Exploring European Eel Anguilla anguilla (L.) Habitat Differences Using Otolith Analysis in Central-Western Mediterranean Rivers and Coastal Lagoons from Sardinia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Locations
2.2. Eel Samples
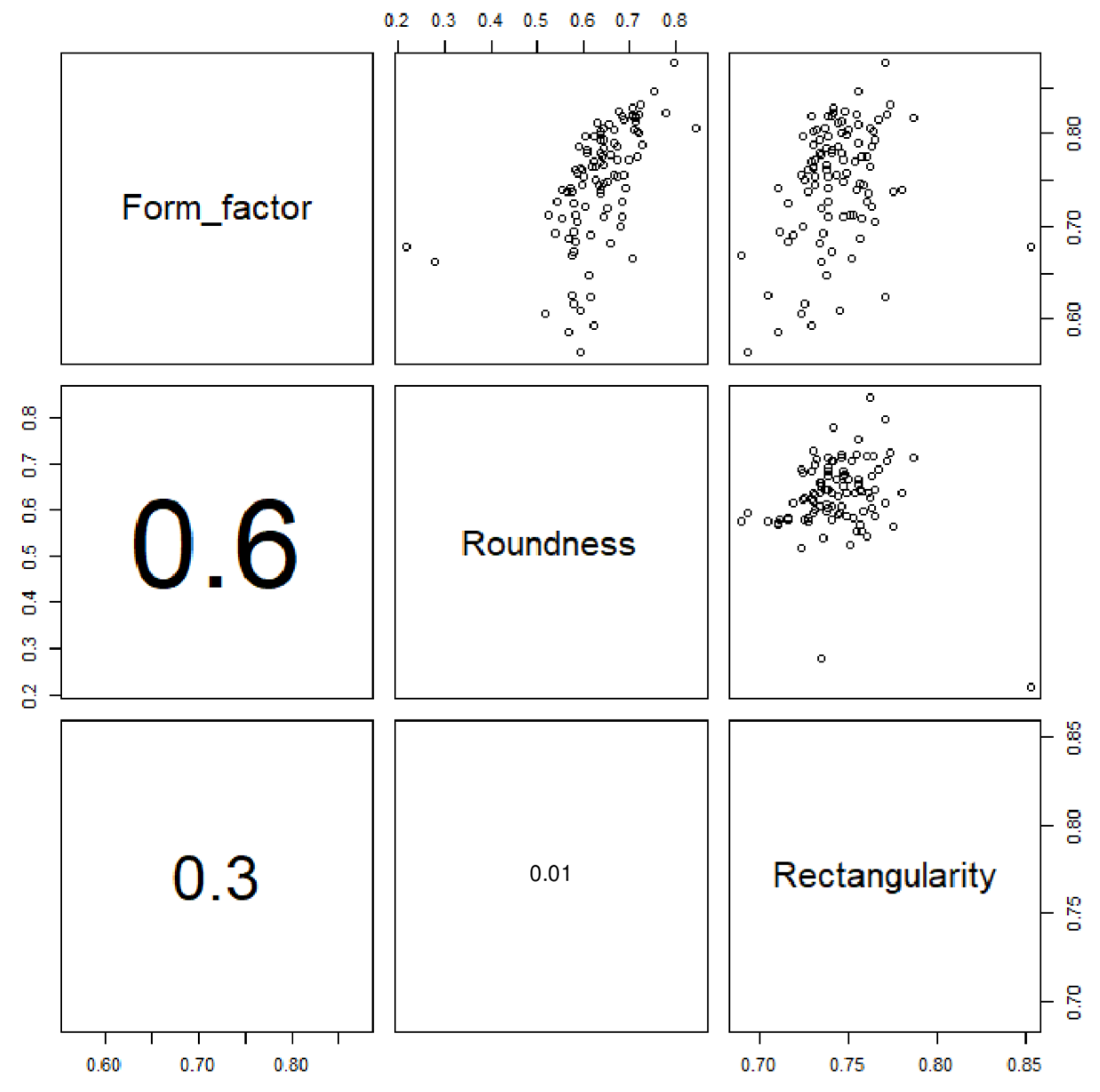
2.3. Otolith Extraction and Shape Analysis
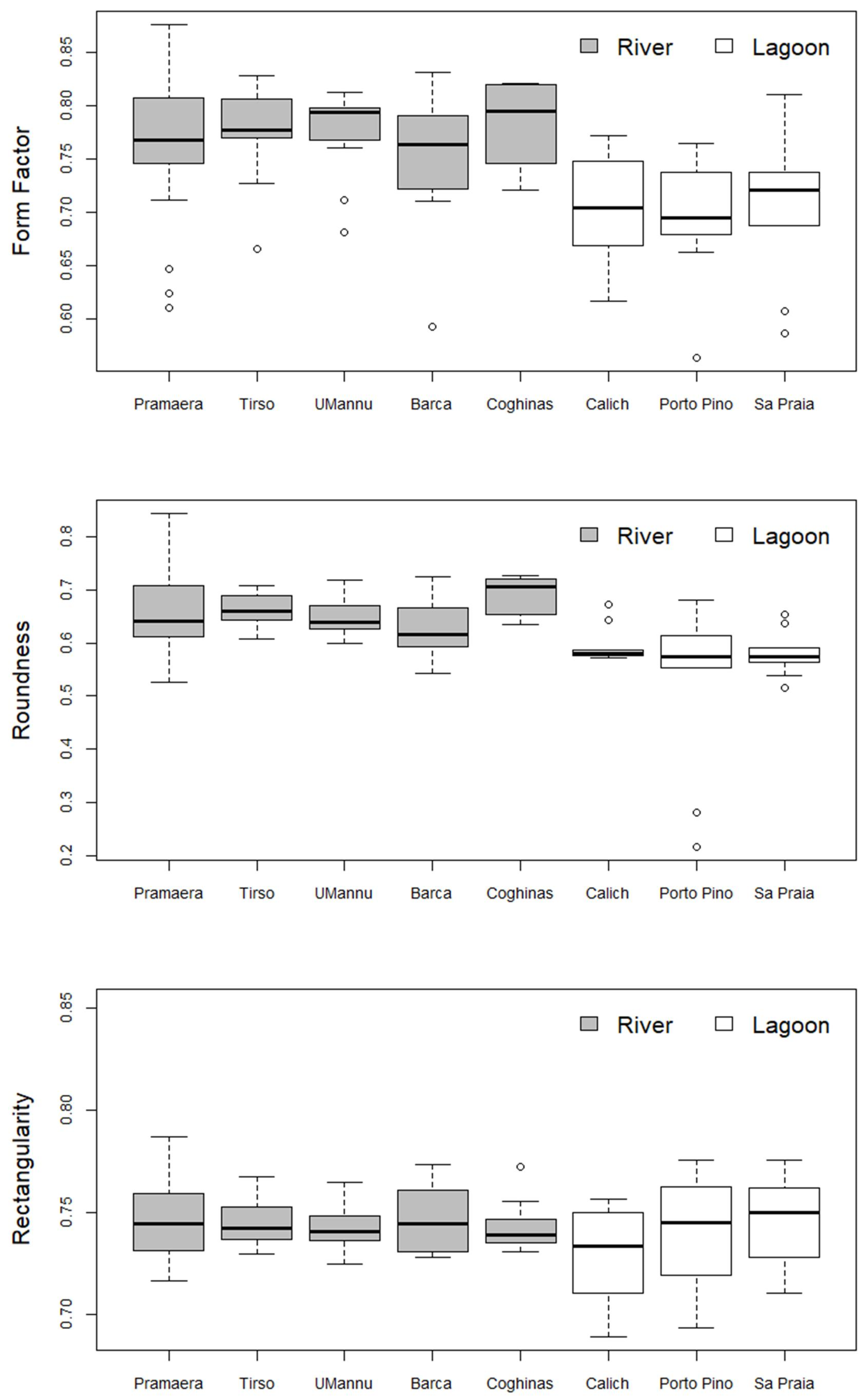
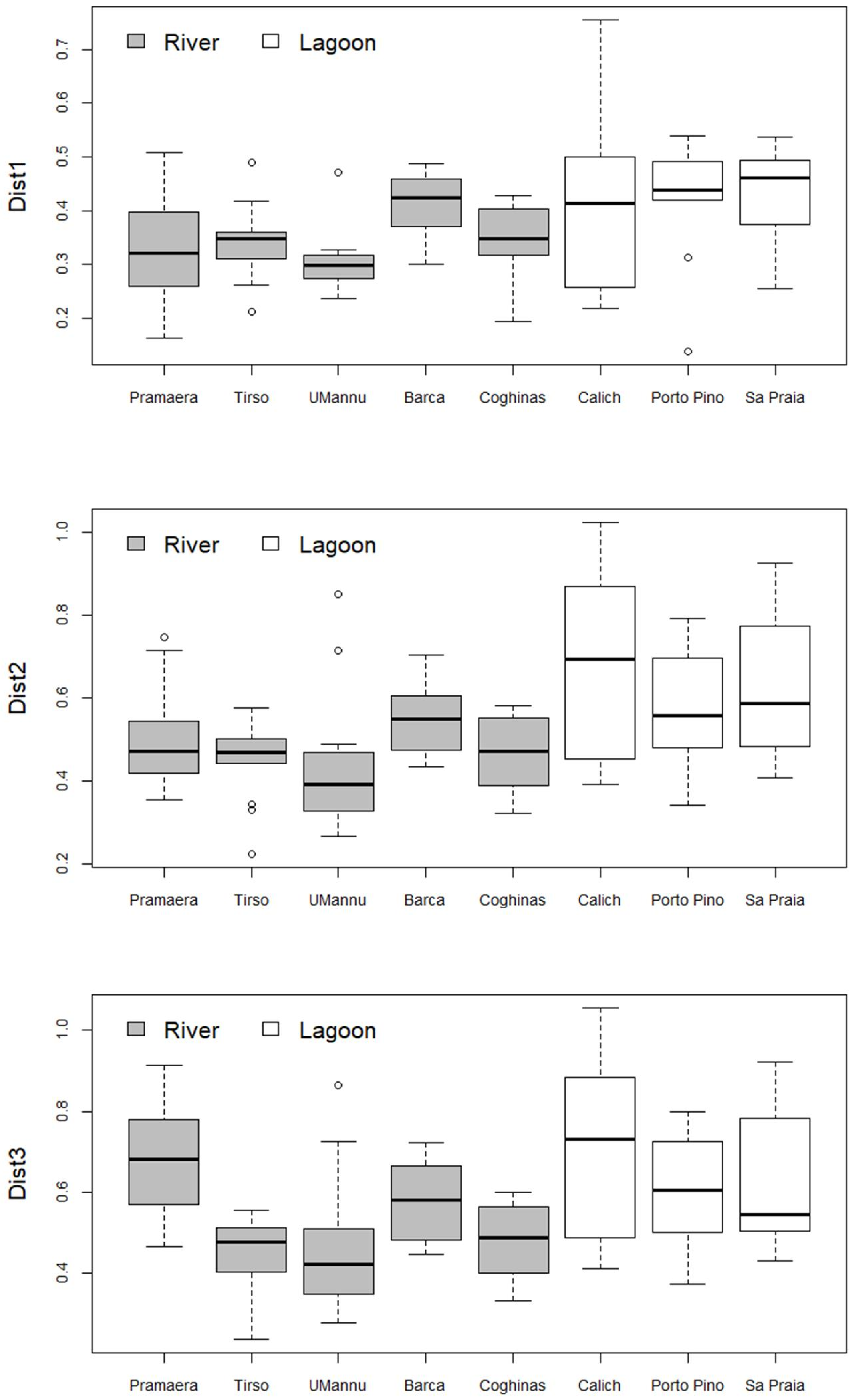
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydın, İ. Balık Larvalarında Otolit; Makale, Sümae Yunus Araştırma Bülteni: Trabzon, Turkey, 2006; Volume 2, p. 6. [Google Scholar]
- Campana, E.S.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Thorrold, S.R.; Campana, S.E.; Jones, C.M.; Swart, P.K. Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 1997, 61, 2909–2919. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B.M. Interactive effects of temperature and salinity on otolith chemistry: Challenges for determining environmental histories of fish. Can. J. Fish. Aquat. Sci. 2002, 59, 1796–1808. [Google Scholar] [CrossRef]
- Lecomte-Finiger, R. Growth history and age at recruitment of European glass eels (Anguilla anguilla) as revealed by otolith microstructure. Mar. Biol. 1992, 114, 205–210. [Google Scholar] [CrossRef]
- Mille, T.; Mahe, K.; Villanueva, M.C.; De Pontual, H.; Ernande, B. Sagittal otolith morphogenesis asymmetry in marine fishes. J. Fish Biol. 2015, 87, 646–663. [Google Scholar] [CrossRef]
- Morat, F.; Letourneur, Y.; Nérini, D.; Banaru, D.; Batjakas, I.E. Discrimination of red mullet populations (Teleostean, Mullidae) along multi-spatial and ontogenetic scales within the Mediterranean basin on the basis of otolith shape analysis. Aquat. Living Resour. 2012, 25, 27–39. [Google Scholar] [CrossRef]
- Paul, K.; Oeberst, R.; Hammer, C. Evaluation of otolith shape analysis as a tool for discriminating adults of Baltic cod stocks. J. Appl. Ichthyol. 2013, 29, 743–750. [Google Scholar] [CrossRef]
- Ozpicak, M.; Saygin, S.; Aydın, A.; Hancer, E.; Yilmaz, S.; Polat, N. Otolith shape analyses of Squalius cephalus (Linnaeus, 1758) (Actinopterygii: Cyprinidae) inhabiting four inland water bodies of the middle Black Sea region, Turkey. Iran. J. Ichthyol. 2018, 5, 293–302. [Google Scholar]
- Zhao, B.; Liu, J.; Song, J.; Cao, L.; Dou, S. Otolith shape analysis for stock discrimination of two Collichthys genus croaker (Pieces: Sciaenidae) from the northern Chinese coast. J. Oceanol. Limnol. 2018, 36, 981–989. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yazicioglu, O.; Saygin, S.; Polat, N. Relationships of otolith dimensions with body length of European perch, Perca fluviatilis L., 1758 from Lake Ladik, Turkey. Pak. J. Zool. 2014, 46, 1231–1238. [Google Scholar]
- Başusta, N.; Khan, U. Sexual dimorphism in the otolith shape of shidrum, Umbrina cirrosa (L.), in the eastern Mediterranean Sea: Fish size–otolith size relationships. J. Fish Biol. 2021, 99, 164–174. [Google Scholar] [CrossRef]
- Gagliano, M.; McCormick, M.I. Feeding history influences otolith shape in tropical fish. Mar. Ecol. Progr. Ser. 2004, 278, 291–296. [Google Scholar] [CrossRef]
- Mille, T.; Mahé, K.; Cachera, M.; Villanueva, M.; de Pontual, H.; Ernande, B. Diet is correlated with otolith shape in marine fish. Mar. Ecol. Prog. Ser. 2016, 555, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E. Photographic Atlas of Fish Otoliths of the Northwest Atlantic Ocean; NRC Research Press: Ottawa, ON, Canada, 2004. [Google Scholar] [CrossRef]
- Bounket, B.; Gibert, P.; Gennotte, V.; Argillier, C.; Carrel, G.; Maire, A.; Logez, M.; Morat, F. Otolith shape analysis and daily increment validation during ontogeny of larval and juvenile European chub Squalius cephalus. J. Fish Biol. 2019, 95, 444–452. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Nakai, I.; Tesch, W. Do all freshwater eels migrate? Nature 1998, 396, 635–636. [Google Scholar] [CrossRef]
- Limburg, K.E.; Wickstrom, H.; Svedang, H.; Elfman, M.; Kristiansson, P. Do stocked freshwater eels migrate? Evidence from the Baltic suggests “yes”. Am. Fish. Soc. Symp. 2003, 33, 275–284. [Google Scholar]
- ICES. Report of the Joint EIFAAC/ICES/GFCM Working Group on Eel (WGEEL); ICES Document CM 2015/ACOM: 18; ICES: Antalya, Turkey, 2015. [Google Scholar]
- Antunes, C.; Tesch, F.-W. A critical consideration of the metamorphosis zone when identifying daily rings in otoliths of European eel, Anguilla anguilla (L.). Ecol. Freshw. Fish 1997, 6, 102–107. [Google Scholar] [CrossRef]
- Dekker, W. Status of the European eel stock and fisheries. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 237–254. [Google Scholar]
- Bertin, L. Eels: A Biological Study; Cleaver-Hume Press: London, UK, 1956. [Google Scholar]
- Helfman, G.S.; Facey, D.E.; Hales, L.S.; Bozeman, E.L. Reproductive ecology of the American eel. Am. Fish. Soc. Symp. 1987, 1, 42–56. [Google Scholar]
- Vollestad, L.A. Geographic Variation in Age and Length at Metamorphosis of Maturing European Eel: Environmental Effects and Phenotypic Plasticity. J. Anim. Ecol. 1992, 61, 41–48. [Google Scholar] [CrossRef]
- Thillart, G.v.D.; van Ginneken, V.; Korner, F.; Heijmans, R.; van der Linden, R.; Gluvers, A. Endurance swimming of European eel. J. Fish Biol. 2004, 65, 312–318. [Google Scholar] [CrossRef]
- Edeline, E.; Dufour, S.; Elie, P. Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar. Ecol. Prog. Ser. 2005, 304, 191–199. [Google Scholar] [CrossRef]
- Edeline, E.; Lambert, P.; Rigaud, C.; Elie, P. Effects of body condition and water temperature on Anguilla anguilla glass eel migratory behavior. J. Exp. Mar. Biol. Ecol. 2006, 331, 217–225. [Google Scholar] [CrossRef]
- Tesch, F.W. The eel; Blackwell Science: Oxford, UK, 2003; p. 408. [Google Scholar]
- Daverat, F.; Beaulaton, L.; Poole, R.; Lambert, P.; Wickström, H.; Andersson, J.; Aprahamian, M.; Hizem, B.; Elie, P.; Yalçın-Özdilek, S.; et al. One century of eel growth: Changes and implications. Ecol. Fresh. Fish 2012, 21, 325–336. [Google Scholar] [CrossRef]
- Krueger, W.H.; Oliveira, K. Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ. Biol. Fishes 1982, 55, 381–389. [Google Scholar] [CrossRef]
- Jessop, B.; Shiao, J.; Iizuka, Y.; Tzeng, W. Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Mar. Ecol. Prog. Ser. 2004, 272, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Izzo, C.; Doubleday, Z.A.; Schultz, A.G.; Woodcock, S.H.; Gillanders, B.M. Contribution of water chemistry and fish condition to otolith chemistry: Comparisons across salinity environments. J. Fish Biol. 2015, 86, 1680–1698. [Google Scholar] [CrossRef]
- D’iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Begg, G.A.; Campana, S.E.; Fowler, A.J.; Suthers, I.M. Otolith research and application: Current directions in innovation and implementation. Mar. Freshw. Res. 2005, 56, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E. Otolith science entering the 21st century. Mar. Freshw. Res. 2005, 56, 485–495. [Google Scholar] [CrossRef] [Green Version]
- ICES. Workshop on Age Reading of European and American Eel (WKAREA); ICES CM 2009\ACOM 48; ICES: Bordeaux, France, 2009; p. 66. [Google Scholar]
- Capoccioni, F.; Costa, C.; Aguzzi, J.; Menesatti, P.; Lombarte, A.; Ciccotti, E. Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla anguilla, L.) local stocks. J. Exp. Mar. Biol. Ecol. 2011, 397, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Panfili, J.; Boulenger, C.; Musseau, C.; Crivelli, A.J. Extreme variability in European eel growth revealed by an extended mark and recapture experiment in southern France and implications for management. Can. J. Fish. Aquat. Sci. 2022, 79, 631–641. [Google Scholar] [CrossRef]
- Pike, C.; Crook, V.; Gollock, M. Anguilla anguilla. In The IUCN Red List of Threatened Species: e.T60344A 152845178; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- ICES. European eel (Anguilla anguilla) throughout Its Natural Range; Report; ICES Advice: Copenhagen, Denmark, 2022. [Google Scholar] [CrossRef]
- Pujolar, J.M.; Jacobsen, M.W.; Als, T.D.; Frydenberg, J.; Munch, K.; Jónsson, B.; Jian, J.B.; Cheng, L.; Maes, G.E.; Bernatchez, L.; et al. Genome-wide single-generation signatures of local selection in the panmictic European eel. Mol. Ecol. 2014, 23, 2514–2528. [Google Scholar] [CrossRef]
- Panfili, J.; Ximénès, M.-C.; Crivelli, A.J. Sources of Variation in Growth of the European Eel (Anguilla anguilla) Estimated from Otoliths. Can. J. Fish. Aquat. Sci. 1994, 51, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Melià, P.; Bevacqua, D.; Crivelli, A.J.; Panfili, J.; De Leo, G.A.; Gatto, M. Sex differentiation of the European eel in brackish and freshwater environments: A comparative analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- De Leo, G.A.; Gatto, M.; Mateo, M.; Lambert, P.; Tetard, S.; Castonguay, M.; Ernande, B.; Drouineau, H.; Rigaud, C.; Daverat, F.; et al. A size and age-structured model of the European eel (Anguilla anguilla L.). Can. J. Fish. Aquat. Sci. 1995, 52, 1351–1367. [Google Scholar] [CrossRef]
- Daverat, F.; Limburg, K.; Thibault, I.; Shiao, J.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Schmidt, J. Breeding Places and Migrations of the Eel. Nature 1923, 111, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.J.; Bonhommeau, S.; Munk, P.; Castonguay, M.; Hanel, R.; McCleave, J.D. A century of research on the larval distributions of the Atlantic eels: A re-examination of the data. Biol. Rev. 2015, 90, 1035–1064. [Google Scholar] [CrossRef]
- Chang, Y.-L.K.; Feunteun, E.; Miyazawa, Y.; Tsukamoto, K. New clues on the Atlantic eels spawning behavior and area: The Mid-Atlantic Ridge hypothesis. Sci. Rep. 2020, 10, 15981. [Google Scholar] [CrossRef]
- Trancart, T.; Tudorache, C.; Thillart, G.v.D.; Acou, A.; Carpentier, A.; Boinet, C.; Gouchet, G.; Feunteun, E. The effect of thermal shock during diel vertical migration on the energy required for oceanic migration of the European silver eel. J. Exp. Mar. Biol. Ecol. 2015, 463, 168–172. [Google Scholar] [CrossRef]
- Righton, D.; Westerberg, H.; Feunteun, E.; Økland, F.; Gargan, P.; Amilhat, E.; Metcalfe, J.; Lobon-Cervia, J.; Sjöberg, N.; Simon, J.; et al. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci. Adv. 2016, 2, e1501694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, R.M.; Piper, A.T.; Aarestrup, K.; Azevedo, J.M.N.; Cowan, G.; Don, A.; Gollock, M.; Ramallo, S.R.; Velterop, R.; Walker, A.; et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci. Rep. 2022, 12, 15362. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Dias, E.; López, R.; Antunes, C. Regional Population Structure of the European Eel at the Southern Limit of Its Distribution Revealed by Otolith Shape Signature. Fishes 2022, 7, 135. [Google Scholar] [CrossRef]
- Podda, C.; Palmas, F.; Pusceddu, A.; Sabatini, A. When the Eel Meets Dams: Larger Dams’ Long-Term Impacts on Anguilla anguilla (L., 1758). Front. Environ. Sci. 2022, 10, 876369. [Google Scholar] [CrossRef]
- De Waele, J.; Martina, M.L.; Sanna, L.; Cabras, S.; Cossu, Q.A. Flash flood hydrology in karstic terrain: Flumineddu Canyon, central-east Sardinia. Geomorphology 2010, 120, 162–173. [Google Scholar] [CrossRef]
- Sabatini, A.; Podda, C.; Frau, G.; Cani, M.V.; Musu, A.; Serra, M.; Palmas, F. Restoration of native Mediterranean brown trout Salmo cettii Rafinesque, 1810 (Actinopterygii: Salmonidae) populations using an electric barrier as a mitigation tool. Eur. Zool. J. 2018, 85, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Palmas, F.; Righi, T.; Musu, A.; Frongia, C.; Podda, C.; Serra, M.; Splendiani, A.; Barucchi, V.C.; Sabatini, A. Pug-Headedness Anomaly in a Wild and Isolated Population of Native Mediterranean Trout Salmo trutta L., 1758 Complex (Osteichthyes: Salmonidae). Diversity 2020, 12, 353. [Google Scholar] [CrossRef]
- Podda, C.; Palmas, F.; Frau, G.; Chessa, G.; Culurgioni, J.; Diciotti, R.; Fois, N.; Sabatini, A. Environmental influences on the recruitment dynamics of juvenile European eels, Anguilla anguilla, in a small estuary of the Tyrrhenian Sea, Sardinia, Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1638–1648. [Google Scholar] [CrossRef]
- AA.VV. Carta Ittica Della Sardegna–D.G.R. N. 2/28, 428. del 20/01/2022; Regione Autonoma della Sardegna (ADA/STNPF)/Università degli Studi di Cagliari (DISVA): Cagliari, Italy, 2022. [Google Scholar]
- AA.VV. Piano di Salvaguardia e Valorizzazione dei Laghi Salsi; Università degli Studi di Cagliari: Cagliari, Italy, 2010; p. 317. [Google Scholar]
- Rossi, R.; Cannas, A. Eel fishing management in a hypersaline lagoon of Southern Sardinia. Fish. Res. 1984, 2, 285–298. [Google Scholar] [CrossRef]
- Gilderhus, P.A.; Marking, L.L. Comparative efficacy of 16 anesthetic chemicals on rainbow trout. N. Am. J. Fish. Manag. 1987, 7, 288–292. [Google Scholar] [CrossRef]
- Colombo, G.; Grandidr, G. Histological study of the development and sex differentiation of the gonad in the European eel. J. Fish Biol. 1996, 48, 493–512. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Mérigot, B.; Letourneur, Y.; Lecomte-Finiger, R. Characterization of local populations of the common sole Solea solea (Pisces, Soleidae) in the NW Mediterranean through otolith morphometrics and shape analysis. Mar. Biol. 2007, 151, 997–1008. [Google Scholar] [CrossRef]
- Stransky, C.; MacLellan, E.S. Species separation and zoogeography of redfish and rockfish (genus Sebastes) by otolith shape analysis. Can. J. Fish. Aquat. Sci. 2005, 62, 2265–2276. [Google Scholar] [CrossRef]
- Morat, F.; Gibert, P.; Reynaud, N.; Testi, B.; Favriou, P.; Raymond, V.; Carrel, G.; Maire, A. Spatial distribution, total length frequencies and otolith morphometry as tools to analyse the effects of a flash flood on populations of roach (Rutilus rutilus). Ecol. Freshw. Fish 2018, 27, 421–432. [Google Scholar] [CrossRef]
- Iwata, H. Tutorial for SHAPE v.1.3. 2006, p. 21. Available online: http://lbm.ab.a.u–tokyo.ac.jp/~iwata/shape/tutorial.pdf (accessed on 20 September 2021).
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Crampton, J.S. Elliptic Fourier shape analysis of fossil bivalves: Some practical considerations. Lethaia 1995, 28, 179–186. [Google Scholar] [CrossRef]
- Ramsay, J.O.; Silveman, B.W. Functional Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2005; p. 310. [Google Scholar]
- Titus, K.; Mosher, J.A.; Williams, B.K. Chance-corrected Classification for Use in Discriminant Analysis: Ecological Applications. Am. Midl. Nat. 1984, 111, 1. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R-project.org (accessed on 28 October 2021).
- Milošević, D.; Bigović, M.; Mrdak, D.; Milašević, I.; Piria, M. Otolith morphology and microchemistry fingerprints of European eel, Anguilla anguilla (Linnaeus, 1758) stocks from the Adriatic Basin in Croatia and Montenegro. Sci. Total. Environ. 2021, 786, 147478. [Google Scholar] [CrossRef]
- Panfili, J.; Ximénès, M.C. Évaluation de l’âge et de la croissance de l’anguille européenne (Anguilla anguilla L.) en milieu continental: Méthodologies, validation, application en Méditerranée et comparaisons en Europe. Bull. Fr. Pêche Piscic. 1994, 335, 43–66. [Google Scholar] [CrossRef] [Green Version]
- Pujolar, J.M.; Maes, G.E.; Vancoillie, C.; Volckaert, F.A.M. Growth rate correlates to individual heterozygosity in the European eel, Anguilla anguilla L. Evolution 2005, 59, 189–199. [Google Scholar]
- Melia, P.; Bevacqua, D.; Crivelli, A.J.; De Leo, G.A.; Panfili, J.; Gatto, M. Age and growth of Anguilla anguilla in the Camargue lagoons. J. Fish Biol. 2006, 68, 876–890. [Google Scholar] [CrossRef]
- Boulenger, C.; Acou, A.; Trancart, T.; Crivelli, A.J.; Feunteun, E. Length-weight relationships of the silver European eel, Anguilla anguilla (Linnaeus, 1758), across its geographic range. J. Appl. Ichthyol. 2015, 31, 427–430. [Google Scholar] [CrossRef]
- Rossi, R.; Colombo, G. Sex Ratio, Age and Growth of Silver Eels, in Two Brackish Lagoons in the Northern Adriatic (Valli of Comacchio and Valle Nuova). Arch. Oceanogr. Limnol. 1976, 18, 227–310. [Google Scholar]
- Gordo, L.S.; Jorge, M.I. Age and growth of the european eel, Anguilla anguilla (Linnaeus, 1758) in the Aveiro Lagoon, Portugal. Sci. Mar. 1991, 55, 389–395. [Google Scholar]
- Aprahamian, M.W.; Walker, A.M.; Williams, B.; Bark, A.; Knights, B. On the application of models of European eel (Anguilla anguilla) production and escapement to the development of Eel Management Plans: The River Severn. ICES J. Mar. Sci. 2007, 64, 1472–1482. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, W. Biology and Management of European Eel (Anguilla anguilla, L.) in the Shannon Estuary, Ireland. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2003. [Google Scholar]
- Mann, R.H.K.; Blackburn, J.H. The biology of the eel Anguilla anguilla (L.) in an English chalk stream and interactions with juvenile trout Salmo trutta L. and salmon Salmo salar L. Hydrobiologia 1991, 218, 65–76. [Google Scholar] [CrossRef]
- Rasmussen, G.; Therkildsen, B. Food, Growth and Production of Anguilla anguilla in a Small Danish Stream. Rapp. Procès-Verbaux Réun. Cons. Int. Pour Explor. Mer. 1979, 174, 32–40. [Google Scholar]
- Moriarty, C. Age determination and growth rate of eels, Anguilla anguilla (L). J. Fish Biol. 1983, 23, 257–264. [Google Scholar] [CrossRef]
- Vøllestad, L.A.; Jonsson, B. Life-History Characteristics of the European Eel Anguilla anguilla in the Imsa River, Norway. Trans. Am. Fish. Soc. 1986, 115, 864–871. [Google Scholar] [CrossRef]
- Daverat, F.; Tomás, J. Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar. Ecol. Prog. Ser. 2006, 307, 247–257. [Google Scholar] [CrossRef]
- Simon, J.; Ubl, C.; Dorow, M. Growth of European eel Anguilla anguilla along the southern Baltic coast of Germany and implication for the eel management. Environ. Biol. Fishes 2013, 96, 1073–1086. [Google Scholar] [CrossRef]
- Patey, G.; Couillard, C.M.; Drouineau, H.; Verreault, G.; Pierron, F.; Lambert, P.; Baudrimont, M.; Couture, P. Early back-calculated size-at-age of Atlantic yellow eels sampled along ecological gradients in the Gironde and St. Lawrence hydrographical systems. Can. J. Fish. Aquat. Sci. 2018, 75, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E.; Neilson, J.D. Microstructure of Fish Otoliths. Can. J. Fish. Aquat. Sci. 1985, 42, 1014–1032. [Google Scholar] [CrossRef]
- Wilson, J.R.R. Depth-related changes in sagitta morphology in six macrourid fishes of the Pacific and Atlantic Oceans. Copeia 1985, 4, 1011–1017. [Google Scholar] [CrossRef]
- Aguirre, H.; Lombarte, A. Ecomorphological comparisons of sagittae in Mullus barbatus and M. surmuletus. J. Fish Biol. 1999, 55, 105–114. [Google Scholar] [CrossRef]
- Morales-Nin, B. Influence of environmental factors on microstructure of otoliths of three demersal fish species caught off Namibia. S. Afr. J. Mar. Sci. 1987, 5, 255–262. [Google Scholar] [CrossRef]
- Gonzalez-Salas, C.; Lenfant, P. Interannual variability and intraannual stability of the otolith shape in European anchovy Engraulis encrasicolus (L.) in the Bay of Biscay. J. Fish Biol. 2007, 70, 35–49. [Google Scholar] [CrossRef]
- Schiavina, M.; Bevacqua, D.; Melià, P.; Crivelli, A.J.; Gatto, M.; De Leo, G.A. A user-friendly tool to assess management plans for European eel fishery and conservation. Environ. Model. Softw. 2015, 64, 9–17. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A demographic model for the conservation and management of the European eel: An application to a Mediterranean coastal lagoon. ICES J. Mar. Sci. 2019, 76, 2164–2178. [Google Scholar] [CrossRef]
- Whigham, D.F.; Baldwin, A.H.; Barendregt, A. Chapter 18—Tidal Freshwater Wetlands. In Coastal Wetlands, 2nd ed.; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 619–640. [Google Scholar] [CrossRef]
- Podda, C.; Sabatini, A.; Palmas, F.; Pusceddu, A. Hard times for catadromous fish: The case of the European eel (Anguilla anguilla, L. 1758). Adv. Oceanogr. Limnol. 2021, 12, 9997. [Google Scholar] [CrossRef]
- Porceddu, R.; Podda, C.; Mulas, G.; Palmas, F.; Picci, L.; Scano, C.; Spiga, S.; Sabatini, A. Changes in Dendritic Spine Morphology and Density of Granule Cells in the Olfactory Bulb of Anguilla anguilla (L., 1758): A Possible Way to Understand Orientation and Migratory Behavior. Biology 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
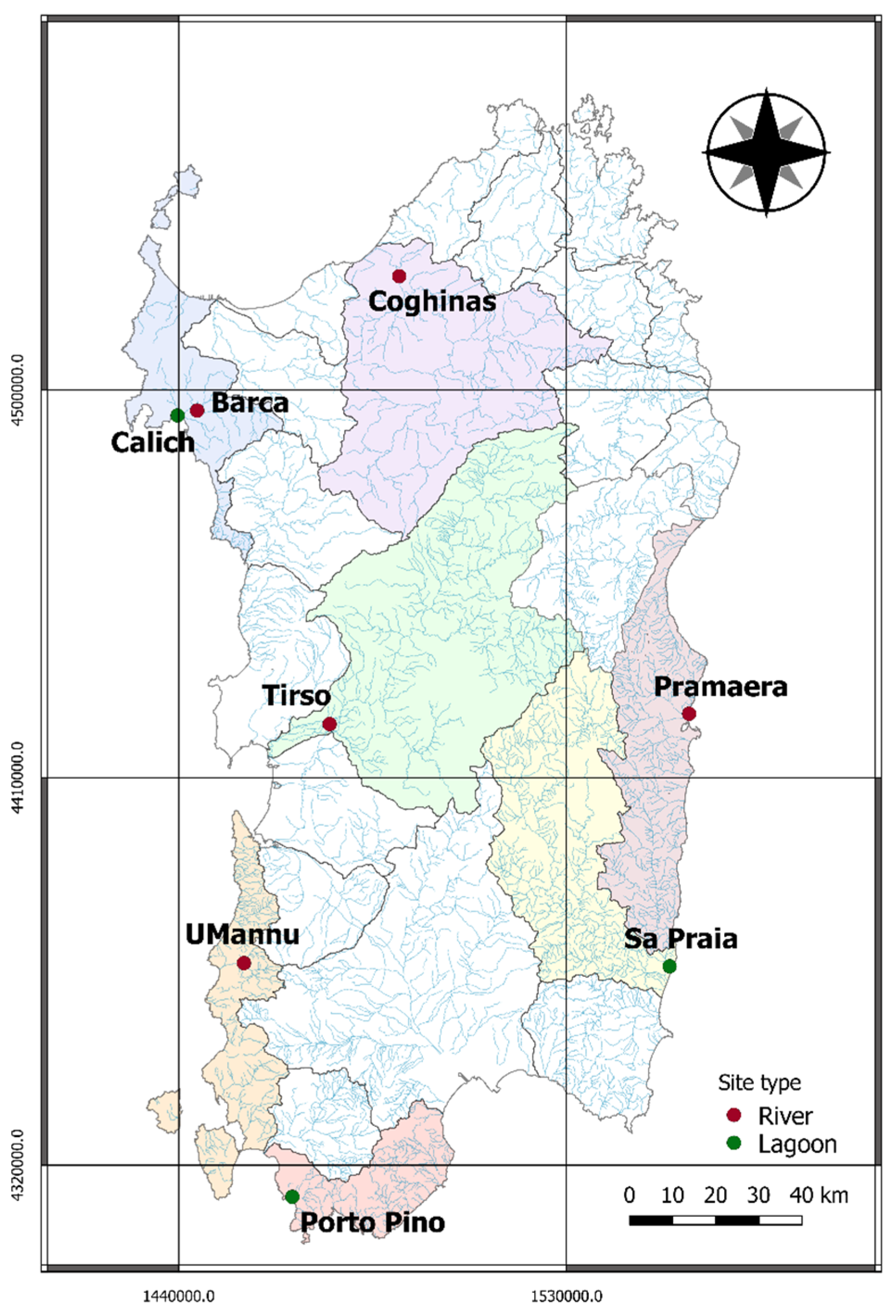


| Site | River (R) Lagoon (L) | Regime (Only R) | Area (km2) | Dams (Yes/No) (Only R) 1 | Number of Eels |
|---|---|---|---|---|---|
| Pramaera | R | Perennial | 184 * | no | 26 |
| Tirso | R | Perennial | 2043 * | yes | 13 |
| Coghinas | R | Perennial | 1836 * | yes | 10 |
| Barca | R | Intermittent | 355 * | yes | 10 |
| UMannu (Mannu di Fluminumaggiore) | R | Intermittent | 126 * | no | 11 |
| Calich | L | 0.9 | 10 | ||
| Porto Pino | L | 0.5 | 10 | ||
| Sa Praia | L | 0.86 | 10 |
| Females | Males | Undifferentiated | ||||
|---|---|---|---|---|---|---|
| Site | TL (cm) | TW (g) | TL (cm) | TW (g) | TL (cm) | TW (g) |
| Pramaera | 49.50–65.00 | 190.00–497.70 | 31.10–41.90 | 50.80–152.20 | 6.80–31.60 | 0.21–51.00 |
| Tirso | / | / | / | / | 16.30–32.30 | 6.70–71.90 |
| UMannu | / | / | 26.40–34.20 | 20.0–58.40 | / | / |
| Barca | 77.20 | 945.65 | 29.30–43.20 | 43.78–143.50 | 28.00 | 31.00 |
| Coghinas | / | / | / | / | 11.00–20.50 | 1.22–10.66 |
| Calich | 47.30–56.00 | 229.97–450.51 | 33.40–38.40 | 68.70–101.95 | 27.50–30.60 | 40.02–50.40 |
| Porto Pino | 26.50–75.50 | 24.50–636.80 | / | / | / | / |
| Sa Praia | 56.00–56.50 | 326.30–342.80 | 32.00–39.50 | 51.18–104.40 | 32.1 | 43.58 |
| Site | Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia |
|---|---|---|---|---|---|---|---|---|
| Tirso | 0.21 | |||||||
| UMannu | 0.76 | 0.86 | ||||||
| Barca | 1.29 | 1.47 | 0.78 | |||||
| Coghinas | 2.52 | 2.59 | 3.16 | 3.18 | ||||
| Calich | 2.14 | 2.34 | 1.97 | 1.28 | 2.84 | |||
| Porto Pino | 1.98 | 2.18 | 1.93 | 1.33 | 2.52 | 0.32 | ||
| Sa Praia | 2.06 | 2.26 | 2.05 | 1.47 | 2.42 | 0.43 | 0.16 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.767 ± 0.063 | ns | ns | ns | ns | ns | * | ns |
| Tirso | 1 | 0.777 ± 0.044 | ns | ns | ns | ns | * | ns |
| UMannu | 1 | 1 | 0.794 ± 0.041 | ns | ns | ns | ns | ns |
| Barca | 1 | 1 | 1 | 0.763 ± 0.067 | ns | ns | ns | ns |
| Coghinas | 1 | 1 | 1 | 1 | 0.794 ± 0.038 | * | * | ns |
| Calich | 0.14 | 0.08 | 0.19 | 1 | 0.04 | 0.704 ± 0.055 | ns | ns |
| Porto Pino | 0.03 | 0.02 | 0.06 | 0.90 | 0.01 | 1 | 0.695 ± 0.055 | ns |
| Sa Praia | 0.44 | 0.24 | 0.49 | 1 | 0.14 | 1 | 1 | 0.720 ± 0.071 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.642 ± 0.073 | ns | ns | ns | ns | ns | * | * |
| Tirso | 1 | 0.660 ± 0.032 | ns | ns | ns | * | * | ** |
| UMannu | 1 | 1 | 0.639 ± 0.040 | ns | ns | ns | ns | ns |
| Barca | 1 | 1 | 1 | 0.616 ± 0.051 | ns | ns | ns | ns |
| Coghinas | 1 | 1 | 1 | 0.30 | 0.705 ± 0.035 | ** | *** | *** |
| Calich | 0.07 | 0.03 | 0.39 | 1 | 0.002 | 0.580 ± 0.034 | ns | ns |
| Porto Pino | 0.03 | 0.01 | 0.19 | 1 | <0.001 | 1 | 0.573 ± 0.015 | ns |
| Sa Praia | 0.01 | 0.007 | 0.12 | 1 | <0.001 | 1 | 1 | 0.574 ± 0.041 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.744 ± 0.018 | ns | ns | ns | ns | ns | ns | ns |
| Tirso | 1 | 0.742 ± 0.012 | ns | ns | ns | ns | ns | ns |
| UMannu | 1 | 1 | 0.741 ± 0.012 | ns | ns | ns | ns | ns |
| Barca | 1 | 1 | 1 | 0.745 ± 0.016 | ns | ns | ns | ns |
| Coghinas | 1 | 1 | 1 | 1 | 0.739 ± 0.013 | ns | ns | ns |
| Calich | 1 | 1 | 1 | 1 | 1 | 0.733 ± 0.024 | ns | ns |
| Porto Pino | 1 | 1 | 1 | 1 | 1 | 1 | 0.745 ± 0.044 | ns |
| Sa Praia | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.750 ± 0.021 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.326 ± 0.088 | ns | ns | ns | ns | ns | ns | ns |
| Tirso | 1 | 0.347 ± 0.069 | ns | ns | ns | ns | ns | ns |
| UMannu | 1 | 1 | 0.298 ± 0.061 | ns | ns | ns | ns | * |
| Barca | 0.45 | 1 | 0.15 | 0.424 ± 0.060 | ns | ns | ns | ns |
| Coghinas | 1 | 1 | 1 | 1 | 0.347 ± 0.68 | ns | ns | ns |
| Calich | 1 | 1 | 0.73 | 1 | 1 | 0.414 ± 0.166 | ns | ns |
| Porto Pino | 0.39 | 1 | 0.07 | 1 | 1 | 1 | 0.439 ± 0.116 | ns |
| Sa Praia | 0.07 | 0.61 | 0.03 | 1 | 1 | 1 | 1 | 0.460 ± 0.086 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.496 ± 0.126 | ns | ns | ns | ns | ns | ns | ns |
| Tirso | 1 | 0.469 ± 0.099 | ns | ns | ns | ns | ns | ns |
| UMannu | 1 | 1 | 0.390 ± 0.184 | ns | ns | * | ns | ns |
| Barca | 1 | 1 | 0.49 | 0.549 ± 0.088 | ns | ns | ns | ns |
| Coghinas | 1 | 1 | 1 | 1 | 0.471 ± 0.088 | ns | ns | ns |
| Calich | 1 | 0.36 | 0.04 | 1 | 0.89 | 0.694 ± 0.223 | ns | ns |
| Porto Pino | 1 | 1 | 0.31 | 1 | 1 | 1 | 0.556 ± 0.145 | ns |
| Sa Praia | 1 | 0.51 | 0.07 | 1 | 1 | 1 | 1 | 0.587 ± 0.188 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 0.693 ± 0.154 | ** | * | ns | ns | ns | ns | ns |
| Tirso | 0.007 | 0.476 ± 0.095 | ns | ns | ns | ns | ns | ns |
| UMannu | 0.01 | 1 | 0.421 ± 0.180 | ns | ns | ns | ns | ns |
| Barca | 1 | 1 | 1 | 0.581 ± 0.100 | ns | ns | ns | ns |
| Coghinas | 0.10 | 1 | 1 | 1 | 0.488 ± 0.094 | ns | ns | ns |
| Calich | 1 | 0.08 | 0.13 | 1 | 0.46 | 0.729 ± 0.234 | ns | ns |
| Porto Pino | 1 | 0.45 | 0.66 | 1 | 1 | 1 | 0.605 ± 0.140 | ns |
| Sa Praia | 1 | 0.52 | 0.75 | 1 | 1 | 1 | 1 | 0.546 ± 0.168 |
| Pramaera | Tirso | UMannu | Barca | Coghinas | Calich | Porto Pino | Sa Praia | |
|---|---|---|---|---|---|---|---|---|
| Pramaera | 6.00 ± 3.50 | * | ns | ns | **** | ns | ns | ns |
| Tirso | 0.01 | 3.58 ± 0.84 | ns | ns | ns | **** | ** | ns |
| UMannu | 0.33 | 1 | 3.70 ± 1.33 | ns | ns | ** | ns | ns |
| Barca | 1 | 0.87 | 1 | 5.29 ± 1.03 | * | ns | ns | ns |
| Coghinas | <0.0001 | 1 | 1 | 0.03 | 7.60 ± 2.86 | **** | **** | *** |
| Calich | 0.72 | <0.0001 | 0.002 | 0.31 | <0.0001 | 9.15 ± 1.77 | ns | ns |
| Porto Pino | 1 | 0.002 | 0.57 | 1 | <0.0001 | 1 | 6.81 ± 2.16 | ns |
| Sa Praia | 1 | 0.57 | 0.65 | 1 | <0.001 | 1 | 1 | 5.90 ± 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podda, C.; Culurgioni, J.; Diciotti, R.; Palmas, F.; Amilhat, E.; Faliex, E.; Morat, F.; Fois, N.; Sabatini, A. Exploring European Eel Anguilla anguilla (L.) Habitat Differences Using Otolith Analysis in Central-Western Mediterranean Rivers and Coastal Lagoons from Sardinia. Fishes 2023, 8, 386. https://doi.org/10.3390/fishes8080386
Podda C, Culurgioni J, Diciotti R, Palmas F, Amilhat E, Faliex E, Morat F, Fois N, Sabatini A. Exploring European Eel Anguilla anguilla (L.) Habitat Differences Using Otolith Analysis in Central-Western Mediterranean Rivers and Coastal Lagoons from Sardinia. Fishes. 2023; 8(8):386. https://doi.org/10.3390/fishes8080386
Chicago/Turabian StylePodda, Cinzia, Jacopo Culurgioni, Riccardo Diciotti, Francesco Palmas, Elsa Amilhat, Elisabeth Faliex, Fabien Morat, Nicola Fois, and Andrea Sabatini. 2023. "Exploring European Eel Anguilla anguilla (L.) Habitat Differences Using Otolith Analysis in Central-Western Mediterranean Rivers and Coastal Lagoons from Sardinia" Fishes 8, no. 8: 386. https://doi.org/10.3390/fishes8080386
APA StylePodda, C., Culurgioni, J., Diciotti, R., Palmas, F., Amilhat, E., Faliex, E., Morat, F., Fois, N., & Sabatini, A. (2023). Exploring European Eel Anguilla anguilla (L.) Habitat Differences Using Otolith Analysis in Central-Western Mediterranean Rivers and Coastal Lagoons from Sardinia. Fishes, 8(8), 386. https://doi.org/10.3390/fishes8080386







