Abstract
Leptin (Lep) plays a crucial role in controlling food intake and maintaining energy balance in mammals. While several studies have shown the presence of multiple leptin genes in teleosts, limited information is currently available on how sex steroid hormones regulate the expression of these genes in fish. In this study, we used two previously expressed and purified leptin proteins to incubate four tissues in vitro (hypothalamic-pituitary-gonadal axis and liver) and used the RT-qPCR method to detect the expression of genes related to growth and reproduction in tongue sole (Cynoglossus semilaevis). The results showed that both recombinant LepA and LepB proteins of tongue sole almost entirely suppressed the expression of genes related to growth and reproduction in the hypothalamic-pituitary-gonadal axis and liver, while LepB may have had a positive role on steroid synthesis in gonads. Further, a high concentration of LepA facilitated the expression of IGF-Ι in liver. At the same time, we utilized human leptin to incubate four tissues in vitro; although most of them had trends similar to those stimulated with tongue sole leptins, there were still some differences, indicating differences among leptin homologs between humans and fishes. To our knowledge, this is the first study to explore the function of tongue sole LepA and LepB within the hypothalamic-pituitary-gonadal axis and liver in vitro. Our results provide a valuable resource and foundation for future studies.
Key Contribution:
The roles of leptins in regulating the growth and reproduction of tongue sole were shown; suggesting the need for further research and possible applications for sole culture.
1. Introduction
Leptin, a 16 kDa class-I helical cytokine, is encoded by the obese gene and functions as an adipostat. It regulates body weight by circulating in levels proportional to adipose tissue and by increasing after meals in mammals [,]. Of all teleosts, leptin was first found in pufferfish, Takifugu rubripes, in 2005 []. Subsequently, leptin was discovered in many other fish species, for example yellow cheek carp (Elopichthys bambusa) [], yellow catfish (Pelteobagrus fulvidraco) [], rainbow trout (Oncorhynchus mykiss) [], zebrafish (Danio rerio) [], grass carp (Ctenopharyngodon idella) [], goldfish (Carassius auratus) [], common carp (Cyprinus carpio) [], and tongue sole (C. semilaevis) []. Although it was well-known that leptin regulated nutrient acquisition [], adipostasis, and glucose homeostasis [], it has also been shown to possess other regulative functions, such as sexual maturation [], immune regulation [], and reproduction [].
In mammals, leptin plays a role in the initiation of puberty and the regulation of menstrual cycles. Low levels of leptin can lead to delayed puberty and menstrual irregularities, while high levels of leptin can lead to early puberty. Leptin also affects the release of gonadotropin-releasing hormone (Gnrh) from the hypothalamus, which ultimately regulates the secretion of luteinizing hormone (Lh) and follicle-stimulating hormone (Fsh) from the pituitary gland. These hormones are essential for the development and maturation of ovarian follicles and the production of estrogen and progesterone [,,]. However, leptin has been found to have both stimulatory and inhibitory effects on the HPG axis in fishes, depending on the species and the reproductive stage [,,]. In chub mackerel (Scomber japonicus), leptin administration has been shown to increase the expression of GnRH and to stimulate the release of Lh and Fsh, leading to enhanced reproductive activity []. In European sea bass (Dicentrarchus labrax), recombinant mouse leptin increases the release of Lh in sea bass pituitary cell culture during the late pre-pubertal, early post-pubertal, and adult stages []. Similarly, in an in vitro study on female rainbow trout, it was found that leptin protein directly stimulated the release of Fsh and Lh by acting on the pituitary []. On the other hand, in certain situations, leptin can also inhibit the HPG axis, potentially acting as a negative feedback signal to regulate reproductive processes []. Although the exact mechanisms and effects of leptin on the HPG axis in fish are still not fully understood, its role in regulating reproductive function is clear.
In addition, leptin inhibits the production of glucose in mammal liver, which helps to lower blood sugar levels. Leptin also stimulates fatty acid oxidation in the liver, promoting the breakdown of stored fats. This is important for maintaining healthy liver function and preventing conditions such as fatty liver disease []. Although the maturation of oocytes can be affected by Lh and Fsh in females [], other hormones like insulin-like growth factors (Igf-1), which are mainly produced by the liver [], could also mediate or modulate maturational oocytes [,]. For example, Igf-1 promoted zebrafish oocyte maturation, through the Egf (epidermal growth factor)-like ligands/Egfr/Mapk pathway []. Thus, leptin can act as a signaling molecule that regulates the expression of igf-1 in the liver and igf-1 can subsequently modulate maturational oocytes. Therefore, leptin plays a role in regulating metabolism and reproductive function in both organs. In addition, the function of LepB may be to regulate glucose homeostasis and adiposity in zebrafish [], while LepA plays a critical role in modulating anxiety, aggression, fear, and circadian rhythm behaviors in zebrafish []. Thus, these two types of leptin genes were studied in tongue sole to further exploit their functions.
Tongue sole (C. semilaevis) is one of the most economically important species in northern China []. The species demonstrates significant sexual differences in growth, with females being able to grow two to four times larger than males. Therefore, studying sex differentiation in this species holds immense potential for its application in aquaculture. Meanwhile, the leptin gene in tongue sole was first identified in 2018 []. However, few studies were performed to investigate the role of the leptin gene in the hypothalamic-pituitary-gonadal (HPG) axis, or the regulation of reproductive function in males or females. Therefore, in this study, the expression of growth and reproduction-related genes in different tissues was detected using RT-qPCR, while the tissues were incubated with two tongue sole leptins and human leptin. This method can give us a better understanding of the regulatory role of leptin expression. This study preliminarily explored the potential roles of the leptin gene in the reproduction of tongue sole. The findings will provide new basic knowledge for the development of stable ovary maturation and spawning regulation technology in tongue soles.
2. Materials and Methods
2.1. Experimental Animals
A total of 24 female tongue soles (body length 45 ± 5 cm; weight 550 ± 50 g) were purchased from a fish farm in Haiyang City, Shandong Province. All fish were held temporarily for 7 days at 24 °C in two open flow 3000 L tanks with semidiameters of 3 m and were fed 2% body weight commercial feed twice per day. The tanks also utilized bottom pumping and water exchange (salinity about 26; photoperiod 6 L:18 D; oxygen above 5 mg/L). All fishes were anesthetized by MS-222 (100 mg/L). After this, the liver, hypothalamus, pituitary gland, and ovaries were sampled and washed by L-15 medium (Gibco, New York, NY, USA). These four tissues were cut into 1 mm3 pieces and washed with L-15 medium again. Fragments of four tissues (30 mg/mL) were placed into a 24-well cell culture plate and were cultured in an L-15 medium with 10% fetal bovine serum (FBS, Gibco, Auckland, New Zealand), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 ug/mL Neomycin (Sangon Biotec, Shanghai, China) at 25 °C. After being cultured for 6 h, the supernatant was removed and L-15 media (without FBS) with different concentrations of recombinant leptin-a or leptin-b protein (csLepA; csLepB) of tongue sole (1 nmol/L, 10 nmol/L, or 100 nmol/L) or human leptin (10 nmol/L) were added separately. PBS was added into an L-15 medium, and served as a control group for each tissue. The csLepA and csLepB of tongue sole were expressed and purified in our previous study [,]. Finally, fragments of four tissues incubated in three types of leptins were collected separately after being cultivated for 24 h and were stored in liquid nitrogen for the subsequent RNA extraction. The selection of incubation time and csLeps concentration were based on a study in tilapia (Oreochromis mossambicus) []. Each concentration of recombinant protein and each sample has at least three biological replicates and three technical replicates. The detailed experimental flowchart is shown in Supplementary Figure S1.
2.2. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
The total RNA of all samples was extracted by TRIzol Reagent (Takara, Beijing, China) according to the manufacturer’s instructions. Meanwhile, following the manufacturer’s instructions, the first strand of cDNA chain was produced using a PrimeScriptTM RT reagent Kit with a gDNA Eraser (Code No. RR047A) (Takara, Beijing, China), with a total of 1 ug RNA added to every sample. Subsequently, 25 vital genes related to the HPG axis were detected by RT-qPCR (Table 1). Their amplification efficiencies were between 0.9–1.12, which agrees with our previous studies. The detailed thermal cycle program used is described here: 94 °C for 2 min, 40 cycles of 94 °C for 15 s; 55 °C for 15 s; and 57 °C for 30 s. 18S rRNA was used as a standard [] and was calculated using the 2−ΔΔCT method. All primers are shown in Table 1. Each sample had three negative amplification controls to confirm the lack of DNA in qPCR reactions.

Table 1.
Primers used in this study.
2.3. Statistic Analysis
Each experiment had at least three biological replicates and each biological sample also had three technical replicates. Data analysis was conducted using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± S.E.M. A p-value ˂ 0.05 and |fold change| > 2 was considered as significant, which was tested using an ANOVA.
3. Results
3.1. Hypothalamus
After being incubated with different concentrations of recombinant LepA or LepB proteins (csLepA & csLepB), nine genes (gnrh, gnrh2, gnrh3, kisspeptin2 (kiss2), kisspeptin2 receptor (kiss2r), pituitary adenylate cyclase-activating polypeptide (pacap), lepa, lepb, leptin receptor (lepr)) that related to hypothalamus were downregulated. Only the gonadotropin-inhibitory hormone (gnih) gene was upregulated significantly by about 2 times when incubated with 10 nmol/L csLepA. Meanwhile, the same regulatory trends were observed in the regulatory treatment stimulated by human leptin. Briefly, the vast majority of the tested genes were significantly downregulated by the human leptin stimulation. However, the expression of gnih was dramatically increased by 2.75 times when induced with human leptin, compared with the control group. Moreover, the gnihr, gnih, lepa, lepb, and lepr genes had the highest degree of downregulation in the hypothalamus, especially the gnihr gene which was downregulated at least 5 times when incubated with 10 nmol/L csLepA. Additionally, the downregulation of gnrh, pacap, gnrh3 and kiss2 was dose-dependent, i.e., the higher the concentration of csLepA, the greater the degree of downregulation (Figure 1). In short, almost all of these nine genes—related to reproduction—exhibited negative regulation with csLepA and csLepB and only gnih exhibited positive regulation.
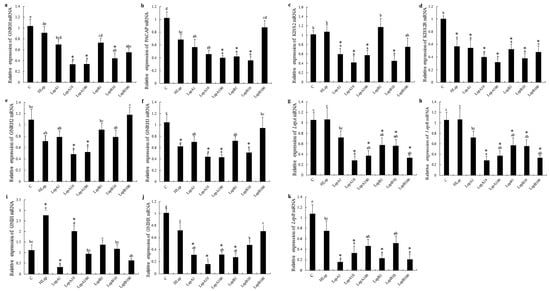
Figure 1.
The expression of eleven genes (a–k) in the hypothalamus that are associated with growth and reproduction after incubation with recombinant tongue sole leptins and human leptin (p < 0.05). C represents the control groups, which only added a culture medium; HLep represents human leptin (10 nmol/L in culture medium); and LepA and LepB represent tongue sole leptins A and B followed by the dose used (1, 10, or 100 nmol/L). (*) denotes a fold-change > 2 with statistically significant differences with respect to the Control. Different letters do not affect reading without the explanations.
3.2. Pituitary
Similarly, follicle-stimulating hormone (fsh), luteinizing hormone (lh), growth hormone (gh), and gonadotropin (gth) were all downregulated post csLepA and csLepB incubation. Interestingly, the significant downregulation of the lh, gth, and gh genes was also found in the pituitaries of tongue sole during human leptin stimulation. In addition, gth, lh, and gth had similar expression trends, whereby the higher the concentration of recombination LepA and LepB, the lower expression of these three genes. However, lh showed an opposite trend during csLepB incubation. Whether at high or low concentrations of csLepA and csLepB, gh was almost significantly downregulated, 10 nmol/L csLepA minimized the expression level of gh by at least 70%, while 10 nmol/L csLepB increased slightly the expression level of gh (Figure 2).
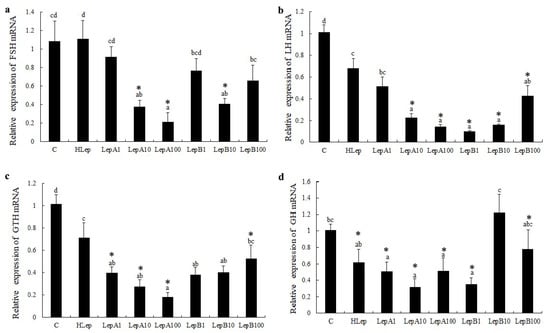
Figure 2.
The expression of four genes (a–d) that are associated with growth and reproduction in the pituitary after incubation with recombinant tongue sole leptins and human leptin (p < 0.05). C represents the control groups, which only added culture medium; HLep represents human leptin (10 nmol/L in culture medium); LepA and LepB represent tongue sole leptins A and B followed by the dose used (1, 10, or 100 nmol/L). (*) denotes a fold-change > 2 with statistically significant differences with respect to the Control. Different letters do not affect reading without the explanations.
3.3. Ovary
The expression of ten genes associated with steroid synthesis and adjustment was quite different. The expression of 3β-hydroxysteroid dehydrogenase (3b-hsd) and steroidogenic acute regulatory protein (star) had a similar trend—a slight increase and then dose-dependent reductions, induced with csLepA. Interestingly, it seems that the expression of most genes were dose-dependent reductions when induced with csLepA, like star, 3b-hsd, 17β-hydroxysteroid dehydrogenase (17b-hsd), P450 17α-hydroxylase/17,20-lyase (p450c17), lepr, follicle stimulating hormone receptor (fshr), and lepa (Figure 3). Moreover, P450 aromatase (p450arom) and P450 cholesterol-side-chain-cleavage (p450scc) had almost no response to stimulation with csLepA; at the same time, 17b-hsd, lepa, and star had almost no response to the stimulation of csLepB. The highest expression of fshr, lepb, p450c17, and 3b-hsd occurred when they were incubated with 100nmol/L csLepB, since they increased in a dose-dependent fashion (Figure 3). Of note, almost all of the tested genes showed insignificant expression in the ovary stimulated by human leptin, which might suggest differences between human and tongue sole leptin in certain regulatory roles.
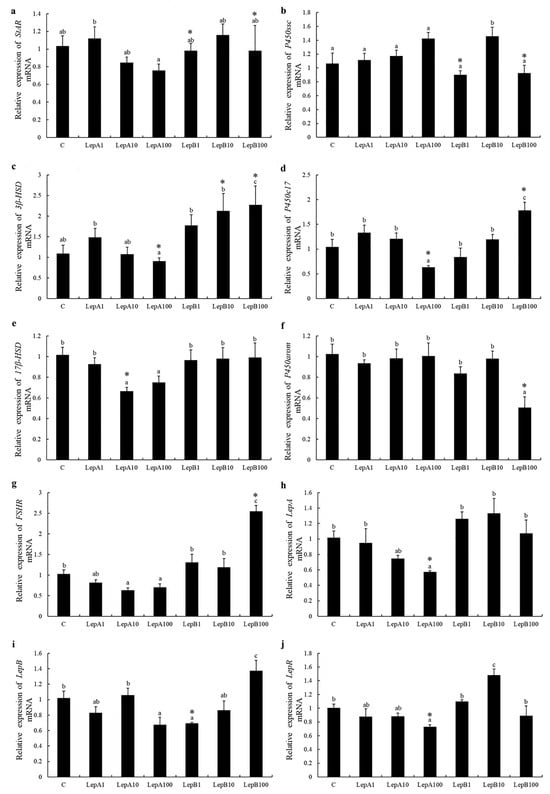
Figure 3.
The expression of ten genes (a–j) in the ovary that are associated with growth and reproduction following incubation with recombinant tongue sole leptins (p < 0.05). C represents the control groups, which only added culture medium; LepA and LepB represent tongue sole leptins A and B followed by the dose used (1, 10, or 100 nmol/L). (*) denotes a fold-change > 2 with statistically significant differences with respect to the Control. Different letters do not affect reading without the explanations.
3.4. Liver
In liver, the expression of lepa, lepb, lepr growth hormone receptor 1 (ghr1), and ghr2 almost all decreased by 60%, regardless of the different concentrations of csLepA and csLepB stimulation. Meanwhile, similar expression patterns were observed following stimulation by human leptin. The result showed that all of these genes were significantly downregulated. Noticeably, the expression of insulin-like growth factor (igf-1) increased sharply (at least 10 times compared with the control group) when incubated with 100 nmol/L csLepA (Figure 4). The fold-change heatmaps for all of these genes for the four tissues are shown in Supplementary Figures S2–S5.
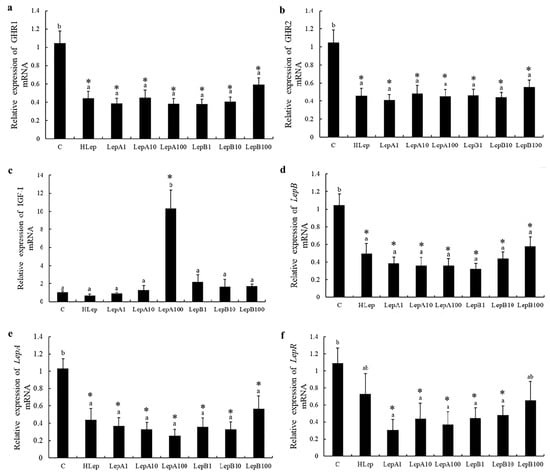
Figure 4.
The expression of six genes (a–f) in the liver that are associated with growth and reproduction after incubation with recombinant tongue sole leptins and human leptin (p < 0.05). C represents the control group, which only added culture medium; Help represents human leptin (10 nmol/L in culture medium); LepA and LepB represent tongue sole leptins A and B followed by the dose used (1, 10, or 100 nmol/L). (*) denotes a fold-change > 2 with statistically significant differences with respect to the Control. Different letters do not affect reading without the explanations.
4. Discussion
Despite extensive exploration of leptin’s function in the HPG axis of mammals, its role in fish reproduction is still not fully understood. In this study, we utilized expressed and purified tongue sole leptin proteins and human leptin to stimulate the HPG axis and liver in vitro and studied the expression of reproduction and growth-related genes, providing some basic information on the reproductive function of leptin in tongue sole.
In tongue sole, after 24 h of cultivation in vitro, two types of exogenous leptin proteins inhibited the expression of LepA, LepB, and LepR, and caused inhibition of genes related to GnRHs, pacap and reproduction (kiss2 and kiss2r), while upregulating GnIH in the hypothalamus. This result was similar to those of other previously studied species. For example, in mammals, proper concentration of leptin administered in the short term, could accelerate puberty in normal mice and reverse infertility in the ob/ob mouse [], while overexpression of leptin in female mice causes hypothalamic hypogonadism []. Thus, leptin may suppress most of the genes that are related to growth and reproduction in vitro in the hypothalamus of tongue sole. However, only gnrh2 was significantly upregulated after 100 nmol/L csLepB stimulation in tongue sole. Also, gnih was significantly upregulated when incubated with 10 nmol/L csLepA. In mammals, the expression of gnrh was increased by 9% when mice were injected with leptin []. Currently, there are only a few studies on the impact of leptin on hypothalamic function in vitro in teleosts. Leptin has only been shown to induce the expression of gnrh2 in the hypothalamus in vitro in pikeperch (Sander lucioperca). []. Our findings preliminarily showed that leptin is involved in growth, reproduction, and other related regulatory systems in tongue sole. Our findings are also the first to show the key roles of leptin genes in fish development.
Interestingly, the expression of fsh, lh, gth, and gh were also downregulated in the pituitaries of tongue sole, which was different from the results of other studies. For example, the release of Lh in European sea bass (D. labrax) pituitary cells was enhanced, induced by a high concentration of mouse recombinant leptin (10−6 M) []. Other studies have shown that human leptin had different effects on the release of Fsh and Lh at different stages of the sexual cycle in rainbow trout (Onchorynchus mykiss) pituitaries in vitro (cultured for 48 h), where Lh release was higher in mature rather than immature individuals []. In our study, the expression of fsh was slightly upregulated in the pituitaries induced with human leptin, while it was downregulated significantly during the tongue sole csLepA and csLepB incubation. The reason for this difference may be that the leptin gene was highly conserved in mammals [], while homology of leptin between mammals and teleosts, and within the teleost group, was very low []; thus, there may be differences in applying mammalian recombinant leptin to fish. Moreover, the expression of tongue sole gh in the pituitary was upregulated significantly when induced with 10 nM csLepB, while it was downregulated significantly when induced with csLepA, which was consistent with the result in tilapia (O. mossambicus) []. The expression of gh mRNA in tilapia was also suppressed in the pituitary treated with rtLepA in vitro, while the basal GH secretion was not affected by rtLepA. Thus, the csLepA and csLepB hormones could suppress the expression of reproduction- and growth-related genes in the pituitary.
Gnrh and Gnih play central roles in the neuroendocrine control of the reproductive process in vertebrates, including fishes, particularly by stimulating synthesis and release of gonadotropins. In detail, the synthesis and secretion of Gth subunits (glycoprotein a, GPa; FSHb; and LHb) in the pituitary gland are generally regulated by Gnrh. Research in several fish species has shown that Gnrh can increase the expression of gths that are crucial in the regulation of gonadal maturation in fishes []. Gnrh can upregulate the expression of gths in striped bass (Morone saxatilis) [], black porgy (Acanthopagrus schlegeli) [], spotted scat (Scatophagus argus) [], and pompano (Trachinotus ovatus) []. After this, sex steroids are secreted in the gonads by the stimulation of gths, which are responsible for gonadal maturation and reproduction []. Consequently, steroids give feedback to the brain and pituitary to maintain homeostatic regulation of reproductive function and behavior that could potentially inhibit the synthesis or secretion of Gnrh from the brain []. In this study, csLeps downregulated almost all gnrh-related genes in the hypothalamus, and csLeps also downregulated gths in the pituitary. Due to the fact that the two tissues were incubated separately, rather than in vivo, the specific regulatory mechanism is not yet known. Additionally, we mainly focused on the preliminary exploration of the regulated roles of leptin genes in the separated hypothalamus and pituitary organ; we found that the leptin genes have potential roles in these two organs. According to the close connection between the hypothalamus and pituitary in vivo, we hypothesized the key regulatory roles of leptin genes during oocyte maturation through the HPG axis. This will serve as the next step in our future study to predict and verify the detailed mechanism in vivo.
Leptin in tongue sole can also directly act upon the ovaries. The pathway of steroidogenesis is crucial during oocyte growth and maturation; thus, in this study, we detected the expression of related genes that take part in the steroidogenic pathway (Δ4 and Δ5 pathways) when exposed to csLepA and csLepB. star, an essential regulator in the rapid control of steroidogenesis by gonadotropic hormone, facilitates the production of steroid hormones by regulating the transportation of cholesterol to the mitochondria. P450scc catalyzes the initial and crucial step in steroidogenesis []. While these two genes have different responses to the two types of leptins, star was upregulated significantly at 1 nmol/L csLepA and downregulated significantly at 100 nmol/L csLepA in the current study. This decrease was dose-dependent, while this gene had almost no response to csLepB, indicating that csLepA was involved in and played an important role in the first step of the steroid synthesis process (Figure 5). In contrast, p450ssc had almost no response to csLepA, while it was upregulated significantly at 10 nmol/L csLepB. This suggests that csLepB may have had a positive effect on the p450ssc that was treated with the synthetic steroid precursor, pregnenolone, by performing side chain cleavage of cholesterol []. Additionally, pregnenolone can be transformed into progesterone through the action of 3b-Hsd (Δ4 pathway), or it can be converted into 17α-hydroxypregnenolone using P450c17 (Δ5 pathway) []. Thus, 3b-hsd and P450c17 play vital roles in steroid hormone synthesis. In our study, the high concentrations of csLepA inhibited the expression of these genes while high concentrations of csLepB increased the expression of 3b-hsd and p450c17. Finally, the formation of estradiol-17b is achieved through 17b-Hsd facilitating the conversion of androstenedione to testosterone and P450arom (Cyp19a) promoting the conversion of testosterone to estradiol-17b [,]. In our study, leptin had a negative effect on the expression of these two genes—17b-hsd was downregulated significantly when treated with 10 and 100 nmol/L csLepA, while p450arom (cyp19a) was significantly downregulated at a high concentration of csLepB stimulation (100 nmol/L). The current findings, which focused solely on the expression of the P450 aromatase gene, do not provide conclusive evidence that this expression resulted in the inhibition of estrogens. Furthermore, fshr, lepa, lepb, and lepr had a similarly expressed trend to 3b-hsd, which was downregulated when treated with csLepA, and upregulated during csLepB stimulation. In summary, LepA seems to play a negative role in the expression of genes that are related to the steroid synthesis process, and LepB seems to play positive roles in tongue sole ovarian maturation. However, the expression of all of these genes were not only dominated by recombined leptin proteins, but also were affected by the concentration of steroids, in vitro culture factors, different stages of ovarian development, and different species. For example, in Atlantic salmon (Salmo salar) parr hepatocytes, no expression of either lepa1 or lepa2 was observed when treated with an aromatase inhibitor, while lepa2 expression was upregulated when co-incubated with a testosterone and aromatase inhibitor, indicating that teleost androgens and estrogen can in turn directly control the regulation of leptin at the transcriptional level []. Leptin significantly increased the expression of p450 in cumulus cells from large antral follicles in vitro compared to that stimulated in vivo []. Thus, we detected the expression of some steroid synthesis-related genes following treatment with leptin in ovary fragments of tongue sole; amore research should be done in these areas.
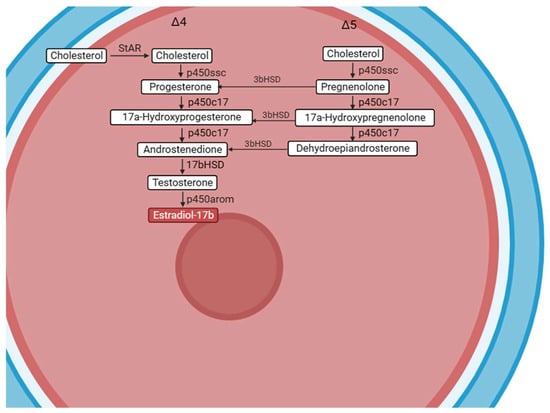
Figure 5.
Pathways of steroid synthesis in an oocyte, showing the steroidogenic pathway (Δ4 and Δ5 pathways) involved in the production of estradiol-17β (in red color) during oocyte growth (vitellogenesis).
Leptin, a hormone, is produced and released by adipose cells in mammals []; while it is mainly produced by the liver in most teleosts [,,,], adipose tissue may also contribute to leptin production []. In the liver of mammals, the Gh-Igf-1 axes have effects on adipolytic, anti-fibrotic, and anti-inflammatory processes [,]. The specific pathway of action is the Gh secreted by the pituitary gland, which binds to the Ghr on hepatocytes and stimulates the production of Igf-1 []. In tilapia liver, low doses of recombinant tilapia LepA upregulated the expression of igf-1 and igf-2 and increased ghr1 and ghr2 mRNA abundance in vitro at higher concentrations, while only the hepatic expression of igf-1 was enhanced by rtLepA in vivo []. In tongue sole liver, however, the expression of ghr1, ghr2, lepa, lepb, lepr, and igf-1 were all downregulated following csLepA and csLepB stimulation. However, the expression of igf-1 was nearly 10 times higher than the control group when exposed to 100 nmol/L csLepA. Similarly, in our previous study, testosterone was observed to decrease the expressed levels of ghr1, ghr2, and igf-2 mRNA abundance, while increasing the expression of the igf-1 in tongue sole liver []. Regardless, our study suggested that leptins suppressed expression of the genes related with growth and metabolism in the liver in vitro and only high concentrations of csLepA increased IGF-Ι mRNA abundance.
Currently, the function of leptin in the hypothalamus, pituitary, gonads, and liver is well-studied in mammals, while the effects of leptin in these tissues has been studied in only a few fish species due to the restriction of homology of leptin. In our study, two leptins could directly act on four tissues and affect gene expression related to growth and reproduction, with most genes being suppressed in the hypothalamus, pituitary, and liver. However, most genes associated with steroid synthesis were suppressed by csLepA, while they were upregulated during csLepB incubation in ovaries, indicating a positive effect on steroid synthesis. It seems that these results contradict the results in higher vertebrates. In mammals, leptin not only has a stimulative effect at the hypothalamic level, but it also directly affects the anterior pituitary []. The release of Gnrh by hypothalamic neurons stimulates the release of pituitary gonadotropins, such as Lh and Fsh that play a positive role in regulating the production of sex hormones and germ cells []. While there were many different results in teleosts, the reason for this difference may be that the response of leptin to nutritional status varies among different fish species [,,], and the stimulation response of leptin to the HPG axis varies under different developmental states and seasons [,].
5. Conclusions
Herein, we used human leptin and tongue sole csLepA and csLepB that were expressed and purified previously to incubate tissues of the HPG axis and liver in vitro. After this, the expression of growth- and reproduction-related genes was detected by RT-qPCR. Both csLepA and csLepB proteins showed almost complete suppression of expression of these genes related to growth and reproduction in the HPG axis and liver. However, csLepB may play a positive role on steroid synthesis in gonads, and high concentration of csLepA facilitated the expression of igf-1 in liver. The findings of this study revealed the key functions of leptin regulating growth- and reproduction-related gene expression in the HPG axis and liver in teleosts, which provides new insight into the processes of ovary maturation and spawning regulation technology in tongue sole at the molecular level. However, understanding of the role of leptin in tongue sole is still in its early stages, and further research is needed to elucidate its specific functions and mechanisms of action.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8120608/s1, Figure S1: The experimental flowchart; Figure S2: The expression of genes in the hypothalamus that are associated with growth and reproduction after incubation with recombinant tongue sole leptins and human leptin (p < 0.05). C refers to the control group that only added culture medium; HLep indicates human leptin ((10 nmol/L in culture medium); LepA and LepB indicate tongue sole leptins A and B followed by the dose used (1, 10 or 100 nmol/L).; Figure S3: The expression of genes in the pituitary that are associated with growth and reproduction after incubating with recombinant tongue sole leptins and human leptin (p < 0.05). C refers to the control group that only added culture medium; HLep indicates human leptin ((10 nmol/L in culture medium); LepA and LepB indicate tongue sole leptins A and B followed by the dose used (1, 10 or 100 nmol/L).; Figure S4: The expression of genes in the ovary that are associated with growth and reproduction after incubating with recombinant tongue sole leptins and human leptin (p < 0.05). C refers to the control group that only added culture medium; LepA and LepB indicate tongue sole leptins A and B followed by the dose used (1, 10 or 100 nmol/L).; Figure S5: The expression of genes in the liver that are associated with growth and reproduction after incubating with recombinant tongue sole leptins and human leptin (p < 0.05). C refers to the control group that only added culture medium; HLep indicates human leptin ((10nmol/L in culture medium); LepA and LepB indicate tongue sole leptins A and B followed by the dose used (1, 10 or 100 nmol/L).
Author Contributions
Conceptualization, X.C. and Y.Z.; methodology, X.C.; software, Y.Z.; validation, X.C., B.W. and Y.Z.; formal analysis, A.C.; investigation, Y.J.; resources, Z.M.; data curation, Z.M.; writing—original draft preparation, Y.Z.; writing—review and editing, X.C.; visualization, Y.L.; supervision, Y.X.; project administration, Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32072993 and 32072949), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD51), Taishan Industrial Experts Program, and Earmarked Fund for China Agriculture Research System (CARS-47).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (Approval code: YSFRI-2022020, and date: 3 July 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Saladin, R.; De Vos, P.; Guerre-Millo, M.; Leturque, A.; Girard, J.; Staels, B.; Auwerx, J. Transient increase in obese gene expression after food intake or insulin administration. Nature 1995, 377, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Gao, J.; Wu, H.; Cheng, X.; Zhang, Z.; Song, R.; Li, S.; Zhou, J.; Li, C.; Zeng, G. Molecular characterization and expression pattern of leptin in yellow cheek carp (Elopichthys bambusa) and its transcriptional changes in response to fasting and refeeding. Biology 2023, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Tan, X.Y.; Wu, K.; Zhuo, M.Q.; Song, Y.F.; Chen, Q.L. Regulation and mechanism of leptin on lipid metabolism in ovarian follicle cells from yellow catfish Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 2015, 222, 116–123. [Google Scholar] [CrossRef]
- Murashita, K.; Uji, S.; Yamamoto, T.; Rønnestad, I.; Kurokawa, T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 377–384. [Google Scholar] [CrossRef]
- Michel, M.; Page-McCaw, P.S.; Chen, W.; Cone, R.D. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, 3084–3089. [Google Scholar] [CrossRef]
- Li, G.G.; Liang, X.F.; Xie, Q.; Li, G.; Yu, Y.; Lai, K. Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen. Comp. Endocrinol. 2010, 166, 117–127. [Google Scholar] [CrossRef]
- De Pedro, N.; Martínez-Alvarez, R.; Delgado, M.J. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J. Endocrinol. 2006, 188, 513–520. [Google Scholar] [CrossRef]
- Huising, M.O.; Geven, E.J.; Kruiswijk, C.P.; Nabuurs, S.B.; Stolte, E.H.; Spanings, F.A.; Verburg-van Kemenade, B.M.; Flik, G. Increased leptin expression in common Carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology 2006, 147, 5786–5797. [Google Scholar] [CrossRef]
- YZhang, B.; Wang, X.; Liu, Y.; Xu, B.; Shi, Y.; Jiang, A.; Cui, Z.; Zhang, R.; Sun, R. Recombinant expression and bioactivity analysis of two leptin genes of Cynoglossus semilaevis. SCXueBao 2019, 43, 2279–2289. [Google Scholar]
- Del Vecchio, G.; Murashita, K.; Verri, T.; Gomes, A.S.; Rønnestad, I. Leptin receptor-deficient (knockout) zebrafish: Effects on nutrient acquisition. Gen. Comp. Endocrinol. 2021, 310, 113832. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, K.; Rizwan, M.Z.; Grattan, D.R.; Horsfield, J.A.; Tups, A. Leptin regulates glucose homeostasis via the canonical Wnt pathway in the zebrafish. Faseb. J. 2022, 36, e22207. [Google Scholar] [CrossRef] [PubMed]
- Trombley, S.; Schmitz, M. Leptin in fish: Possible role in sexual maturation in male Atlantic salmon. Fish. Physiol. Biochem. 2013, 39, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Mariano, G.; Stilo, R.; Terrazzano, G.; Coccia, E.; Vito, P.; Varricchio, E.; Paolucci, M. Effects of recombinant trout leptin in superoxide production and NF-κB/MAPK phosphorylation in blood leukocytes. Peptides 2013, 48, 59–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar]
- Suter, K.J.; Pohl, C.R.; Wilson, M.E. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: Potential signals for the initiation of puberty. J. Clin. Endocrinol. Metab. 2000, 85, 808–814. [Google Scholar] [CrossRef]
- Plant, T.M. Leptin, growth hormone, and the onset of primate puberty. J. Clin. Endocrinol. Metab. 2001, 86, 458–460. [Google Scholar] [CrossRef]
- Ghizzoni, L.; Mastorakos, G. Interactions of leptin, GH, and cortisol in normal children. Ann. N. Y. Acad. Sci. 2003, 997, 56–63. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, C.; Yi, S.; Wan, S.; Chen, B.; Gao, Z. Leptin genes in blunt snout bream: Cloning, phylogeny and expression correlated to gonads development. Int. J. Mol. Sci. 2015, 16, 27609–27624. [Google Scholar] [CrossRef]
- Bagivalu Lakshminarasimha, A.; Puvvada, M.; Hammerschmidt, M.; Michel, M. Leptin modulates oocyte maturation by a central and a direct pathway in zebrafish. J. Endocrinol. 2022, 254, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weil, C.; Le Bail, P.Y.; Sabin, N.; Le Gac, F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 2003, 130, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ohga, H.; Ito, K.; Kakino, K.; Mon, H.; Kusakabe, T.; Lee, J.M.; Matsuyama, M. Leptin is an important endocrine player that directly activates gonadotropic cells in teleost fish, Chub Mackerel. Cells 2021, 10, 3505. [Google Scholar] [CrossRef] [PubMed]
- Peyon, P.; Zanuy, S.; Carrillo, M. Action of leptin on in vitro luteinizing hormone release in the European sea bass (Dicentrarchus labrax). Biol. Reprod. 2001, 65, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Peyon, P.; Vega-Rubín de Celis, S.; Gómez-Requeni, P.; Zanuy, S.; Pérez-Sánchez, J.; Carrillo, M. In vitro effect of leptin on somatolactin release in the European sea bass (Dicentrarchus labrax): Dependence on the reproductive status and interaction with NPY and GnRH. Gen. Comp. Endocrinol. 2003, 132, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef] [PubMed]
- Schirman-Hildesheim, T.D.; Gershon, E.; Litichever, N.; Galiani, D.; Ben-Aroya, N.; Dekel, N.; Koch, Y. Local production of the gonadotropic hormones in the rat ovary. Mol. Cell Endocrinol. 2008, 282, 32–38. [Google Scholar] [CrossRef]
- Conchillo, M.; Prieto, J.; Quiroga, J. Insulin-like growth factor I (IGF-I) and liver cirrhosis. Rev. Esp. Enferm. Dig. 2007, 99, 156–164. [Google Scholar]
- Patiño, R.; Yoshizaki, G.; Thomas, P.; Kagawa, H. Gonadotropic control of ovarian follicle maturation: The two-stage concept and its mechanisms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 427–439. [Google Scholar] [CrossRef]
- Mukherjee, D.; Majumder, S.; Moulick, S.R.; Pal, P.; Mallick, B.; Chakraborty, A.; Gupta, S. Signaling pathways in insulin- and IGF-I mediated oocyte maturation in lower vertebrates. Indian. J. Biochem. Biophys. 2014, 51, 520–526. [Google Scholar]
- Xie, L.; Tang, Q.; Yang, L.; Chen, L. Insulin-like growth factor I promotes oocyte maturation through increasing the expression and phosphorylation of epidermal growth factor receptor in the zebrafish ovary. Mol. Cell Endocrinol. 2016, 419, 198–207. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ding, Y.; Nowik, N.; Jager, C.; Eeza, M.N.H.; Alia, A.; Baelde, H.J.; Spaink, H.P. Leptin deficiency affects glucose homeostasis and results in adiposity in zebrafish. J. Endocrinol. 2021, 249, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sarasamma, S.; Chen, J.R.; Juniardi, S.; Sampurna, B.P.; Liang, S.T.; Lai, Y.H.; Lin, G.M.; Hsieh, M.C.; Hsiao, C.D. Zebrafish mutants carrying leptin a (lepa) gene deficiency display obesity, anxiety, less aggression and fear, and circadian rhythm and color preference dysregulation. Int. J. Mol. Sci. 2018, 19, 4038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Cheng, P.; Chen, S. Identification of potential blind-side hypermelanosis-related lncRNA-miRNA-mRNA regulatory network in a flatfish species, Chinese tongue sole (Cynoglossus semilaevis). Front. Genet. 2021, 12, 817117. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Wang, B.; Liu, X.; Liu, Q.; Song, X.; Shi, B.; Ren, K. Leptin and leptin receptor genes in tongue sole (Cynoglossus semilaevis): Molecular cloning, tissue distribution and differential regulation of these genes by sex steroids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 224, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Baltzegar, D.A.; Mankiewicz, J.; Taylor, J.; Yamaguchi, Y.; Lerner, D.T.; Seale, A.P.; Grau, E.G.; Breves, J.P.; Borski, R.J. Control of leptin by metabolic state and its regulatory interactions with pituitary growth hormone and hepatic growth hormone receptors and insulin like growth factors in the tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2017, 240, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xin, N.; Zhai, Y.; Jiang, L.; Zhai, J.; Zhang, Q.; Qi, J. Reference gene selection for quantitative real-time RT-PCR normalization in the half-smooth tongue sole (Cynoglossus semilaevis) at different developmental stages, in various tissue types and on exposure to chemicals. PLoS ONE 2014, 9, e91715. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Wei, Y.; Zhang, Y.; Liang, M. Effects of different dietary DHA:EPA ratios on gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis). Br. J. Nutr. 2017, 118, 179–188. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Xu, Y.; Zhang, Y.; Liu, X. In vitro effects of tongue sole LPXRFa and kisspeptin on relative abundance of pituitary hormone mRNA and inhibitory action of LPXRFa on kisspeptin activation in the PKC pathway. Anim. Reprod. Sci. 2019, 203, 1–9. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Liu, X.; Xu, Y.; Song, X.; Shi, B. Molecular characterization of kiss2 and differential regulation of reproduction-related genes by sex steroids in the hypothalamus of half-smooth tongue sole (Cynoglossus semilaevis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 213, 46–55. [Google Scholar] [CrossRef]
- Ji, X.S.; Chen, S.L.; Jiang, Y.L.; Xu, T.J.; Yang, J.F.; Tian, Y.S. Growth differences and differential expression analysis of pituitary adenylate cyclase activating polypeptide (PACAP) and growth hormone-releasing hormone (GHRH) between the sexes in half-smooth tongue sole Cynoglossus semilaevis. Gen. Comp. Endocrinol. 2011, 170, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [PubMed]
- Yura, S.; Ogawa, Y.; Sagawa, N.; Masuzaki, H.; Itoh, H.; Ebihara, K.; Aizawa-Abe, M.; Fujii, S.; Nakao, K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J. Clin. Investig. 2000, 105, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Duan, J.; Duff, E.; Choi, Y.H.; Hartzell, D.L.; Della-Fera, M.A.; Baile, C.A. Gene expression in arcuate nucleus-median eminence of rats treated with leptin or ciliary neurotrophic factor. Biofactors 2007, 31, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, F.J.; Wuertz, S. Insights into kisspeptin- and leptin-signalling on GnRH mRNA expression in hypothalamic organ cultures of immature pikeperch Sander lucioperca. Int. Aquat. Res. 2016, 8, 191–196. [Google Scholar] [CrossRef][Green Version]
- Ahima, R.S.; Osei, S.Y. Leptin signaling. Physiol. Behav. 2004, 81, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. Int. Rev. Cytol. 2003, 225, 131–185. [Google Scholar]
- Hassin, S.; Gothilf, Y.; Blaise, O.; Zohar, Y. Gonadotropin-I and -II subunit gene expression of male striped bass (Morone saxatilis) after gonadotropin-releasing hormone analogue injection: Quantitation using an optimized ribonuclease protection assay. Biol. Reprod. 1999, 58, 1233–1240. [Google Scholar] [CrossRef][Green Version]
- An, K.W.; Nelson, E.R.; Habibi, H.R.; Choi, C.Y. Molecular characterization and expression of three GnRH forms mRNA during gonad sex-change process, and effect of GnRHa on GTH subunits mRNA in the protandrous black porgy (Acanthopagrus schlegeli). Gen. Comp. Endocrinol. 2008, 159, 38–45. [Google Scholar] [CrossRef]
- Chen, H.P.; Cui, X.F.; Wang, Y.R.; Li, Z.Y.; Tian, C.X.; Jiang, D.N.; Zhu, C.H.; Zhang, Y.; Li, S.S.; Li, G.L. Identification, functional characterization, and estrogen regulation on gonadotropin-releasing hormone in the spotted scat, Scatophagus argus. Fish. Physiol. Biochem. 2020, 46, 1743–1757. [Google Scholar] [CrossRef]
- Ren, X.; Huang, Y.; Li, X.; Li, Z.; Yang, H.; He, R.; Zhong, H.; Li, G.; Chen, H. Identification and functional characterization of gonadotropin-releasing hormone in pompano (Trachinotus ovatus). Gen. Comp. Endocrinol. 2022, 316, 113958. [Google Scholar] [CrossRef] [PubMed]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, A.J.; Clarke, I.J. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol. Reprod. 2001, 64, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. The role of the StAR protein in steroidogenesis: Challenges for the future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50 (Suppl. S1), S195–S219. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Sudhakumari, C.C.; Chang, X.T.; Kobayashi, T.; Oba, Y.; Guan, G.; Yoshiura, Y.; Yoshikuni, M.; Nagahama, Y. Ovarian carbonyl reductase-like 20beta-hydroxysteroid dehydrogenase shows distinct surge in messenger RNA expression during natural and gonadotropin-induced meiotic maturation in nile tilapia. Biol. Reprod. 2002, 67, 1080–1086. [Google Scholar] [CrossRef]
- Fouad Mansour, M.; Pelletier, M.; Boulet, M.M.; Mayrand, D.; Brochu, G.; Lebel, S.; Poirier, D.; Fradette, J.; Cianflone, K.; Luu-The, V.; et al. Oxidative activity of 17β-hydroxysteroid dehydrogenase on testosterone in male abdominal adipose tissues and cellular localization of 17β-HSD type 2. Mol. Cell Endocrinol. 2015, 414, 168–176. [Google Scholar] [CrossRef]
- Hojo, Y.; Hattori, T.A.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.T.; Mukai, H.; Morrison, J.H.; Janssen, W.G.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef]
- Trombley, S.; Rocha, A.; Schmitz, M. Sex steroids stimulate leptin gene expression in Atlantic salmon parr hepatocytes in vitro. Gen. Comp. Endocrinol. 2015, 221, 156–164. [Google Scholar] [CrossRef]
- Kumar, P.A.; Sivakumar, A.V.N.; Pathipati, D.; Chakravarthi, V.P.; Brahmaiah, K.V.; Rao, V.H. Leptin induced in vitro development of ovarian follicles in sheep is related to the expression of P450 aromatase and steroidogenesis. Theriogenology 2019, 136, 1–6. [Google Scholar] [CrossRef]
- Flier, J.S.; Maratos-Flier, E. Lasker lauds leptin. Cell 2010, 143, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Soengas, J.L. Leptin signalling in teleost fish with emphasis in food intake regulation. Mol. Cell Endocrinol. 2021, 526, 111209. [Google Scholar] [CrossRef] [PubMed]
- Angotzi, A.R.; Stefansson, S.O.; Nilsen, T.O.; Rathore, R.M.; Rønnestad, I. Molecular cloning and genomic characterization of novel leptin-like genes in salmonids provide new insight into the evolution of the Leptin gene family. Gen. Comp. Endocrinol. 2013, 187, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Baltzegar, D.A.; Breves, J.P.; Lerner, D.T.; Seale, A.P.; Grau, E.G.; Borski, R.J. Prolactin is a major inhibitor of hepatic Leptin A synthesis and secretion: Studies utilizing a homologous Leptin A ELISA in the tilapia. Gen. Comp. Endocrinol. 2014, 207, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Won, E.T.; Baltzegar, D.A.; Picha, M.E.; Borski, R.J. Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): Control of feeding and regulation by nutritional state. Gen. Comp. Endocrinol. 2012, 178, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, C.; Johansson, M.; Angotzi, A.R.; Rønnestad, I.; Jönsson, E.; Björnsson, B.T.; Gutiérrez, J.; Navarro, I.; Capilla, E. Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. Gen. Comp. Endocrinol. 2015, 210, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Utz, A.L.; Yamamoto, A.; Hemphill, L.; Miller, K.K. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J. Clin. Endocrinol. Metab. 2008, 93, 2507–2514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaves, V.E.; Júnior, F.M.; Bertolini, G.L. The metabolic effects of growth hormone in adipose tissue. Endocrine 2013, 44, 293–302. [Google Scholar] [CrossRef]
- Carotti, S.; Guarino, M.P.L.; Valentini, F.; Porzio, S.; Vespasiani-Gentilucci, U.; Perrone, G.; Zingariello, M.; Gallo, P.; Cicala, M.; Picardi, A.; et al. Impairment of GH/IGF-1 axis in the liver of patients with HCV-related chronic hepatitis. Horm. Metab. Res. 2018, 50, 145–151. [Google Scholar] [CrossRef]
- Elias, C.F.; Purohit, D. Leptin signaling and circuits in puberty and fertility. Cell Mol. Life Sci. 2013, 70, 841–862. [Google Scholar] [CrossRef]
- Landry, D.; Cloutier, F.; Martin, L.J. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod. Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Frøiland, E.; Jobling, M.; Björnsson, B.T.; Kling, P.; Ravuri, C.S.; Jørgensen, E.H. Seasonal appetite regulation in the anadromous Arctic charr: Evidence for a role of adiposity in the regulation of appetite but not for leptin in signalling adiposity. Gen. Comp. Endocrinol. 2012, 178, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Flik, G. Leptin in teleostean fish, towards the origins of leptin physiology. J. Chem. Neuroanat. 2014, 61–62, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Johnson, T.M.; Londraville, R.L. Evidence for leptin expression in fishes. J. Exp. Zool. 2000, 286, 718–724. [Google Scholar] [CrossRef]
- Won, E.T.; Douros, J.D.; Hurt, D.A.; Borski, R.J. Leptin stimulates hepatic growth hormone receptor and insulin-like growth factor gene expression in a teleost fish, the hybrid striped bass. Gen. Comp. Endocrinol. 2016, 229, 84–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).