Sex Determination and Male Differentiation in Southern Swordtail Fishes: Evaluation from an Evolutionary Perspective
Abstract
:1. Introduction
2. Sex Chromosomes in Swordtails
3. H-Y Antigen in Swordtails
4. Chromosomes Versus Sex-Determining Genes
| Main Group | MSD Factor | Description | Fish in Which the MSD Factor Is Detected | Reference |
|---|---|---|---|---|
| Transcription factors | Dmy | Dmy (MSD in medaka) arose from duplication of the autosomal Dmrt1 (doublesex and mab-3 related transcription factor 1) gene. It acts through activation of Gsdf (see below) during early development of the gonads. Dmrt1 is well conserved during evolution and plays central roles in testis differentiation in animals rowing from mammals to insects. In birds it seems to be the MSD factor. | Oryzias latipes (medaka) Cynoglossus semilaevis (chinese tongue sole) | [72,73,74] |
| Sox3 | Sox3 shares homology with SRY, which is the MSD factor in most mammals. In fish it acts through activation of Gsdf as found for Dmy in the medaka (se above). | Oryzias dancena | [75] | |
| Sdy | Sdy (sexually dimorphic on the Y chromosome) seems to be the MSD in the rainbow trout and other salmonids. | Oncorhynchus mykiss (rainbow trout) | [76,77] | |
| TGF-β related factors | Gsdf | Gsdf (gonadal soma derived growth factor) seems to be the MSD factor and has shown to be necessary for testis differentiation in fx the medaka-related luzon rice fish from the Philippines. | Oryzias luzonensis (luzon rice fish) | [78] |
| df6Y | This growth and differentiation factor encoded by a gene on the factor encoded by a gene on the Y chromosome seems to be the MSD in the shortlived killifish from Zimbabve. | Nothobranchius furzeri (turquoise killifish) | [79] | |
| Bmpr1bb | This factor belonging to the group of Bone morphogenetic protein receptors is a strong MSD candidate in the hering. There are only a few genes in the sex-determining region of the Y chromosome, and BMP’s are part of the testis differentiation cascade in many species. | Clupea harengus (atlantic herring) | [80] | |
| AMH | Anti-müllerian hormone (AMH) is secreted from the Sertoli cells and is known to induce regression of the Müllerian ducts in male mammals and birds. However, studies in fish have shown that AMH may be the MSD factor in several teleosts. | Odontesthes hatcheri (patagonian pejerry) | [81] | |
| AMHR2 | Also expression of the AMH receptor 2 is necessary for normal sex differentiation in vertebrates, and. seems to be the MSD in tiger pufferfish and a couple of closely related species. | Takifugu rubripes (fugu = tiger pufferfish) | [82] |
5. Possible Effects of Hormones on Sex Determination or Differentiation in Swordtails
6. Sex Reversal or Sex Change in Swordtails?
7. More Than One Male Phenotype
8. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwak, H.-I.; Bae, M.-O.; Lee, M.-H.; Lee, Y.-S.; Lee, B.-J.; Kang, K.-S.; Chae, C.-H.; Sung, H.-J.; Shin, J.-S.; Kim, J.-H.; et al. Effects of nonylphenol, bisphenol A and their mixture on the viviparous swordtail fish (Xiphophorus helleri). Environ. Toxicol. Chem. 2001, 20, 787–795. [Google Scholar] [CrossRef]
- Meyer, M.K.; Wischnath, L.; Foerster, W. Lebendgebärende Zierfische. Arten der Welt; Baensch, H.A., Ed.; MERGUS: Osnabrück, Germany; MERGUS: Hong Kong, China, 1985; pp. 392–393. [Google Scholar]
- Baroiller, J.-F.; D’Cotta, H. The Reversible Sex of Gonochoristic Fish: Insights and Consequences. Sex. Dev. 2016, 10, 242–266. [Google Scholar] [CrossRef]
- Rosen, D.E.; Bailey, R.M. The poecilid fishes (Cyprinodontiformes), their structure, zoogeography and systematics. Bull. Am. Mus. Natur. Hist. 1963, 126, 1–176. [Google Scholar]
- Wourms, J.P. Viviparity: The Maternal-Fetal Relationship in Fishes. Am. Zool. 1981, 21, 473–515. [Google Scholar] [CrossRef] [Green Version]
- Grier, H.J. Cellular Organization of the Testis and Spermatogenesis in Fishes. Am. Zool. 1981, 21, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Grier, H.J.; Uribe, M.C.; Nostro, F.L.L.; Mims, S.D.; Parenti, L.R. Conserved form and function of the germinal epithelium through 500 million years of vertebrate evolution. J. Morphol. 2016, 277, 1014–1044. [Google Scholar] [CrossRef]
- Gul, M.; Hildorf, S.; Dong, L.; Thorup, J.; Hoffmann, E.R.; Jensen, C.F.S.; Sønksen, J.; Cortes, D.; Fedder, J.; Andersen, C.Y.; et al. Review of injection techniques for spermatogonial stem cell transplantation. Hum. Reprod. Updat. 2020, 26, 368–391. [Google Scholar] [CrossRef]
- Pudney, J. Spermatogenesis in nonmammalian vertebrates. Microsc. Res. Tech. 1995, 32, 459–497. [Google Scholar] [CrossRef]
- Basolo, A.L.; Culumber, Z.W.; Tobler, M.; Braasch, I.; Peterson, S.M.; Desvignes, T.; McCluskey, B.M.; Batzel, P.; Postlethwait, J.H.; Cui, R.; et al. Genetic Linkage and Color Polymorphism in the Southern Platyfish (Xiphophorus maculatus): A Model System for Studies of Color Pattern Evolution. Zebrafish 2006, 3, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Vallowe, H.H. Some physiological aspects of reproduction in Xiphophorus maculatu. Biol. Bull. 1953, 104, 240–249. [Google Scholar] [CrossRef]
- Basolo, A.L. Female Preference Predates the Evolution of the Sword in Swordtail Fish. Science 1990, 250, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.G.; Martinez, T.Y.F.; de León, F.J.G.; Ryan, M.J. Shared Preferences by Predators and Females for Male Ornaments in Swordtails. Am. Nat. 2001, 158, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.G.; Evans, C.S. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc. Natl. Acad. Sci. USA 1998, 95, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Schartl, M.; Walter, R.B.; Meyer, A. Comprehensive phylogenetic analysis of all species of swordtails and platies (Pisces: Genus Xiphophorus) uncovers a hybrid origin of a swordtail fish, Xiphophorus monticolus, and demonstrates that the sexually selected sword originated in the ancestral lineage of the genus, but was lost again secondarily. BMC Evol. Biol. 2013, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.; Morrissey, J.M.; Schartl, M. Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature 1994, 368, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; Murray: London, UK, 1871. [Google Scholar]
- Graves, J.A.M.; Ferguson-Smith, M.A.; McLaren, A.; Mittwoch, U.; Renfree, M.B.; Burgoyne, P. The evolution of mammalian sex chromosomes and the origin of sex determining genes. Philos. Trans. R. Soc. B Biol. Sci. 1995, 350, 305–312. [Google Scholar] [CrossRef]
- Schartl, M. Sex chromosome evolution in non-mammalian vertebrates. Curr. Opin. Genet. Dev. 2004, 14, 634–641. [Google Scholar] [CrossRef]
- Torsvik, T.H.; Cocks, L.R.M. Earth geography from 400 to 250 Ma: A palaeomagnetic, faunal and facies review. J. Geol. Soc. 2004, 161, 555–572. [Google Scholar] [CrossRef]
- Grützner, F.; Rens, W.; Tsend-Ayush, E.; El-Mogharbel, N.; O’Brien, P.C.M.; Jones, R.C.; Ferguson-Smith, M.A.; Graves, J.A.M. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 2004, 432, 913–917. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Llamas, B.; Soubrier, J.; Rawlence, N.J.; Worthy, T.H.; Wood, J.; Lee, M.S.Y.; Cooper, A. Data from: Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution. Science 2014, 344, 898–900. [Google Scholar] [CrossRef]
- Ishijima, J.; Uno, Y.; Nishida, C.; Matsuda, Y. Genomic Structures of the kW1 Loci on the Z and W Chromosomes in Ratite Birds: Structural Changes at an Early Stage of W Chromosome Differentiation. Cytogenet. Genome Res. 2014, 142, 255–267. [Google Scholar] [CrossRef]
- Geffroy, B.; Wedekind, C. Effects of global warming on sex ratios in fishes. J. Fish Biol. 2020, 97, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Lampert, K.; Schartl, M. The origin and evolution of a unisexual hybrid: Poecilia formosa. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2901–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultheis, C.; Böhne, A.; Schartl, M.; Volff, J.; Galiana-Arnoux, D. Sex Determination Diversity and Sex Chromosome Evolution in Poeciliid Fish. Sex. Dev. 2009, 3, 68–77. [Google Scholar] [CrossRef]
- Ross, J.A.; Urton, J.R.; Boland, J.; Shapiro, M.D.; Peichel, C.L. Turnover of Sex Chromosomes in the Stickleback Fishes (Gasterosteidae). PLoS Genet. 2009, 5, e1000391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, G. Vergleichende Untersuchungen am drei Subspecies von Xiphophorus helleri Heckel (Pisces). Zeitsch. Zool. Syst. Evolutionsforsch. 1964, 2, 185–271. [Google Scholar] [CrossRef]
- Kallman, K.D.; Bao, I.Y. Female heterogamety in the swordtail, Xiphophorus alvarezi Rosen (Pisces, Poeciliidae), with comments on a natural polymorphism affecting sword coloration. J. Exp. Zool. 1987, 243, 93–102. [Google Scholar] [CrossRef]
- Kallman, K.D.; Schreibman, M.P. The origin and possible genetic control of new, stable pigment patterns in the poeciliid fish Xiphophorus maculatus. J. Exp. Zool. 1971, 176, 147–168. [Google Scholar] [CrossRef]
- Kallman, K.D. Genetic control of size at maturity in Xiphophorus. In Ecology and Evolution of Livebearing Fishes (Poecilidae); Meffe, G.K., Snelson, F.F., Eds.; Drenta Hall: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- Lampert, K.P.; Schmidt, C.; Fischer, P.; Volff, J.-N.; Hoffmann, C.; Muck, J.; Lohse, M.J.; Ryan, M.J.; Schartl, M. Determination of onset of sexual maturation and mating behaviour by melanocortin receptor 4 polymorphisms. Curr. Biol. 2010, 20, 1729–1734. [Google Scholar] [CrossRef]
- Schultheis, C.; Zhou, Q.; Froschauer, A.; Nanda, I.; Selz, Y.; Schmidt, C.; Matschl, S.; Wenning, M.; Veith, A.-M.; Naciri, M.; et al. Molecular analysis of the sex-determining region of the platyfish Xiphophorus maculates. Zebrafish 2006, 3, 299–309. [Google Scholar] [CrossRef]
- Borowski, R.L. The evolutionary genetics of Xiphophorus. In Evolutionary Genetics of Fishes; Turner, B.J., Ed.; Plenum Press: New York, NY, USA, 1984; pp. 235–310. [Google Scholar]
- Kavumpurath, S.; Pandian, T.J. Production of a YY female guppy, Poecilia reticulata, by endocrine sex-reversal and progeny testing. Aquaculture 1993, 118, 183–189. [Google Scholar] [CrossRef]
- Franchini, P.; Jones, J.C.; Xiong, P.; Kneitz, S.; Gompert, Z.; Warren, W.C.; Walter, R.B.; Meyer, A.; Schartl, M. Long-term experimental hybridisation results in the evolution of a new sex chromosome in sword tail fish. Nature Commun. 2018, 9, 5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochvíl, L.; Stöck, M.; Rovatsos, M.; Bullejos, M.; Herpin, A.; Jeffries, D.L.; Peichel, C.L.; Perrin, N.; Valenzuela, N.; Pokorná, M.J.; et al. Expanding the classical paradigm: What we have learnt from vertebrates about sex chromosome evolution. Phil. Trans. R. Soc. B 2021, 376, 20200097. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.A.; Clark, F.E.; Roberts, R.B.; Xu, L.; Tao, W.; Zhou, Q.; Wang, D.; Kocher, T.D. Origin of a giant sex chromosome. Mol. Biol. Evol. 2020, 38, 1554–1569. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D.; Bergero, R.; Graham, C.; Gardner, J.; Keegan, K. How did the guppy Y chromosome evolve? PLoS Genet. 2021, 17, e1009704. [Google Scholar] [CrossRef]
- Sember, A.; Nguyen, P.; Perez, M.F.; Altmanová, M.; Ráb, P.; Cioffi, M.d.B. Multiple sex chromosomes in teleost fishes from a cytogenetic perspective: State of the art and future challenges. Phil. Trans. R. Soc. B 2021, 376, 20200098. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Salzburger, W.; Schartl, M. Hybrid origin of a swordtail species (Teleostei: Xiphophorus clemenciae) driven by sexual selection. Mol. Ecol. 2006, 15, 721–730. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.C.; Perez-Sato, J.-A.; Meyer, A. A phylogeographic investigation of the hybrid origin of a species of swordtail fish from Mexico. Mol. Ecol. 2012, 21, 2692–2712. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.C.; Fan, S.; Franchini, P.; Schartl, M.; Meyer, A. The evolutionary history of Xiphophorus fish and their sexually selected sword: A genome-wide approach using restriction site-associated DNA sequencing. Mol. Ecol. 2013, 22, 2986–3001. [Google Scholar] [CrossRef] [Green Version]
- Wachtel, S.S. H-Y Antigen and the Biology of Sex Determination; Grune & Stratton: New York, NY, USA, 1983. [Google Scholar]
- Eichwald, E.J.; Silmser, C.R. Untitled communication. Transplant. Bull. 1955, 2, 148–149. [Google Scholar]
- Goldberg, E.H.; Boyse, E.A.; Bennett, D.; Scheid, M.; Carswell, E.A. Serological demonstration of H-Y (male) antigen on mouse sperm. Nature 1971, 232, 478–480. [Google Scholar] [CrossRef]
- Bradley, M.P.; Ebensperger, C.; Wiberg, U.H. Determination of the serological sex-specific (Sxs) antigen (“H-Y antigen”) in birds and mammals using high-titer antisera and a sensitive urease ELISA. Hum. Genet. 1987, 76, 352–356. [Google Scholar] [CrossRef]
- Meck, J.M.; Goldberg, E.H. Serological detection of H-Y antigen in humans with a cellular radioimmunobinding assay and monoclonal antibody. J. Immunol. Methods 1984, 73, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Guichard, A.; Reyss-Brion, M.; Scheib, D. Induction of H-Y antigen in the gonads of male quail embryos by diethylstilbestrol. Differentiation 1980, 16, 129–133. [Google Scholar] [CrossRef]
- Fraccaro, M.; Mayerová, A.; Wolf, U.; Bühler, E.; Gebauer, J.; Gilgenkrantz, S. Correlation between the number of sex chromosomes and the H-Y antigen titer. Hum. Genet. 1982, 61, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wolf, U.; Fraccaro, M.; Mayerová, A.; Hecht, T.; Maraschio, P.; Hameister, H. A gene controlling H-Y antigen on the X chromosome. Tentative assignment by deletion mapping to Xp223. Hum. Genet. 1980, 54, 149–154. [Google Scholar] [CrossRef]
- Nakamura, D.; Wachtel, S.S.; Kallman, K. H-Y antigen and the evolution of heterogamety. J. Hered. 1984, 75, 353–358. [Google Scholar] [CrossRef]
- Zenzes, M.T.; Wolf, U.; Günther, E.; Engel, W. Studies on the function of H-Y antigen: Dissociation and reorganization experiments on rat gonadal tissue. Cytogenet. Cell Genet. 1978, 20, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Zenzes, M.T.; Bauknecht, T.; Wolf, U.; Siebers, J.W.; Engel, W. Appearance of hCG-receptor after conversion of newborn ovarian cells into testicular structures by H-Y antigen in vitro. Hum. Genet. 1978, 45, 203–207. [Google Scholar] [CrossRef]
- Urban, E.; Zenzes, M.T.; Müller, U.; Wolf, U. Cell reorganization in vitro of heterosexual gonadal cocultures. Differentiation 1981, 18, 161–168. [Google Scholar] [CrossRef]
- Ohno, S.; Nagai, Y.; Ciccarese, S. Testicular cells lysostripped of H-Y antigen organize ovarian follicle-like aggregates. Cytogenet. Cell Genet. 1978, 20, 351–364. [Google Scholar] [CrossRef]
- Fedder, J.; Hansen, L.G.; Hjort, T. Reduced level of sex-specific antigen (H-Y antigen) on lymphocytes in some patients with bilateral cryptorchidism. Arch. Androl. 1989, 22, 67–75. [Google Scholar] [CrossRef]
- Fedder, J.; Kristensen, I.B.; Friedrich, U.; Agger, A.O. H-Y antigen og testisudvikling hos pige med XY-karyotype. Ugeskr. Læger 1989, 151, 1060–1062. [Google Scholar] [PubMed]
- Fedder, J.; Hjort, T. Evidence for more than one male-specific antigen in human. In New Concepts in Reproduction, Recent Developments in Fertility and Sterility Series; Boutaleb, Y., Gzouli, A., Eds.; The Parthenon Publishing Group Ltd.: Carnforth, UK, 1991; Volume 6, Chapter 16; pp. 117–120. [Google Scholar]
- Mohammadi, A.A.; Tetro, J.A.; Filion, L.G. Epitope selection to male specific antigens for sex selection in swine. J. Reprod. Immunol. 2011, 89, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meadows, L.R.; Haan, J.M.M.D.; Sherman, N.E.; Chen, Y.; Blokland, E.; Shabanowitz, J.; Agulnik, A.I.; Hendrickson, R.C.; Bishop, C.E.; et al. Human H-Y: A male-specific histocompatibility antigen derived from the SMCY protein. Science 1995, 269, 1588–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Q.; Kay, T.; Depincé, A.; Adolfi, M.; Schartl, M.; Guiguen, Y.; Herpin, A. Evolution of master sex determiners: TGF-β signalling pathways at regulatory crossroads. Phil. Trans. R. Soc. B 2021, 376, 20200091. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, P.B.; Kjartansdóttir, K.R.; Fedder, J. Care of women with XY karyotype. A clinical practice guideline. Fertil. Steril. 2010, 94, 105–113. [Google Scholar] [CrossRef]
- Berglund, A.; Johannsen, T.H.; Stochholm, K.; Viuff, M.H.; Fedder, J.; Main, K.M.; Gravholt, C.H. Incidence, prevalence, diagnostic delay and clinical presentation of female 46,XY disorder of sex development. J. Clin. Endocrinol. Metab. 2016, 101, 4532–4540. [Google Scholar] [CrossRef] [Green Version]
- Berglund, A.; Johannsen, T.; Stochholm, K.; Aksglaede, L.; Fedder, J.; Viuff, M.; Main, K.; Gravholt, C. Incidence, prevalence, diagnostic delay, morbidity, mortality and socioeconomic status in males with 46,XX disorders of sex development—A nationwide study. Hum. Reprod. 2017, 32, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, A.T.; Smith, C.A. Sex reversal in birds. Sex. Dev. 2016, 10, 288–300. [Google Scholar] [CrossRef]
- Ferguson-Smith, M. The evolution of sex chromosomes and sex determination in vertebrates and the key role of DMRT1. Sex. Dev. 2007, 1, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Jost, A.; Vigier, B.; Prepin, J.; Perchellet, J.P. Studies on sex differentiation in mammals. Rec. Prog. Hormone Res. 1973, 29, 1–41. [Google Scholar] [CrossRef]
- Josso, N.; Picard, J.-Y.; Tran, D. The Antimüllerian hormone. Rec. Prog. Hormone Res. 1976, 33, 117–167. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A.; et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin-I, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef] [Green Version]
- Yano, A.; Guyomard, R.; Nicol, B.; Jouanno, E.; Quillet, E.; Klopp, C.; Cabau, C.; Bouchez, O.; Fostier, A.; Guiguen, Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 2012, 22, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichwald, K.; Petzold, A.; Koch, P.; Downie, B.R.; Hartmann, N.; Pietsch, S.; Baumgart, M.; Chalopin, D.; Felder, M.; Bens, M.; et al. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell 2015, 163, 1527–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafati, N.; Chen, J.; Herpin, A.; Pettersson, M.E.; Han, F.; Feng, C.; Wallerman, O.; Rubin, C.-J.; Péron, S.; Cocco, A.; et al. Reconstruction of the birth of a male sex chromosome present in Atlantic herring. Proc. Natl. Acad. Sci. USA 2020, 117, 24359–24368. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Kamiya, T.; Kai, W.; Tasumi, S.; Oka, A.; Matsunaga, T.; Mizuno, N.; Fujita, M.; Suetake, H.; Suzuki, S.; Hosoya, S.; et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu). PLoS Genet. 2012, 8, e1002798. [Google Scholar] [CrossRef] [Green Version]
- Zaborski, P.; Dorizzi, M.; Pieau, C. H-Y antigen expression in temperature sex-reversed turtles (Emys orbicularis). Differentiation 1982, 22, 73–78. [Google Scholar] [CrossRef]
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending genders: The biology of natural sex change in fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef]
- Pechan, P.; Shapiro, D.Y.; Tracey, M. Increased H-Y antigen levels associated with behaviourally induced, female-to-male sex reversal in a coral-reef fish. Differentiation 1986, 31, 106–110. [Google Scholar] [CrossRef]
- Muncaster, S.; Norberg, B.; Andersson, E. Natural sex change in the temperate protogynous Ballan wrasse Labrus bergylta. J. Fish Biol. 2013, 82, 1858–1870. [Google Scholar] [CrossRef]
- Shapiro, D.Y. Size, maturation and the social control of sex reversal in the coral reef fish Anthias squamipinnis. J. Zool. Lond. 1981, 193, 105–128. [Google Scholar] [CrossRef]
- Casas, L.; Saborido-Rey, F.; Ryu, T.; Michell, C.; Ravasi, T.; Irigoien, X. Sex change in clownfish: Molecular insights from transcriptome analysis. Sci. Rep. 2016, 6, 35461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, R.R. Sex change in fishes: Hypotheses, evidence and objections. Environ. Biol. Fish. 1988, 2, 81–90. [Google Scholar] [CrossRef]
- Essenberg, J.M. Complete sex-reversal in the viviparous teleost Xiphophorus helleri. Biol. Bull. 1926, 60, 98–111. [Google Scholar] [CrossRef]
- Essenberg, J.M. Sex-differentiation in the viviparous teleost, Xiphophorus helleri Heckel. Biol. Bull. 1923, 45, 46–96. [Google Scholar] [CrossRef]
- Gomel’skiy, B.I.; Fetisov, A.N. Sex ratio at various stages of ontogeny on the swordtail, Xiphophorus helleri (Cyprinodontiformes, Poecilidae), in connection with the problem of sex redifferentiation. J. Ichthyol. 1978, 17, 689–692. [Google Scholar]
- Kozak, E.C.; Uetz, G.W. Male courtship signal modality and female mate preference in the wolf spider Schizocosa ocreata: Results of digital multinodal playback studies. Curr. Zool. 2019, 65, 705–711. [Google Scholar] [CrossRef]
- Cummings, M.E.; DeLeon, F.J.G.; Mollaghan, D.M.; Ryan, M.J. Is UV ornamentation an amplifier in swordtails? Zebrafish 2006, 3, 91–100. [Google Scholar] [CrossRef]
- Maderspacher, F. Reproductive Strategies: How big is your love? Curr. Biol. 2010, 20, R925–R928. [Google Scholar] [CrossRef] [Green Version]
- Boulton, K.; Sinderman, B.; Pearce, M.R.; Earley, R.L.; Wilson, A.J. He who dares only wins sometimes: Physiological stress and contest behaviour in Xiphophorus helleri. Behaviour 2012, 149, 977–1002. [Google Scholar] [CrossRef]
- Boulton, K.; Walling, C.A.; Grimmer, A.J.; Rosenthal, G.G.; Wilson, A.J. Phenotypic and genetic integration of personality and growth under competition in the sheepshead swordtail, Xiphophorus birchmanni. Evolution 2017, 72, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, J.J. Socially induced inhibition of genetically determined maturation in the platyfish, Xiphophorus maculatus. Science 1977, 195, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, R.L. Social control of adult size in males of Xiphophorus variatus. Nature 1973, 245, 332–335. [Google Scholar] [CrossRef]
- Borowsky, R. Social inhibition of maturation in natural populations of Xiphophorus variatus (Pisces: Poeciliidae). Science 1978, 201, 933–935. [Google Scholar] [CrossRef]
- Boulton, K.; Rosenthal, G.G.; Grimmer, A.J.; Walling, C.A.; Wilson, A.J. Sex-specific plasticity and genotype x sex-interactions for age and size of maturity in the sheepshead swordtail Xiphophorus birchmanni. J. Evol. Biol. 2016, 29, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.L. Seasonal trends in body size of adult male mosquitofish, Gambusia affinis, with evidence for their social control. Environ. Biol. Fish 1985, 14, 251–258. [Google Scholar] [CrossRef]
- Farr, J.A. The effects of juvenile social interaction on growth rate, size and age at maturity, and adult social behaviour in Girardinus metallicus Poey (Pisces: Poeciliidae). Z. Tierpsychol. 1980, 52, 247–268. [Google Scholar] [CrossRef]

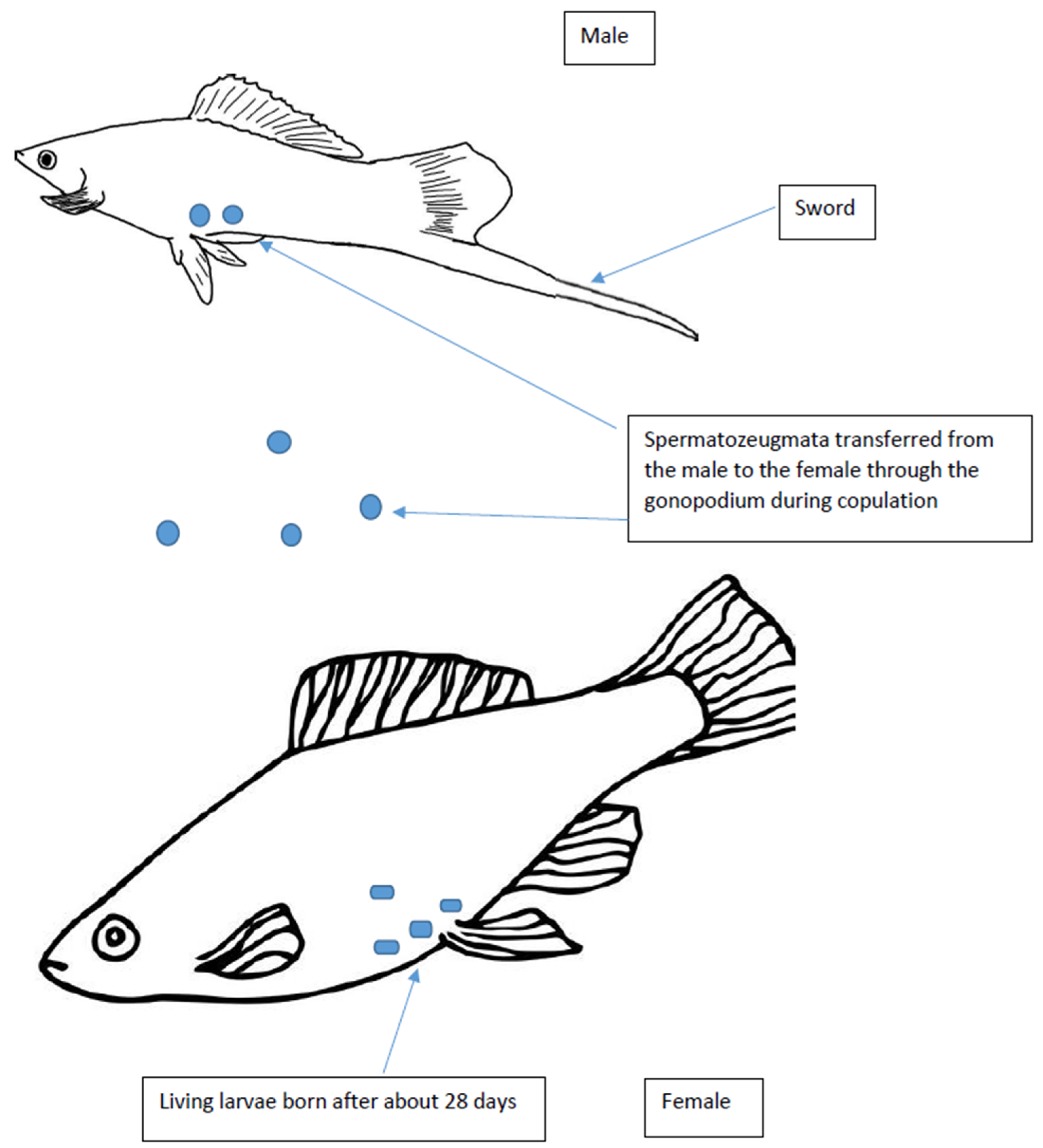


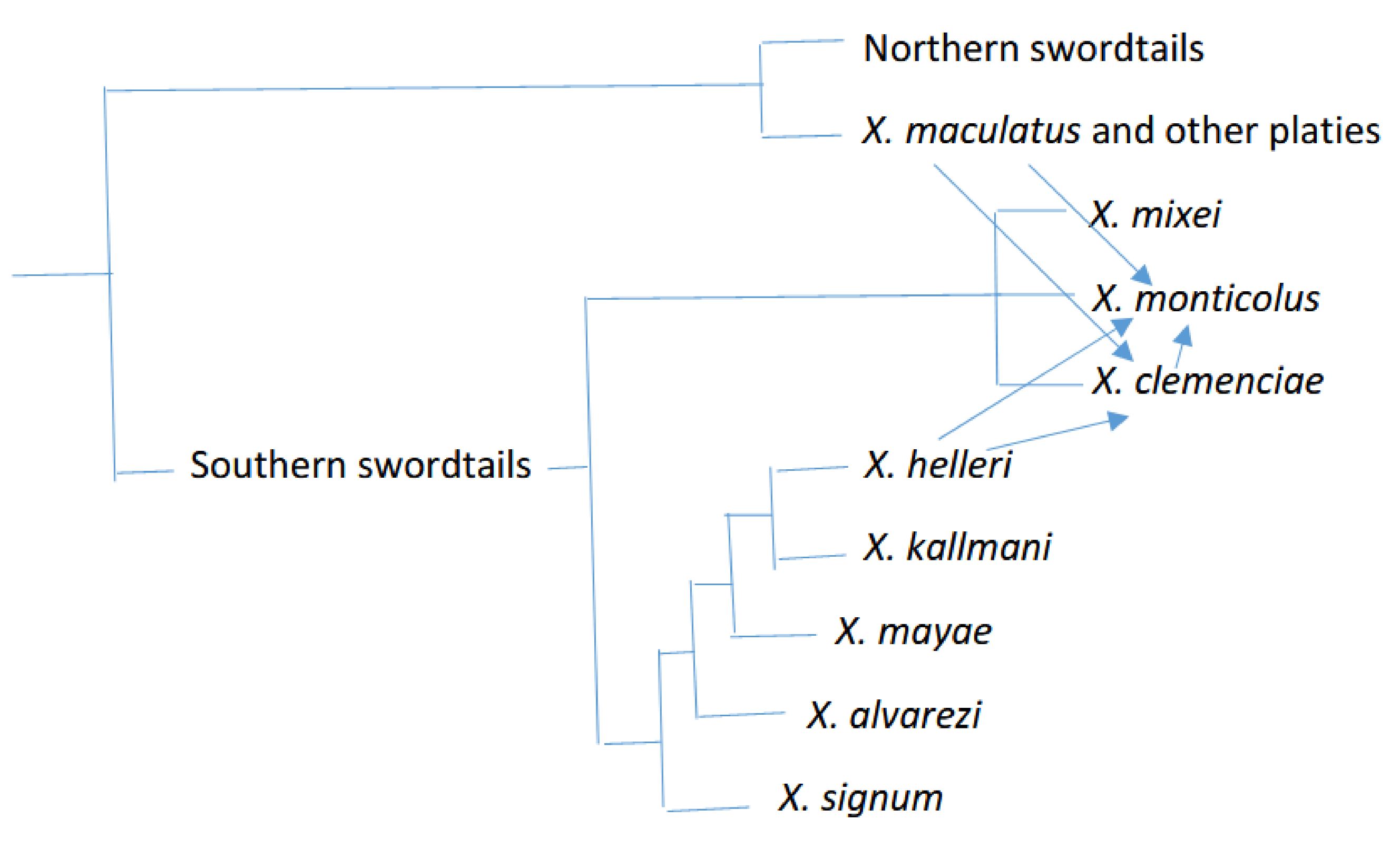
| Fish Group | Sex Determination Mechanisms/Sex Chromosomes | Male Phenotype | Reproduction |
|---|---|---|---|
| Xiphophorus species | Different systems with heterogametry in males and females, respectively. Also polyfactorial systems suggested. | Swordtails usually have elongation of the ventral rays of the tail. Males smaller or equal to female size. | Internal fertilization. Living larvae born. |
| Poecilia species not belonging to Xiphophorus | Male heterogametry (XX-XY) detected in several species, incl. guppies and mollies. The Y chromosome degenerated in some species. | Males often smaller than females, and in fx. guppies males show more colours. | Internal fertilization. Living larvae born. |
| Teleosts not belonging to Poecilia | Huge variations including genetic (GSD) and environmental sex determination (ESD). Sex chromosomes may be homomorphic or heteromorphic, and species with multiple sex chromosomes occur. | Huge variations. Sequential hermaphroditism found in several species. | The majority of species produce eggs, which are fertilized outside the body. |
| Fish group | Species | Sex Chromosome Systems | References |
|---|---|---|---|
| Southern swordtails | * X. helleri ** X. alvarezi X. signum X. mayae X. kallmani X. clemenciae X. monticolus X. mixei | * Female ZZ-ZW heterogametry or a polyfactorial sex determination system ** Female ZZ-ZW heterogametry (studies on sword colour) | * [26] * [28] ** [29] |
| Northern swordtails | X. birchmani 1 * X. cortezi 1 X. malinche 1 X. continens 2 X. montezumae 2 ** X. nezahualcoyotl 2 X. multilineatus 3 *** X. nigrensis 3 X. pygmaeus 3 | * XX-XY system. Pure XX males occur due to autosomal modifiers. ** XX-XY system. Two Y chromosomes: Y and Y’. XY females may occur due to an autosomal modifier *** XX-XY system. XY are males. XX usually are females but may be males due to autosomal modifiers. | * [30] ** [26] *** [31] *** [32] |
| Platies | X. couchianus X. gordoni X. meyeri X. andersi X. evelynae * X. maculatus X. milleri ** X. variatus X. xiphidum | * ♀:XX, XW or YW. ♂: XY or YY. ** XX-XY system. Male heterogametry suggested from inheritance of sex- linked pigment phenotypes. | * [33] ** [34] |
| Non-Xiphophorus species included in this paper | * Gasterosteus wheatlandi ** Poecilia reticulata (guppy) | * X1X2Y sex determination system. ** XX-XY system, sensitive to hormones. | * [27] ** [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedder, J. Sex Determination and Male Differentiation in Southern Swordtail Fishes: Evaluation from an Evolutionary Perspective. Fishes 2023, 8, 407. https://doi.org/10.3390/fishes8080407
Fedder J. Sex Determination and Male Differentiation in Southern Swordtail Fishes: Evaluation from an Evolutionary Perspective. Fishes. 2023; 8(8):407. https://doi.org/10.3390/fishes8080407
Chicago/Turabian StyleFedder, Jens. 2023. "Sex Determination and Male Differentiation in Southern Swordtail Fishes: Evaluation from an Evolutionary Perspective" Fishes 8, no. 8: 407. https://doi.org/10.3390/fishes8080407
APA StyleFedder, J. (2023). Sex Determination and Male Differentiation in Southern Swordtail Fishes: Evaluation from an Evolutionary Perspective. Fishes, 8(8), 407. https://doi.org/10.3390/fishes8080407







