Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Biometrical Parameters
2.3. Blood Collection and 11KT Plasma Measurements
2.4. Tissue Indices and Ultrasound
2.5. Histology
2.6. RT-PCR
2.7. Reproduction
2.8. Data Analysis
3. Results
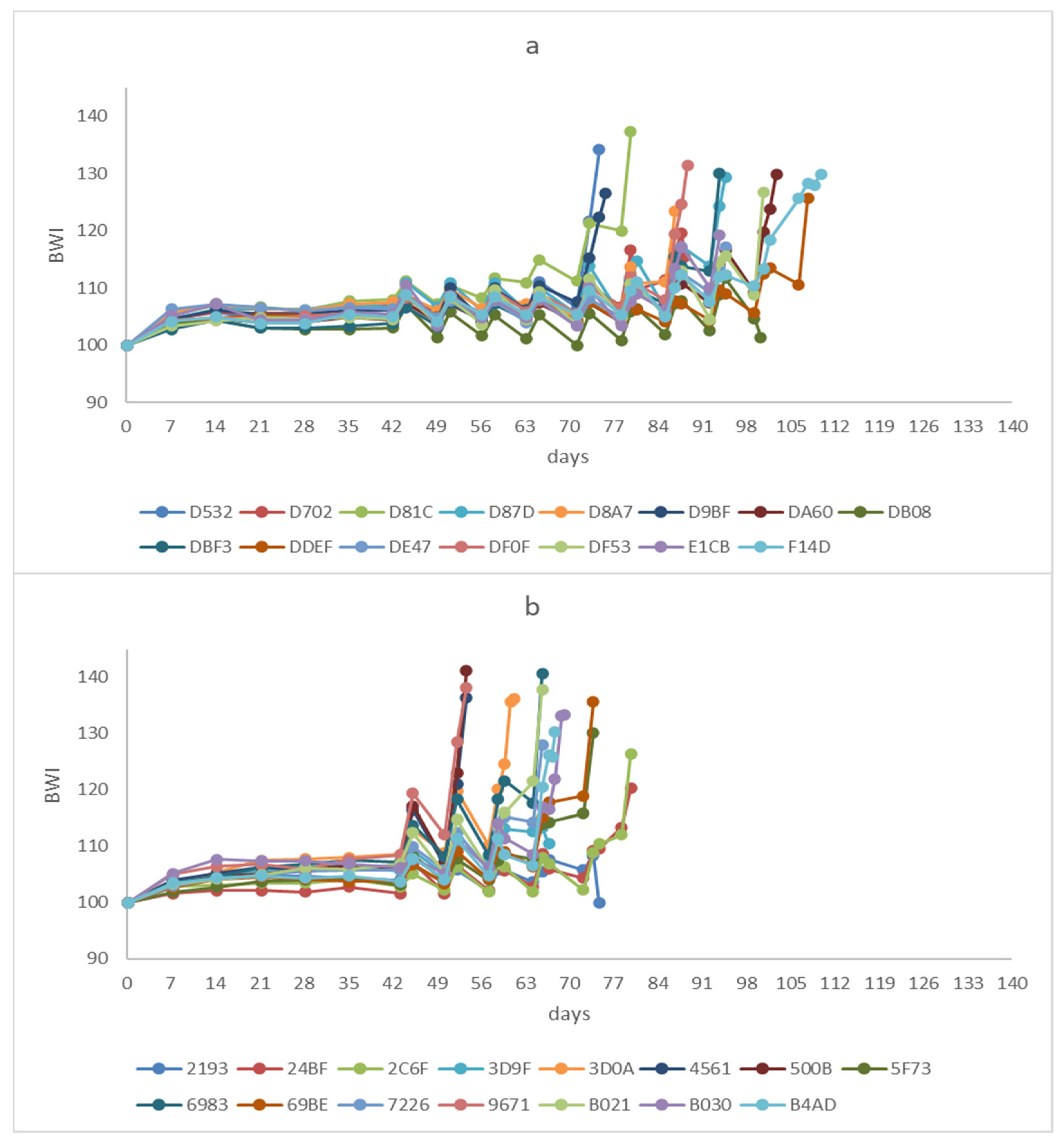
3.1. Simulated Migration
3.2. Portrait Pictures and Biometry
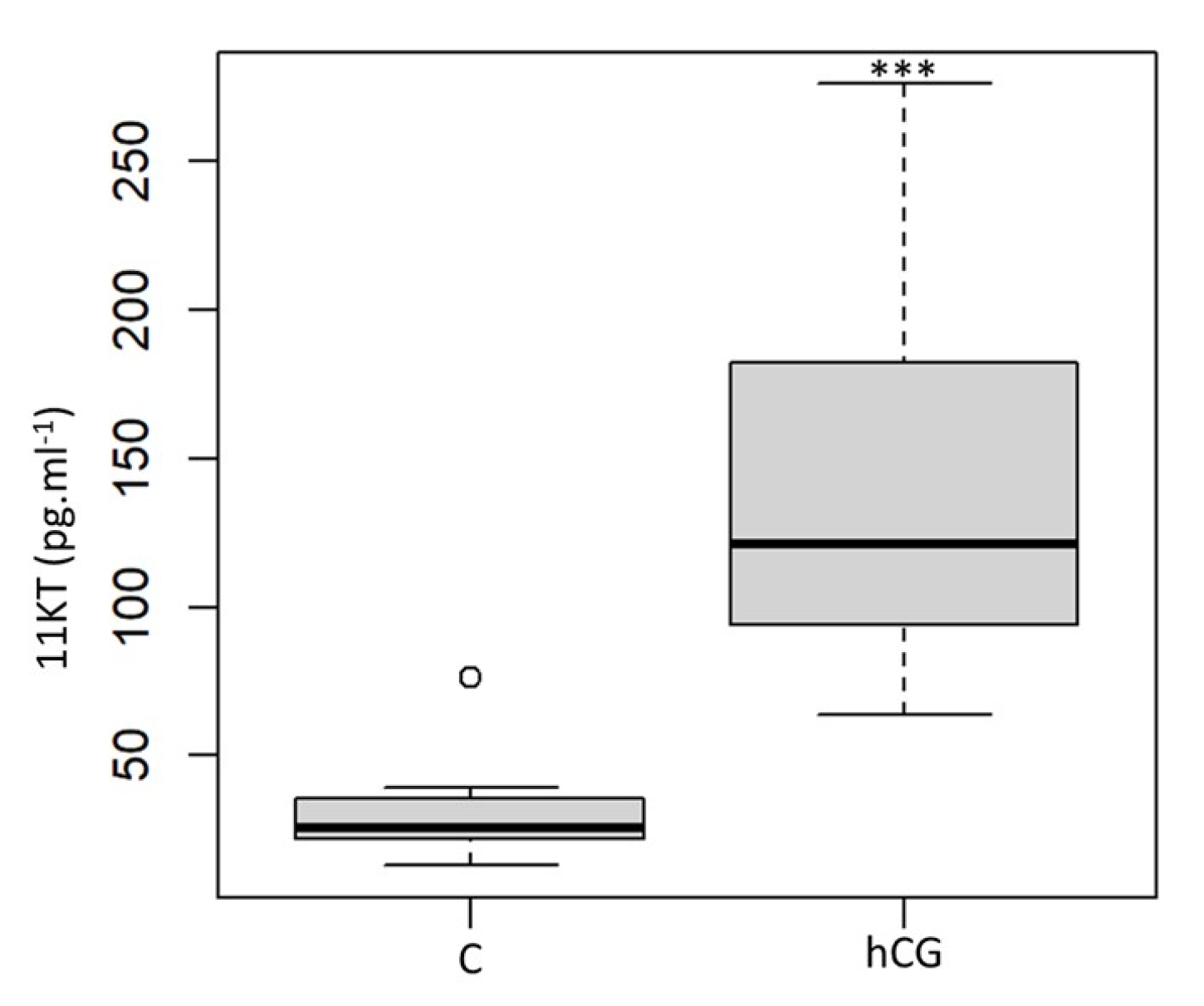
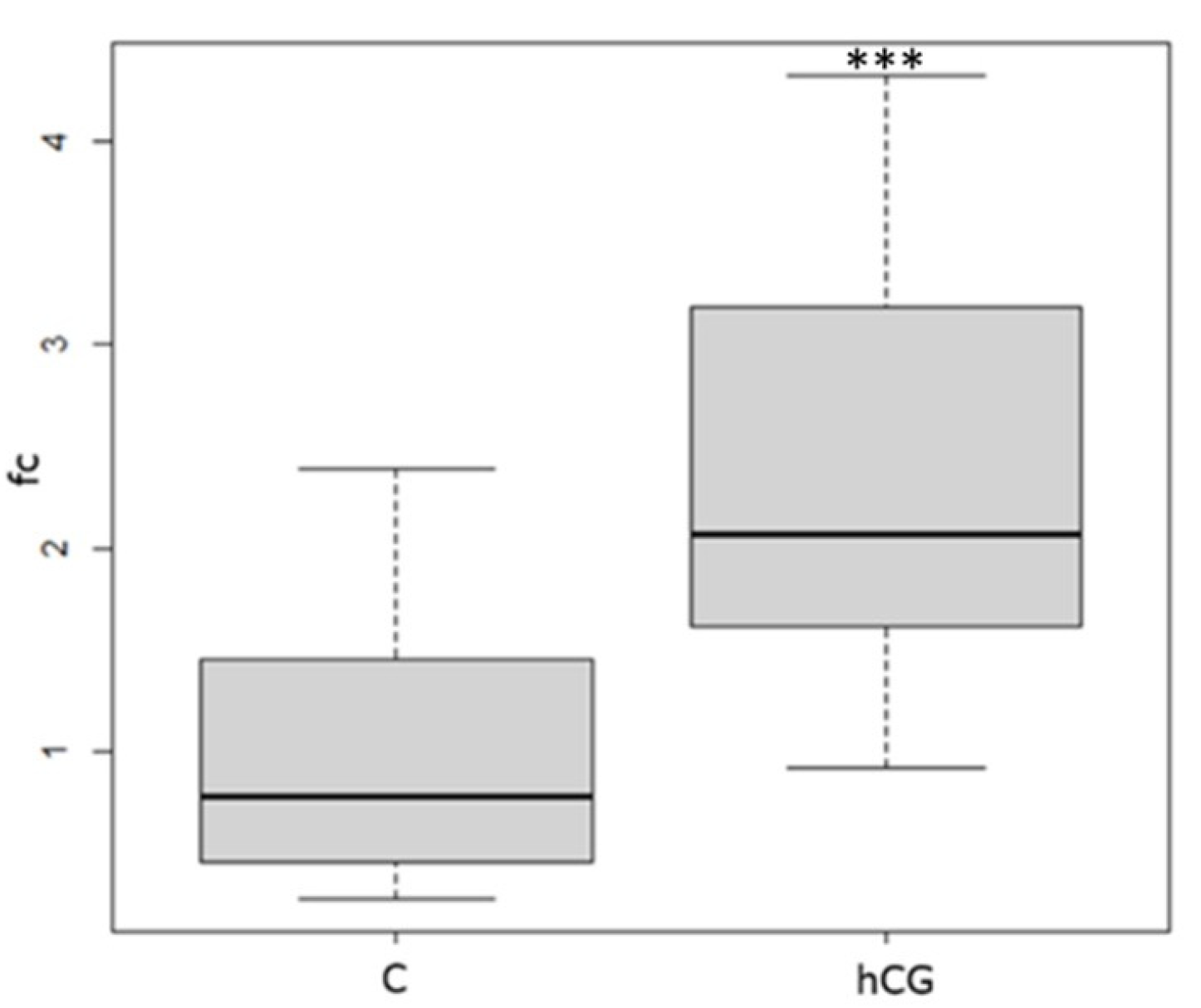
3.3. Blood Plasma 11-Ketotestosterone
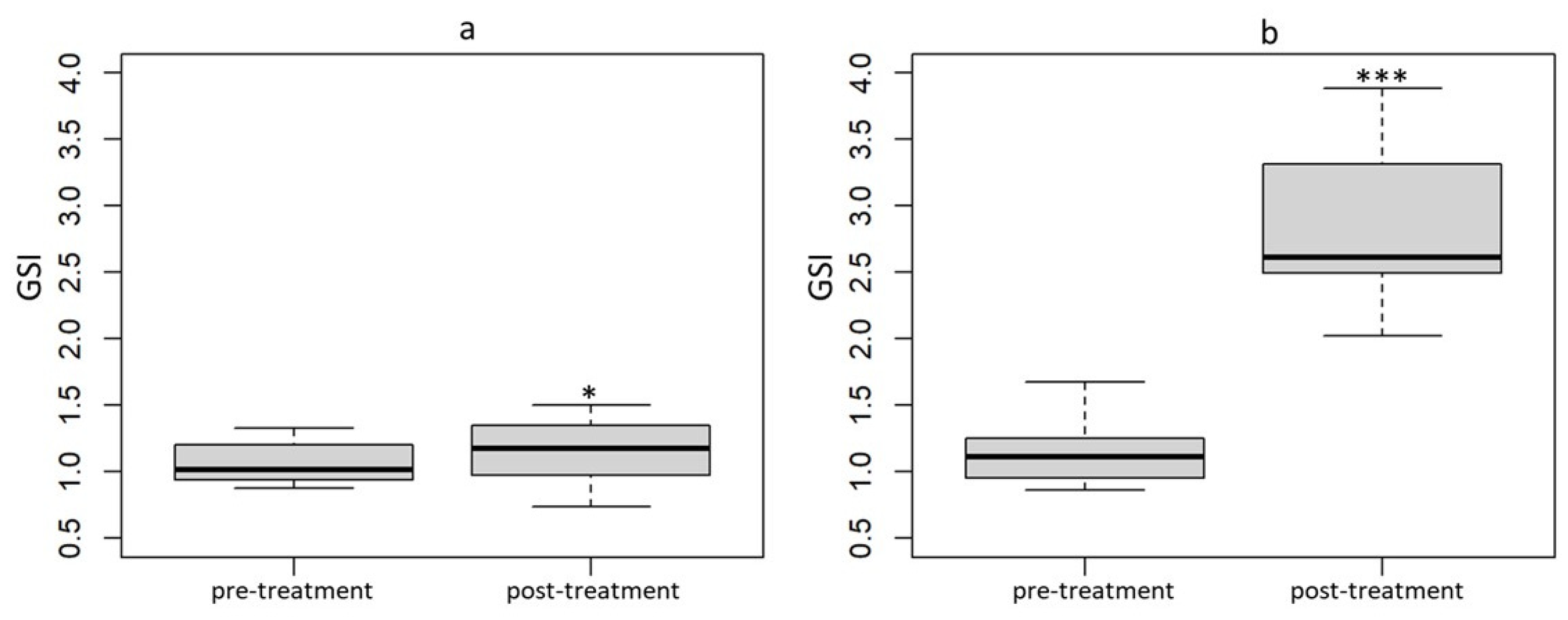
3.4. Ultrasound and Dissection
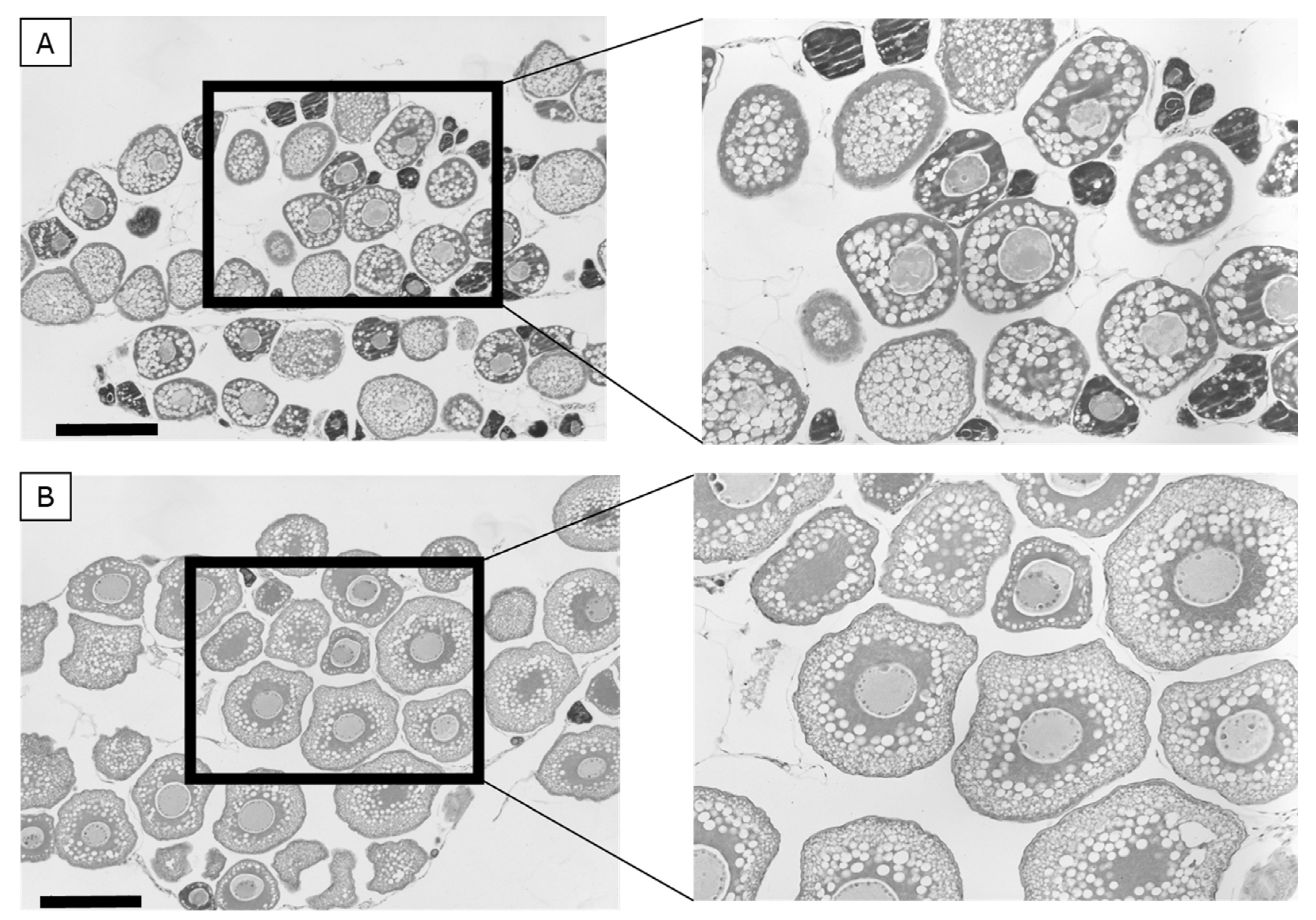
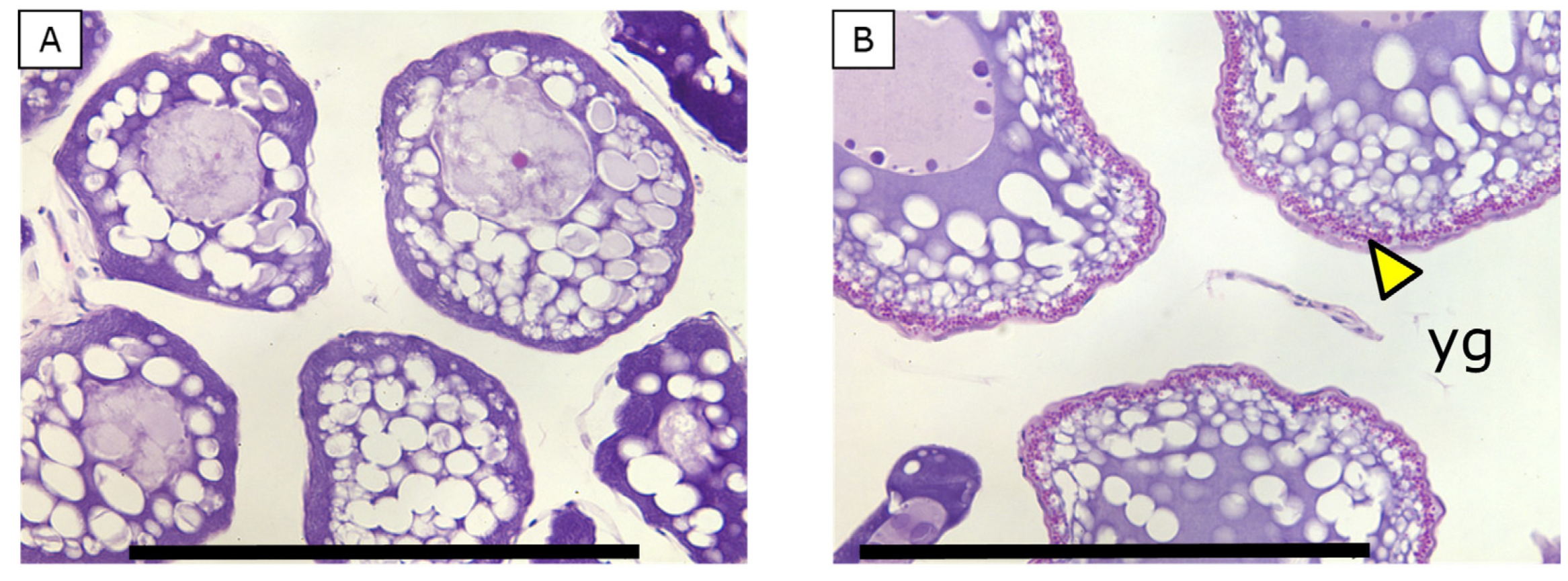
3.5. Oocyte Histology
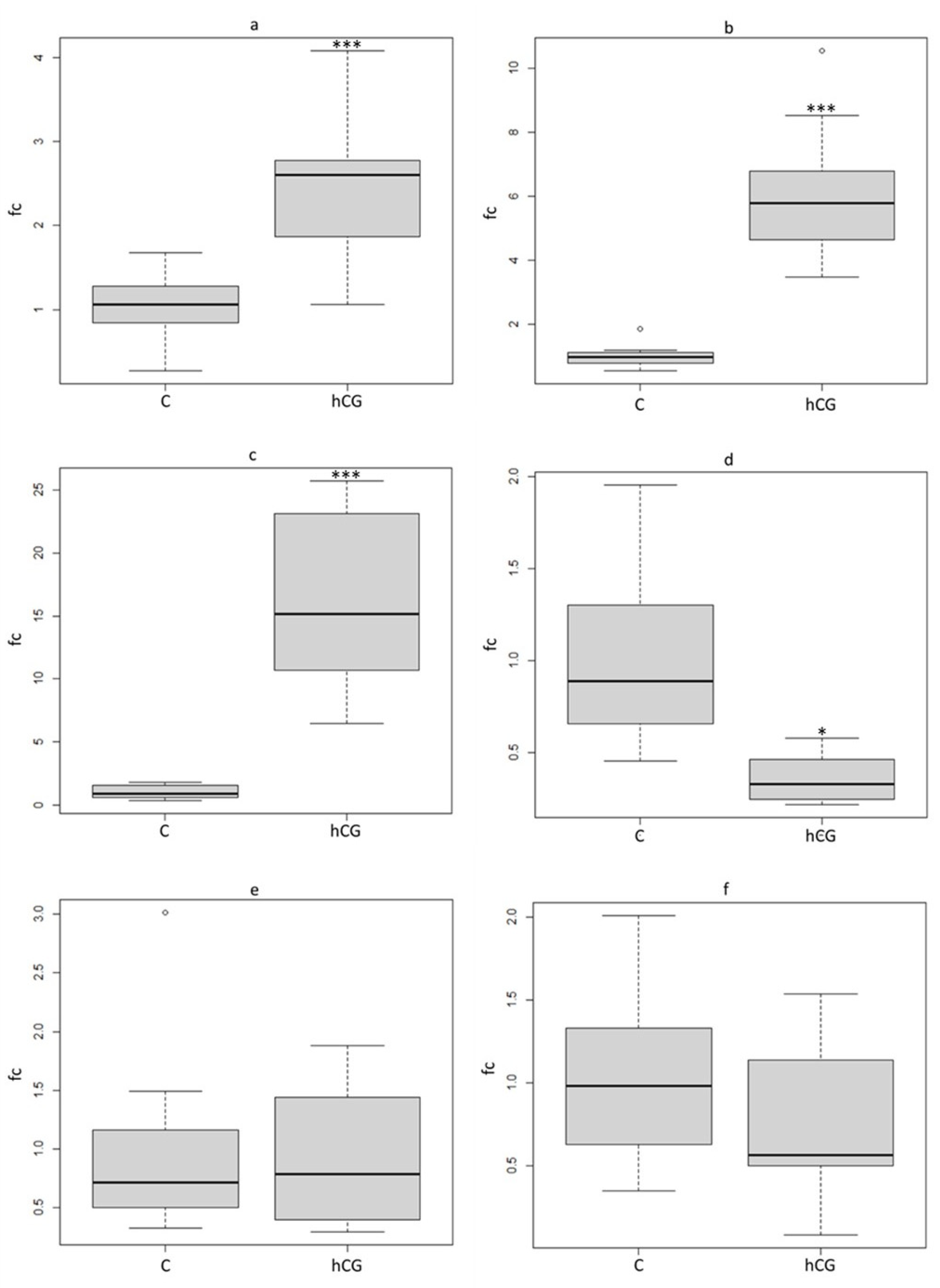
3.6. RT-PCR
3.7. Reproduction Parameters
4. Discussion
4.1. hCG Induction of Silvering
4.2. hCG Induction of Vitellogenesis
4.3. hCG Effects on Reproductive Success
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazeto, Y.; Suzuki, H.; Ozaki, Y.; Gen, K. C-terminal peptide (hCTP) of human chorionic gonadotropin enhances in vivo biological activity of recombinant Japanese eel follicle-stimulating hormone and luteinizing hormone produced in FreeStyle 293-F cell lines. Gen. Comp. Endocrinol. 2021, 306, 113731. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.D.; Wierman, M.E.; Shupnik, M.A.; Chin, W.W. Molecular biology of the pituitary gonadotropins. Endocr. Rev. 1990, 11, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Molés, G.; Hausken, K.; Carrillo, M.; Zanuy, S.; Levavi-Sivan, B.; Gómez, A. Generation and use of recombinant gonadotropins in fish. Gen. Comp. Endocrinol. 2020, 299, 113555. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.; Dickey, J.T.; Campbell, B. Biochemistry and physiology of fish gonadotropins. Fish. Physiol. Biochem. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Aizen, J.; Kowalsman, N.; Kobayashi, M.; Hollander, L.; Sohn, Y.C.; Yoshizaki, G.; Niv, M.Y.; Levavi-Sivan, B. Experimental and computational study of inter- and intra- species specificity of gonadotropins for various gonadotropin receptors. Mol. Cell. Endocrinol. 2012, 364, 89–100. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Tosaka, R.; Gen, K.; Yokoyama, M.; Miura, C.; Miura, T.; Adachi, S.; Yamauchi, K. Molecular characterization and gene expression of Japanese eel (Anguilla japonica) gonadotropin receptors. Zool. Sci. 2012, 29, 204–211. [Google Scholar] [CrossRef]
- Minegishi, Y.; Dirks, R.P.; de Wijze, D.L.; Brittijn, S.A.; Burgerhout, E.; Spaink, H.P.; van den Thillart, G.E.E.J.M. Quantitative bioassays for measuring biologically functional gonadotropins based on eel gonadotropic receptors. Gen. Comp. Endocrinol. 2012, 178, 145–152. [Google Scholar] [CrossRef]
- Lokman, P.M.; Wylie, M.J.; Downes, M.; Di Biase, A.; Damsteegt, E.L. Artificial induction of maturation in female silver eels, Anguilla australis: The benefits of androgen pre-treatment. Aquaculture 2015, 437, 111–119. [Google Scholar] [CrossRef]
- Aroua, S.; Schmitz, M.; Baloche, S.; Vidal, B.; Rousseau, K.; Dufour, S. Endocrine evidence that silvering, a secondary metamorphosis in the eel, is a pubertal rather than a metamorphic event. Neuroendocrinology 2005, 82, 221–232. [Google Scholar] [CrossRef]
- Divers, S.L.; McQuillan, H.J.; Matsubara, H.; Todo, T.; Lokman, P.M. Effects of reproductive stage and 11-ketotestosterone on LPL mRNA levels in the ovary of the shortfinned eel. J. Lipid Res. 2010, 51, 3250–3258. [Google Scholar] [CrossRef]
- Rainuzzo, J.R.; Reitan, K.I.; Olsen, Y. The significance of lipids at early stages of marine fish: A review. Aquaculture 1997, 155, 103–115. [Google Scholar] [CrossRef]
- Asanuma, H.; Ohashi, H.; Matsubara, H.; Ijiri, S.; Matsubara, T.; Todo, T.; Adachi, S.; Yamauchi, K. 11-ketotestosterone potentiates estrogen-induced vitellogenin production in liver of Japanese eel (Anguilla japonica). Fish. Physiol. Biochem. 2003, 28, 383–384. [Google Scholar] [CrossRef]
- Montserrat, N.; González, A.; Méndez, E.; Piferrer, F.; Planas, J.V. Effects of follicle stimulating hormone on estradiol-17β production and P-450 aromatase (CYP19) activity and mRNA expression in brown trout vitellogenic ovarian follicles in vitro. Gen. Comp. Endocrinol. 2004, 137, 123–131. [Google Scholar] [CrossRef]
- Lafont, A.-G.; Rousseau, K.; Tomkiewicz, J.; Dufour, S. Three nuclear and two membrane estrogen receptors in basal teleosts, Anguilla sp.: Identification, evolutionary history and differential expression regulation. Gen. Comp. Endocrinol. 2016, 235, 177–191. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yoshikuni, M.; Yamashita, M.; Tokumoto, T.; Katsu, Y. 4 Regulation of oocyte growth and maturation in fish. Cur Top. Dev. Biol. 1995, 30, 103–145. [Google Scholar] [CrossRef]
- Wallace, R.A. Vitellogenesis and oocyte growth in nonmammalian vertebrates. In Oogenesis; Springer: Boston, MA, USA, 1985; pp. 127–177. [Google Scholar] [CrossRef]
- Sire, M.-F.; Babin, P.J.; Vernier, J.-M. Involvement of the lysosomal system in yolk protein deposit and degradation during vitellogenesis and embryonic development in trout. J. Exp. Zool. 1994, 269, 69–83. [Google Scholar] [CrossRef]
- Jéhannet, P.; Palstra, A.P.; Giménez Nebot, I.; Swinkels, W.; Heinsbroek, L.T.N.; Komen, H. Recombinant FSH and LH treatment to induce oocyte development in vitro and in vivo in the European eel Anguilla anguilla. Fishes 2023, 8, 123. [Google Scholar] [CrossRef]
- Brydges, N.M.; Boulcott, P.; Ellis, T.; Braithwaite, V.A. Quantifying stress responses induced by different handling methods in three species of fish. Appl. Anim. Behav. Sci. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. In Reproductive Biotechnology in Finfish Aquaculture, 1st ed.; Lee, C.-S., Donaldson, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3–24. [Google Scholar] [CrossRef]
- Adachi, S.; Ijiri, S.; Kazeto, Y.; Yamauchi, K. Oogenesis in the Japanese eel Anguilla japonica. In Eel Biology, 1st ed.; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 502–518. [Google Scholar]
- Palstra, A.P.; Bouwman, L.J.; Jéhannet, P.; Kruijt, L.; Schipper, H.; Blokland, M.H.; Swinkels, W.; Heinsbroek, L.T.N.; Lokman, P.M. Steroid implants for the induction of vitellogenesis in feminized European silver eels (Anguilla anguilla L.). Front. Genet. 2022, 13, 969202. [Google Scholar] [CrossRef]
- Fontaine, M. Sur la maturation complète des organes génitaux de l’anguille mâle et l’émission spontanée de ses produits sexuels. CR Acad. Sci. Paris 1936, 202, 55. [Google Scholar]
- Furuhashi, M.; Shikone, T.; Fares, F.A.; Sugahara, T.; Hsueh, A.J.; Boime, I. Fusing the carboxy-terminal peptide of the chorionic gonadotropin (CG) beta-subunit to the common alpha-subunit: Retention of O-linked glycosylation and enhanced in vivo bioactivity of chimeric human CG. Mol. Endocrinol. 1995, 9, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Chia, J.H.Z.; Kazeto, Y.; Wylie, M.J.; Lokman, P.M. Induction of oocyte development in previtellogenic eel, Anguilla australis. Gen. Comp. Endocrinol. 2020, 291, 113404. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Tosaka, R.; Sago, K.; Hatanaka, R.; Ijiri, S.; Adachi, S. The relationship between the developmental stage of oocytes in various seasons and the quality of the egg obtained by artificial maturation in the feminized Japanese eel Anguilla japonica. Aquac. Sci. 2010, 58, 269–278. [Google Scholar] [CrossRef]
- Böhm, T.; Graziano, M.; Blom, E.; Brittijn, S.A.; Dirks, R.P.; Palstra, A.P. Simulated migration of feminized eels to stimulate and predict the sexual maturation response. In Proceedings of the ICBF2016, San Marcos, TX, USA, 12–18 June 2016. [Google Scholar]
- Ijiri, S.; Tsukamoto, K.; Chow, S.; Kurogi, H.; Adachi, S.; Tanaka, H. Controlled reproduction in the Japanese eel (Anguilla japonica), past and present. Aquac. Eur. 2011, 36, 13–17. [Google Scholar]
- Mes, D.; Dirks, R.P.; Palstra, A.P. Simulated migration under mimicked photothermal conditions enhances sexual maturation of farmed European eel (Anguilla anguilla). Aquaculture 2016, 452, 367–372. [Google Scholar] [CrossRef]
- Fulton, T.W. Rate of Growth of Sea Fishes; Neill & Company: Edinburg, UK, 1902. [Google Scholar]
- Palstra, A.; van den Thillart, G. Artificial maturation and reproduction of European eel. In Spawning Migration of European eel. Reproduction Index, a Useful Tool for Conservation Management, 1st ed.; van den Thillart, G., Dufour, S., Rankin, C., Eds.; Springer: Heidelberg, Germany, 2009; pp. 309–331. [Google Scholar]
- Pankhurst, N.W. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish. Biol. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Bureau du Colombier, S.; Jacobs, L.; Gesset, C.; Elie, P.; Lambert, P. Ultrasonography as a non-invasive tool for sex determination and maturation monitoring in silver eels. Fish. Res. 2015, 164, 50–58. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jéhannet, P.; Kruijt, L.; Damsteegt, E.L.; Swinkels, W.; Heinsbroek, L.T.N.; Lokman, P.M.; Palstra, A.P. A mechanistic model for studying the initiation of anguillid vitellogenesis by comparing the European eel (Anguilla anguilla) and the shortfinned eel (A. australis). Gen. Comp. Endocrinol. 2019, 279, 129–138. [Google Scholar] [CrossRef]
- Maugars, G.; Dufour, S. Demonstration of the coexistence of duplicated LH receptors in teleosts, and their origin in ancestral actinopterygians. PLoS ONE 2015, 10, e0135184. [Google Scholar] [CrossRef]
- Setiawan, A.N.; Lokman, P.M. The use of reference gene selection programs to study the silvering transformation in a freshwater eel Anguilla australis: A cautionary tale. BMC Mol. Biol. 2010, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Zadmajid, V.; Falahatimarvast, A.; Damsteegt, E.L.; Setiawan, A.N.; Ozaki, Y.; Shoae, A.; Lokman, P.M. Effects of 11-ketotestosterone and temperature on inhibin subunit mRNA levels in the ovary of the shortfinned eel, Anguilla australis. Comp. Bioch Phys. B 2015, 187, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, H.; Tanaka, H.; Ohta, H.; Okuzawa, K.; Hirose, K. In vitro effects of 17α-hydroxyprogesterone and 17α, 20β-dihydroxy-4-pregnen-3-one on final maturation of oocytes at various developmental stages in artificially matured Japanese eel Anguilla japonica. Fish. Sci. 1995, 61, 1012–1015. [Google Scholar] [CrossRef]
- Palstra, A.P.; Cohen, E.G.H.; Niemantsverdriet, P.R.W.; van Ginneken, V.J.T.; van den Thillart, G.E.E.J.M. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture 2005, 249, 533–547. [Google Scholar] [CrossRef]
- Tanaka, H. Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish. Sci. 2015, 81, 11–19. [Google Scholar] [CrossRef]
- Han, Y.-S.; Liao, I.-C.; Huang, Y.-S.; He, J.-T.; Chang, C.-W.; Tzeng, W.-N. Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 2003, 219, 783–796. [Google Scholar] [CrossRef]
- Pelster, B. Swimbladder function and the spawning migration of the European eel Anguilla anguilla. Front. Physiol. 2015, 5, 486. [Google Scholar] [CrossRef]
- Yamada, Y.; Zhang, H.; Okamura, A.; Tanaka, S.; Horie, N.; Mikawa, N.; Utoh, T.; Oka, H.P. Morphological and histological changes in the swim bladder during maturation of the Japanese eel. J. Fish. Biol. 2001, 58, 804–814. [Google Scholar] [CrossRef]
- Balm, S.P.; Durif, C.; van Ginneken, V.; Antonissen, E.; Boot, R.; van Den Thillart, G.; Verstegen, M. Silvering of European eel (Anguilla anguilla L.): Seasonal changes of morphological and metabolic parameters. Anim. Biol. 2007, 57, 63–77. [Google Scholar] [CrossRef]
- Lokman, P.M.; Vermeulen, G.J.; Lambert, J.G.D.; Young, G. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish. Physiol. Biochem. 1998, 19, 325–338. [Google Scholar] [CrossRef]
- Rohr, D.H.; Lokman, P.M.; Davie, P.S.; Young, G. 11-Ketotestosterone induces silvering-related changes in immature female short-finned eels, Anguilla australis. Comp. Biochem. Phys. A 2001, 130, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Thomson-Laing, G.; Jasoni, C.L.; Lokman, P.M. The effects of migratory stage and 11-ketotestosterone on the expression of rod opsin genes in the shortfinned eel (Anguilla australis). Gen. Comp. Endocrinol. 2018, 257, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, R.A.; McKinley, R.S.; Kaak, G.; Dutil, J.-D.; Reid, K.B.; McGrath, K.J. Plasma non-esterified fatty acid profiles and 17beta-oestradiol levels of juvenile immature and maturing adult American eels in the St Lawrence River. J. Fish. Biol. 2001, 59, 364–379. [Google Scholar] [CrossRef]
- Tzchori, I.; Degani, G.; Hurvitz, A.; Moav, B. Cloning and developmental expression of the cytochrome P450 aromatase gene (CYP19) in the European eel (Anguilla anguilla). Gen. Comp. Endocrinol. 2004, 138, 271–280. [Google Scholar] [CrossRef]
- Yan, L.; Swanson, P.; Dickhoff, W.W. A two-receptor model for salmon gonadotropins (GTH I and GTH II). Biol. Reprod. 1992, 47, 418–427. [Google Scholar] [CrossRef]
- Miwa, S.; Yan, L.; Swanson, P. Localization of two gonadotropin receptors in the salmon gonad by in vitro ligand autoradiography. Biol. Reprod. 1994, 50, 629–642. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Miura, T.; Miura, C.; Yamaguchi, S.; Trant, J.M.; Adachi, S.; Yamauchi, K. Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): Production of biologically active recombinant Fsh and Lh by drosophila S2 cells and their differential actions on the reproductive biology. Biol. Reprod. 2008, 79, 938–946. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.; Tian, Z.; Sun, A.; Dong, Y.; Dong, T.; Hu, H. Effects of 11-tetotestosterone on development of the previtellogenic ovary in the Sterlet, Acipenser ruthenus. Front. Endocrinol. 2020, 11, 115. [Google Scholar] [CrossRef]
- Perazzolo, L.M.; Coward, K.; Davail, B.; Normand, E.; Tyler, C.R.; Pakdel, F.; Schneider, W.J.; Le Menn, F. Expression and localization of messenger ribonucleic acid for the vitellogenin receptor in ovarian follicles throughout oogenesis in the Rainbow trout, Oncorhynchus mykiss. Biol. Reprod. 1999, 60, 1057–1068. [Google Scholar] [CrossRef]

| Abv. | Gene | Accession Number | Primer Sequence (5′-3′) | Size (bp) | Temp °C | Ref. |
|---|---|---|---|---|---|---|
| 18S | 18 s ribosomal RNA | FM946133 | FW: GTACACACGGCCGGTACAGT RV: GGTAGGCGCAGAAAGTACCA | 302 | 60 | [37] |
| cyp19 | Aromatase cytochrome P450 | KF990052 | FW: CGCACCTACTTTGCTAAAGCTC RV: AGGTTGAGGATGTCCACCTG | 137 | 62 | [35] |
| elf-1 | Elongation factor 1 | EU407825 | FW: CCCCTGCAGGATGTCTACAA RV: AGGGACTCATGG TGCATTTC | 152 | 64 | [37] |
| esr-1 | Estrogen receptor 1 | LN879034 | FW: GGCATGGCCGAGATTTTC RV: GCACCGGAGTTGAGCAGTAT | 116 | 62 | [35] |
| fshr | Follicle-stimulating hormone receptor | LN831181 | FW: CCTGGTCGAGATAACAATCACC RV: CCTGAAGGTCAAACAGAAAGTCC | 173 | 63 | [38] |
| l36 | 60 s ribosomal protein l36 | G | FW: CCTGACCAAGCAGACCAAGT RV: TCTCTTTGCACGGATGTGAG | 160 | 62 | [37] |
| lhr-1 | Luteinizing hormone 1 | LN831182 | FW: GCGGAAACACAGGGAGAAC RV: GGTTGAGGTACTGGAAATCGAAG | 155 | 60 | [36] |
| lhr-2 | Luteinizing hormone 2 | LN831183 | FW: TCAACAACCTCACCAATCTCTCT RV: GCAGTGAAGAAATAGCCGACA | 162 | 62 | [18] |
| lpl | lipoprotein lipase | XM035416270 | FW: TGATGCTGATTGCTACTTCTGG RV: ATGCTCTCCTGCTGCTTCTT | 115 | 62 | This study |
| vtgr | Vitellogenin receptor | G | FW: TCTGAACGAACCCCAGGA RV: TTTGGGGAGTGCTTGTTGA | 140 | 59 | [35] |
| BL (cm) | BW (g) | K | BGI | EI | |
|---|---|---|---|---|---|
| C pre-treatment | 58 ± 3 | 347 ± 47 | 0.17 ± 0.00 | 0.18 ± 0.00 | 7.26 ± 1.27 |
| C post-treatment | 58 ± 3 | 323 ± 45 (−) | 0.16 ± 0.00 (−) | 0.17 ± 0.00 (−) | 7.04 ± 1.26 |
| hCG pre-treatment | 58 ± 3 | 336 ± 37 | 0.18 ± 0.00 | 0.18 + 0.00 | 7.59 ± 1.10 |
| hCG post-treatment | 58 ± 3 | 329 ± 37 (−) | 0.17 ± 0.00 (−) | 0.18 ± 0.00 | 9.11 ± 0.92 (+) |
| Days to Reach BWI 110 | CPE Injections Mature | Hours after Final DHP Injection | % Floating Eggs | |
|---|---|---|---|---|
| C group | 90 ± 11 | 14 ± 2 | 12 ± 1 | 33 ± 24 |
| hCG group | 65 ± 9 | 10 ± 1 | 12 ± 1 | 39 ± 26 |
| Larval Longevity | |||||
|---|---|---|---|---|---|
| Eggs | Embryos | Larvae | Average (dph) | Max (dph) | |
| C group | 12 | 9 | 9 | 5 | 9 |
| hCG group | 12 | 11 | 11 | 5 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palstra, A.P.; van de Ven, I.; Jéhannet, P.; Kruijt, L.; Schipper, H.; Swinkels, W.; Heinsbroek, L.T.N. Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla). Fishes 2023, 8, 281. https://doi.org/10.3390/fishes8060281
Palstra AP, van de Ven I, Jéhannet P, Kruijt L, Schipper H, Swinkels W, Heinsbroek LTN. Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla). Fishes. 2023; 8(6):281. https://doi.org/10.3390/fishes8060281
Chicago/Turabian StylePalstra, Arjan P., Ida van de Ven, Pauline Jéhannet, Leo Kruijt, Henk Schipper, William Swinkels, and Leon T. N. Heinsbroek. 2023. "Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla)" Fishes 8, no. 6: 281. https://doi.org/10.3390/fishes8060281
APA StylePalstra, A. P., van de Ven, I., Jéhannet, P., Kruijt, L., Schipper, H., Swinkels, W., & Heinsbroek, L. T. N. (2023). Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla). Fishes, 8(6), 281. https://doi.org/10.3390/fishes8060281







