The Effects of Grapevine (Vitis vinifera L.) Leaf Extract on Growth Performance, Antioxidant Status, and Immunity of Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Experimental Design and Feed Formulation
2.3. Growth Performance
2.4. Determination of Innate Immune and Antioxidant Parameters
2.5. Sampling for Gene Expression Study
2.6. RNA Isolation and Laboratory Methods of Gene Expression
2.7. Data Analysis
3. Results
3.1. Growth Performance
3.2. Innate Immune Parameters
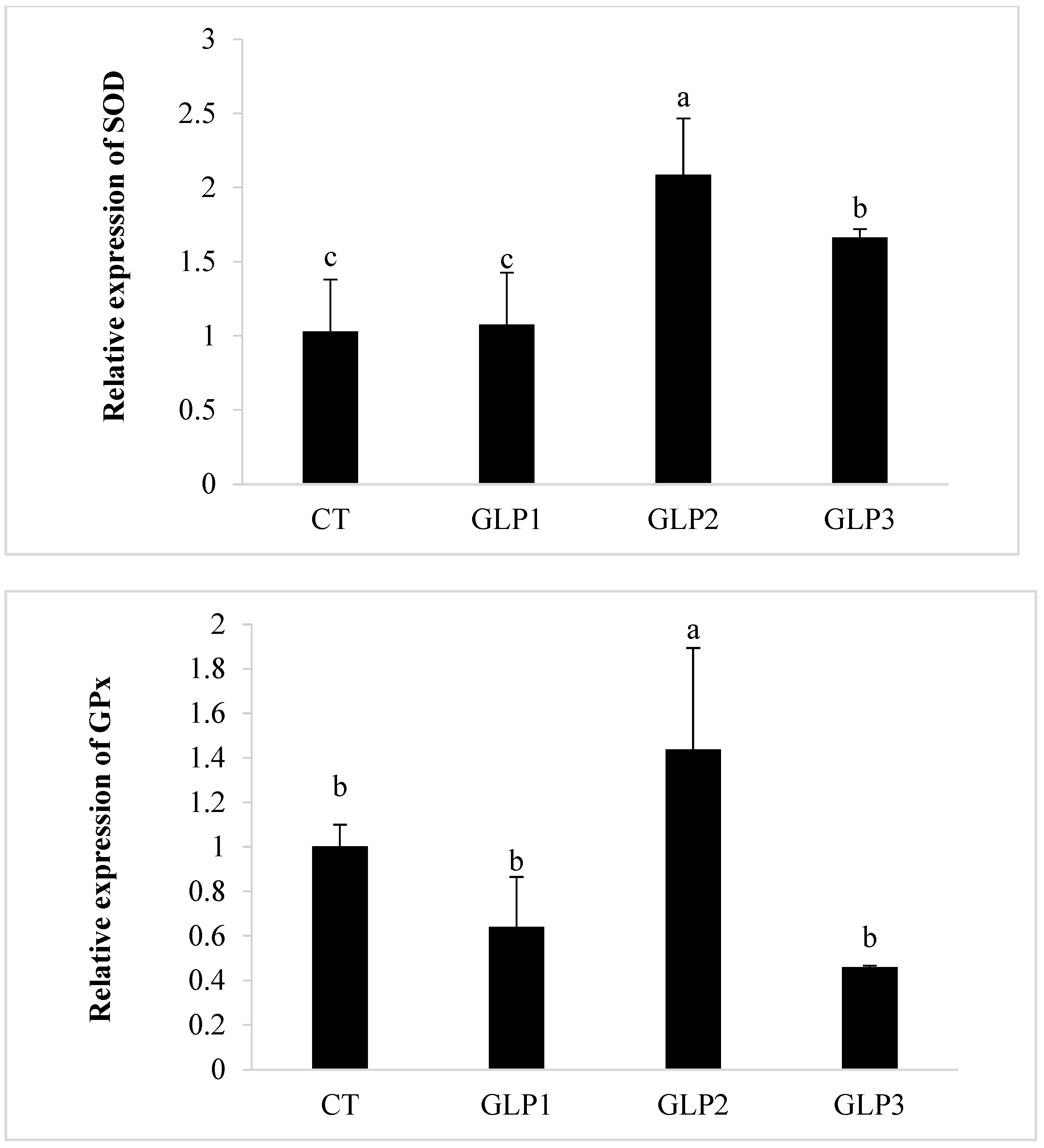
3.3. Antioxidant Enzyme Activity
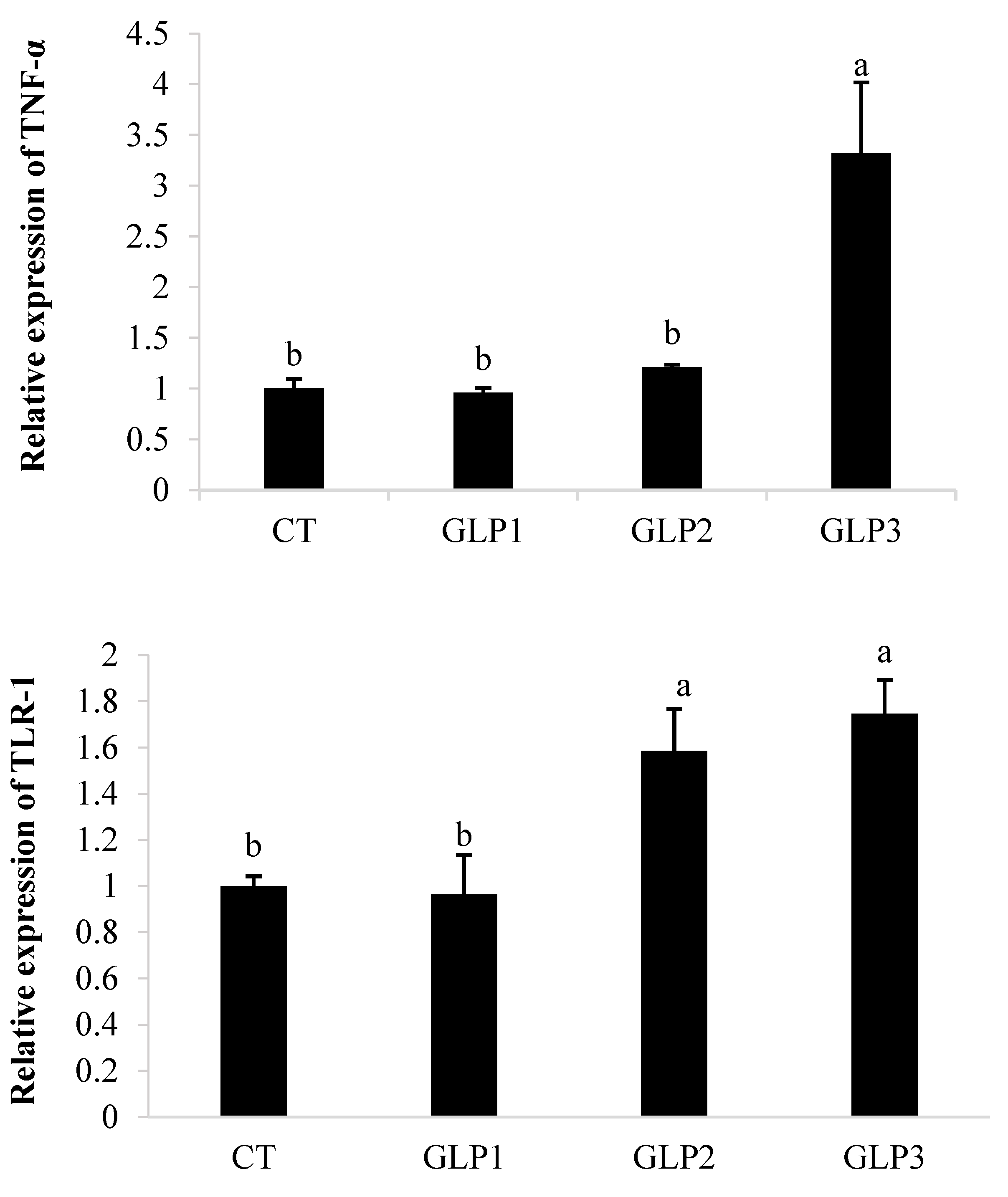
3.4. Immune and Oxidative Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2013, 3, 232–249. [Google Scholar]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2016, 101, 605–628. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence; InTech: London, UK, 2012; Volume 2, pp. 23–48. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Martins, L.A.M.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.C.; Moreira, J.C.F.; Gottfried, C.; Guma, F.C.R. Resveratrol Induces Pro-oxidant Effects and Time-Dependent Resistance to Cytotoxicity in Activated Hepatic Stellate Cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Olivares-Marin, I.K.; Canizal-García, M.; González-Hernández, J.C.; Nava, G.M.; Madrigal-Perez, L.A. Resveratrol induces mitochondrial dysfunction and decreases chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. J. Bioenerg. Biomembr. 2017, 49, 241–251. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.O.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H. The Gene Regulatory Roles of Herbal Extracts on the Growth, Immune System, and Reproduction of Fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Effects of chestnut (Castanea sativa) polyphenols on growth, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) culture under biofloc system. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Jahazi, A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Hoseinifar, H.; Jahazi, A.; Nikdehghan, N.; Van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols from agricultural by-products on mucosal and humoral immune and antioxidant responses of convict cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Omnes, M.-H.; Le Goasduff, J.; Le Delliou, H.; Le Bayon, N.; Quazuguel, P.; Robin, J.H. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquac. Rep. 2017, 6, 21–27. [Google Scholar] [CrossRef]
- Sicuro, B.; Daprà, F.; Gai, F.; Palmegiano, G.B.; Schiavone, R.; Zilli, L.; Vilella, S. Olive oil by-product as a natural antioxidant in gilthead sea bream (Sparus aurata) nutrition. Aquac. Int. 2010, 18, 511–522. [Google Scholar] [CrossRef]
- Sicuro, B.; Barbera, S.; Daprà, F.; Gai, F.; Gasco, L.; Paglialonga, G.; Palmegiano, G.B.; Vilella, S. The olive oil by-product in ’Rainbow trout Onchorynchus mykiss (Walbaum)’ farming: Productive results and quality of the product. Aquac. Res. 2010, 41, e475–e486. [Google Scholar] [CrossRef]
- Sicuro, B.; Badino, P.; Daprà, F.; Gai, F.; Galloni, M.; Odore, R.; Palmegiano, G.B.; Macchi, E. Physiological effects of natural olive oil antioxidants utilization in rainbow trout (Onchorynchus mykiss) feeding. Aquacult. Int. 2010, 18, 415–431. [Google Scholar] [CrossRef]
- Orso, G.; Solovyev, M.M.; Facchiano, S.; Tyrikova, E.; Sateriale, D.; Kashinskaya, E.; Pagliarulo, C.; Hoseinifar, H.S.; Simonov, E.; Varricchio, E.; et al. Chestnut shell tannins: Effects on intestinal inflammation and dysbiosis in Zebrafish. Animals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Antiinflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Aleström, P.; Winther-Larsen, H.C. Zebrafish offer aquaculture research their services. In Genomics in Aquaculture, 1st ed.; MacKenzie, S., Jentoft, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 165–194. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Johns Hopkins University: Methuen, MA, USA, 1959. [Google Scholar]
- Holbech, H.; Andersen, L.; Petersen, G.I.; Korsgaard, B.; Pedersen, K.L.; Bjerregaard, P. Development of an ELISA for vitellogenin in whole body homogenate of zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. 2001, 130, 119–131. [Google Scholar] [CrossRef]
- Yousefi, S.; Hoseinifar, S.H.; Paknejad, H.; Hajimoradloo, A. The effects of dietary supplement of galactooligosaccharide on innate immunity, immune related genes expression and growth performance in zebrafish (Danio rerio). Fish Shellfish Immunol. 2018, 73, 192–196. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]
- Clerton, P.; Troutaud, D.; Verlhac, V.; Gabaudan, J.; Deschaux, P. Dietary vitamin E and rainbow trout (Oncorhynchus mykiss) phagocyte functions: Effect on gut and on head kidney leucocytes. Fish Shellfish Immunol. 2001, 11, 1–13. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs, and total immunoglobulin level in serum. In Fish Disease Diagnosis and Prevention Methods; Siwicki, A.K., Anderson, D.P., Waluga, J., Eds.; Wydawnictwo Instytutu Rybactwa Strodladowego: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Zou, H.K.; Hoseinifar, S.H.; Kolangi Miandare, H.; Hajimoradloo, A. Agaricus bisporus powder improved cutaneous mucosal and serum immune parameters and up-regulated intestinal cytokines gene expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2016, 58, 380–386. [Google Scholar]
- Hoseinifar, S.H.; Khalili, M.; Rufchaei, R.; Raeisi, M.; Attar, M.; Cordero, H.; Esteban, M.Á. Effects of date palm fruit extracts on skin mucosal immunity, immune related genes expression and growth performance of common carp (Cyprinus carpio) fry. Fish Shellfish Immunol. 2015, 47, 706–711. [Google Scholar] [CrossRef]
- Orso, G.; Imperatore, R.; Coccia, E.; Ashouri, G.; Paolucci, M. Lamiaceae as Feed Additives in Fish Aquaculture. Fishes 2022, 7, 349. [Google Scholar] [CrossRef]
- Dresch, R.R.; Dresch, M.K.; Guerreiro, A.F.; Biegelmeyer, R.; Holzschuh, M.H.; Rambo, D.F.; Henriques, A.T. Phenolic Compounds from the Leaves of Vitis labrusca and Vitis vinifera L. as a Source of Waste Byproducts: Development and Validation of LC Method and Antichemotactic Activity. Food Anal. Methods 2013, 7, 527–539. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-guided recovery of bioactive flavonol compounds from Greco di Tufo vine leaves. Molecules 2019, 24, 3630–3640. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Paolucci, M.; Sansone, F.; Mencherini, T.; Pacifico, S.; Volpe, M.G. Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Appl. Sci. 2021, 11, 5827. [Google Scholar] [CrossRef]
- Segner, H.; Reiser, S.; Ruane, N.; Rösch, R.; Steinhagen, D.; Vehanen, T. Welfare of Fishes in Aquaculture; FAO Fisheries and Aquaculture Circular No. 1189; FAO: Budapest, Hungary, 2019. [Google Scholar]
- Parrillo, L.; Coccia, E.; Volpe, M.G.; Siano, F.; Pagliarulo, C.; Scioscia, E.; Varricchio, E.; Safari, O.; Eroldogan, T.; Paolucci, M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 2017, 473, 161–168. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. Reactive Oxygen Species (ROS) in Living Cells; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and Activators of SOD, GSH-Px, and CAT, Enzyme Inhibitors and Activators, Murat Senturk; IntechOpen: 2017. Available online: https://www.intechopen.com/chapters/52877 (accessed on 2 April 2023).
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, D.; Wang, J.; Qi, Q. The effects of TPT and dietary quercetin on growth, hepatic oxidative damage and apoptosis in zebrafish. Ecotoxicol. Environ. Saf. 2021, 224, 112697. [Google Scholar] [CrossRef] [PubMed]
- Pês, T.S.; Saccol, E.M.H.; Ourique, G.M.; Londero, É.P.; Gressler, L.T.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F.; Gonçalves, P.B.D.; Neto, J.R.; et al. Quercetin in the diet of silver catfish: Effects on antioxidant status, blood parameters and pituitary hormone expression. Aquaculture 2016, 458, 100–106. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 2009, 30, 2–12. [Google Scholar]
- Long, M.; Lin, W.; Hou, J.; Guo, H.; Li, L.; Li, D.; Tang, R.; Yang, F. Dietary supplementation with selenium yeast and tea polyphenols improve growth performance and nitrite tolerance of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 68, 74–83. [Google Scholar] [CrossRef]
- Yang, G.; Yu, R.; Geng, S.; Xiong, L.; Yan, Q.; Kumar, V.; Wen, C.; Peng, M. Apple polyphenols modulates the antioxidant defense response and attenuates inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture 2021, 534, 736284. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Adeshina, I.; Jenyo-Oni, A.; Ajani, E.K.; Emikpe, B.O. Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to Listeria monocytogenes infection. Fish Shellfish Immunol. 2018, 78, 346–354. [Google Scholar] [CrossRef]
- Adeshina, I.; Jenyo-Oni, A.; Emikpe, B.O.; Ajani, E.K.; Abdel-Tawwab, M. Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (B.), to Aeromonas hydrophila infection. J. World Aquac. Soc. 2019, 50, 390–405. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Itav, S.; Elinav, E. Integration of innate immune signaling. Trends Immunol. 2016, 37, 84–101. [Google Scholar] [CrossRef]
- Grayfer, L.; Walsh, J.G.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev. Comp. Immunol. 2008, 32, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; Dawood, M.A.; Assar, D.H.; Omar, A.A.; Elbialy, Z.I.; Farrag, F.A.; Shukry, M.; Zayed, M.M. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 2020, 515, 734589. [Google Scholar] [CrossRef]
- Kurian, A.; Van Doan, H.; Tapingkae, W.; Elumalai, P. Modulation of mucosal parameters, innate immunity, growth and resistance against Streptococcus agalactiae by enrichment of Nile tilapia (Oreochromis niloticus) diet with Leucas aspera. Fish Shellfish Immunol. 2019, 97, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Rashidian, G.; Ghafarifarsani, H.; Jahazi, M.A.; Soltani, M.; Van Doan, H.; El-Haroun, E.; Paolucci, M. Effects of Apple (Malus pomila) Pomace-Derived Pectin on the Innate Immune Responses, Expressions of Key Immune-Related Genes, Growth Performance, and Digestive Enzyme Activity of Rainbow Trout (Oncorhynchus mykiss). Animals 2021, 11, 2117. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Thamizharasan, S.; Devi, G.; Van Doan, H.; Ajith Kumar, T.T.; Hoseinifar, S.H.; Balasundaram, C. Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol. 2020, 106, 675–684. [Google Scholar] [CrossRef] [PubMed]

| Proximate Composition (%) | |
|---|---|
| Dry matter | 93.6 |
| Crude protein | 38.9 |
| Crude lipid | 15.0 |
| Ash | 11 |
| Primer Name | Primer Sequence | Application | Accession Number |
|---|---|---|---|
| TLR-1 q-PCRF | CAGAGCGAATGGTGCCACTAT | immune | AY389444 |
| TLR-1 q-PCRR | GTGGCAGAGGCTCCAGAAGA | ||
| TNF-α-PCRF | CTGCTTCACGCTCCATAAGA | immune | AY427649 |
| TNF-α-PCRR | CTGGTCCTGGTCATCTCTCC | ||
| GPx q-PCRF | CCAAGTAAACCAGCGGCTTCT | antioxidant | NM_001007281 |
| GPx q-PCRR | TGATGTGCAGTGGACGGTTTAT | ||
| SOD q-PCRF | GGGTGGCAATGAGGAAAG | antioxidant | BC055516 |
| SOD q-PCRR | GCCCACATAGAAATGCACAG | ||
| CAT q-PCRF | GCATGTTGGAAAGACGACAC | antioxidant | AJ007505 |
| CAT q-PCRR | GTGGATGAAAGACGGAGACA | ||
| Gapdh q-PCRF | GTGGAGTCTACTGGTGTCTTC | Housekeeping gene | BC083506 |
| Gapdh q-PCRR | GTGCAGGAGGCATTGCTTACA |
| CT | GLE1 | GLE2 | GLE3 | |
|---|---|---|---|---|
| IW (mg) | 124.1 ± 9.01 a | 115.5 ± 7.09 a | 130.2 ± 10.07 a | 110.3 ± 11.06 a |
| FW (mg) | 366.3 ± 5.7 b | 348.6 ± 9.2 b | 394.2 ± 3.9 a | 339.3 ± 10.3 b |
| WG (mg) | 242.3 ± 16.3 b | 265.6 ± 19.7 b | 293.6 ± 9.9 a | 229.7 ± 15.6 b |
| SGR (% d−1) | 1.57 ± 0.13 b | 1.68 ± 0.16 b | 1.88 ± 0.08 a | 1.49 ± 0.19 b |
| WBE | CT | GLE1 | GLE2 | GLE3 |
|---|---|---|---|---|
| Total protein (g dL−1) | 0.10 ± 0.01 c | 0.45 ± 0.02 b | 0.51 ± 0.02 a | 0.48 ± 0.05 ab |
| Total Ig (g dL−1) | 0.06 ± 0.005 b | 0.28 ± 0.009 a | 0.31 ± 0.010 a | 0.31 ± 0.033 a |
| Lysozyme (U mL−1) | 5.10 ± 0.20 c | 7.40 ± 0.45 b | 9.30 ± 0.21 a | 8.20 ± 0.65 ab |
| Skin mucus | ||||
| Total protein (g dL−1) | 0.09 ± 0.005 b | 0.12 ± 0.015 a | 0.10 ± 0.01 a | 0.10 ± 0.04 a |
| Total Ig (g dL−1) | 0.058 ± 0.008 | 0.079 ± 0.008 | 0.066 ± 0.012 | 0.060 ± 0.017 |
| Lysozyme (U mL−1) | 11.10 ± 0.43 | 11.76 ± 0.28 | 11.20 ± 0.52 | 10.90 ± 0.40 |
| WBE | CT | GLE1 | GLE2 | GLE3 |
|---|---|---|---|---|
| CAT (U mL−1) | 9.91 ± 0.74 c | 14.44 ± 0.46 b | 16.59 ± 0.29 a | 15.93 ± 0.87 ab |
| SOD (U mL−1) | 520.97 ± 2.03 b | 725.05 ± 11.67 a | 718.29 ± 3.83 a | 724.36 ± 9.62 a |
| GPx (U mL−1) | 42.55 ± 1.02 b | 96.64 ± 1.59 a | 96.49 ± 1.59 a | 95.50 ± 2.36 a |
| MDA (µmol/mL) | 4.60 ± 0.30 b | 2.49 ± 0.72 a | 2.34 ± 0.90 a | 2.35 ± 0.95 a |
| Skin mucus | ||||
| CAT (U mL−1) | 10.09 ± 0.36 b | 11.62 ± 0.65 a | 11.57 ± 0.41 a | 9.77 ± 0.51 b |
| SOD (U mL−1) | 530.86 ± 8.03 ab | 558.16 ± 13.04 a | 548.46 ± 27.69 aa | 520.36 ± 15.51 b |
| GPx (U mL−1) | 42.70 ± 2.40 | 42.53 ± 1.12 | 43.39 ± 1.40 | 42.35 ± 2.10 |
| MDA (µmol/mL) | 4.48 ± 0.84 | 4.06 ± 0.36 | 4.43 ± 0.18 | 4.44 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoseinifar, S.H.; Fazelan, Z.; El-Haroun, E.; Yousefi, M.; Yazici, M.; Van Doan, H.; Paolucci, M. The Effects of Grapevine (Vitis vinifera L.) Leaf Extract on Growth Performance, Antioxidant Status, and Immunity of Zebrafish (Danio rerio). Fishes 2023, 8, 326. https://doi.org/10.3390/fishes8060326
Hoseinifar SH, Fazelan Z, El-Haroun E, Yousefi M, Yazici M, Van Doan H, Paolucci M. The Effects of Grapevine (Vitis vinifera L.) Leaf Extract on Growth Performance, Antioxidant Status, and Immunity of Zebrafish (Danio rerio). Fishes. 2023; 8(6):326. https://doi.org/10.3390/fishes8060326
Chicago/Turabian StyleHoseinifar, Seyed Hossein, Zohreh Fazelan, Ehab El-Haroun, Morteza Yousefi, Metin Yazici, Hien Van Doan, and Marina Paolucci. 2023. "The Effects of Grapevine (Vitis vinifera L.) Leaf Extract on Growth Performance, Antioxidant Status, and Immunity of Zebrafish (Danio rerio)" Fishes 8, no. 6: 326. https://doi.org/10.3390/fishes8060326
APA StyleHoseinifar, S. H., Fazelan, Z., El-Haroun, E., Yousefi, M., Yazici, M., Van Doan, H., & Paolucci, M. (2023). The Effects of Grapevine (Vitis vinifera L.) Leaf Extract on Growth Performance, Antioxidant Status, and Immunity of Zebrafish (Danio rerio). Fishes, 8(6), 326. https://doi.org/10.3390/fishes8060326