The Effects of Porphyra yezoensis Polysaccharides on Intestinal Health of Spotted Sea Bass, Lateolabrax maculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.2. Feeding and Management
2.3. Sample Collection
2.4. Growth Performance Analysis
2.5. Intestinal Digestive Enzyme Activity and Antioxidant Capacity Analysis
2.6. Preparation and Observation of Intestinal Tissue Section
2.7. Intestinal Microbes Analysis
2.8. Statistical Analysis
3. Results
3.1. The Effect of PPs on the Growth Performance of Spotted Sea Bass
3.2. The Effects of PPs on Intestinal Physiological and Biochemical Indexes
3.2.1. The Effects of PPs on the Activity of Intestinal Digestive Enzymes
3.2.2. The Effects of PPs on Intestinal Antioxidant Capacity
3.3. The Effects of PPs on Intestinal Tissue Morphology
3.4. The Effects of PPs on Intestinal Microbes
3.4.1. The Effects of PPs on Intestinal Microbial OTUs
3.4.2. The Effects of PPs on Intestinal Microbial Alpha Diversity
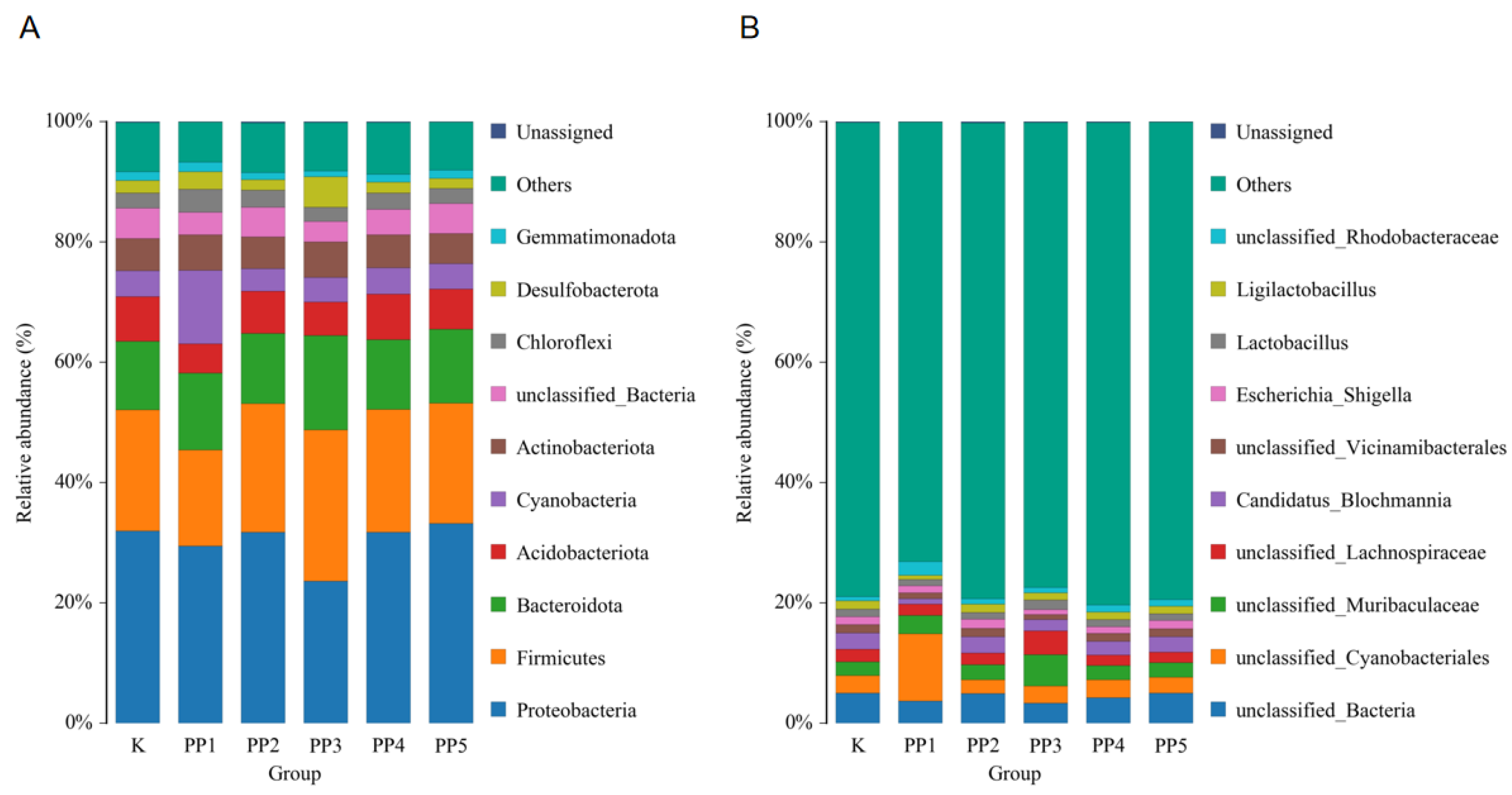
3.4.3. The Effects of PPs on Species Composition and Abundance of Intestinal Microbes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Julia, K.; Jerry, W.; Cani, P.D.; García-Ródenas, C.L.; Tom, M.; Annick, M.; Jacqueline, W.; Freddy, T.; Robert-Jan, B. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, 196. [Google Scholar]
- Abreu, A.M.; Masayuki, F.; Moshe, A. TLR signaling in the gut in health and disease. J. Immunol. 2005, 174, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, X.; Wu, H.; Xiao, J.; Gao, Y.; Zhang, Y. Role of intestinal inflammation in predisposition of Edwardsiella tarda infection in zebrafish (Danio rerio). Fish Shellfish Immunol. 2014, 41, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, W.; Ran, C.; Hu, J.; Zhou, Z. Abrupt suspension of probiotics administration may increase host pathogen susceptibility by inducing gut dysbiosis. Sci. Rep. 2016, 6, 23214. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zou, S.; Zhai, L.; Wang, Y.; Zhang, F.; An, L.; Yang, G. Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 2017, 71, 35–42. [Google Scholar] [CrossRef]
- Sayyaf, D.B.; Castaldelli, G.; Giari, L. Histopathological and ultrastructural assessment of two mugilid species infected with myxozoans and helminths. J. Fish Dis. 2018, 41, 299–307. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Reinoso, W.C.; Koboziev, I.; Furr, K.L.; Grisham, M.B. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology 2016, 23, 67–80. [Google Scholar] [CrossRef]
- Rombout, J.H.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Gough, E.K. The impact of mass drug administration of antibiotics on the gut microbiota of target populations. Infect. Dis. Poverty 2022, 11, 76. [Google Scholar] [CrossRef]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (Macrobrachium nipponense), and provokes human health risk. Sci. Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Sun, C.; Ji, Y.; Abdolmaleky, H.; Zhou, J.; Wang, S.; Bao, C. Herbal medicine improves gastrointestinal health in mice via modulation of intestinal tight junctions and gut microbiota and inhibition of inflammation. Biomed. Pharmacother. 2021, 138, 111426. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Chen, F.; Dai, D. The regulation of host intestinal microbiota by polyphenols in the development and prevention of chronic kidney disease. Front. Immunol. 2019, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Huang, H.; Liu, S.; Liu, F.; Tu, Q.; Yin, Y.; He, S. Resveratrol improves growth performance, intestinal morphology, and microbiota composition and metabolism in mice. Front. Microbiol. 2021, 12, 726878. [Google Scholar] [CrossRef]
- Layla, A.N.; Aaron, K. Soy isoflavones and gastrointestinal health. J. Curr. Nutr. Rep. 2020, 9, 193–201. [Google Scholar]
- Feng, Y.Q.; Song, Y.T.; Zhou, J.; Duan, Y.Q.; Kong, T.Y.; Ma, H.L.; Zhang, H.H. Recent progress of Lycium barbarum polysaccharides on intestinal microbiota, microbial metabolites and health: A review. Crit. Rev. Food Sci. Nutr. 2022, 28, 21–24. [Google Scholar]
- Song, B.; Zheng, C.; Zha, C.; Hu, S.; Yang, X.; Wang, L.; Xiao, H. Dietary leucine supplementation improves intestinal health of mice through intestinal SIgA secretion. J. Appl. Microbiol. 2020, 128, 574–583. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2020, 140, 109858. [Google Scholar] [CrossRef]
- Lai, Y.; Fang, Q.; Guo, X.; Lei, H.; Zhou, Q.; Wu, N.; Song, C. Effect of polysaccharides from Dictyophora indusiata on regulating gut microbiota and short-chain fatty acids in mice. J. Food Meas. Charact. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Hu, X.; Liu, R.; Zhao, Z.; Wang, S.; Zhang, R.; Guo, K.; Luo, L. Dietary supplementation with Lycium barbarum polysaccharides conducive to maintaining the health of Luciobarbus capito via the enhancement of enzyme activities and the modulation of gut microbiota. Int. J. Biol. Macromol. 2023, 232, 123500. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, S.; Wang, Q.; Wang, D.; Wu, Q.; Xie, S.; Zou, J. Effects of partial replacement of dietary flour meal with seaweed polysaccharides on the resistance to ammonia stress in the intestine of hybrid snakehead (Channa maculatus female × Channa argus male). Fish Shellfish Immunol. 2022, 127, 271–279. [Google Scholar] [CrossRef]
- Jyotsna; Vijayakumar, P.; Dhas, S.T.; Mani, R.; Raguraman, V. Antiviral activity of sulfated polysaccharides from Sargassum ilicifolium against fish Betanodavirus infection. Aquac. Int. 2021, 29, 1049–1067. [Google Scholar] [CrossRef]
- Rajendran, P.; Subramani, A.P.; Michael, D. Polysaccharides from marine macroalga, Padina gymnospora improve the nonspecific and specific immune responses of Cyprinus carpio and protect it from different pathogens. Fish Shellfish. Immunol. 2016, 58, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Hu, L.; Liu, S.; Yu, H.; Xing, R.; Li, R.; Wang, X.; Li, P. In vitro prebiotic effects of seaweed polysaccharides. J. Oceanol. Limnol. 2018, 36, 926–932. [Google Scholar] [CrossRef]
- Hasan, A.M.B.; Tuan, N.T.; Zhang, Y.; Hu, H.; Lin, H.; Zhang, M.; Liang, H.; Zhang, Y.; Li, S. Effects of dietary supplementation of Gracilaria lemaneiformis-derived sulfated polysaccharides on the growth, antioxidant capacity, and innate immunity of rabbitfish (Siganus canaliculatus). Fish Shellfish. Immunol. 2023, 139, 108933. [Google Scholar]
- Peixoto, J.M.; Salas-Leitón, E.; Pereira, F.L.; Queiroz, A.; Magalhães, F.; Pereira, R.; Abreu, H.; Reis, P.A.; Gonçalves, J.F.M.; Ozório, R.O.A. Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax). Aquac. Rep. 2016, 3, 189–197. [Google Scholar] [CrossRef]
- Abdelrhman, A.M.; Mohamed, A.; Al-Zahaby, M.A.; Sharawy, Z.Z.; Nazmi, H.; Zaki, M.A.A.; Ahmed, N.H.; Ahmed, S.R.; El-Haroun, E.; Van, D.H.; et al. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Yoshida, T.; Notoya, M.; Kikuchi, N.; Miyata, M. Catalogue of species of Porphyra in the world, with special reference to the type locality and bibliography. J. Nat. Hist. Res. 1997, 3, 5–18. [Google Scholar]
- Lahaye, M.; Jegou, D. Chemical and physical-chemical characteristics of dietary fibres from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J. Appl. Phycol. 1993, 5, 195–200. [Google Scholar] [CrossRef]
- Geng, Y. Structure and bioactivities of Porphyrans and Oligoporphyrans. Curr. Pharm. Des. 2019, 25, 1163–1171. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y. The growth performance and nonspecific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary Porphyra yezoensis polysaccharide supplementation. Fish Shellfish Immunol. 2019, 87, 615–619. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Zhou, Q.; Huang, X.; Huang, Z.; Li, T.; Wu, Q.; Zhou, C.; Ma, Z.; Lin, H. Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 2022, 207, 850–858. [Google Scholar] [CrossRef]
- Martin, S.A.M.; Dehler, C.E.; Król, E. Transcriptomic responses in the fish intestine. Dev. Comp. Immunol. 2016, 64, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.; Mai, K.; Li, H.; Zhang, C.; Zhang, L.; Duan, Q.; Tan, B.; Xu, W.; Ma, H.; Zhang, W.; et al. Effects of dietary protein to energy ratios on growth and body composition of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 2003, 230, 507–516. [Google Scholar] [CrossRef]
- Young, S.G.; Fong, L.G.; Beigneux, A.P.; Allan, C.M.; He, C.; Jiang, H.; Nakajima, K.; Meiyappan, M.; Birrane, G.; Ploug, M. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 2019, 30, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.A.; Zaichik, B.T.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B. Enzyme immunoassay for detection of Sudan I dye and its application to the control of foodstuffs. J. Anal. Chem. 2016, 71, 944–948. [Google Scholar] [CrossRef]
- Nauseef, W.M. Nox enzymes in immune cells. Semin. Immunopathol. 2008, 30, 195–208. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; Calvaruso, G.; Lauricella, M.; Giuliano, M.; Bellavia, G.; D’Anneo, A.; Vento, R.; Tesoriere, G. Apoptosis induced in hepatoblastoma HepG2 cells by the proteasome inhibitor MG132 is associated with hydrogen peroxide production, expression of Bcl-XS and activation of caspase-3. Int. J. Oncol. 2002, 21, 857–865. [Google Scholar] [CrossRef]
- Christophe, G.; Buc, C.P. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar]
- Iskusnykh, I.Y.; Zakharova, A.A.; Dhruba, P. Glutathione in brain disorders and aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2016, 524, 13–30. [Google Scholar] [CrossRef]
- Wu, Y.; Huo, Y.; Xu, L.; Xu, Y.; Wang, X.; Zhou, T. Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 165, 2116–2125. [Google Scholar] [CrossRef]
- Zheng, M.; Ma, M.; Yang, Y.; Liu, Z.; Liu, S.; Hong, T.; Ni, H.; Jiang, Z. Structural characterization and antioxidant activity of polysaccharides extracted from Porphyra haitanensis by different methods. Int. J. Biol. Macromol. 2023, 242, 125003. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef]
- Schug, H.; Yue, Y.; Krese, R.; Fischer, S.; Kortner, T.M.; Schirmer, K. Time- and concentration-dependent expression of immune and barrier genes in the RTgutGC fish intestinal model following immune stimulation. Fish Shellfish Immunol. 2019, 88, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Buckland, A.; Sheaves, M. Fish gut content analysis: Robust measures of diet composition. Fish Fish. 2014, 15, 170–177. [Google Scholar] [CrossRef]
- Wu, N.; Song, Y.; Wang, B.; Zhang, X.; Zhang, X.; Wang, Y.; Cheng, Y.; Chen, D.; Xia, X.; Lu, Y.; et al. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef] [PubMed]
- Caspary, W.F. Physiology and pathophysiology ofintes-tinal absorption. Am. J. Clin. Nutr. 1992, 55, 299–308. [Google Scholar] [CrossRef]
- Liu, A.; Kim, E.; Cui, J.; Li, J.; Lee, Y.; Zhang, G. Laminaria Japonica polysaccharide improved the productivities and systemic health of ducks by mediating the gut microbiota and metabolome. J. Agric. Food Chem. 2023, 71, 7382–7395. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.; Christophe, A.J.; Amandine, A.; Raphaël, S.; Antoine, G.; Lucie, B.; Sébastien, V. Ecological specialization within a carnivorous fish family is supported by a herbivorous microbiome shaped by a combination of gut traits and specific diet. Front. Mar. Sci. 2021, 8, 91. [Google Scholar]
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: Diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef]
- Pérez, T.; Balcázar, J.L.; Ruiz-Zarzuela, I.; Halaihel, N.; Vendrell, D.; DeBlas, I.; Múzquiz, J.L. Host-microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010, 3, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.M.; Vazoller, R.F.; Pazinato, J.M.; Mendes, L.W.; Paulo, E.N. Molecular characterization of the archaeal community in an amazonian wetland soil and culture-dependent isolation of Methanogenic Archaea. Diversity 2010, 2, 1026–1047. [Google Scholar]
- Pélissier, R.; Couteron, P. An operational, additive framework for species diversity partitioning and Beta-diversity analysis. J. Ecol. 2007, 95, 294–300. [Google Scholar] [CrossRef]
- Chen, P.; Tong, M.; Zeng, H.; Zheng, B.; Hu, X. Structural characterization and in vitro fermentation by rat intestinal microbiota of a polysaccharide from Porphyra haitanensis. Food Res. Int. 2021, 147, 110546. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wang, Y.; Huang, Y.; Wang, C. Effects of alternate feeding between fish meal and novel protein diets on the intestinal health of juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 23, 101023. [Google Scholar] [CrossRef]
- Elaine, p.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Paul, R.R.; Fitzgerald, G.F.; Catherine, S. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar]
- Cheng, W.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Wei, X.; Sun, C.; Chi, M.; Bi, W.; Yang, B.; et al. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, L.G. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Han, S.; Liu, C.; Liu, F. Effect of two seaweed polysaccharides on intestinal microbiota in mice evaluated by illumina PE250 sequencing. Int. J. Biol. Macromol. 2018, 112, 796–802. [Google Scholar] [CrossRef]



| Ingredient (%) | Content |
|---|---|
| Fish meal a | 49.0 |
| Soybean meal a | 23.5 |
| Wheat flour | 15.0 |
| Yeast powder a | 3.0 |
| Fish oil | 3.0 |
| Soybean oil | 2.0 |
| Lecithin | 1.0 |
| Vitamin premix b | 0.6 |
| Mineral premix c | 0.8 |
| Choline | 0.6 |
| Ca(H2PO4)2 | 1.2 |
| Antioxidant | 0.3 |
| Proximate composition (%) | |
| Crude protein | 48.01 |
| Crude fat | 8.60 |
| Crude ash | 12.22 |
| Carbohydrate | 10.80 |
| Gross energy (kj/g) | 16.68 |
| Groups | Growth Performance | |||
|---|---|---|---|---|
| WG (%) | SGR (%) | CF (%) | FCR | |
| K | 5.72 ± 0.06 a | 3.66 ± 0.02 a | 1.46 ± 0.09 a | 1.04 ± 0.02 a |
| PP1 | 6.63 ± 0.56 bc | 3.91 ± 0.14 bc | 1.79 ± 0.22 b | 0.99 ± 0.03 a |
| PP2 | 6.23 ± 0.05 ab | 3.80 ± 0.01 ab | 1.51 ± 0.07 a | 0.99 ± 0.05 a |
| PP3 | 7.07 ± 0.30 c | 4.01 ± 0.07 c | 1.57 ± 0.09 a | 1.00 ± 0.04 a |
| PP4 | 7.13 ± 0.32 c | 4.03 ± 0.08 c | 2.04 ± 0.05 c | 1.00 ± 0.05 a |
| PP5 | 6.74 ± 0.41 bc | 3.93 ± 0.10 bc | 1.78 ± 0.06 b | 1.01 ± 0.09 a |
| Groups | Digestive Enzymes | ||
|---|---|---|---|
| AMS (U/mgprot) | LPS (U/gprot) | TRS (U/mgprot) | |
| K | 0.45 ± 0.18 a | 1.23 ± 0.42 a | 758.31 ± 78.34 a |
| PP1 | 0.55 ± 0.09 a | 1.05 ± 0.39 a | 1136.13 ± 228.34 ab |
| PP2 | 0.66 ± 0.08 ab | 1.15 ± 0.36 a | 833.00 ± 110.18 ab |
| PP3 | 0.91 ± 0.23 b | 1.58 ± 0.71 a | 1038.81 ± 569.55 ab |
| PP4 | 0.71 ± 0.12 ab | 1.47 ± 0.59 a | 1354.13 ± 310.48 b |
| PP5 | 0.69 ± 0.07 ab | 1.27 ± 0.30 a | 719.37 ± 123.00 a |
| Groups | Antioxidant Capacity | ||
|---|---|---|---|
| CAT (μmol/gprot) | GSH (U/mgprot) | MDA (nmol/mgprot) | |
| K | 1.11 ± 0.03 a | 31.84 ± 1.21 a | 0.79 ± 0.32 b |
| PP1 | 0.88 ± 0.46 a | 33.73 ± 5.31 a | 0.38 ± 0.07 a |
| PP2 | 0.93 ± 0.24 a | 36.07 ± 5.01 ab | 0.49 ± 0.23 ab |
| PP3 | 0.91 ± 0.15 a | 44.20 ± 4.99 b | 0.37 ± 0.18 a |
| PP4 | 0.92 ± 0.13 a | 36.74 ± 6.38 ab | 0.47 ± 0.16 ab |
| PP5 | 0.82 ± 0.35 a | 32.37 ± 5.70 a | 0.55 ± 0.45 ab |
| Groups | Intestinal Tissue Morphology | |||
|---|---|---|---|---|
| Villus Height (μm) | Villus Width (μm) | Crypt Depth (μm) | Muscular Thickness (μm) | |
| K | 549.28 ± 36.82 a | 69.51 ± 10.33 b | 86.85 ± 6.46 a | 229.48 ± 19.74 a |
| PP1 | 597.72 ± 17.42 b | 66.60 ± 5.89 ab | 91.22 ± 7.94 a | 219.41 ± 10.75 a |
| PP2 | 629.62 ± 22.14 bc | 66.38 ± 5.43 ab | 88.77 ± 5.54 a | 218.53 ± 20.03 a |
| PP3 | 586.91 ± 59.97 ab | 60.81 ± 4.24 a | 90.87 ± 7.14 a | 220.76 ± 53.86 a |
| PP4 | 647.89 ± 28.19 c | 59.55 ± 1.54 a | 91.25 ± 3.37 a | 208.37 ± 26.79 a |
| PP5 | 606.72 ± 28.54 bc | 63.04 ± 2.34 ab | 86.98 ± 3.76 a | 212.56 ± 15.69 a |
| Groups | Alpha Diversity | |||
|---|---|---|---|---|
| ACE | Chao1 | Simpson | Shannon | |
| K | 1885.67 ± 54.05 ab | 1885.67 ± 54.05 ab | 1.00 ± 0 b | 10.16 ± 0.04 a |
| PP1 | 2227.48 ± 350.49 b | 2227.34 ± 350.45 b | 0.98 ± 0.01 a | 9.50 ± 0.57 a |
| PP2 | 1965.33 ± 67.28 ab | 1965.33 ± 67.28 ab | 1.00 ± 0 b | 10.20 ± 0.04 a |
| PP3 | 1664.07 ± 311.40 a | 1664.00 ± 311.34 a | 0.99 ± 0.01 b | 9.41 ± 1.29 a |
| PP4 | 1903.00 ± 67.01 ab | 1903.00 ± 67.01 ab | 1.00 ± 0 b | 10.25 ± 0.06 a |
| PP5 | 1860.73 ± 67.08 ab | 1860.67 ± 66.97 ab | 1.00 ± 0 b | 10.17 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Zhou, S.; Huang, Z.; Ma, J.; Kong, L.; Lin, Y.; Long, Z.; Qin, H.; Liu, L.; Zhao, Y.; et al. The Effects of Porphyra yezoensis Polysaccharides on Intestinal Health of Spotted Sea Bass, Lateolabrax maculatus. Fishes 2023, 8, 419. https://doi.org/10.3390/fishes8080419
Lin H, Zhou S, Huang Z, Ma J, Kong L, Lin Y, Long Z, Qin H, Liu L, Zhao Y, et al. The Effects of Porphyra yezoensis Polysaccharides on Intestinal Health of Spotted Sea Bass, Lateolabrax maculatus. Fishes. 2023; 8(8):419. https://doi.org/10.3390/fishes8080419
Chicago/Turabian StyleLin, Hao, Sishun Zhou, Zhangfan Huang, Jianrong Ma, Lumin Kong, Yi Lin, Zhongying Long, Huihui Qin, Longhui Liu, Yanbo Zhao, and et al. 2023. "The Effects of Porphyra yezoensis Polysaccharides on Intestinal Health of Spotted Sea Bass, Lateolabrax maculatus" Fishes 8, no. 8: 419. https://doi.org/10.3390/fishes8080419
APA StyleLin, H., Zhou, S., Huang, Z., Ma, J., Kong, L., Lin, Y., Long, Z., Qin, H., Liu, L., Zhao, Y., & Li, Z. (2023). The Effects of Porphyra yezoensis Polysaccharides on Intestinal Health of Spotted Sea Bass, Lateolabrax maculatus. Fishes, 8(8), 419. https://doi.org/10.3390/fishes8080419