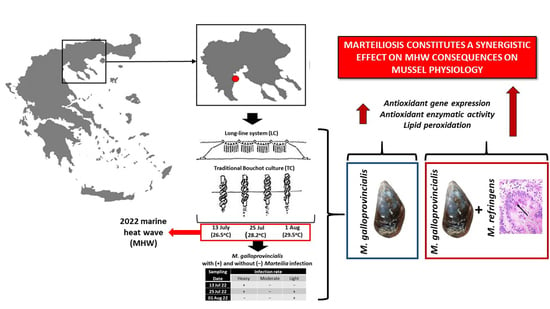
Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence
Abstract
1. Introduction
2. Materials and Methods
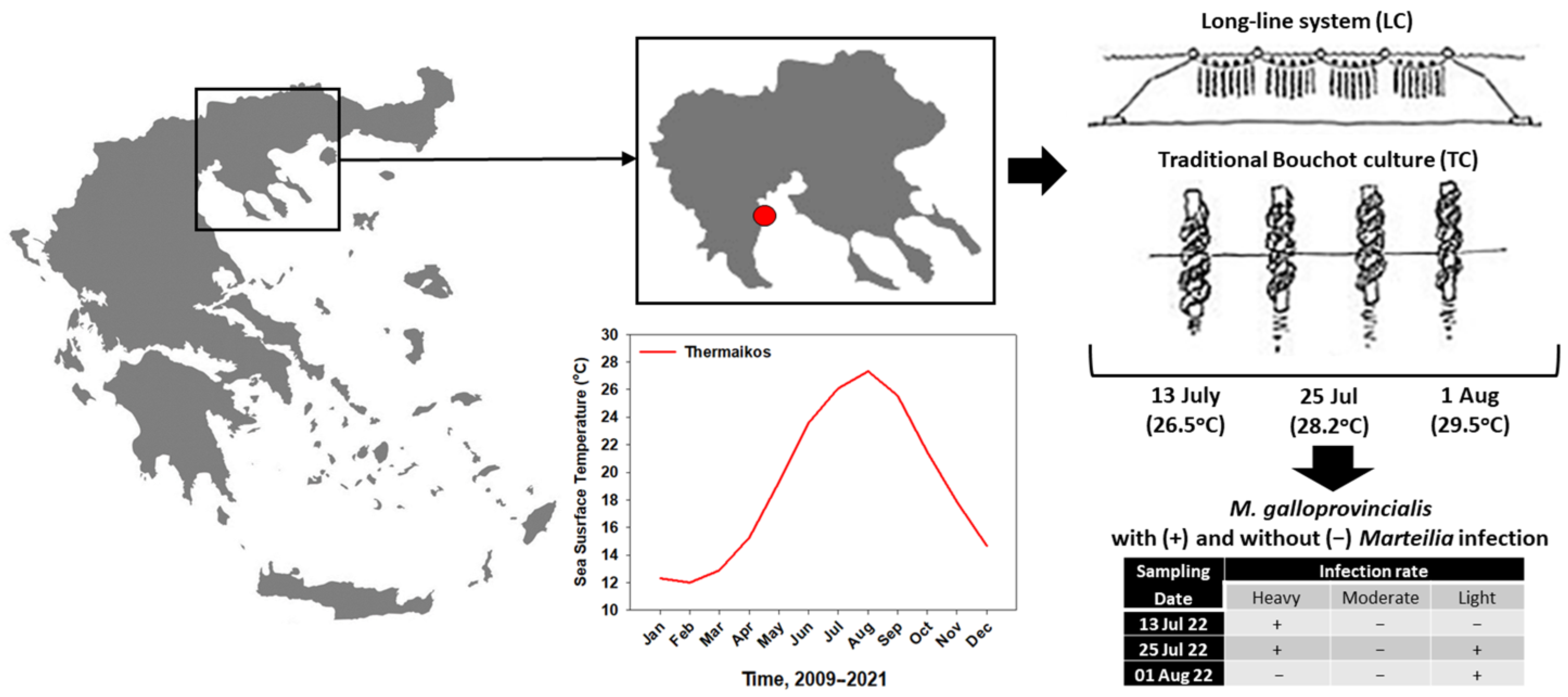
2.1. Sampling Procedure
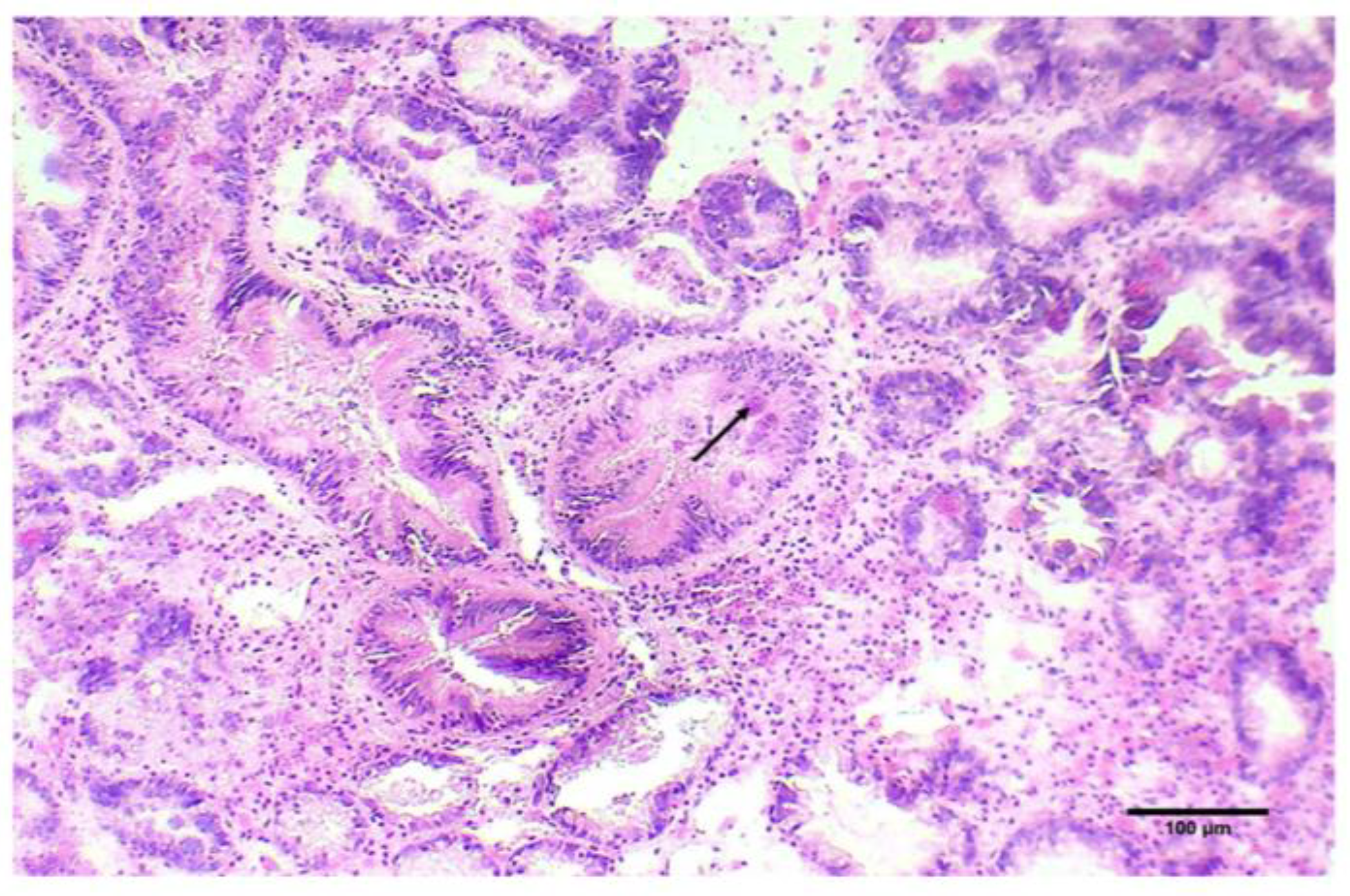
2.2. Histopathogical Detection of M. refringens
2.3. RNA Extraction and cDNA Synthesis
2.4. Gene Expression Analysis
2.5. Assays of Antioxidant Enzymes and Lipid Peroxidation (TBARS)
2.6. Statistical Analysis
3. Results
3.1. Marteilia Detection
3.2. Gene Expression
3.3. Enzymatic Activity
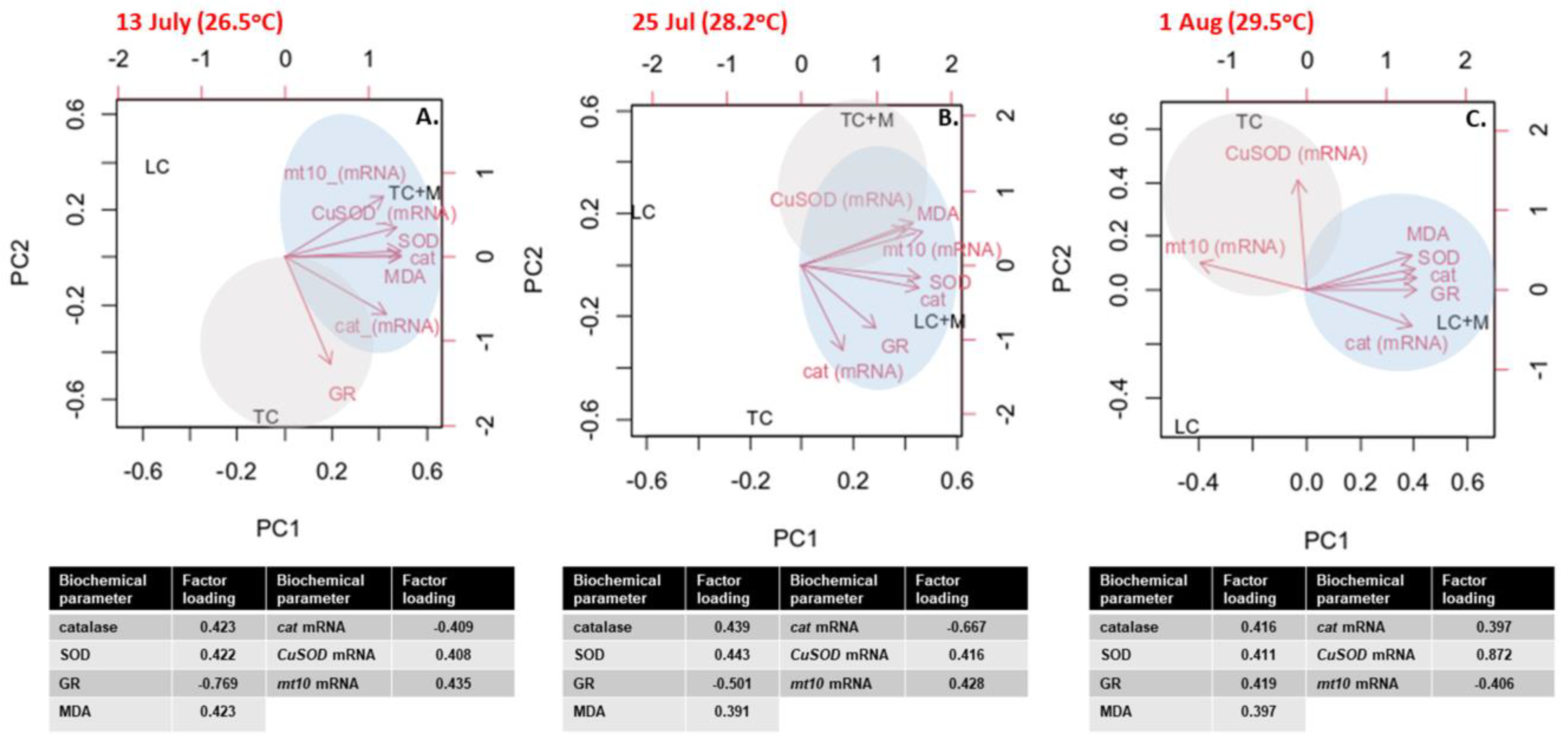
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lattos, A.; Chaligiannis, I.; Papadopoulos, D.; Giantsis, I.A.; Petridou, E.I.; Vafeas, G.; Staikou, A.; Michaelidis, B. How Safe to Eat Are Raw Bivalves? Host Pathogenic and Public Health Concern Microbes within Mussels, Oysters, and Clams in Greek Markets. Foods 2021, 10, 2793. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Gentry, R.R.; Halpern, B.S. Global change in marine aquaculture production potential under climate change. Nat. Ecol. Evol. 2018, 2, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Zgouridou, A.; Tripidaki, E.; Giantsis, I.A.; Theodorou, J.A.; Kalaitzidou, M.; Raitsos, D.E.; Lattos, A.; Mavropoulou, A.M.; Sofianos, S.; Karagiannis, D.; et al. The current situation and potential effects of climate change on the microbial load of marine bivalves of the Greek coastlines: An integrative review. Environ. Microbiol. 2022, 24, 1012–1034. [Google Scholar] [CrossRef] [PubMed]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D.M. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Chapman, E.J.; Byron, C.J.; Lasley-Rasher, R.; Lipsky, C.; Stevens, J.R.; Peters, R. Effects of climate change on coastal ecosystem food webs: Implications for aquaculture. Mar. Environ. Res. 2020, 162, 105103. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia infected Mussels from Thermaikos gulf, Greece. Animals 2022, 12, 2805. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Feidantsis, K.; Georgoulis, I.; Karagiannis, D.; Carella, F.; Michaelidis, B. Investigation of the highly endangered Pinna nobilis’ mass mortalities: Seasonal and temperature patterns of health status, antioxidant and heat stress responses. Mar. Environ. Res. 2023, 188, 105977. [Google Scholar] [CrossRef] [PubMed]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean aquaculture in a changing climate: Temperature effects on pathogens and diseases of three farmed fish species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.; Shinn, A.P.; Grenfell, S.E.; Bron, J.E.; Burnell, G.; Cook, E.J.; Crumlish, M.; Culloty, S.; Davidson, K.; Ellis, R.P.; et al. Review of climate change impacts on marine aquaculture in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 389–421. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Pourmozaffar, S.; Tamadoni Jahromi, S.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Abrari Lazarjani, S. The role of salinity in physiological responses of bivalves. Rev. Aquac. 2020, 12, 1548–1566. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. Bivalve immune responses and climate changes: Is there a relationship? Invertebr. Surviv. J. 2011, 8, 70–77. [Google Scholar]
- Canesi, L.; Gallo, G.; Gavioli, M.; Pruzzo, C. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 2002, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.; Raftos, D. Immune responses to infectious diseases in bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Dailianis, S. Production of superoxides and nitric oxide generation in haemocytes of mussel Mytilus galloprovincialis (Lmk.) after exposure to cadmium: A possible involvement of Na þ/H þ exchanger in the induction of cadmium toxic effects. Fish Shellfish Immunol. 2009, 27, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Choi, K. The known and unknown sources of reactive oxygen and nitrogen species in haemocytes of marine bivalve molluscs. Fish Shellfish Immunol. 2015, 42, 91–97. [Google Scholar] [CrossRef]
- Lambert, C.; Soudant, P.; Jegaden, M.; Delaporte, M.; Labreuche, Y.; Moal, J.; Samain, J. In vitro modulation of reactive oxygen and nitrogen intermediate (ROI/RNI) production in Crassostrea gigas hemocytes. Aquaculture 2007, 270, 413–421. [Google Scholar] [CrossRef]
- Box, A.; Capó, X.; Tejada, S.; Catanese, G.; Grau, A.; Deudero, S.; Sureda, A.; Valencia, J.M. Reduced antioxidant response of the fan mussel Pinna nobilis related to the presence of Haplosporidium pinnae. Pathogens 2020, 9, 932. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. MicroRNAs and their role on fish oxidative stress during xenobiotic environmental exposures. Ecotoxicol. Environ. Saf. 2018, 148, 995–1000. [Google Scholar] [CrossRef]
- Barber, S.C.; Mead, R.J.; Shaw, P.J. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 1051–1067. [Google Scholar] [CrossRef]
- Monari, M.; Matozzo, V.; Foschi, J.; Cattani, O.; Serrazanetti, G.P.; Marin, M.G. Effects of high temperatures on functional responses of haemocytes in the clam Chamelea gallina. Fish Shellfish Immunol. 2007, 22, 98–114. [Google Scholar] [CrossRef]
- Abele, D.; Burlando, B.; Viarengo, A.; Pörtner, H.O. Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 425–435. [Google Scholar] [CrossRef]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC, 2021: Annex VII: Glossary. [Matthews, J.B.R.; Möller, V.; van Diemen, R.; Fuglestvedt, J.S.; Masson-Delmotte, V.; Méndez, C.; Semenov, S.; Reisinger, A. (Eds.)]; In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023; pp. 2215–2256. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Knust, R. Climate change affects marine fishes through the Oxygen Limitation of Thermal Tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Behavioural, metabolic and molecular stress indicators in the marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. 2007, 293, R911–R921. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Lazou, A.; Michaelidis, B. Metabolic and molecular stress responses of sublittoral bearded horse mussel Modiolus barbatus to warming sea water: Implications for vertical zonation. J. Exp. Biol. 2008, 211, 2889–2898. [Google Scholar] [CrossRef]
- Katsikatsou, M.; Anestis, A.; Pörtner, H.O.; Vratsistas, A.; Aligizaki, K.; Michaelidis, B. Field studies and projections of climate change effects on the bearded horse mussel Modiolus barbatus in the Gulf of Thermaikos, Greece. Mar. Ecol. Prog. Ser. 2012, 449, 183–196. [Google Scholar] [CrossRef][Green Version]
- Kroeker, K.J.; Gaylord, B.; Hill, T.M.; Hosfelt, J.D.; Miller, S.H.; Sanford, E. The Role of Temperature in Determining Species’ Vulnerability to Ocean Acidification: A Case Study Using Mytilus galloprovincialis. PLoS ONE 2014, 9, e100353. [Google Scholar] [CrossRef]
- Galimany, E.; Ramón, M.; Ibarrola, I. Feeding behavior of the mussel Mytilus galloprovincialis (L.) in a Mediterranean estuary: A field study. Aquaculture 2011, 314, 236–243. [Google Scholar] [CrossRef]
- Shaw, B.L.; Battle, H.I. The Gross and Microscopic Anatomy of the Digestive Tract of the Oyster Crassostrea Virginica (Gmelin). Can. J. Zool. 1957, 35, 325–347. [Google Scholar] [CrossRef]
- Howard, D.W.; Lewis, E.J.; Keller, B.J.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks and Crustaceans; NOAA Technical Memorandum NOS NCCOS 5; NOAA: Silver Spring, MD, USA, 2004; 218p.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Fasulo, S. Effects of Oxygen Availability on Oxidative Stress Biomarkers in the Mediterranean Mussel Mytilus galloprovincialis. Mar. Biotechnol. 2017, 19, 614–626. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, Z.; Wu, H.; Liu, F.; Zhao, J. Molecular characterization of a manganese superoxide dismutase and copper/zinc superoxide dismutase from the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2013, 34, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Dondero, F.; Piacentini, L.; Marsano, F.; Rebelo, M.; Vergani, L.; Venier, P.; Viarengo, A. Gene transcription profiling in pollutant exposed mussels (Mytilus spp.) using a new low-density oligonucleotide microarray. Gene 2006, 376, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Pereiro, P.; Costa, M.M.; Figueras, A.; Novoa, B. Evaluation of reference genes of Mytilus galloprovincialis and Ruditapes philippinarum infected with three bacteria strains for gene expression analysis. Aquat. Living Resour. 2015, 27, 147–152. [Google Scholar] [CrossRef]
- Salach, J.I. Preparation of Monoamine Oxidase from Beef Liver Mitochondria. Methods Enzymol. 1978, 53, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oOxidation. Methods Enzymol. 1990, 186, 209–220. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Biomembranes—Part C: Biological Oxidations. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Gagnaire, B.; Frouin, H.; Moreau, K.; Thomas-Guyon, H.; Renault, T. Effects of temperature and salinity on haemocyte activities of the Pacific oyster, Crassostrea gigas (Thunberg). Fish Shellfish Immunol. 2006, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.C.; Houston, C.T.; Alcantar, C.Y.; Milshteyn, L.; Brazil, C.A.; Zepeda, O.G. Interactive effects of multiple stressors on the physiological performance of the invasive mussel Mytilus galloprovincialis. Mar. Environ. Res. 2022, 178, 105665. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Hajer, A.; Sforzini, S.; Oliveri, C.; Boussetta, H.; Viarengo, A. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 160, 23–29. [Google Scholar] [CrossRef]
- Regoli, F.; Winston, G.W. Applications of a new method for measuring the total oxyradical scavenging capacity in marine invertebrates. Mar. Environ. Res. 1998, 46, 439–442. [Google Scholar] [CrossRef]
- Feidantsis, K.; Giantsis, I.A.; Vratsistas, A.; Makri, S.; Pappa, A.Z.; Drosopoulou, E.; Anestis, A.; Mavridou, E.; Exadactylos, A.; Vafidis, D.; et al. Correlation between intermediary metabolism, Hsp gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R264–R281. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Mangoni, O.; Mollica, M.P.; Cavaliere, G.; Trinchese, G.; Aniello, F.; De Vico, G. Assessment of the health status of mussels Mytilus galloprovincialis along the campania coastal areas: A multidisciplinary approach. Front. Physiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Dang, V.T.; Speck, P.; Benkendorff, K. Influence of elevated temperatures on the immune response of abalone, Haliotis rubra. Fish Shellfish Immunol. 2012, 32, 732–740. [Google Scholar] [CrossRef]
- Virvilis, C.; Angelidis, P. Presence of the parasite Marteilia sp. in the flat oyster (Ostrea edulis L.) in Greece. Aquaculture 2006, 259, 1–5. [Google Scholar] [CrossRef]
- Karagiannis, D.; Angelidis, P. Infection of cultured mussels Mytilus galloprovincialis by the protozoan Marteilia sp. in the Thermaikos Gulf (N Greece). Bull. Eur. Assoc. Fish Pathol. 2007, 27, 131–141. [Google Scholar]
- Karagiannis, D.; Michaelidis, B.; Theodoridis, A.; Angelidis, P.; Feidantsis, K.; Staikou, A. Field studies on the effects of Marteilia sp. on growth of mussel Mytilus galloprovincialis in Thermaikos Gulf. Mar. Environ. Res. 2018, 142, 116–123. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michaelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: Metabolic and physiological parameters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 57–66. [Google Scholar] [CrossRef]
- Fuentes, J.; López, J.; Mosquera, E.; Vázquez, J.; Villalba, A.; Álvarez, G. Growth, mortality, pathological conditions and protein expression of Mytilus edulis and M. galloprovincialis crosses cultured in the Ría de Arousa (NW of Spain). Aquaculture 2002, 213, 233–251. [Google Scholar] [CrossRef]
- Elgharsalli, R.; Seguineau, C.; Arzul, I.; Aloui-Bejaoui, N.; Quere, C.; Moal, J. Effect of infection by the protistan parasite Marteilia refringens on the enzyme activity and energy reserves of oyster Ostrea stentina (Payraudeau, 1826) in Tunisia. J. Mar. Biol. Assoc. UK 2018, 98, 161–170. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.I. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem.-Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Taylor, A.M.; Maher, W.A.; Ubrihien, R.P. Mortality, condition index and cellular responses of Anadara trapezia to combined salinity and temperature stress. J. Exp. Mar. Biol. Ecol. 2017, 497, 172–179. [Google Scholar] [CrossRef]
- Matozzo, V.; Chinellato, A.; Munari, M.; Bressan, M.; Marin, M.G. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 2013, 72, 34–40. [Google Scholar] [CrossRef]
- Lannig, G.; Flores, J.F.; Sokolova, I.M. Temperature-dependent stress response in oysters, Crassostrea virginica: Pollution reduces temperature tolerance in oysters. Aquat. Toxicol. 2006, 79, 278–287. [Google Scholar] [CrossRef]
- Amorim, V.E.; Gonçalves, O.; Capela, R.; Fernández-Boo, S.; Oliveira, M.; Dolbeth, M.; Arenas, F.; Cardoso, P.G. Immunological and oxidative stress responses of the bivalve Scrobicularia plana to distinct patterns of heatwaves. Fish Shellfish Immunol. 2020, 106, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Soudant, P.; Chu, F.L.E.; Volety, A. Host-parasite interactions: Marine bivalve molluscs and protozoan parasites, Perkinsus species. J. Invertebr. Pathol. 2013, 114, 196–216. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, M.; Whang, I.; Nikapitiya, C.; Oh, C.; Choi, C.Y.; Lee, J. Transcriptional analysis of disk abalone (Haliotis discus discus) antioxidant enzymes against marine bacteria and virus challenge. Fish Shellfish Immunol. 2011, 31, 155–160. [Google Scholar] [CrossRef]
- Labreuche, Y.; Lambert, C.; Soudant, P.; Boulo, V.; Huvet, A.; Nicolas, J. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes Infect. 2006, 8, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Boo, S.; Duarte, C.; Guévélou, E.; Sousa, J.; Freitas, R.; Joaquim, S.; Costas, B.; Magalhães, L.; Matias, D.; Cruz, A. Effect of the alveolate parasite Perkinsus olseni infection on sexual maturation and spawning efficiency of the clam Ruditapes decussatus. Parasitology 2023, 150, 321–328. [Google Scholar] [CrossRef]
- Haffnerl, P. Experimental transmission of Marteilia refringens with special consideration of its life cycle. Dis. Aquat. Org. 1998, 34, 135–144. [Google Scholar]
- Audemard, C.; Barnaud, A.; Collins, C.M.; Le Roux, F.; Sauriau, P.G.; Coustau, C.; Blachier, P.; Berthe, F.C.J. Claire ponds as an experimental model for Marteilia refringens life-cycle studies: New perspectives. J. Exp. Mar. Bio. Ecol. 2001, 257, 87–108. [Google Scholar] [CrossRef]
- Morga, B.; Renault, T.; Faury, N.; Chollet, B.; Arzul, I. Cellular and molecular responses of haemocytes from Ostrea edulis during in vitro infection by the parasite Bonamia ostreae. Int. J. Parasitol. 2011, 41, 755–764. [Google Scholar] [CrossRef]
- Genard, B.; Miner, P.; Nicolas, J.L.; Moraga, D.; Boudry, P.; Pernet, F.; Tremblay, R. Integrative Study of Physiological Changes Associated with Bacterial Infection in Pacific Oyster Larvae. PLoS ONE 2013, 8, e64534. [Google Scholar] [CrossRef]
- Callaway, R.; Burdon, D.; Deasey, A.; Mazik, K.; Elliott, M. The riddle of the sands: How population dynamics explains causes of high bivalve mortality. J. Appl. Ecol. 2013, 50, 1050–1059. [Google Scholar] [CrossRef]



| Target Gene | Forward Primer (5′→3′) | Amplicon Size (bp) | GenBank Accession No. | Reference |
|---|---|---|---|---|
| Reverse Primer (5′→3′) | ||||
| Catalase (cat) | CTCTGACCGTGGAACCCCTGA | 193 | AY743716.2 | [35] |
| ATCACGGATGGCATAATCTGGA | ||||
| Cu/Zn superoxide dismutase (Cu/Zn sod) | AGGCGCAATCCATTTGTTAC | 212 | JN863296.1 | [36] |
| CATGCCTTGTGTGAGCATCT | ||||
| Metallothionein-10 (mt-10) | GGGCGCCGACTGTAAATGTTC | 93 | AY566248.1 | [37] |
| CACGTTGAAGGCCCTGTACACC | ||||
| Elongation factor (EF1-α) | GATATGCGCCAGTCTTGGAT | 223 | AB162021 | [38] |
| CTCATGTCTCGGACAGCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Giantsis, I.A.; Georgoulis, I.; Karagiannis, D.; Michaelidis, B. Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence. Fishes 2023, 8, 408. https://doi.org/10.3390/fishes8080408
Lattos A, Papadopoulos DK, Feidantsis K, Giantsis IA, Georgoulis I, Karagiannis D, Michaelidis B. Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence. Fishes. 2023; 8(8):408. https://doi.org/10.3390/fishes8080408
Chicago/Turabian StyleLattos, Athanasios, Dimitrios K. Papadopoulos, Konstantinos Feidantsis, Ioannis A. Giantsis, Ioannis Georgoulis, Dimitrios Karagiannis, and Basile Michaelidis. 2023. "Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence" Fishes 8, no. 8: 408. https://doi.org/10.3390/fishes8080408
APA StyleLattos, A., Papadopoulos, D. K., Feidantsis, K., Giantsis, I. A., Georgoulis, I., Karagiannis, D., & Michaelidis, B. (2023). Antioxidant Defense of Mytilus galloprovincialis Mussels Induced by Marine Heatwaves in Correlation with Marteilia Pathogen Presence. Fishes, 8(8), 408. https://doi.org/10.3390/fishes8080408