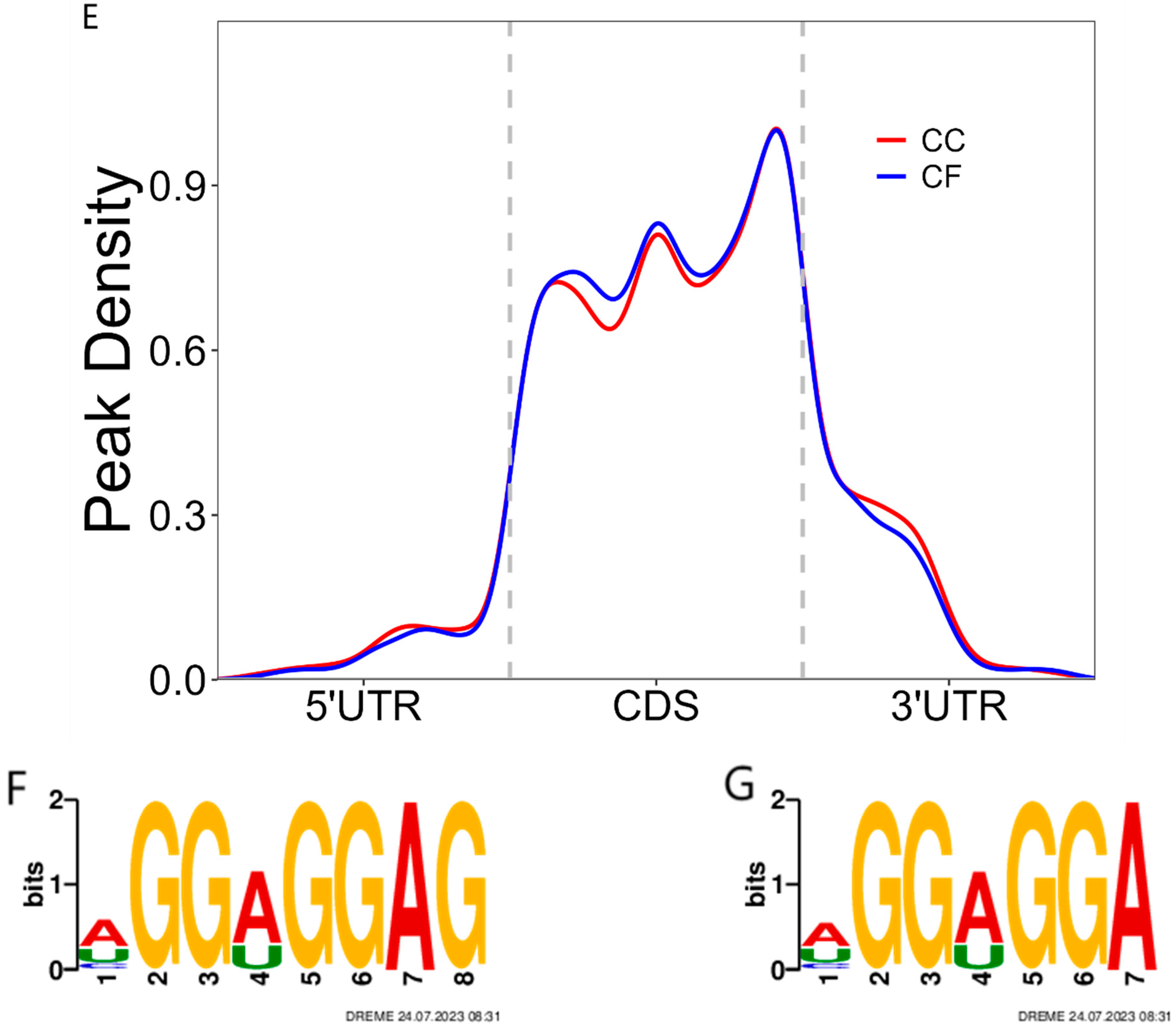
3.3. The Identification of the m6A Peak and Motif Analysis
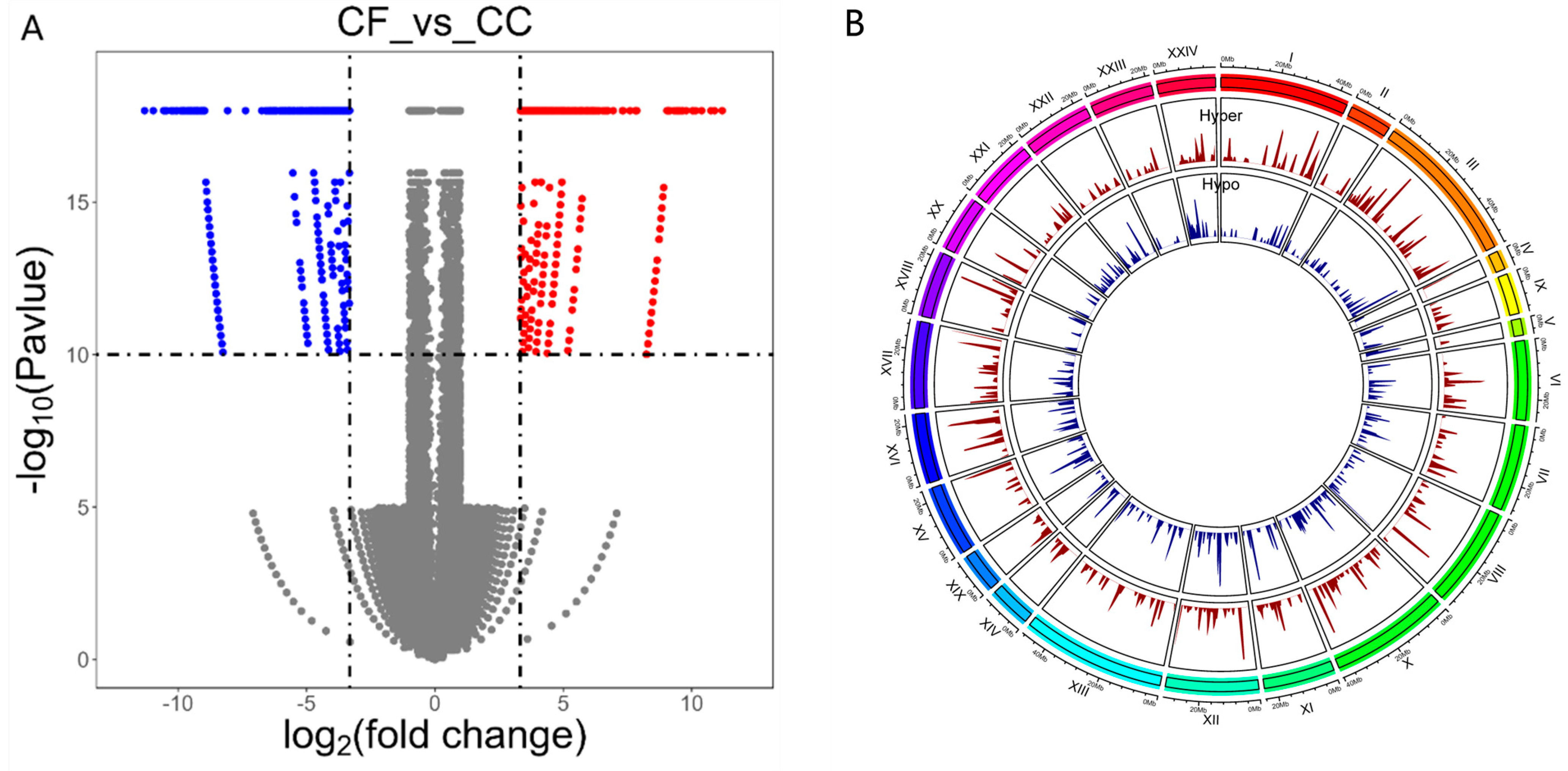
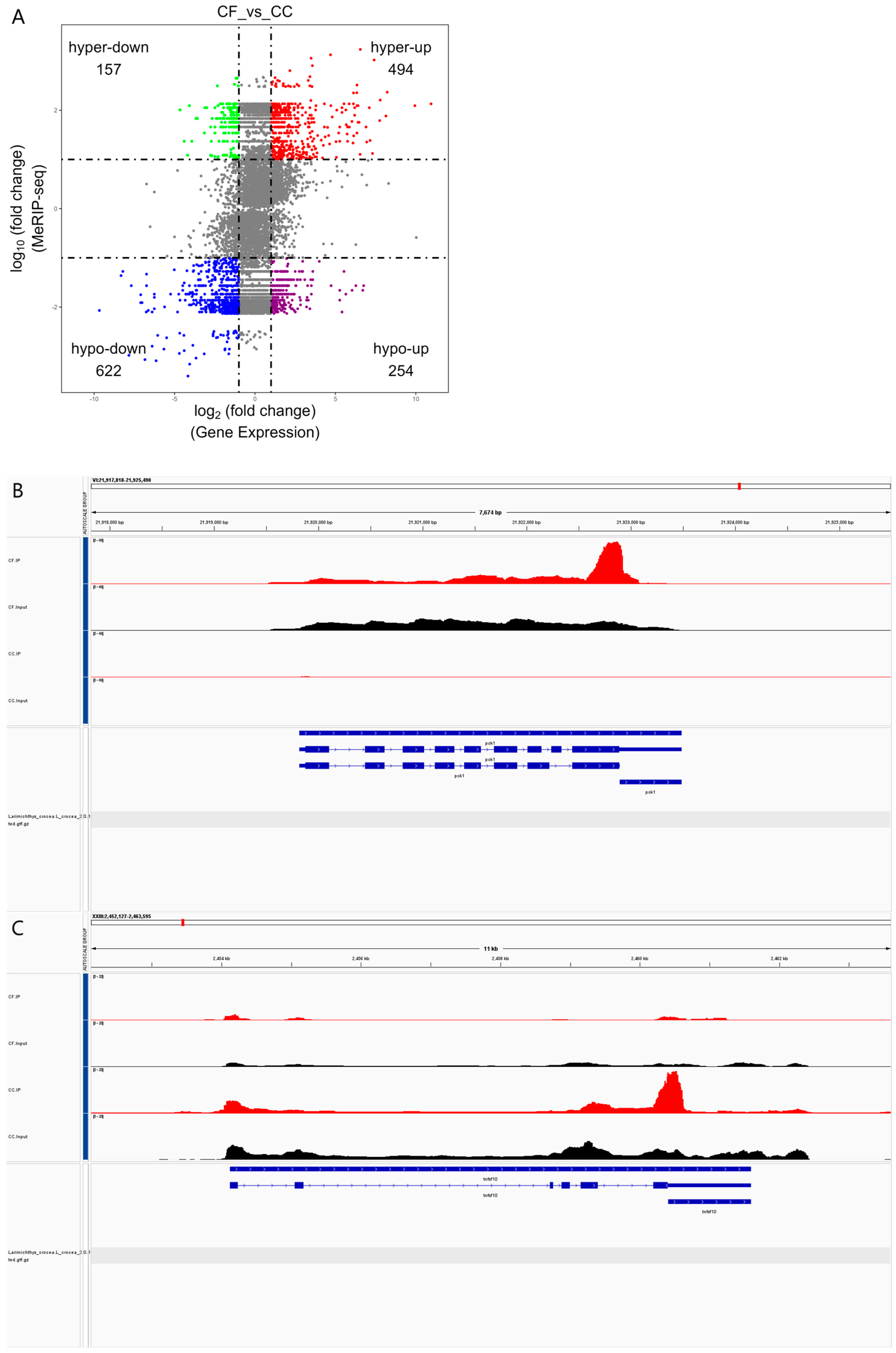
3.4. Difference in m6A Peak Analysis
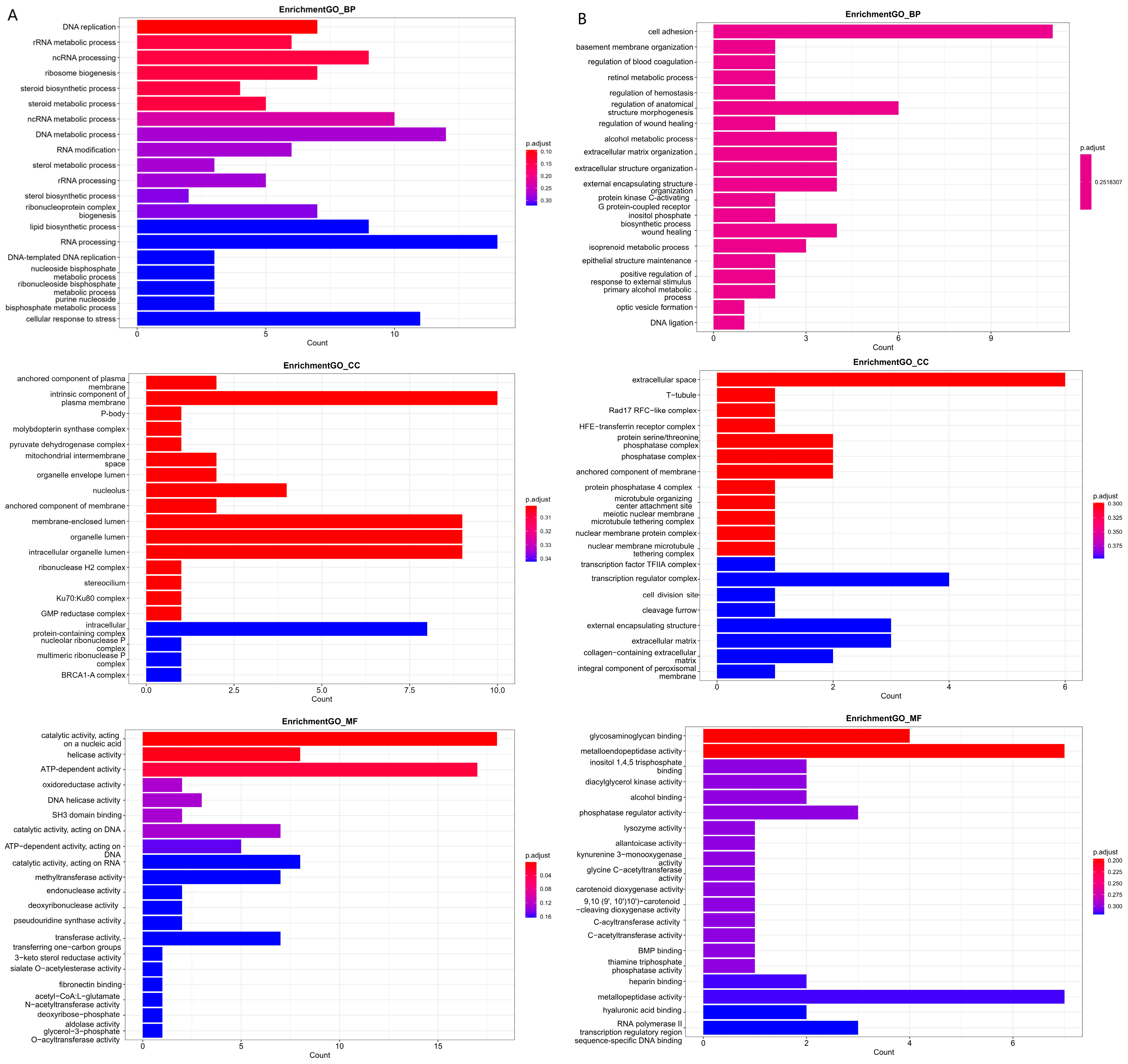
3.5. Differential m6A Methylation Gene GO Analysis
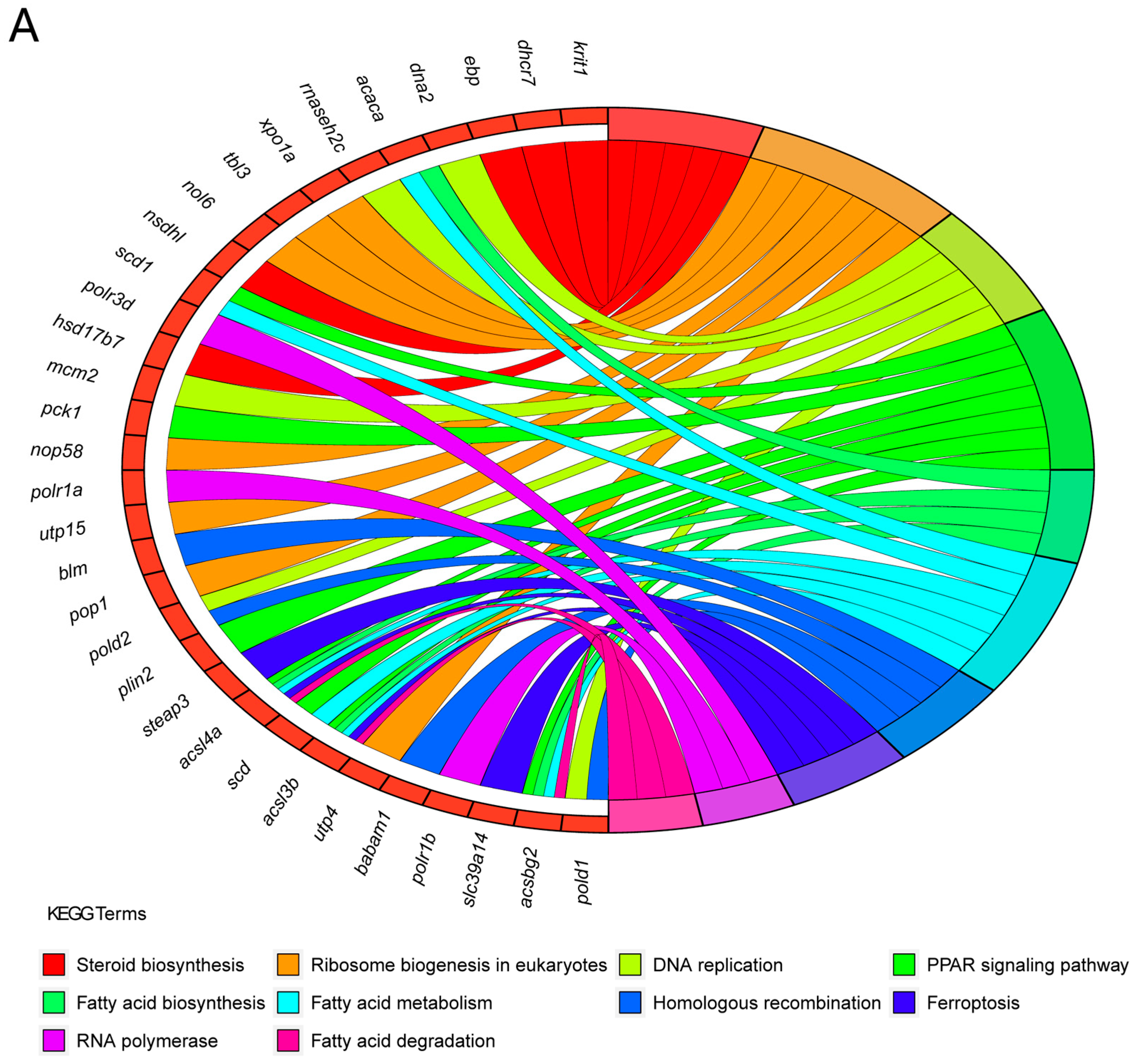
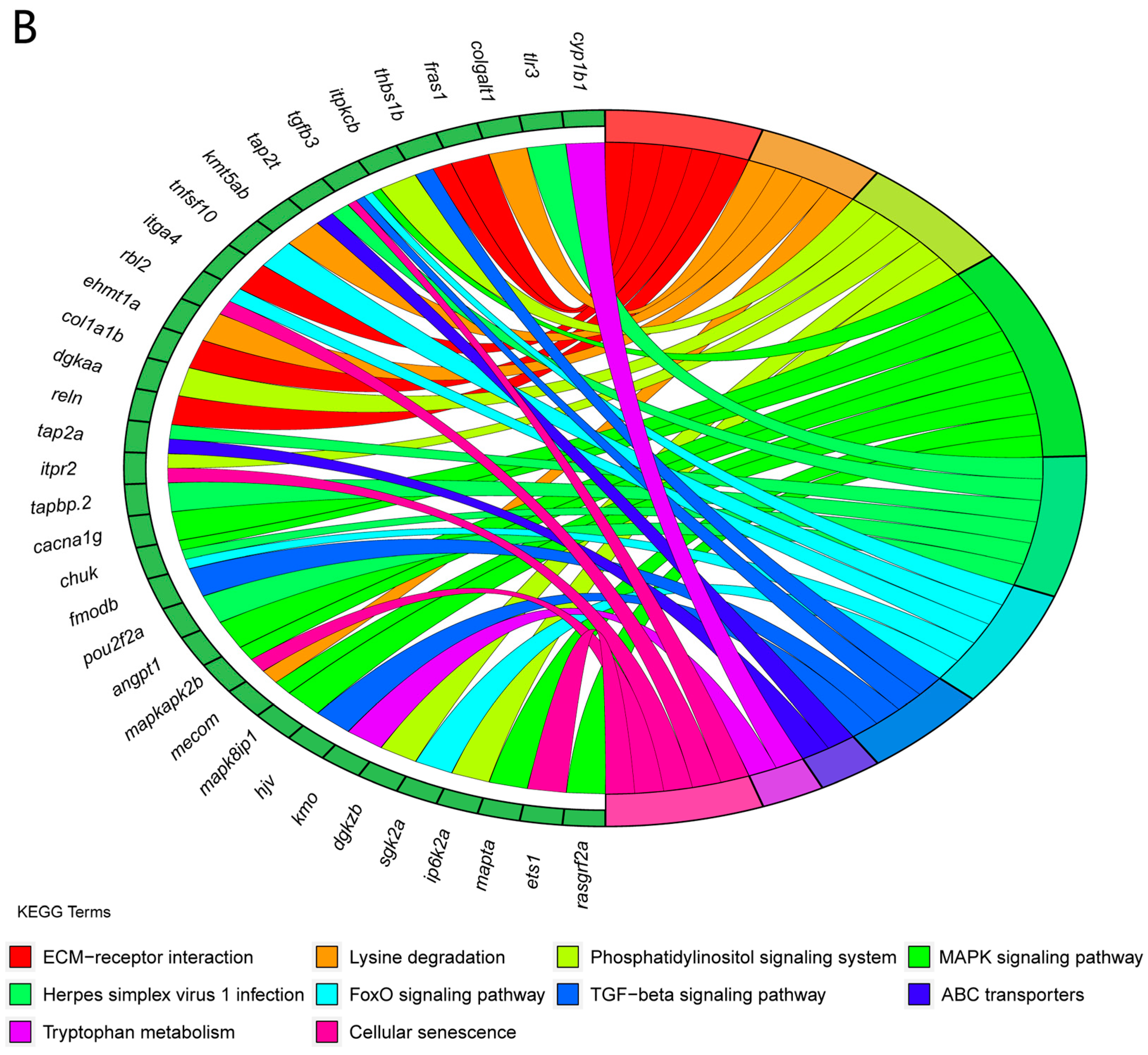
3.6. An Analysis of the KEGG Functional Enrichment Pathways
3.7. An Integrated Analysis of MeRIP and RNA Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold shock and fish. J. Fish Biol. 2010, 73, 1491–1530. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; Mccauley, R.W. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Zerai, D.B.; Fitzsimmons, K.M.; Collier, R.J. Transcriptional response of delta-9-desaturase gene to acute and chronic cold stress in nile tilapia, Oreochromis niloticus. World Aquacult. Soc. 2010, 41, 800–806. [Google Scholar] [CrossRef]
- Kuo, C.M.; Hsieh, S.L. Comparisons of physiological and biochemical responses between milkfish (Chanos chanos) and grass carp (Ctenopharyngodon idella) to cold shock. Aquaculture 2006, 251, 525–536. [Google Scholar] [CrossRef]
- Green, B.S.; Fisher, R. Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J. Exp. Mar. Biol. Ecol. 2004, 299, 115–132. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.H.; Song, W.; Xie, Z.L.; Zhang, H. The effect of copper domestication on oxidative damage in large yellow croaker under low temperature stress based on transcriptome analysis. J. Fish. Sci. China 2022, 29, 1425–1436. [Google Scholar]
- Liu, J.F. Farming and Biology of Yellow Croaker; Xiamen University Press: Xiamen, China, 2013; pp. 8–14. [Google Scholar]
- Lu, D.L. The Mechanism of Hunger Enhancing Cold Stress Tolerance in Zebrafish (Danio rerio). Ph.D. Thesis, East China Normal University, Shanghai, China, 2019. [Google Scholar]
- Zeng, L.; Song, W.; Xie, Z.L.; Wang, Y.H.; Xiong, Y.F.; Zhang, H. Based on metabolomics analysis, the adaptation mechanism of yellow croaker to low temperature and hunger stress. J. Fish. China 2023, 47, 86–97. [Google Scholar]
- Xu, H. Preliminary Exploration of the Effect and Mechanism of Starvation Stress on the Low-Temperature Tolerance of Large Yellow Croaker. Master’s Thesis, Jimei University, Fujian, China, 2015. [Google Scholar]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Zhang, X.; Xia, L. RNA m6A methylation regulators in sepsis. Mol. Cell. Biochem. 2023, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, J.; Gao, X.; Zhang, S.; Jaffrey, S.R.; Qian, S.B. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef]
- Lu, Z.K. A Study on Heat Stress in Hu Sheep Based on RNA Seq and MeRIP Seq. Ph.D. Thesis, Gansu Agricultural University, Gansu, China, 2020. [Google Scholar]
- Xiang, Y.; Laurent, B.; Hsu, C.H.; Nachtergaele, S.; Lu, Z.; Sheng, W.; Xu, C.; Chen, H.; Ouyang, J.; Wang, S. RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature 2017, 543, 573–576. [Google Scholar] [CrossRef]
- Fan, X.T. Studies on the Response Mechanism of Hunger Stress to Reproductive Development, Cell Growth, Autophagy and DNA Methylation in Zebrafish. Ph.D. Thesis, Northwest A&F University, Shanxi, China, 2021. [Google Scholar]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. Bioinformatics 2010, 26, 1968–1971. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W. Model-based analysis of ChIP-Seq (MACS). Methods Mol. Biol. 2008, 1150, 81–95. [Google Scholar] [CrossRef]
- Bailey, T.L. DREME: Motif discovery in transcription factor ChIP-seq data. Bioinformatics 2011, 27, 1653–1659. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Consortium, T.G.O. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar]
- Draghici, S.; Khatri, P.; Tarca, A.L.; Amin, K.; Done, A.; Voichita, C.; Georgescu, C.; Romero, R. A systems biology approach for pathway level analysis. Genome Res. 2007, 17, 1537–1545. [Google Scholar] [CrossRef]
- Anders, S. Analysing RNA-Seq data with the DESeq package. Mol. Biol. 2010, 43, 1–17. [Google Scholar]
- Shao, Y.; Duan, X.; Zhao, X.; Lv, Z.; Li, C. Global N6-methyladenosine methylation analysis reveals the positive correlation between m6A modification and mRNA abundance during Apostichopus japonicus disease development. Dev. Comp. Immunol. 2022, 133, 104434. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Kharas, M.G. Emerging links between m6A and misregulated mRNA methylation in cancer. Genome Med. 2017, 9, 2. [Google Scholar] [CrossRef]
- Zhang, C.X.; Chen, Y.S.; Sun, B.F.; Wang, L.; Yang, Y.; Ma, D.Y.; Lv, J.H.; Heng, J.; Ding, Y.Y.; Xue, Y.Y. m6A modulates haematopoietic stem and progenitor cell specification. Nature 2017, 549, 273–276. [Google Scholar] [CrossRef]
- Sun, J.H. Differential Expression Analysis and Lineage Identification of Low-Temperature Transcriptome Genes in the Red Finned Pufferfish. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA modifications in gene expression regulation. Cell 2017, 169, 1187. [Google Scholar] [CrossRef]
- Budna, J.; Celichowski, P.; Bryja, A.; Dyszkiewicz-Konwińska, M.; Jeseta, M.; Bukowska, D.; Antosik, P.; Brüssow, K.P.; Bruska, M.; Nowicki, M. Significant Down-regulation of “biological adhesion” genes in porcine oocytes after IVM. Int. J. Mol. Sci. 2017, 18, 2685. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Ye, Y.T.; Wu, P.; Cai, C.F.; Cao, X.M.; Cheng, Z.Q.; Wang, Y.L. Biological evolution based on steroid synthesis pathways and utilization of feed resources through their products. Feed Ind. 2023, 44, 1–10. [Google Scholar]
- Ye, Y.T.; Cai, C.F.; Xu, F.; Dong, J.J.; Zhang, B.T.; Xiao, P.Z. Feeding oxidized fish oil upregulates the expression of cholesterol and bile acid synthesis gene pathways in the intestinal mucosa of grass carp. J. Aquat. Biol. 2015, 39, 90–100. [Google Scholar]
- Gao, M.M.; Ye, Y.T.; Wu, P.; Cai, C.F.; Lin, X.X.; Wu, D.W.; He, J.; Zhang, B.T.; Xiao, P.Z. The effect of feeding oxidized fish oil on differential expression of genes involved in cholesterol and bile acid synthesis pathways in the gastrointestinal mucosa of yellow catfish. Genom. Appl. Biol. 2017, 36, 1912–1925. [Google Scholar]
- Long, Y.; Song, G.; Yan, J.; He, X.; Li, Q.; Cui, Z. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genom. 2013, 14, 612. [Google Scholar] [CrossRef]
- Lemberger, T. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol. 1996, 12, 335–363. [Google Scholar] [CrossRef]
- Miao, T.; Wan, Z.X.; Sun, L.N.; Li, X.N.; Xing, L.L.; Bai, Y.C.; Wang, F.; Yang, H.S. Extracellular matrix remodeling and matrix metalloproteinases (ajMMP-2 like and ajMMP-16 like) characterization during intestine regeneration of sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 212, 12–23. [Google Scholar] [CrossRef]
- Chen, M.; Meng, Q.; Qin, Y.; Liang, P.; Tan, P.; He, L.; Zhou, Y.; Chen, Y.; Huang, J.; Wang, R.F.; et al. TRIM14 Inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol. Cell 2016, 64, 105–119. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Wang, D.; Xu, J.; Xu, B.; Ma, B.S.; Zhu, X.Y. Transcriptome analysis of the liver and pancreas of crayfish under hypoxic stress. Freshw. Fish. 2022, 52, 26–35. [Google Scholar]
- Li, X.; Cui, Z.; Liu, Y.; Song, C.; Shi, G.; Yue, G. H Transcriptome analysis and discovery of genes involved in immune pathways from hepatopancreas of microbial challenged mitten crab eriocheir sinensis. PLoS ONE 2013, 8, e68233. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Xu, M.; Liu, Q.; Ye, H.; Zou, C.; Ye, C. Liver transcriptome analysis and de novo annotation of the orange-spotted groupers (Epinephelus coioides) under cold stress. Comparative biochemistry and physiology. Part D Genom. Proteom. 2019, 29, 264–273. [Google Scholar]
- Xu, D.; You, Q.; Chi, C.; Luo, S.; Song, H.; Luo, B.; Takeuchi, Y. Transcriptional response to low temperature in the yellow drum (Nibea albiflora) and identification of genes related to cold stress. Comparative Biochemistry and Physiology. Part D Genom. Proteom. 2018, 28, 80–89. [Google Scholar]
- Hu, J.W.; You, F.; Wang, Q.; Weng, S.D.; Liu, H.; Wang, L.J.; Zhang, P.J.; Tan, X.G.; Xu, P. Transcriptional responses of olive flounder (Paralichthys olivaceus) to low temperature. PLoS ONE 2014, 9, e108582. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Liu, R.; He, M.N.; Che, T.D.; Jin, L.; Deng, L.M.; Tian, S.L.; Li, Y.; Lu, H.F. mRNA N6-methyladenosine methylation of postnatal liver development in pig. PLoS ONE 2017, 12, e0173421. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Zhu, G. Profiling of RNA N6-methyladenosine methylation during follicle selection in chicken ovary. Poult. Sci. 2019, 98, 6117–6124. [Google Scholar] [CrossRef]
| Primer Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | TCCTCGGTATGGAATCTTGC | GAGTATTTACGCTCAGGTGGG |
| mettl3 | GCAGAGCAAGAAGGTGAGCAGAG | TCCCGTGGTCGCAGAACTCC |
| mettl14 | TGCCCAACTCCCACCTATCCG | CCTCTGTCTCCGCCTCTTCCTG |
| fto | CCTCATGCTGCCACTGGAATCTG | TGAACTGAAGCGTGCGATGTCTC |
| alkbh5 | CGGTGTTTGTCCTGCCTGTGAG | ACGCCGCTGCTTGATGTCTTG |
| Sample | Raw Reads | Q30 | Clean Reads | Clean Ratio | Mapped Reads |
|---|---|---|---|---|---|
| CF.IP | 42,980,372 | 90.97% | 42,939,500 | 99.90% | 27,123,510 |
| CC.IP | 47,704,922 | 90.96% | 47,656,038 | 99.90% | 32,767,664 |
| CF.Input | 77,079,832 | 93.42% | 75,403,704 | 97.83% | 57,529,263 |
| CC.Input | 90,898,260 | 93.74% | 89,057,952 | 97.98% | 65,467,101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Q.; Xie, Z.; Li, L.; Han, X.; Song, W. A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea). Fishes 2024, 9, 41. https://doi.org/10.3390/fishes9010041
Ji Q, Xie Z, Li L, Han X, Song W. A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea). Fishes. 2024; 9(1):41. https://doi.org/10.3390/fishes9010041
Chicago/Turabian StyleJi, Qun, Zhengli Xie, Lizhen Li, Xulei Han, and Wei Song. 2024. "A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea)" Fishes 9, no. 1: 41. https://doi.org/10.3390/fishes9010041
APA StyleJi, Q., Xie, Z., Li, L., Han, X., & Song, W. (2024). A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea). Fishes, 9(1), 41. https://doi.org/10.3390/fishes9010041






