Quantifying the Neural and Behavioral Correlates of Repeated Social Competition in the Fighting Fish Betta splendens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
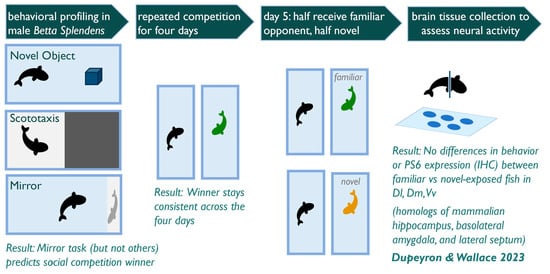
2.2. Experimental Design
2.3. Behavioral Scoring
2.4. Tissue Processing
2.5. Imaging and Cell Quantification
2.6. Statistical Analysis
3. Results
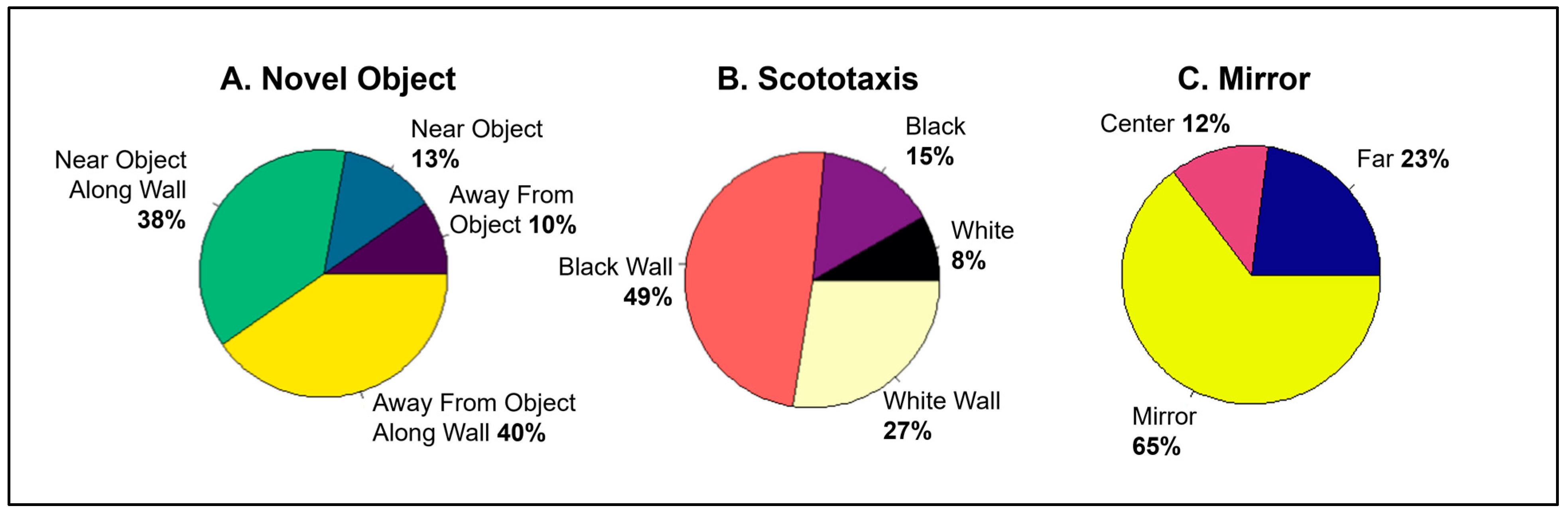
3.1. Is Individual Behavior Predictable and Consistent across Contexts?
3.2. Does Behavior in Other Contexts Predict Social Competition?
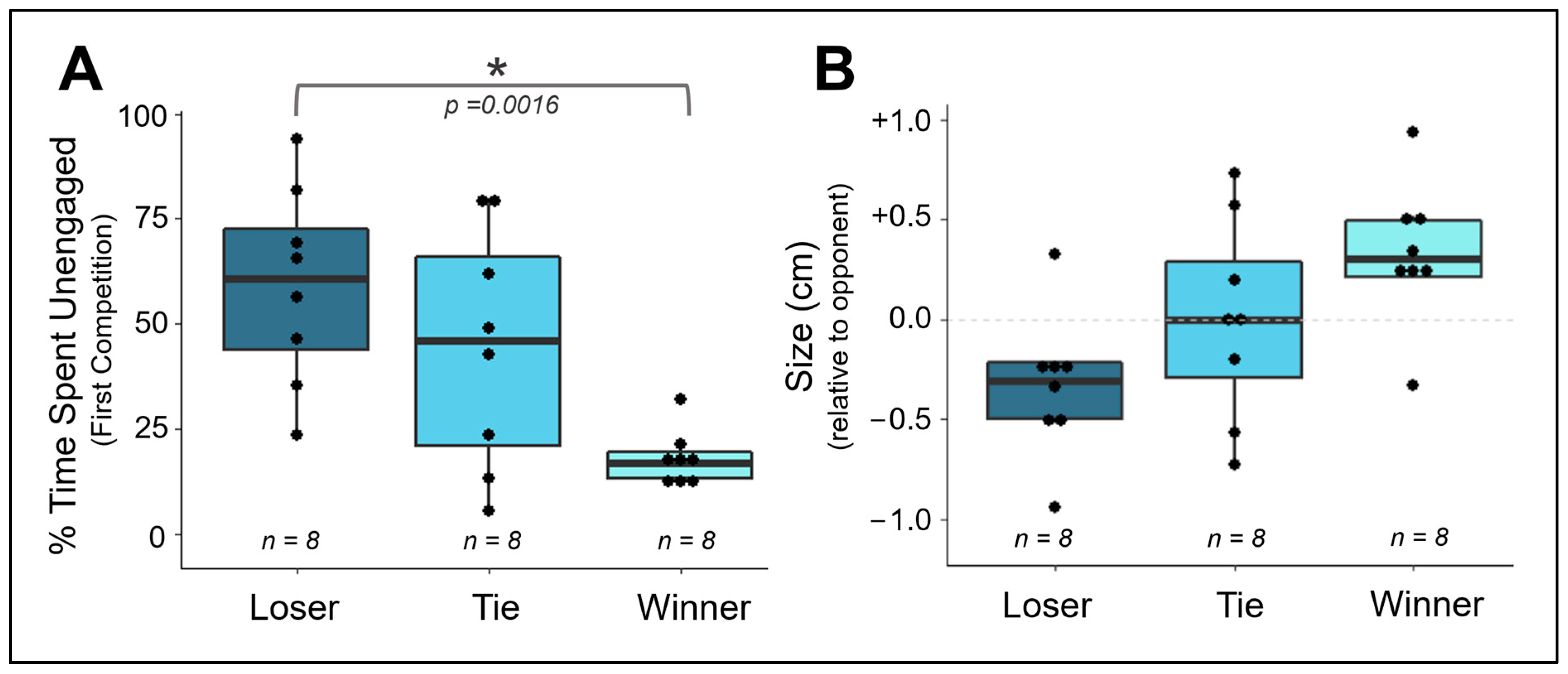
3.3. Does Competitive Behavior Remain Consistent over Repeated Exposures?
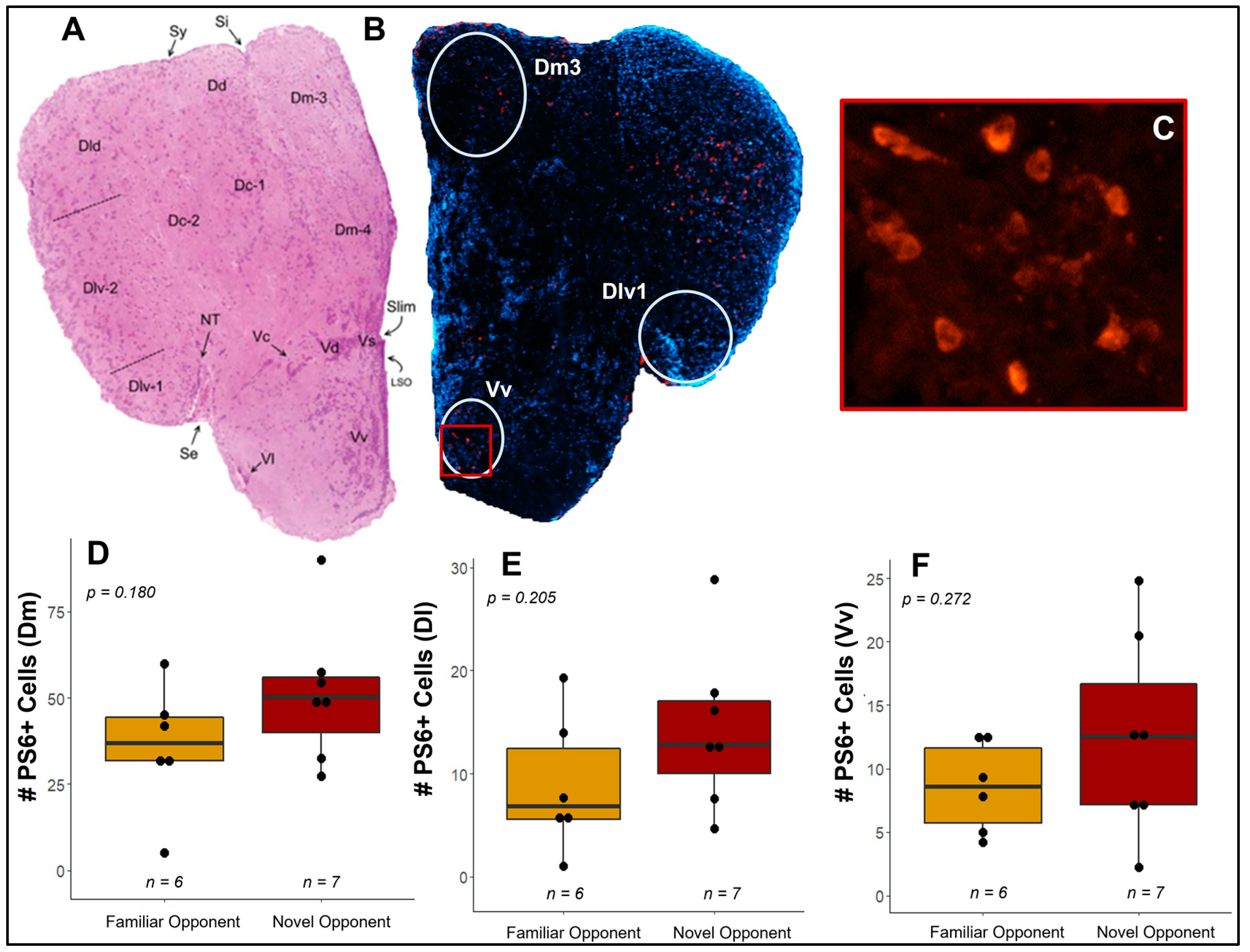
3.4. Do Competitive Behavior and Neural Responses Differ between Individuals Exposed to A Novel vs. Familiar Opponent?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shettleworth, S.J. Cognition, Evolution, and Behavior; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Haley, M.P.; Deutsch, C.J.; Le Boeuf, B.J. Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Anim. Behav. 1994, 48, 1249–1260. [Google Scholar] [CrossRef]
- Funghi, C.; Leitão, A.V.; Ferreira, A.C.; Mota, P.G.; Cardoso, G.C. Social Dominance in a Gregarious Bird is Related to Body Size but not to Standard Personality Assays. Ethology 2014, 121, 84–93. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Dale, J. A socially enforced signal of quality in a paper wasp. Nature 2004, 432, 218–222. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Hegner, R.E.; Dufty, A.M., Jr.; Ball, G.F. The “Challenge Hypothesis”: Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am. Nat. 1990, 136, 829–846. [Google Scholar] [CrossRef]
- Formica, V.; Donald, H.; Marti, H.; Irgebay, Z.; Brodie, E. Social network position experiences more variable selection than weaponry in wild subpopulations of forked fungus beetles. J. Anim. Ecol. 2020, 90, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Favre, M.; Martin, J.G.; Festa-Bianchet, M. Determinants and life-history consequences of social dominance in bighorn ewes. Anim. Behav. 2008, 76, 1373–1380. [Google Scholar] [CrossRef]
- Real, L.A. Toward a cognitive ecology. Trends Ecol. Evol. 1993, 8, 413–417. [Google Scholar] [CrossRef]
- Wallace, K.J.; Hofmann, H.A. Decision-making in a social world: Integrating cognitive ecology and social neuroscience. Curr. Opin. Neurobiol. 2021, 68, 152–158. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Sih, A.; Del Giudice, M. Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2762–2772. [Google Scholar] [CrossRef]
- Guillette, L.M.; Reddon, A.R.; Hurd, P.L.; Sturdy, C.B. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees, Poecile atricapillus. Behav. Process. 2009, 82, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.G.; Rodd, F.H. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Anim. Behav. 2008, 76, 911–922. [Google Scholar] [CrossRef]
- Gibelli, J.; Aubin-Horth, N.; Dubois, F. Individual differences in anxiety are related to differences in learning performance and cognitive style. Anim. Behav. 2019, 157, 121–128. [Google Scholar] [CrossRef]
- Colléter, M.; Brown, C. Personality traits predict hierarchy rank in male rainbowfish social groups. Anim. Behav. 2011, 81, 1231–1237. [Google Scholar] [CrossRef]
- David, M.; Auclair, Y.; Cézilly, F. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Anim. Behav. 2011, 81, 219–224. [Google Scholar] [CrossRef]
- Favati, A.; Leimar, O.; Løvlie, H. Personality Predicts Social Dominance in Male Domestic Fowl. PLoS ONE 2014, 9, e103535. [Google Scholar] [CrossRef]
- Wallace, K.J.; Choudhary, K.D.; Kutty, L.A.; Le, D.H.; Lee, M.T.; Wu, K.; Hofmann, H.A. Social ascent changes cognition, behaviour and physiology in a highly social cichlid fish. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200448. [Google Scholar] [CrossRef]
- Devost, I.; Jones, T.B.; Cauchoix, M.; Montreuil-Spencer, C.; Morand-Ferron, J. Personality does not predict social dominance in wild groups of black-capped chickadees. Anim. Behav. 2016, 122, 67–76. [Google Scholar] [CrossRef]
- Castro, N.; Ros, A.F.; Becker, K.; Oliveira, R.F. Metabolic costs of aggressive behaviour in the Siamese fighting fish, Betta splendens. Aggress. Behav. 2006, 32, 474–480. [Google Scholar] [CrossRef]
- Evans, C. Display vigour and subsequent fight performance in the siamese fighting fish, Betta splendens. Behav. Process. 1985, 11, 113–121. [Google Scholar] [CrossRef]
- Portugal, S.J. Siamese fighting fish. Curr. Biol. 2023, 33, R341–R343. [Google Scholar] [CrossRef]
- Wallen, K.; Wojciechowski-Metzlar, C.I. Social conditioning and dominance in male Betta splendens. Behav. Process. 1985, 11, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lissmann, H.W. Die Umwelt des Kampffisches (Betta splendens Regan). J. Comp. Physiol. A 1932, 18, 65–111. [Google Scholar] [CrossRef]
- Newman, S.W. The Medial Extended Amygdala in Male Reproductive Behavior A Node in the Mammalian Social Behavior Network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.L. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 2005, 48, 11–22. [Google Scholar] [CrossRef]
- Kelly, A.M. A consideration of brain networks modulating social behavior. Horm. Behav. 2022, 141, 105138. [Google Scholar] [CrossRef]
- Wallace, K.J.; Chun, E.K.; Manns, J.R.; Ophir, A.G.; Kelly, A.M. A test of the social behavior network reveals differential patterns of neural responses to social novelty in bonded, but not non-bonded, male prairie voles. Horm. Behav. 2023, 152, 105362. [Google Scholar] [CrossRef]
- O’Connell, L.A.; Hofmann, H.A. Evolution of a Vertebrate Social Decision-Making Network. Science 2012, 336, 1154–1157. [Google Scholar] [CrossRef]
- Calvo, R.; Schluessel, V. Neural substrates involved in the cognitive information processing in teleost fish. Anim. Cogn. 2021, 24, 923–946. [Google Scholar] [CrossRef]
- Fan, R.; Reader, S.M.; Sakata, J.T. Alarm cues and alarmed conspecifics: Neural activity during social learning from different cues in Trinidadian guppies. Proc. R. Soc. B Boil. Sci. 2022, 289, 20220829. [Google Scholar] [CrossRef]
- Baran, N.M.; Streelman, J.T. Ecotype differences in aggression, neural activity and behaviorally relevant gene expression in cichlid fish. Genes Brain Behav. 2020, 19, e12657. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Santiago, M.; Jordan, A.; Hofmann, H.A. Neural activity patterns differ between learning contexts in a social fish. Proc. R. Soc. B Boil. Sci. 2022, 289, 20220135. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.M.; Rasia-Filho, A.A. The Cytoarchitecture of the Telencephalon of Betta splendens Regan 1910 (Perciformes: Anabantoidei) with a Stereological Approach to the Supracommissural and Postcommissural Nuclei. Anat. Rec. 2017, 301, 88–110. [Google Scholar] [CrossRef]
- McDonald, C.G.; Paul, D.H.; Hawryshyn, C.W. Visual Sensation of an Ethological Stimulus, the Agonistic Display of Betta splendens, Revealed using Multi-Unit Recordings from Optic Tectum. Environ. Biol. Fishes 2004, 70, 285–291. [Google Scholar] [CrossRef]
- Vu, T.-D.; Iwasaki, Y.; Shigenobu, S.; Maruko, A.; Oshima, K.; Iioka, E.; Huang, C.-L.; Abe, T.; Tamaki, S.; Lin, Y.-W.; et al. Behavioral and brain- transcriptomic synchronization between the two opponents of a fighting pair of the fish Betta splendens. PLoS Genet. 2020, 16, e1008831. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.D.P.; Mayer, P.C.M.; Walsh-Monteiro, A.; Gouveia, A. Effects of menthol on aggressive behavior in Siamese fighting fish (Betta splendens). Psychol. Neurosci. 2015, 8, 290–296. [Google Scholar] [CrossRef]
- Forsatkar, M.N.; Nematollahi, M.A.; Biro, P.A.; Beckmann, C. Individual boldness traits influenced by temperature in male Siamese fighting fish. Physiol. Behav. 2016, 165, 267–272. [Google Scholar] [CrossRef]
- Tudor, M.S.; Lopez-Anido, R.N.; Yocius, C.A.; Conlin, S.M.; Hamlin, H.J. Ecologically relevant arsenic exposure alters female mate preference and anxiety-like behavior in Betta splendens. Heliyon 2019, 5, e02646. [Google Scholar] [CrossRef]
- Bronstein, P.M. Onset of combat in male Betta splendens. J. Comp. Psychol. 1983, 97, 135–139. [Google Scholar] [CrossRef]
- Ramos, A.; Alex, D.; Cardoso, S.D.; Gonçalves, D. Androgens and corticosteroids increase in response to mirror images and interacting conspecifics in males of the Siamese fighting fish Betta splendens. Horm. Behav. 2021, 132, 104991. [Google Scholar] [CrossRef]
- Baenninger, R. Fighting by Betta splendens: Effects on aggressive displaying by conspecifics. Psychon. Sci. 1968, 10, 185–186. [Google Scholar] [CrossRef]
- Meliska, J.A.; Meliska, C.J.; Hoyenga, K.T.; Hoyenga, K.B.; Ward, E.F. Approach tendency and threat display as related to social status of Siamese fighting fish, Betta splendens. Anim. Learn. Behav. 1975, 3, 135–139. [Google Scholar] [CrossRef]
- Hebert, O.L.; Lavin, L.E.; Marks, J.M.; Dzieweczynski, T.L. The effects of 17α-ethinyloestradiol on boldness and its relationship to decision making in male Siamese fighting fish. Anim. Behav. 2014, 87, 203–212. [Google Scholar] [CrossRef]
- Alyan, S. Male Betta splendens are equally aggressive toward neighbors and strangers. J. Ichthyol. 2010, 50, 1066–1069. [Google Scholar] [CrossRef]
- Gheusi, G.; Bluthé, R.-M.; Goodall, G.; Dantzer, R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behav. Process. 1994, 33, 59–87. [Google Scholar] [CrossRef]
- O′Connell, L.A.; Hofmann, H.A. The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J. Comp. Neurol. 2011, 519, 3599–3639. [Google Scholar] [CrossRef]
- Northcutt, R.G. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 2006, 494, 903–943. [Google Scholar] [CrossRef]
- Portavella, M.; Vargas, J.; Torres, B.; Salas, C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 2002, 57, 397–399. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Dadda, M. Guppies Show Behavioural but Not Cognitive Sex Differences in a Novel Object Recognition Test. PLoS ONE 2016, 11, e0156589. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Whitlow, S.M.; Roberts, D.A.; Maruska, K.P. Neural and behavioural correlates of repeated social defeat. Sci. Rep. 2018, 8, 6818. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef]
- Calvo, R.; Hofmann, M.H.; Schluessel, V. Brain areas activated during visual learning in the cichlid fish Pseudotropheus zebra. Brain. Struct. Funct. 2023, 228, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Westrick, S.E.; Hartsough, L.; Hoke, K.L. Differences in neural activity, but not behavior, across social contexts in guppies, Poecilia reticulata. Behav. Ecol. Sociobiol. 2018, 72, 131. [Google Scholar] [CrossRef]
- Tarnowski, B.I.; Spinale, F.G.; Nicholson, J.H. DAPI as a Useful Stain for Nuclear Quantitation. Biotech. Histochem. 1991, 66, 296–302. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 7 September 2022).
- Wallace, K.J. Cowlogdata: An R Package to Analyze and Visualize Observations Generated by the Event Logging Software CowLog. 2020. Available online: github.com/kellyjwallace/cowlogdata (accessed on 7 September 2022).
- Simpson, M. The Display of the Siamese Fighting Fish, Betta splendens. Anim. Behav. Monogr. 1968, 1, 1–73. [Google Scholar] [CrossRef]
- Balaban-Feld, J.; Mitchell, W.A.; Kotler, B.P.; Vijayan, S.; Elem, L.T.T.; Rosenzweig, M.L.; Abramsky, Z. Individual willingness to leave a safe refuge and the trade-off between food and safety: A test with social fish. Proc. R. Soc. B Boil. Sci. 2019, 286, 20190826. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Vranken, N.; Hoge, C.; Lichak, M.R.; Norovich, A.L.; Francis, K.X.; Camacho-Garcia, J.; Bista, I.; Wood, J.; McCarthy, S.; et al. Genomic consequences of domestication of the Siamese fighting fish. Sci. Adv. 2022, 8, eabm4950. [Google Scholar] [CrossRef] [PubMed]
- Forsatkar, M.N.; Nematollahi, M.A.; Brown, C. The toxicological effect of Ruta graveolens extract in Siamese fighting fish: A behavioral and histopathological approach. Ecotoxicology 2016, 25, 824–834. [Google Scholar] [CrossRef]
- Archard, G.A.; Braithwaite, V.A. Variation in aggressive behaviour in the poeciliid fish Brachyrhaphis episcopi: Population and sex differences. Behav. Process. 2011, 86, 52–57. [Google Scholar] [CrossRef]
- Solomon-Lane, T.K.; Hofmann, H.A. Early-life social environment alters juvenile behavior and neuroendocrine function in a highly social cichlid fish. Horm. Behav. 2019, 115, 104552. [Google Scholar] [CrossRef]
- Way, G.P.; Kiesel, A.L.; Ruhl, N.; Snekser, J.L.; McRobert, S.P. Sex differences in a shoaling-boldness behavioral syndrome, but no link with aggression. Behav. Process. 2015, 113, 7–12. [Google Scholar] [CrossRef]
- Lacasse, J.; Aubin-Horth, N. A test of the coupling of predator defense morphology and behavior variation in two threespine stickleback populations. Curr. Zool. 2012, 58, 53–65. [Google Scholar] [CrossRef][Green Version]
- Miklósi, A.; Haller, J.; Csányi, V. The Influence of Opponent-Related and Outcome-Related Memory on Repeated Aggressive Encounters in the Paradise Fish (Macropodus opercularis). Biol. Bull. 1995, 188, 83–88. [Google Scholar] [CrossRef]
- Colyer, S.W.; Jenkins, C. Pheromonal Control of Aggressive Display in Siamese Fighting Fish (Betta splendens). Percept. Mot. Ski. 1976, 42, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Curren, L.J.; Murphy, C.E. Mate Choice and Spawning Success in the Fighting Fish Betta splendens: The Importance of Body Size, Display Behavior and Nest Size. Ethology 2006, 112, 1170–1178. [Google Scholar] [CrossRef]
- Lichak, M.R.; Barber, J.R.; Kwon, Y.M.; Francis, K.X.; Bendesky, A. Care and Use of Siamese Fighting Fish (Betta Splendens) for Research. Comp. Med. 2022, 72, 169–180. [Google Scholar] [CrossRef]
- Dzieweczynski, T.L.; Earley, R.L.; Green, T.M.; Rowland, W.J. Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav. Ecol. 2005, 16, 1025–1030. [Google Scholar] [CrossRef]
- Godwin, J.; Thompson, R. Nonapeptides and Social Behavior in Fishes. Horm. Behav. 2012, 61, 230–238. [Google Scholar] [CrossRef]
- Goldstein, S.R. Observations on the establishment of a stable community of adult male and female siamese fighting fish (Betta splendens). Anim. Behav. 1975, 23, 179–185. [Google Scholar] [CrossRef]
- Cain, N.W.; Baenninger, R. Social organization and the maintenance of aggressive behavior in community-housed male Siamese fighting fish (Betta splendens). Anim. Learn. Behav. 1980, 8, 171–176. [Google Scholar] [CrossRef]
- Wallace, K.J.; Hofmann, H.A. Equal performance but distinct behaviors: Sex differences in a novel object recognition task and spatial maze in a highly social cichlid fish. Anim. Cogn. 2021, 24, 1057–1073. [Google Scholar] [CrossRef]
- Foran, C.M.; Bass, A.H. Preoptic AVT Immunoreactive Neurons of a Teleost Fish with Alternative Reproductive Tactics. Gen. Comp. Endocrinol. 1998, 111, 271–282. [Google Scholar] [CrossRef]
- Luchiari, A.C. How Betta splendens finds its way. Behav. Process. 2016, 124, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Roitblat, H.L.; Tham, W.; Golub, L. Performance of Betta splendens in a radial arm maze. Anim. Learn. Behav. 1982, 10, 108–114. [Google Scholar] [CrossRef]
- Rnic, A. Food and Mirror Image as Reinforcers in Discrimination Reversal Learning with Siamese Fighting Fish (Betta splendens). Ph.D. Thesis, Lakehead University, Thunder Bay, ON, Canada, 2017. [Google Scholar]
- Patton, B.W.; Braithwaite, V.A. Changing tides: Ecological and historical perspectives on fish cognition. WIREs Cogn. Sci. 2015, 6, 159–176. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]


| Behavior | Description |
|---|---|
| ramming/biting | Fish making rapid, targeted contact with the mirror or opponent-facing barrier using their mouth. |
| surface breathing | Fish swimming up to the surface of the water to inhale oxygen. Often, but not always, an emitted bubble can be seen during this behavior. |
| gill flaring | Fish flaring their operculum. To be tallied during the social exposure assay, this behavior must occur in the opponent-facing half of the chamber. |
| tail beating | Fish bending body continually to making the motion of an “S”. This is a conspicuous display that occurs more slowly than simply bending the body to swim forward. To be tallied during the social exposure assay, this behavior had to occur in the opponent-facing half of the chamber. |
| lateral swimming | Fish swimming with body in close proximity to (roughly one fish-width) and in parallel to the mirror or opponent-facing barrier. |
| unengaged (N/A) | Fish that are not engaging in any of the above behaviors, or in the case of the social exposure assay are in the half of the tank far away from the opponent. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupeyron, S.; Wallace, K.J. Quantifying the Neural and Behavioral Correlates of Repeated Social Competition in the Fighting Fish Betta splendens. Fishes 2023, 8, 384. https://doi.org/10.3390/fishes8080384
Dupeyron S, Wallace KJ. Quantifying the Neural and Behavioral Correlates of Repeated Social Competition in the Fighting Fish Betta splendens. Fishes. 2023; 8(8):384. https://doi.org/10.3390/fishes8080384
Chicago/Turabian StyleDupeyron, Solanch, and Kelly J. Wallace. 2023. "Quantifying the Neural and Behavioral Correlates of Repeated Social Competition in the Fighting Fish Betta splendens" Fishes 8, no. 8: 384. https://doi.org/10.3390/fishes8080384
APA StyleDupeyron, S., & Wallace, K. J. (2023). Quantifying the Neural and Behavioral Correlates of Repeated Social Competition in the Fighting Fish Betta splendens. Fishes, 8(8), 384. https://doi.org/10.3390/fishes8080384