Epidemiology of Turbot (Scophthalmus maeoticus) Bacterial Contamination, a Fishery Limiting Factor on the Romanian Black Sea
Abstract
:1. Introduction
2. Materials and Methods
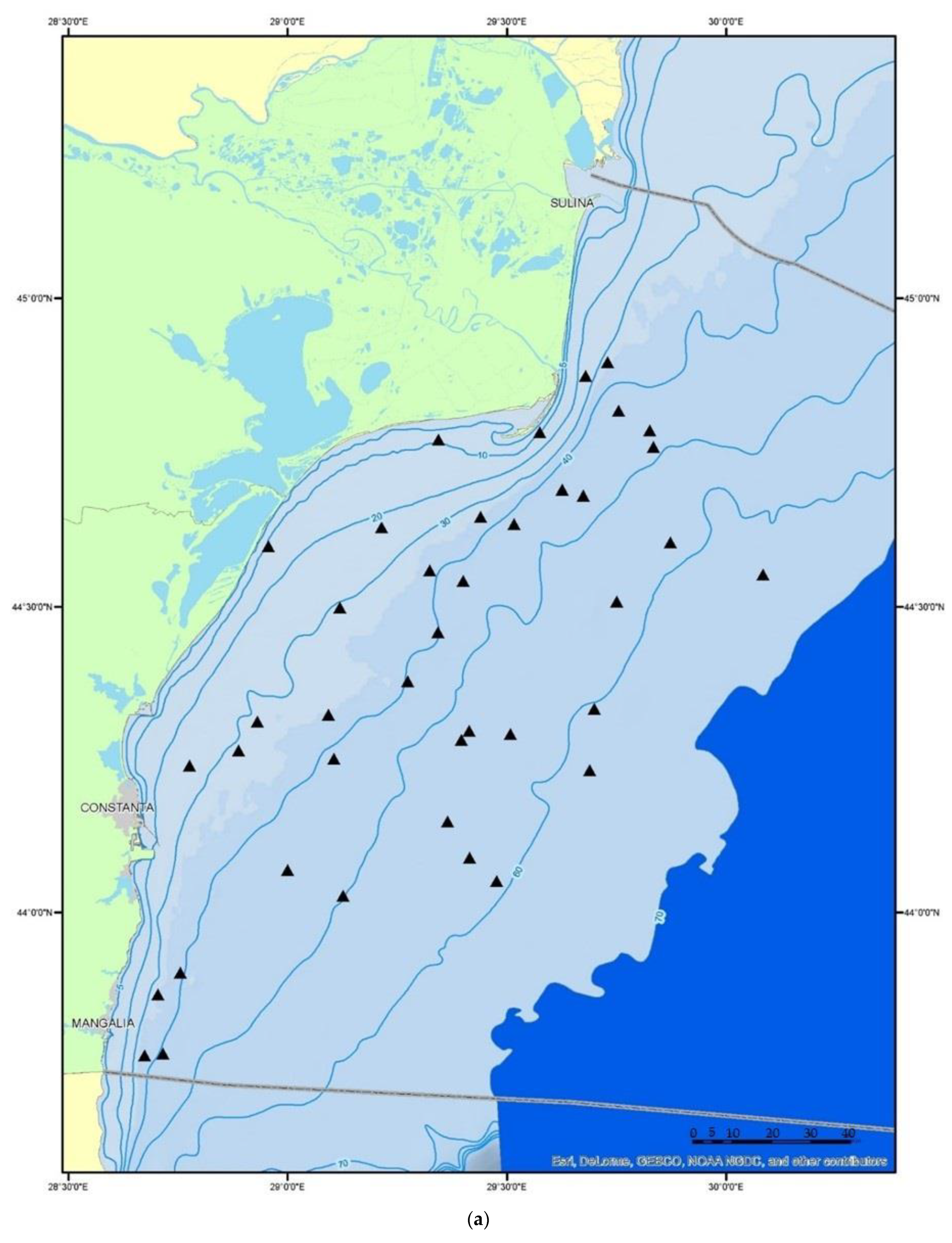
2.1. Fish Sampling
2.2. Determination of Bacterial Diseases
2.3. Determination of Constitutional Diseases
3. Results
3.1. Bacterial Diseases and Their Impact on S. maeoticus Populations from the Romanian Coast
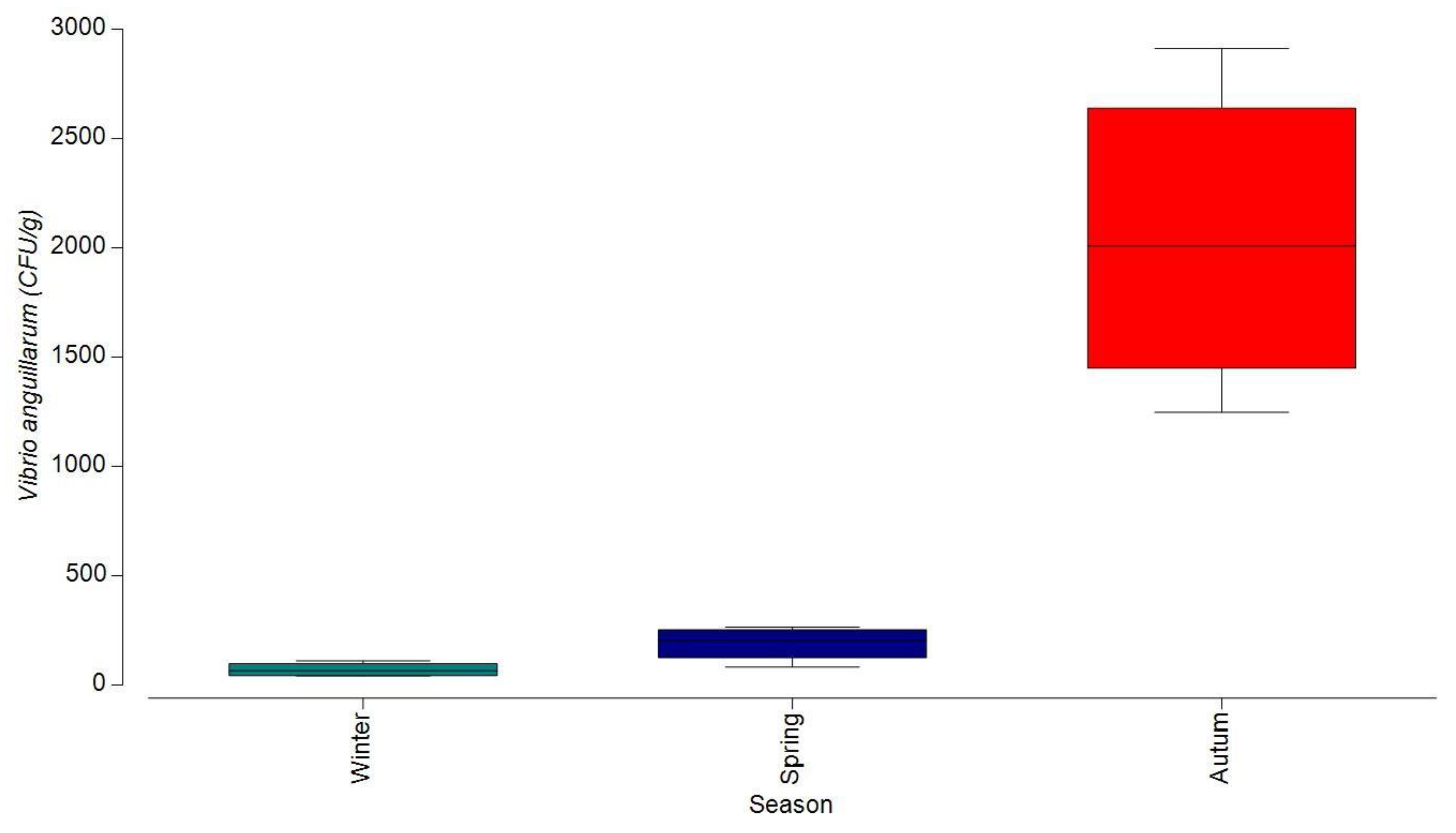
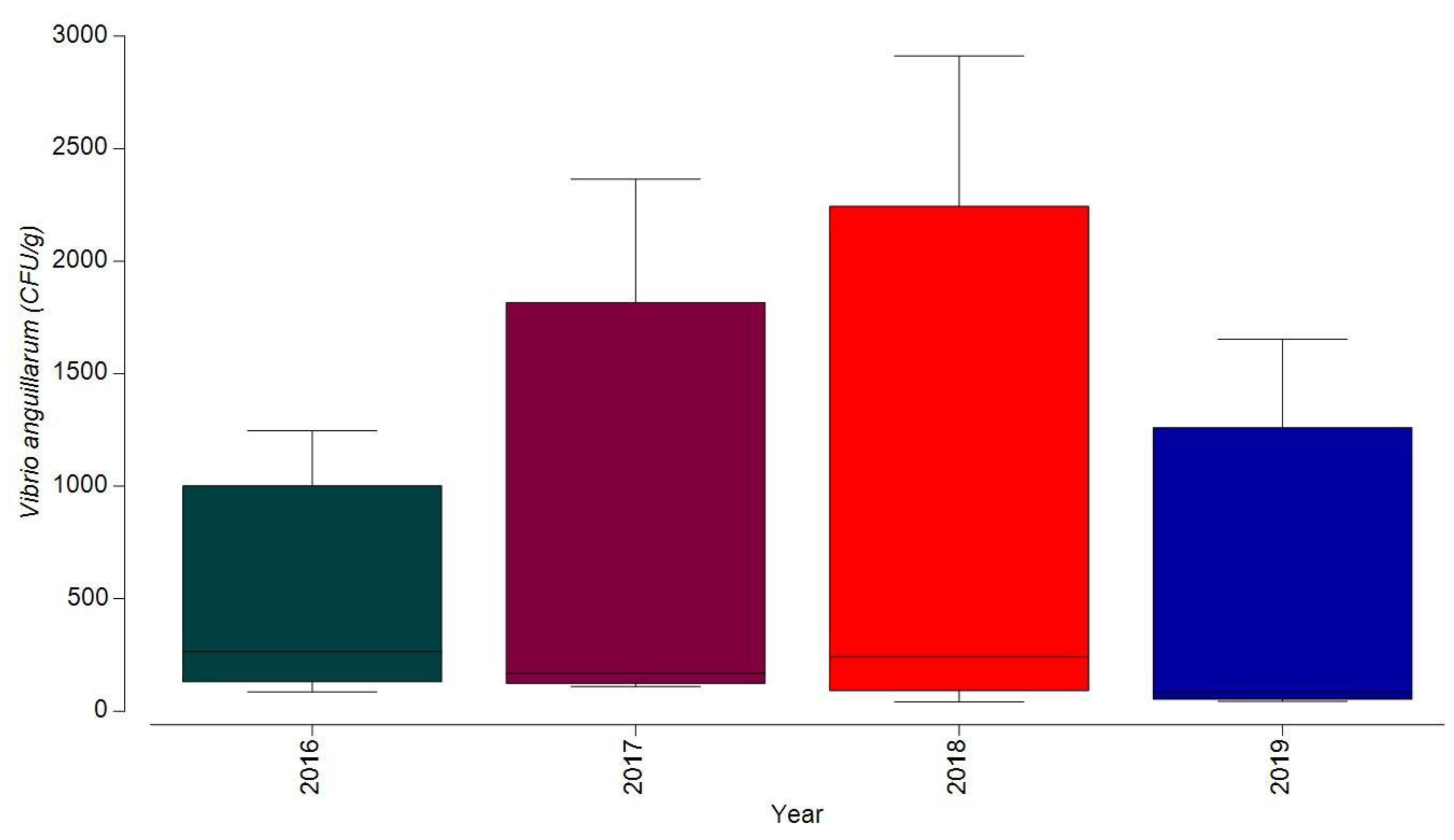
3.1.1. Bacterial Contamination of S. maeoticus with V. anguillarium
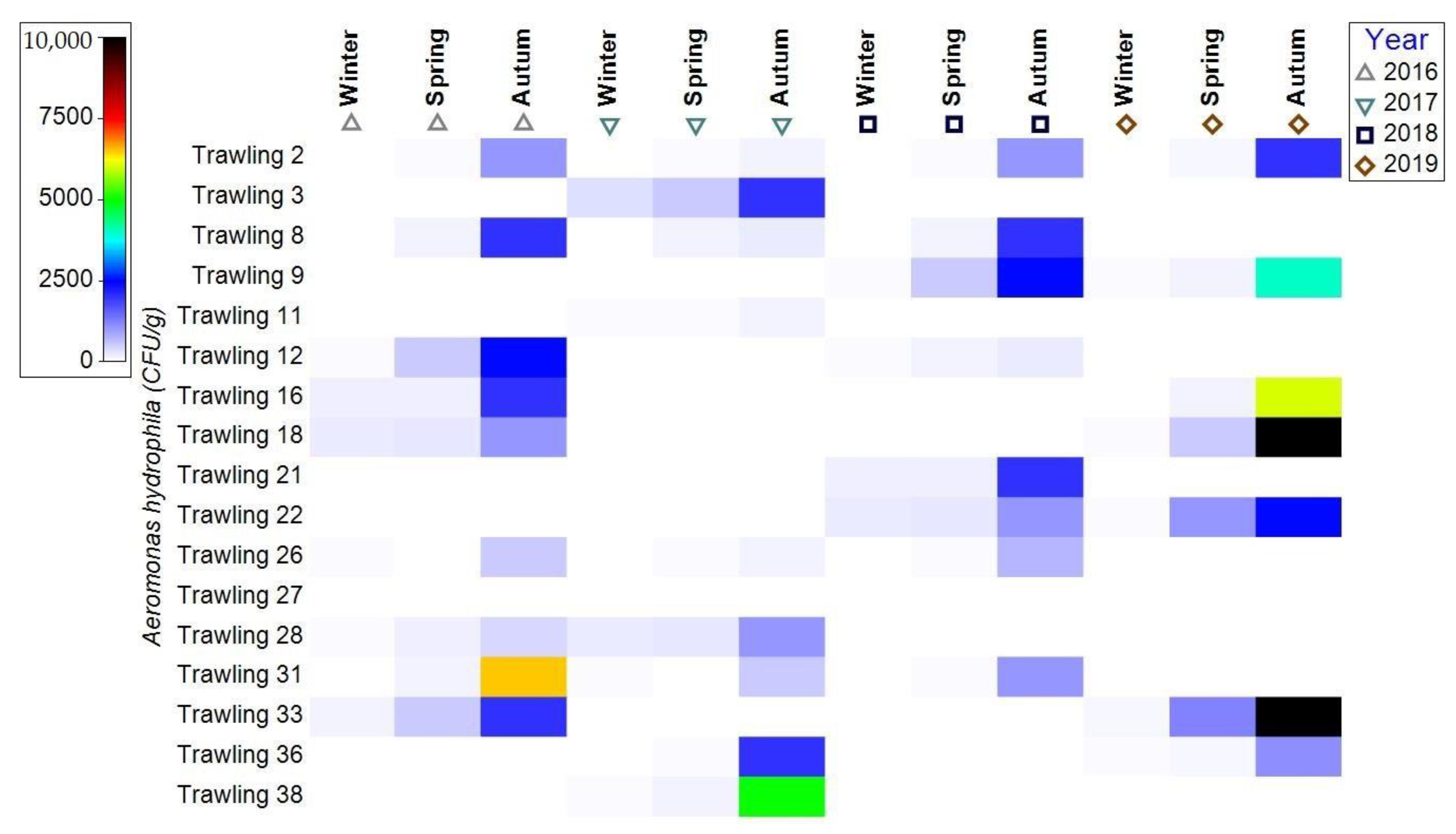
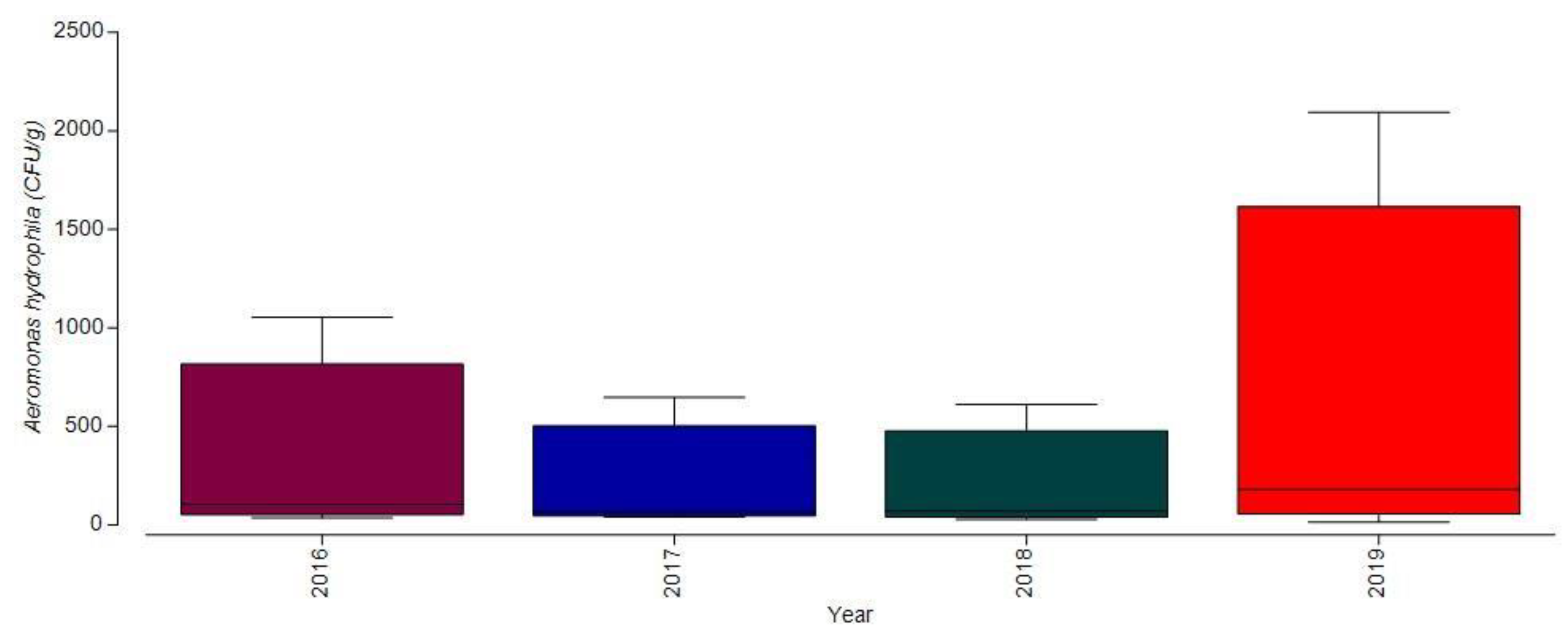
3.1.2. Bacterial Contamination of S. maeoticus with A. hydrophilla
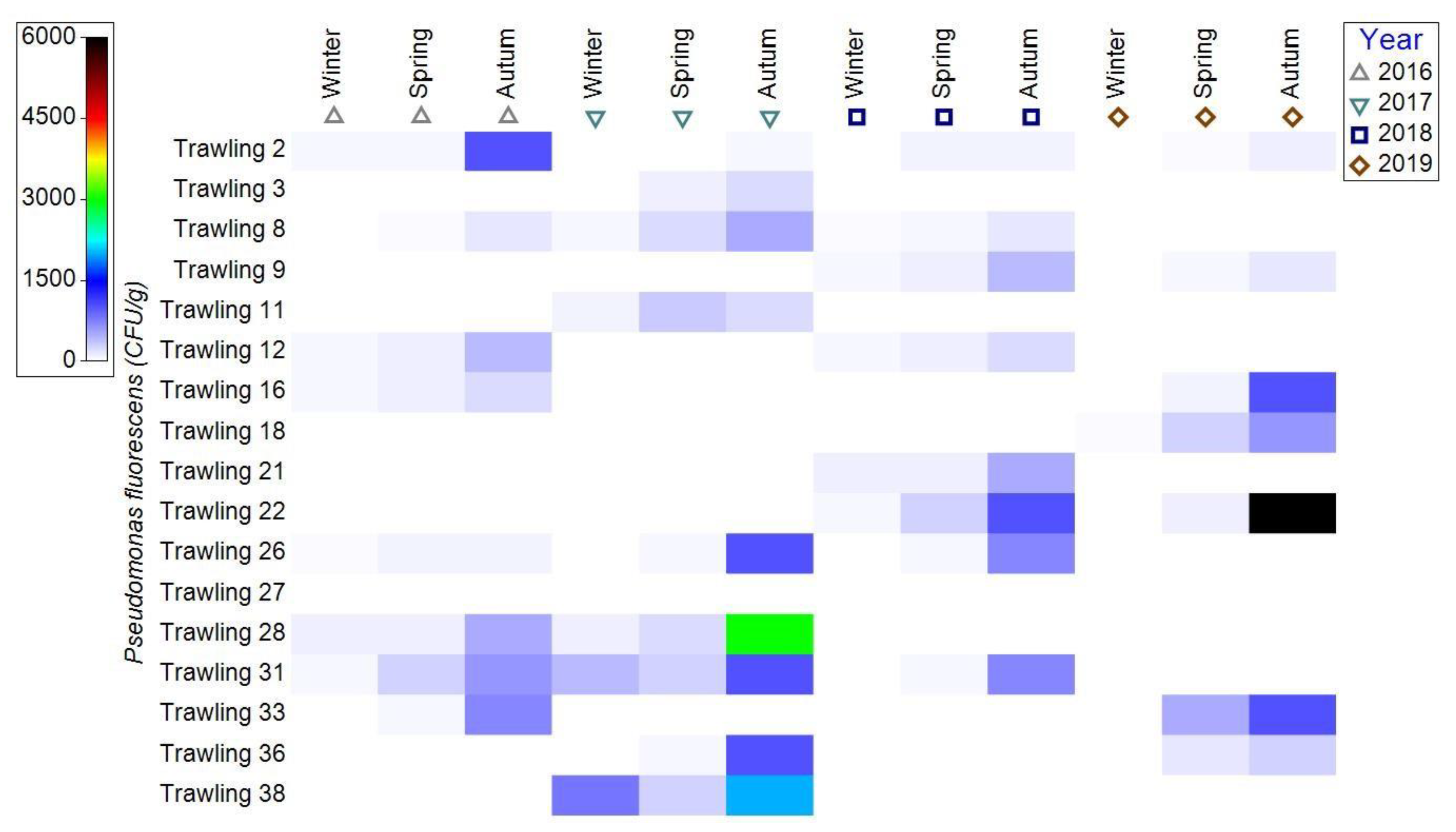
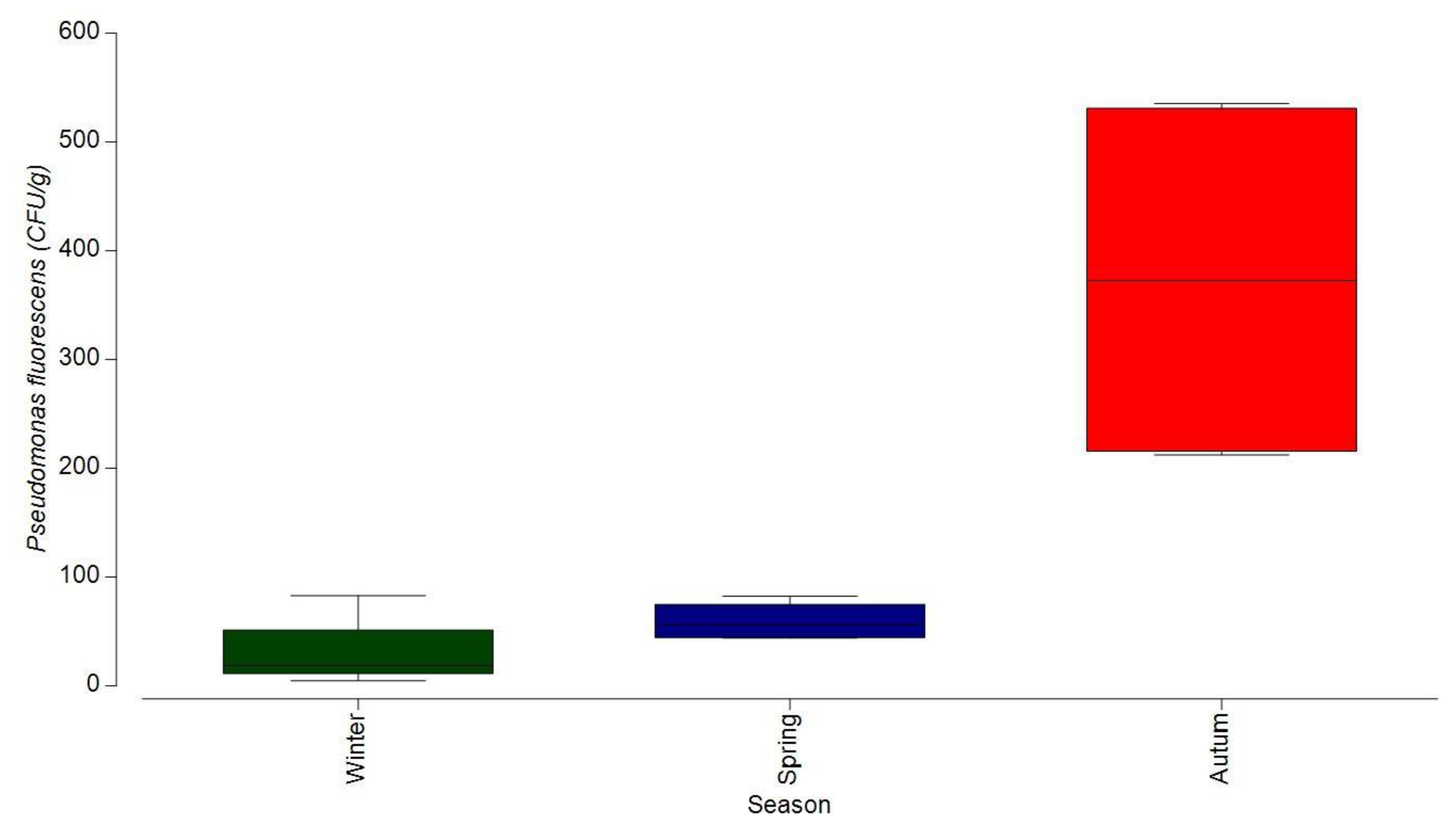
3.1.3. Bacterial Contamination of S. maeoticus with P. fluorescens
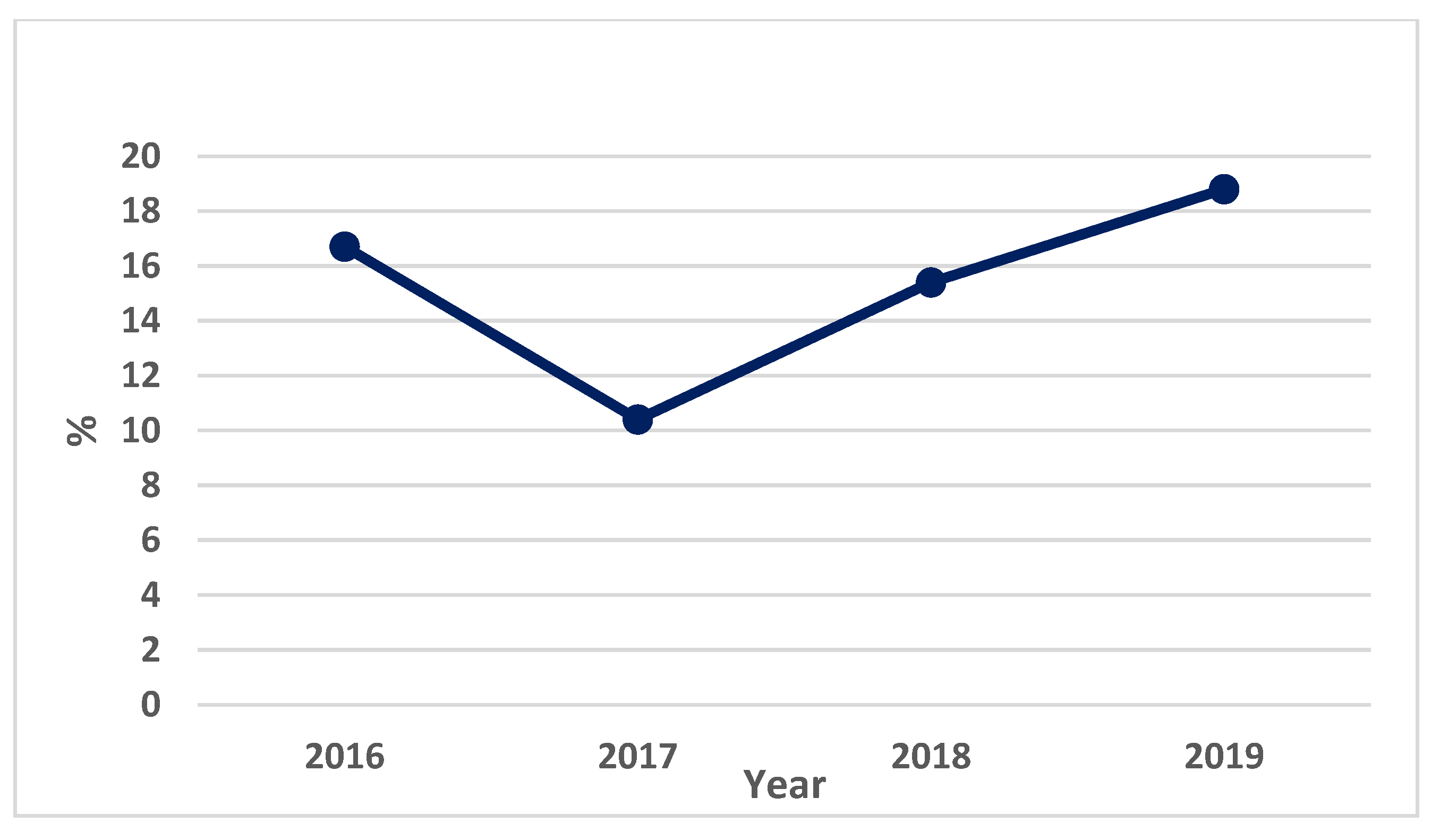
3.2. Constitutional Diseases—Evaluation of Neoplasia from the Romanian Coast in 2016–2019
- -
- the early phase, when the epithelial surface of the center of the lesions is removed, and superficial hemorrhagic ulcers are produced;
- -
- the phase with three distinct zones: a peripheral pale zone; a dark-grey intermediate zone; and a central hemorrhagic zone.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanaychenko, A.N.; Giragosov, V.E.; Gaevskaya, A.V. Epizootological state of the wild Black Sea turbot (kalkan). Visible pathology: Preliminary data. Mar. Ecol. J. 2012, 11, 85–93. [Google Scholar] [CrossRef]
- Lang, T.; Mellergaard, S.; Wosniok, W.; Kadakas, V.; Neumann, K. Spatial distribution of grossly visible diseases and parasites in flounder (Platichthys flesus) from the Baltic Sea: A synoptic survey. ICES J. Mar. Sci. 1999, 56, 138–147. [Google Scholar] [CrossRef]
- Giragosov, V.E.; Khanaychenko, A.N. The state-of art of the Black Sea Turbot spawning population off Crimea (1998–2010). Turk. J. Fish. Aquat. Sci. 2012, 12, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Niță, V.; Nenciu, M.; Galațchi, M. Speciile de Pești de la Litoralul Românesc. Atlas Actualizat/Fish Species of the Romanian Coast. Updated Atlas; INCDM: Constanta, Romania, 2022; 156p, ISBN 978-973-0-36642-6. [Google Scholar]
- Khanaychenko, A.N.; Giragosov, V.E. Black Sea Turbot as indicator of state-of-the-art of the Black Sea Scene, Drivers, pressures, state, impacts, response and recovery indications towards better governance of Black Sea environmental protection, Theses of 3rd Bi-annual BS Sci. In Proceedings of the Conference and UP-GRADE BS-SCENE Project Joint Conference, Odessa, Ukraine, 1–4 November 2011; pp. 209–210. [Google Scholar]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Wiley-Blackwell A John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2011; 544p, ISBN 978-1-119-94946-6. [Google Scholar]
- Rodsaether, M.C.; Olafsen, J.; Rea, J.; Myhre, K.; Steen, J.B. Copper as an initiating factor of vibriosis (Vibrio anguillarum) in eel (Anguilla anguilla). J. Fish Biol. 1977, 10, 17–21. [Google Scholar] [CrossRef]
- Griffiths, E. Environmental regulation of bacterial virulence-implications for vaccine design and production. Trends Biotechnol. 1991, 9, 309–315. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Flardh, B.G.; Nystrom, T.; Moriarty, D.J.W. Growth Limitation and Starvation of Bacteria. In Aquatic Microbiology; Ford, T.E., Ed.; Blackwell Scientific Publications: Boston, MA, USA, 1993; pp. 289–320. [Google Scholar]
- Speare, D.J.; Mirsalimi, S.M. Pathology of the mucous coat of trout skin during an erosive bacterial dermatitis: A technical advance in mucous coat stabilization for ultrastructural examination. J. Comp. Pathol. 1992, 106, 201–211. [Google Scholar] [CrossRef]
- Bermant, M.V.; Podzerova, A.A. Idirova IV Vibriosis Outbreak in Azov Sea Turbot. In Parasitology and Pathology of the Marine Organisms: Proc. Intern. Conference, Kaliningrad; U.S. Department of Commerce: Washington, DC, USA, 1987; pp. 1–61. [Google Scholar]
- Emre, N.; Dedebali, N.; Mefut, A. Rapid Diagnosis for Kalkan Disease; Sakai, M., Itami, T., Eds.; JICA Special Publications: Tokyo, Japan, 2011; Volume 5, pp. 1–68. [Google Scholar]
- Giragosov, V.E.; Khanaychenko, A.N.; Kirin, M.P.; Gutsal, D.K. Record of Scophthalmus rhombus (Pleuronectiformes: Scophthalmidae) near the Crimea. J. Ichthyol. 2012, 52, 127–132. [Google Scholar] [CrossRef]
- Roberts, R.J. Fish Pathology, 2nd ed.; Bailliere Tindall: London, UK, 1989; pp. 1–405. [Google Scholar]
- Radu, G.; Nicolaev, S.; Anton, E. Atlas al Principalelor Specii de Pești din Marea Neagră; Ed. VIROM: Constanta, Romania, 2008; pp. 1–293. (In Romanian) [Google Scholar]
- Buller, N.B. Bacteria from Fish and Other Aquatic Animals: A Practical Identification Manual; CABI Publishing: Oxfordshire, UK, 2004. [Google Scholar]
- Prevot, A.R. Traite de Systematiques Bacterienne; Ed. Dunod: Paris, France, 1961; Volume 1, pp. 1–472, Volume 2, pp. 1–735. [Google Scholar]
- Holt, G.J. The Shorter Bergey”s Manual of Determinative Bacteriology; Ed. Eighth: Baltimore, MD, USA, 1968; pp. 1–176. [Google Scholar]
- Dumitrescu, E. Tumora semnalată la calcanul Scophthalmus maeoticus de la litoralul românesc al Marii Negre. Cercet. Mar. IRCM 1983, 26, 139–142. (In Romanian) [Google Scholar]
- Anderson, J.I.W.; Conroy, D.A. The significance of disease in preliminary attempts to raise flatfish and salmonids in sea water. Bull. Off. Int. Epizoot. 1968, 69, 1129–1137. [Google Scholar]
- Thompson, F.; Iida, T.; Swigs, J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin-Faublée, V.; Rosso, L.; Vigneulle, M.; Flandrois, J.P. The effect of incubation temperature and sodium chloride concentration on the growth kinetics of Vibrio anguillarum and Vibrio anguillarum-related organisms. J. Appl. Bacteriol. 1995, 78, 621–629. [Google Scholar] [CrossRef]
- Maeda, T.; Matsuo, Y.; Furushita, M.; Shiba, T. Seasonal dynamics in a coastal Vibrio community examined by a rapid clustering method based on 16S rDNA. Fish. Sci. 2003, 69, 385–394. [Google Scholar] [CrossRef]
- Guijarro, J.A.; Cascales, D.; García-Torrico, A.I.; García-Domínguez, M.; Méndez, J. Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front. Microbiol. 2015, 6, 700. [Google Scholar] [CrossRef]
- O’Toole, R.; Milton, D.L.; Wolf-Watz, H. Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol. Microbiol. 1996, 19, 625–637. [Google Scholar] [CrossRef]
- Weber, B.; Chen, C.; Milton, D.L. Colonization of fish skin is vital for Vibrio anguillarum to cause disease. Environ. Microbiol. Rep. 2010, 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.L.; Mellergaard, S. Agglutination typing of Vibrio anguillarum isolates from diseased fish and from the environment. Appl. Environ. Microbiol. 1984, 47, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Spanggaard, B.; Huber, I.; Nielsen, J.; Nielsen, T.; Gram, L. Proliferation and location of Vibrio anguillarum during infection of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2000, 23, 423–427. [Google Scholar] [CrossRef]
- Euzeby, J.P. Les modifications apportees à la nomenclature bacterienne durant l’annee 1992 et leur importance en medecine veterinaire. Rev. Med. Vet. 1993, 144, 13–38. [Google Scholar]
- Wiklund, T.; Dalsgaard, I. Occurrence and significance of atypical Aeromonas salmonicida in non-salmonid and salmonid fish species: A review. Dis. Aquat. Org. 1998, 32, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, L.; White, M.R. Diagnosis and Treatment of “Aeromonas hydrophila” Infection of Fish; Aquaculture Extension, Illinois-Indiana Sea Grant Program: West Lafayette, IN, USA, 1991. [Google Scholar]
- Răpuntean, G.; Dancea, Z.; Marica, D.; Oneţiu, O. Caracterizarea Biochimică a Tulpinilor de Aeromonas hydrofila Izolate de la Peşti, din apă şi Alimente; SIMP: Timişoara, Romania, 1992; pp. 81–82. [Google Scholar]
- Lopez, J.R.; Dieguez, A.L.; Doce, A.; De La Roca, E.; De La Herran, R.; Navas, J.I.; Romalde, J.L. Pseudomonas baetica sp. nov., a fish pathogen isolated from wedge sole, Dicologlossa cuneata (Moreau). Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 4, 874–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiklund, T.; Bylund, G. Pseudomonas anguillseptica as a pathogen of salmonids fish in Finland. Dis. Aquat. Org. 1990, 8, 13–19. [Google Scholar] [CrossRef]
- El-Nagar, R.M.A. Bacteriological Studies on Pseudomonas Microorganisms in Cultured Fish. Master’s Thesis, Faculty of Veterinary Medicine, Zagreb University, Zagreb, Croatia, 2010. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens, 2nd ed.; Pseudomonadaceae Representatives; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens. Disease of Farmed and Wild Fish; Springer-Praxis: Chichester, UK, 1999. [Google Scholar]
- Țoțoiu, A.; Radu, G.; Nenciu, M.I.; Patriche, N. Overview of the Health Status of the Main Romanian Black Sea Coast Fish. J. Environ. Prot. Ecol. 2018, 19, 1591–1602. [Google Scholar]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Țoțoiu, A.; Anton, E.; Radu, G.; Danilov, C.S.; Nenciu, M.I.; Patriche, N. Impact of industrial fishing gears on the health status of commercia fish populations at the Romanian Black Sea coast. Cercet. Mar. Rech. Mar. 2017, 47, 273–280. [Google Scholar]
- Daskalov, G.; Ratz, H.J. Assessment of Black Sea Stocks. Scientific, Technical and Economic Committee for Fisheries; STECFOWP-11-06; JRS Scientific and Technical Reports; Publications Office of the EU: Luxembourg, 2011; pp. 1–213. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Diseases of Farmed and Wild Fish, 4th ed.; Springer-Praxis Publishing: New York, NY, USA; Chichester, UK, 2007; Available online: https://link.springer.com/book/10.1007/978-94-007-4884-2 (accessed on 13 July 2023).
- Țoțoiu, A.; Patriche, N. Assessing the Inter-Relations Between Fish Health and Stock Status of the Main Commercial Fish Species. Agric. Life Life Agric. 2018, 1, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Țoțoiu, A.; Nenciu, M.-I.; Nicolae, C.G. Assessing the inter-relations between fish health and stock status on human health and consumer perception. Sci. Pap. Ser. D Anim. Sci. 2018, 61, 268–273. [Google Scholar]
- Durborow, R.M. Health and safety concerns in fisheries and aquaculture. Occup. Med. 1999, 14, 373–406. [Google Scholar]
- Radulescu, I.; Lustun, L.; Voican, V. Bolile Peștilor; Ceres: Bucharest, Romania, 1976; pp. 1–261. [Google Scholar]
- Işıdan, H.; Bolat, Y. A Survey of Viral Hemorrhagic Septicemia (VHS) in Turkey. J. Fish. Aquat. Sci. 2011, 11, 507–513. [Google Scholar]
- Crisafi, F.; Denaro, R.; Genovese, M.; Yakimov, M.; Genovese, L. Application of relative real-time PCR to detect differential expression of virulence genes in Vibrio anguillarum under standard and stressed growth conditions. J. Fish Dis. 2014, 37, 629–640. [Google Scholar] [CrossRef]
- Guanhua, Y.; Wang, C.; Wang, X.; Ma, R.; Zheng, H.; Liu, Q.; Zhang, Y.; Ma, Y.; Wang, Q. Complete genome sequence of the marine fish pathogen Vibrio anguillarum and genome-wide transposon mutagenesis analysis of genes essential for in vivo infection. Microbiol. Rez. 2018, 216, 97–107. [Google Scholar] [CrossRef]
- McCoy, A.J.; Koizumi, Y.; Toma, C.; Higa, N.; Dixit, V.; Taniguchi, S.; Tschopp, J.; Suzuki, T. Cytotoxins of the human pathogen Aeromonas hydrophila trigger via the NLRP3 a inflammasomes, caspase-1 activation in macrophages. Eur. J. Immunol. 2010, 40, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Sindermann, C.J. Pollution-associated diseases and abnormalities of fish and shellfish: A review. Fish. Bull. 1979, 78, 717–749. [Google Scholar]
- Totoiu, A.; Nenciu, M.; Nita, V. Atlas of the Main Diseases Identified in Shellfish and Finfish of the Romanian Coast; INCDM: Constanta, Romania, 2023; pp. 1–140. ISBN 978-973-0-38071-2. [Google Scholar]









| Vibrio anguillarum | Aeromonas hydrophila | Pseudomonas fluorescens | |
|---|---|---|---|
| Colony characteristics | Round colonies, 1–2 mm in diameter, transparent, smooth convex, shiny, with an opaque halo, soft consistency, grey–brown–yellow. | Round colonies, large with regular margins, smooth, opaque, white–grey to yellowish, up to 5 mm in diameter. | Small colonies, 2–3 mm diameter, with regular edges, slightly convex, dirty white, green, opaque, or slightly transparent |
| Pigments in broth | Stir the alkaline peptone water and the broth with the formation of a film on top. | Intense, uniform, uncharacteristic turbidity, without pigment or with brown pigment. Sedimentation tendency. | Turbidity, film on the surface, sometimes green, red, brown, orange pigment. |
| Selective media | TGBS, BSA—small colonies, round, transparent, bright, smooth, soft. | MK—red, large colonies. Colour changes due to the fermentation of sugars: red colonies on MacConkey, red-purple colonies on Levine, brown on Tryptic Soy Agar. | MI—blue-green colonies, with or without a black dot. It does not ferment the sugars, and does not cause discolouration on Levine. |
| Morphological characteristics | Bacilli slightly curved, 0.4–0.6/1–3 µm | Bacilli, coccobacilli, with polar flagellum. | Bacilli, coccobacilli (short, thick rods, with 0.5–0.6/1.5µm) |
| Gram coloration | - | - | - |
| Mobility | - | + | + |
| Glucose | +F | +F | +O |
| Lactose | - | + | - |
| Sucrose | + | + | + - |
| Indole | + | + | - |
| Lysine | - | - | - |
| Urea | - | - | + |
| H2S | - | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țoțoiu, A.; Patriche, N.; Niță, V.; Sîrbu, E.; Dima, F.M.; Nenciu, M.I.; Nistor, V. Epidemiology of Turbot (Scophthalmus maeoticus) Bacterial Contamination, a Fishery Limiting Factor on the Romanian Black Sea. Fishes 2023, 8, 418. https://doi.org/10.3390/fishes8080418
Țoțoiu A, Patriche N, Niță V, Sîrbu E, Dima FM, Nenciu MI, Nistor V. Epidemiology of Turbot (Scophthalmus maeoticus) Bacterial Contamination, a Fishery Limiting Factor on the Romanian Black Sea. Fishes. 2023; 8(8):418. https://doi.org/10.3390/fishes8080418
Chicago/Turabian StyleȚoțoiu, Aurelia, Neculai Patriche, Victor Niță, Elena Sîrbu, Floricel Maricel Dima, Magda Ioana Nenciu, and Veta Nistor. 2023. "Epidemiology of Turbot (Scophthalmus maeoticus) Bacterial Contamination, a Fishery Limiting Factor on the Romanian Black Sea" Fishes 8, no. 8: 418. https://doi.org/10.3390/fishes8080418
APA StyleȚoțoiu, A., Patriche, N., Niță, V., Sîrbu, E., Dima, F. M., Nenciu, M. I., & Nistor, V. (2023). Epidemiology of Turbot (Scophthalmus maeoticus) Bacterial Contamination, a Fishery Limiting Factor on the Romanian Black Sea. Fishes, 8(8), 418. https://doi.org/10.3390/fishes8080418