Feasibility of In Vivo Semen Collection and Description of the Morphology and Ultrastructure of the Spermatozoa of Arapaima gigas (Schinz, 1822)
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Sampling Procedure
2.2. Spermatozoon Ultrastructure
2.2.1. Scanning Electron Microscopy (SEM) of Sperm
2.2.2. Transmission Electron Microscopy (TEM) of Sperm
2.3. Spermatozoon Morphometrics
2.4. Cell Membrane Integrity
3. Results
3.1. Spermatozoon Ultrastructure
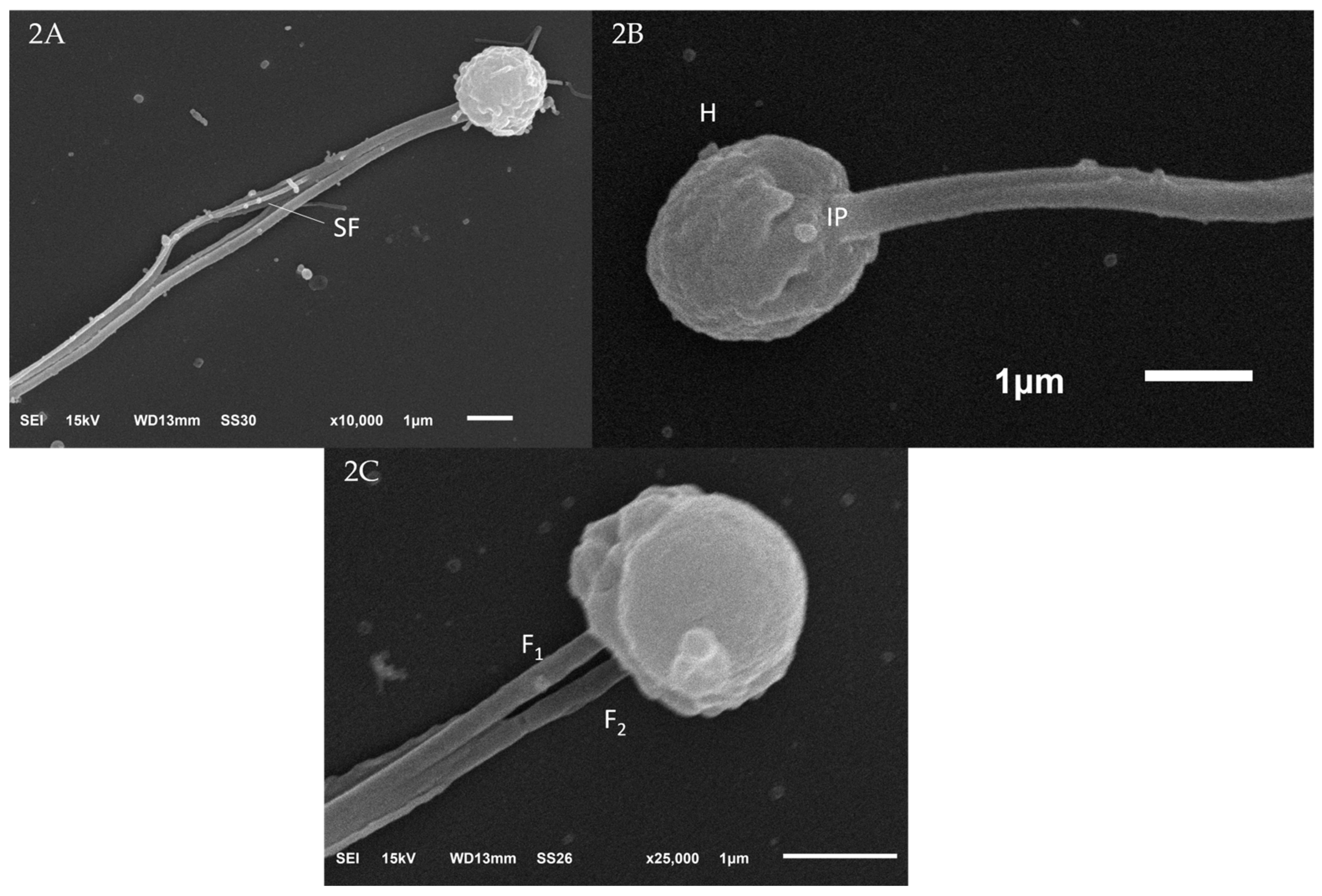
3.1.1. Scanning Electron Microscopy (SEM) of Sperm
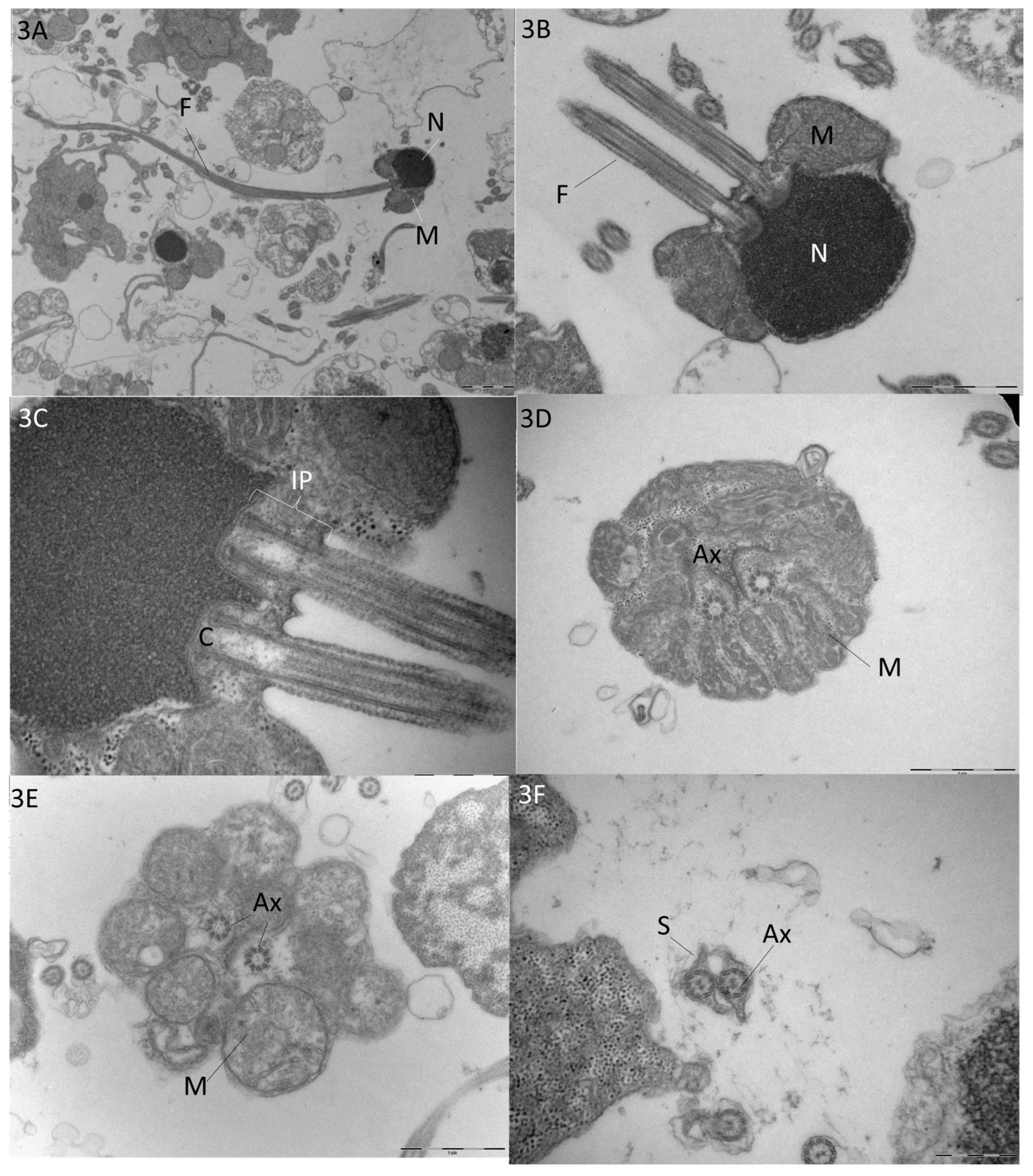
3.1.2. Transmission Electron Microscopy (TEM) of Sperm
3.1.3. Spermatozoon Morphometrics and Cell Membrane Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes; The Natural History Press: Garden City, NY, USA, 1966. [Google Scholar]
- Rocha, M.J.; Arukwe, A.; Kapoor, B.G. Fish Reproduction; Science Publishers: Enfield, CT, USA, 2008. [Google Scholar]
- Sloman, K.A. The Diversity of Fish Reproduction: An Introduction. In Encyclopedia of Fish Physiology—From Genome to Environment; Farrell, A., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume I, pp. 613–615. [Google Scholar]
- Mattei, X. Spermatozoon ultrastructure and its systematic implications in fishes. Can. J. Zool. 1991, 69, 3038–3055. [Google Scholar] [CrossRef]
- Pitnick, S.; Hosken, D.J.; Birkhead, T.R. Sperm morphological diversity. In Sperm Biology: An Evolutionary Perspective; Pitnick, S., Hosken, D.J., Birkhead, T.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 69–149. [Google Scholar]
- Koenig, L.A.; Gallant, J.R. Sperm competition, sexual selection and the diverse reproductive biology of Osteoglossiformes. J. Fish Biol. 2021, 99, 740–754. [Google Scholar] [CrossRef]
- Mattei, X.; Marchand, B.; Quilichini, Y. A biflagellate spermatozoon in the African bonytongue Heterotis niloticus (Teleostei, Osteoglossidae). J. Fish Biol. 2019, 94, 335–338. [Google Scholar] [CrossRef]
- Morrow, E.H. How the sperm lost its tail: The evolution of aflagellate sperm. Biol. Rev. 2004, 79, 795–814. [Google Scholar] [CrossRef]
- Bratton, B.O.; Kramer, B. Patterns of the electric organ discharge during courtship and spawning in the mormyrid fish, Pollimyrus isidori. Behav. Ecol. Sociobiol. 1989, 24, 349–368. [Google Scholar] [CrossRef]
- Montgomerie, R.; Fitzpatrick, J.L. Testes, sperm, and sperm competition. In Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes): Phylogeny, Reproductive System, Viviparity, Spermatozoa; Jamieson, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–53. [Google Scholar]
- Du Plessis, L.; Soley, J.T. Structural peculiarities associated with multiflagellate sperm in the emu, Dromaius novaehollandiae. Theriogenology 2012, 78, 1094–1101. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Castello, L. Nesting habitat of Arapaima gigas (Schinz) in Amazonian floodplains. J. Fish Biol. 2008, 72, 1520–1528. [Google Scholar] [CrossRef]
- Gurdak, D.J.; Stewart, D.J.; Castello, L.; Arantes, C.C. Diversity in reproductive traits of arapaima (Arapaima spp., Müller, 1843) in Amazonian várzea floodplains: Conservation implications. Aquat. Conserv. 2019, 29, 245–257. [Google Scholar] [CrossRef]
- Castello, L. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol. Freshw. Fish 2008, 17, 38–46. [Google Scholar] [CrossRef]
- Fontenele, O. Contribuição para o conhecimento da biologia do Pirarucú, “Arapaima gigas” (Cuvier), em cativeiro (Actinopterygii, Osteoglossidae). Rev. Bras. Biol. 1948, 8, 445–459. [Google Scholar]
- Zelada-Mázmela, R.F.; Gutiérrez, G.A.; Zelada-Mázmela, E. Histological description of the early gonadal development of Arapaima gigas, paiche. J. World. Aquac. Soc. 2022, 53, 754–764. [Google Scholar] [CrossRef]
- Amaral, A.C.; Lima, A.F.; Ganeco-Kirschnik, L.N.; Almeida, F.L. Morphological characterization of pirarucu Arapaima gigas (Schinz, 1822) gonadal differentiation. J. Morphol. 2020, 281, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Godinho, H.P.; Santos, J.E.; Formagio, P.S.; Guimarães-Cruz, R.J. Gonadal morphology and reproductive traits of the Amazonian fish Arapaima gigas (Schinz, 1822). Acta Zool. Stock. 2005, 86, 289–294. [Google Scholar] [CrossRef]
- Núñez, J.; Duponchelle, F. Towards a universal scale to assess sexual maturation and related life history traits in oviparous teleost fishes. Fish Physiol. Biochem. 2009, 35, 167–180. [Google Scholar] [CrossRef]
- Núñez, J.; Chu-Koo, F.; Berland, M.; Arévalo, L.; Ribeyro, O.; Duponchelle, F.; Renno, J. Reproductive success and fry production of the paiche or pirarucu, Arapaima gigas (Schinz), in the region of Iquitos, Perú. Aquac. Res. 2011, 42, 815–822. [Google Scholar] [CrossRef]
- Farrel, A.P.; Randall, D.J. Air-breathing mechanics in two Amazonian teleosts, Arapaima gigas and Hoplerythrinus unitaeniatus. Can. J. Zool. 1978, 56, 939–945. [Google Scholar] [CrossRef]
- Faustino, F.; Silva, R.C.; Hilbig, C.C.; Makino, L.C.; Senhorini, J.A.; Ninhaus-Silveira, A.; Nakaghi, L.S.O. Spermatozoon ultrastructure and semen parameters of Brycon vermelha (Characiformes, Characidae). Anim. Reprod. Sci. 2015, 157, 17–23. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Blom, E. A one-minute live-dead sperm stain by means of eosin-nigrosin. Fertil. Steril. 1950, 1, 176–177. [Google Scholar] [CrossRef]
- Lubzens, E.; Daube, N.; Pekarsky, I.; Magnus, Y.; Cohen, A.; Yusefovich, F.; Feigin, P. Carp (Cyprinus carpio L.) spermatozoa cryobanks—Strategies in research and application. Aquaculture 1997, 155, 13–30. [Google Scholar] [CrossRef]
- Woynárovich, A.; Anrooy, R.V. Field Guide to the Culture of Tambaqui (Colossoma macropomum, Cuvier, 1816); Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Mataveli, M.; Moraes, G.V.; Vargas, L.D.; Toninato, M.J.C.; Sakaguti, E.S.; Barbosa, R.C.; Streit, D.P., Jr.; Merlini, L. Avaliação da qualidade do sêmen de tilápia-do-nilo (Oreochromis niloticus), linhagem chitralada, suplementada com diferentes concentrações de vitamina c. Bol. Inst. Pesca 2007, 33, 1–7. [Google Scholar]
- Król, J.; Żarski, D.; Bernáth, G.; Palińska-Żarska, K.; Krejszeff, S.; Długoński, A.; Horváth, Á. Effect of urine contamination on semen quality variables in Eurasian perch Perca fluviatilis L. Anim. Reprod. Sci. 2018, 197, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F. Characterization of seminal plasma proteins stabilizing the sperm viability in rainbow trout (Oncorhynchus mykiss). Anim. Reprod. Sci. 2007, 97, 151–164. [Google Scholar] [CrossRef]
- Drokin, S.I. Phospholipids and fatty acids of phospholipids of sperm from several freshwater and marine species of fish. Comp. Biochem. Physiol. Part B 1993, 104, 423–428. [Google Scholar] [CrossRef]
- Labbé, C.; Maisse, G. Influence of rainbow trout thermal acclimation on sperm cryopreservation: Relation to change in the lipid composition of the plasma membrane. Aquaculture 1996, 145, 281–294. [Google Scholar] [CrossRef]
- Jaspers, E.J.; Avault, J.W.; Russell, J.D. Spermatozoal morphology and ultrastructure of channel catfish, Ictalurus punctatus. Trans. Am. Fish. Soc. 1976, 105, 475–480. [Google Scholar] [CrossRef]
- Poirier, G.R.; Nicholson, N. Fine-structure of the testicular spermatozoa from the channel catfish, Ictalurus punctatus. J. Ultrastruct. Res. 1982, 80, 104–110. [Google Scholar] [CrossRef]
- Lavoue, S. Was Gondwanan breakup the cause of the intercontinental distribution of Osteoglossiformes? A time-calibrated phylogenetic test combining molecular, morphological, and paleontological evidence. Mol. Phylogenet. Evol. 2016, 99, 34–43. [Google Scholar] [CrossRef]
- Irene, K.F.K.; Tiéhoua, K.; Yaya, S.; Konan, N. Reproduction De Heterotis Niloticus (Cuvier, 1829) De La Riviere Agneby (Cote d’Ivoire). Eur. J. Sci. Res. 2016, 12, 1857–7881. [Google Scholar]
- Monentcham, S.E.; Kouam, J.; Pouomogne, V.; Kestemont, P. Biology and prospect for aquaculture of African bonytongue, Heterotis niloticus (Cuvier, 1829): A review. Aquaculture 2009, 289, 191–198. [Google Scholar] [CrossRef]
- Yao, Z.; Crim, L.W. Spawning of ocean pout (Macrozoarces americanus L.): Evidence in favour of internal fertilization of eggs. Aquaculture 1995, 130, 361–372. [Google Scholar] [CrossRef]
- Burke, M.G.; Leatherland, J.F. Seasonal changes in testicular histology of brown bullheads, Ictalurus nebulosus Lesueur. Can. J. Zool. 1984, 62, 1185–1194. [Google Scholar] [CrossRef]
- Torati, L.S.; Lima, A.F.; Ganeco-Kirschnik, L.N.; Migaud, H. Endoscopy and Cannulation as Non-Invasive Tools to Identify Sex and Monitor Reproductive Development in Arapaima gigas. Copeia 2019, 107, 287–296. [Google Scholar] [CrossRef]
- Farias, I.P.; Leão, A.; Crossa, M.; Almeida, Y.S.; Honczaryk, A.; Verba, J.T.; Hrbek, T. Evidence of polygamy in the socially monogamous Amazonian fish Arapaima gigas (Schinz, 1822) (Osteoglossiformes, Arapaimidae). Neotrop. Ichthyol. 2015, 13, 195–204. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L.; Desjardins, J.K.; Milligan, N.; Montgomerie, R.; Balshine, S. Reproductive-tactic-specific variation in sperm swimming speeds in a shell-brooding cichlid. Biol. Reprod. 2007, 77, 280–284. [Google Scholar] [CrossRef]
- Smith, C.C.; Ryan, M.J. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J. Evol. Biol. 2010, 23, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Berger, B.; Weismann, T.; Patzner, R.A. Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism. Fish Physiol. Biochem. 1996, 15, 167–179. [Google Scholar] [CrossRef]
- Coward, K.; Bromage, N.R.; Hibbitt, O.; Parrington, J. Gamete physiology, fertilization and egg activation in teleost fish. Rev. Fish Biol. Fish. 2002, 12, 33–58. [Google Scholar] [CrossRef]
- Torati, L.S.; Taylor, J.; Mesquita, P.E.C.; Migaud, H. GnRHa implants and size pairing effects on plasma and cephalic secretion sex steroids in Arapaima gigas. Gen. Comp. Endocrinol. 2020, 299, 113614. [Google Scholar] [CrossRef]
- Chu-Koo, F.; Dugue, R.; Alvan Aguilar, M.; Casanova Daza, A.; Alcantara Bocanegra, F.; Chavez Veintemilla, C.; Duponchelle, F.; Renno, J.F.; Tello, S.; Nunez, J. Gender determination in the Paiche or Pirarucu (Arapaima gigas) using plasma vitellogenin, 17β-estradiol, and 11-ketotestosterone levels. Fish Physiol. Biochem. 2009, 35, 125–136. [Google Scholar] [CrossRef]
- Lima, A.F. The influence of sex ratio on the reproduction of pirarucu, Arapaima gigas, in captivity. Acta Amaz. 2018, 48, 38–41. [Google Scholar] [CrossRef]
- Torati, L.S.; Varges, A.P.S.; Galvão, J.A.S.; Mesquita, P.E.C.; Migaud, H. Endoscopy application in broodstock management of Arapaima gigas (Schinz, 1822). J. Appl. Ichthyol. 2016, 32, 353–355. [Google Scholar] [CrossRef]

| Male ID | GnRHa Implanted | Time | Head Length (µm) | Head Width (µm) | Head Area (µm2) | Flagellum Length (µm) | Membrane Integrity (%) |
|---|---|---|---|---|---|---|---|
| M1 | Yes | B | 3.84 ± 0.68 | 3.22 ± 0.44 | 10.60 ± 3.15 | 67.01 ± 5.94 | 66.8 (n = 542) |
| M1 | Yes | C | 2.73 ± 0.26 | 2.38 ± 0.34 | 5.66 ± 1.22 | 72.15 ± 3.92 | 78.8 (n = 330) |
| M3 | No | A | 3.18 ± 0.32 | 2.87 ± 0.24 | 8.30 ± 1.24 | 67.69 ± 5.30 | 78.4 (n = 385) |
| M3 | No | B | 3.58 ± 0.30 | 3.08 ± 0.25 | 9.32 ± 0.98 | 72.44 ± 2.24 | n.a. |
| M4 | Yes | A | 2.33 ± 0.30 | 2.89 ± 0.19 | 8.41 ± 1.27 | 68.81 ± 1.84 | 62.5 (n = 387) |
| M7 | Yes | A | 3.18 ± 0.31 | 2.69 ± 0.26 | 7.42 ± 1.26 | 68.79 ± 4.28 | 70.4 (n = 81) |
| M7 | Yes | C | 3.39 ± 0.54 | 2.74 ± 0.44 | 7.53 ± 2.25 | 63.13 ± 7.94 | 78.8 (n = 142) |
| M8 | Yes | B | 3.36 ± 0.25 | 3.05 ± 0.25 | 8.71 ± 1.63 | 65.62 ± 6.49 | 69.0 (n = 113) |
| Overall mean | 3.32 ± 0.49 | 2.87 ± 0.39 | 8.26 ± 2.19 | 68.34 ± 5.69 | 72.1 (n = 1980) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torati, L.S.; Lopes, J.T.; Lima, A.F.; Puvanendran, V.; Batlouni, S.R.; Ganeco-Kirschnik, L.N. Feasibility of In Vivo Semen Collection and Description of the Morphology and Ultrastructure of the Spermatozoa of Arapaima gigas (Schinz, 1822). Fishes 2024, 9, 24. https://doi.org/10.3390/fishes9010024
Torati LS, Lopes JT, Lima AF, Puvanendran V, Batlouni SR, Ganeco-Kirschnik LN. Feasibility of In Vivo Semen Collection and Description of the Morphology and Ultrastructure of the Spermatozoa of Arapaima gigas (Schinz, 1822). Fishes. 2024; 9(1):24. https://doi.org/10.3390/fishes9010024
Chicago/Turabian StyleTorati, Lucas S., Júlia T. Lopes, Adriana F. Lima, Velmurugu Puvanendran, Sergio R. Batlouni, and Luciana N. Ganeco-Kirschnik. 2024. "Feasibility of In Vivo Semen Collection and Description of the Morphology and Ultrastructure of the Spermatozoa of Arapaima gigas (Schinz, 1822)" Fishes 9, no. 1: 24. https://doi.org/10.3390/fishes9010024
APA StyleTorati, L. S., Lopes, J. T., Lima, A. F., Puvanendran, V., Batlouni, S. R., & Ganeco-Kirschnik, L. N. (2024). Feasibility of In Vivo Semen Collection and Description of the Morphology and Ultrastructure of the Spermatozoa of Arapaima gigas (Schinz, 1822). Fishes, 9(1), 24. https://doi.org/10.3390/fishes9010024







