Abstract
Here, the olive leaf extract (OLE) rich in polyphenols was employed as a prebiotic agent, together with Lactobacillus reuteri and Bacillus clausii, to develop synbiotics. The prebiotic effect of olive leaf extract on the probiotic strains was tested at concentrations of 0, 50, 100, 400, and 1000 μg mL−1, and also 20 and 40 mg mL−1. Olive leaf extract at 40 mg mL−1 showed the best prebiotic activity on L. reuteri and B. clausii. A basal diet and two experimental synbiotic-containing diets were prepared. The synbiotic diets were manufactured by adding to the basal diet 5 × 106 CFU g−1 L. reuteri + 5 × 106 CFU g−1 B. clausii + 0.25 mg g−1 OLE and 1 × 107 CFU g−1 L. reuteri + 1 × 107 CFU g−1 B. clausii + 0.25 mg g−1 OLE. The diets were administered to the freshwater crayfish Astacus astacus (1.35 ± 0.04 g) in an 84-day feeding trial. The diet containing 5 × 106 CFU g−1 L. reuteri + 5 × 106 CFU g−1 B. clausii + 0.25 mg g−1 OLE significantly improved (p < 0.05) final weight, specific growth rate, body condition, and survival rate. A significant growth of Enterobacteriaceae, which include strains with proven beneficial activities for intestinal health and general animal welfare, significantly increased in crayfish fed with synbiotics. The obtained results could be suitable for functional feed development in crayfish farming.
Key Contribution:
Polyphenols extracted from olive leaf were employed as prebiotic agents with Lactobacillus reuteri and Bacillus clausii to develop synbiotics. Synbiotics promoted crayfish growth and gut resident probiotics belonging to the Enterobacteriaceae family, with proven beneficial activities for intestinal health and general animal welfare.
1. Introduction
Freshwater crayfish farming, widely practiced worldwide, faces challenges such as environmental sustainability, animal welfare, and profitability [1]. Diseases are often identified as the main menace to aquaculture activity. Losses can be caused by bacteria, viruses, and parasites responsible for slowing growth, lowering the quality of the final product, and, in the most serious cases, leading to the massive loss of farmed species [2]. Using antibiotics, vaccines, and other conventional pharmacological strategies to manage pathogens is not always desirable nor possible [3,4]. Currently, the aquaculture industry is focusing on developing innovative nutritional supplements and combinations of prebiotics and probiotics, also notorious as synbiotics. It has been reported in several studies carried out on farmed aquatic species that pre- and probiotics can act in synergy to positively influence growth, nutritional and digestive efficiency, carcass composition, and the immune response responsible for resistance to stress [5,6,7,8,9]. Recently, the development of second-generation synbiotics with polyphenols as prebiotic components has been proposed [10].
Polyphenols are a large class of phytocompounds characterized by antioxidant and antimicrobial activities widely exploited in aquaculture [11,12,13,14,15,16]. Plants, parts of plants, and agricultural by-products rich in polyphenols are used as a whole or extracted with solvents and mixed within the aquafeed [17]. For a long time, man has used the different parts (fruits and leaves) of the olive tree (Olea europaea L.) for nutritional and medicinal purposes [18]. Olive by-products, of which leaves represent the most substantial part, are rich in polyphenols such as oleuropein, glucoside-7-flavone, verbascoside, ligstroside, tyrosol, and hydroxytyrosol [19,20,21,22].
Among the probiotics, lactic acid bacteria (LAB) are promising candidates due to their properties capable of inducing growth, improving gut health, and boosting immunity against pathogenic bacteria [23,24,25]. The genus Lactobacillus is the largest of the lactic acid bacteria, with more than 200 recognized species and subspecies with fermentative metabolism and the ability to tolerate oxygen [26]. It is amply reported that Lactobacillus reuteri, belonging to the Lactobacillaceae family, has long been used as a probiotic in humans and other animals [27,28,29,30]. L. reuteri shows diversified and useful biochemical properties, such as tolerance to low pH and bile salts [31] and elevated activity of phytate degradation [32]. L. reuteri produces folate and cobalamin, also known as vitamin B12 [33]. Moreover, it produces a wide range of antimicrobial substances such as lactic acid, hydrogen peroxide [34], reutericyclin [35], and reuterin [36]. The latter is a potent antimicrobial agent also able to shape and model the content and spatial configuration of the gastrointestinal microbiota [37]. Further, its capabilities also extend to the inhibition of several Gram-positive and Gram-negative bacteria, fungi, and protozoa [38]. Among the most classic but always valid probiotic bacteria, there are both sporal and vegetative forms of Bacillus species (Bacillus clausii, Bacillus cereus, Bacillus pumilus), characterized by several different properties such as colonizing, immunostimulating, and antimicrobial activity [39]. B. clausii, a rod-shaped and motile Gram-positive bacterium, is capable of forming spores and is available in the Italian market for human consumption with the trade name of Enterogermina®, provided as spores (2 × 109) suspended in water [40]. The application of Bacillus spp. strains as probiotics not only ameliorate growth performance and pathogen inhibition in fish culture [41,42] but also improve water quality, decomposing and consuming organic material present in the water bodies [43].
Astacus astacus, or noble crayfish, is a native species common to nearly all freshwater bodies in Europe, and also a common human food source [44,45]. In the last years of the 19th century, with the introduction of non-native species, such as Pacifastacus leniusculus and Faxonius limosus, and the simultaneous spread of the highly infectious crayfish plague, transmitted by the oomycete Aphanomyces astaci, A. astacus populations in Europe acutely decreased [45,46,47]. Today, A. astacus is the European autochthonous crayfish species with the highest commercial value and is still of consumer interest because of its abundant meat content and high protein content. A. astacus is considered a luxury food product due to the limited availability, which increased its economic value. It is indeed available in small quantities only in local markets. A. astacus is the only native crayfish species in Estonia, where there are about 20 crayfish farms growing A. astacus. pointing at its potentiality to diversify the aquaculture production [48].
In this context, the purpose of this study was to investigate the adequacy of olive leaf extract rich in polyphenols as prebiotic agents and verify the ability of polyphenols to create functional synergy with the probiotics to develop phytoproduct-based synbiotics. The synbiotics were administered as a dietary supplement to freshwater crayfish A. astacus in feeding trials with the aim of evaluating the effects on the growth performance and the qualitative and quantitative composition of the microbiota with a culture-dependent approach.
2. Materials and Methods
2.1. Prebiotic Agent
The prebiotic employed in this study consisted of olive leaf extract (OLE) (Olea europaea L.) provided by EPO S.r.l., Milan, Italy, as hydroalcoholic dry extract (70% v/v ethanol) (OLE) filter-sterilized (0.45 μm). According to the manufacturer, the olive leaf used for the preparation of polyphenolic extract was harvested from the period of December to February from plants growing in China. The extract was dried by spray-drying.
2.2. Total Phenolic Content
The Folin–Ciocalteu method was employed to measure the total phenolic content in the OLE [49], modified according to [50]. Shortly, 50 µL of OLE were added to 2 mL of dH2O, 50 µL of Folin reagent (1:2 ratio), and 100 µL of 20% Na2CO4. The mixture was quickly vortexed and incubated for 90 min in the dark. Biomate 3 spectrophotometer-Thermo Spectronic (Thermo Fisher, Waltham, MA, USA) was employed to measure absorbance at 765 nm. The assay was carried out in triplicate. The obtained values of the absorbance were interpolated with a standard curve of gallic acid. The results were expressed as mg of gallic acid equivalent (GAE) g−1 of sample.
2.3. HPLC Analysis
HPLC analysis was performed on an HPLC apparatus (LC-4000) (JASCO, Osaka, Tokyo, Japan) consisting of a pump (model PU-2829 plus), a column oven (CO-2060 plus), a UV/Vis Photodiode Array Detector (MD-2818 plus), an autosampler (AS-2059 plus), and a ChromNAV software program (Jasco, Japan). Samples were loaded onto a C18 column of 5-μm particle size, 25 cm × 3.00 mm I.D. (Phenomenex, Torrance, CA, USA) with a guard cartridge manufactured with the same material. For HPLC injection, OLE was filtered with a 0.22 µm syringe filter of cellulose acetate. Chromatographic solvents used were water and acetic acid (97.5:2.5) (A) and acetonitrile (B). The flow rate was 0.8 mL min−1. The linear gradient started with 95% (A) and 5% (B); 75% (A) and 25% (B) after 20 min; 50% (A) e 50% (B) after 35 min; and 20% (A) e 80% (B) after 40 min. The system was rebalanced in 5 min at the initial condition of 95% (A) and 5% (B) ([51] with some modifications). The UV spectrum was acquired in the range of 200–620 nm. The main polyphenols were identified by comparing the retention times and absorption spectra with oleuropein and hydroxytyrosol standards (Sigma-Aldrich, St. Louis, MI, USA).
2.4. Probiotic Strains Tested as Component for Synbiotic Preparation
The probiotic strains used in this study were Lactobacillus reuteri and Bacillus clausii. In detail, probiotic Gram-positive L. reuteri DSM26866 was isolated from the pharmaceutical formulation Reuril PLUS (Pharmaluce, San Marino, Italy). Probiotic Gram-positive B. clausii SIN was isolated from the pharmaceutical formulation Enterogermina® (Sanofy, Milan, Italy). The strains were cultured under aerobic conditions at 37 °C in Luria Bertani (LB) (Thermo Fisher Scientific, Waltham, MA, USA), Lactobacillus MRS (HIMEDIA, Maharashtra, India) agar/broth medium, and Rogosa agar/broth medium (CONDA, Madrid, Spain). Bacterial load was quantified using the Colonies Forming Units (CFU) mL−1 formula, estimated by serial dilutions carried out in triplicate. Bacterial strains were stored at −80 °C in cryotubes with glycerol 10% (Sigma Aldrich, St. Louis, MI, USA) as cryoprotectants, and working cultures were activated at 37 °C for 24–48 h. Probiotic strains were included in the experimental diets in the form of vital dehydrated cell pellets combined with the prebiotic agent (OLE).
2.5. In Vitro Effect of the Prebiotic Agent (OLE) on L. reuteri and B. clausii Growth
The “agar well diffusion” assay, as described in [52] with minor modifications, was performed to evaluate, qualitatively, the activity of OLE against the probiotic strains. The bacterial cells of L. reuteri and B. clausii were grown in LB broth to an optical density (O.D.) of 0.5 at 600 nm. Then, an aliquot of the microbial suspension (200 µL) was spread on the agar plate, where wells (5 mm diameter) were cut. Different concentrations of OLE (0, 1, 2, 4 mg) were placed into the wells. Amoxicillina (AMX) (Aesculapius Farmaceutici S.r.l., Brescia, Italy) at a concentration of 250 µg well−1 was used as a positive control. The hydroalcoholic buffer (70% ethanol: 30% water) was used as a negative control. Plates were incubated at 37 °C for 48 h. Thereafter, the mean diameter of the inhibition zones (MDIZ) (expressed in mm) caused by the natural extract was measured to evaluate the expression of in vitro activities against the tested microorganisms. To quantify the prebiotic effect of the OLE on the probiotic strains, an in vitro growth and survival assay was performed (fitness assay). In particular, the susceptibility of L. reuteri and B. clausii to different concentrations of OLE was determined according to Clinical and Laboratory Standards Institute (CLSI) guidelines by means of the dilution tube method with standard inoculums of 1 × 105 CFU mL−1, [53,54]. OLE was added to tubes at the final concentrations of 0, 50, 100, 400, and 1000 μg mL−1. The bacterial cultures were incubated at 37 °C in a shaking incubator (ES-20, Biosan, Riga, Latvia) at 150 rpm. To evaluate the growth and survival of probiotic strains, during the overall observation period of 144 h, the O.D. was measured at a wavelength of 600 nm with a spectrophotometer (Ultrospec 2000, Pharmacia Biotech, Cologno Monzese, Milan, Italy) at intervals of 6, 24, 48, 72, 120, and 144 h, and aliquots of the diluted bacterial suspensions were spread on LB agar. Finally, the plates were incubated for 48 h at 37 °C to carry out the count of the viable bacterial colonies. The probiotic strains were also tested with amoxicillin as a positive control and the hydroalcoholic buffer as a negative control. To prove the prebiotic effect of OLE at concentrations higher than 1000 μg mL−1 on probiotic strains, we performed a microplate growth assay based on [55] with minor modifications. Briefly, a standard inoculum (1 × 105 CFU mL−1) of each of the probiotic strains was prepared according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [53,54]. OLE was added, achieving final concentrations of 0, 20, and 40 mg mL−1. Microbial culture without OLE was used as a positive control, while the negative control consisted of inoculum medium only (LB broth). The assay was performed in triplicate. The plates were incubated at 37 °C, and absorbance was read at 600 nm after 24, 48, and 72 h with a 680-microplate reader (Bio-Rad, Hercules, CA, USA).
2.6. In Vivo Studies: Experimental Diets
A basal (control) diet was formulated on the basis of the literature on crayfish [56,57,58]. It contained proteins (40%), carbohydrates (52%), and lipids (8%). The chemical composition of the feed was determined by Weende analysis at the Estonian University of Life Sciences (EULS) Institute of Veterinary Medicine and Animal Sciences Laboratory of Animal Nutrition. Weende analysis is a method for the quantitative analysis of macronutrients in feeding stuff for the determination of crude protein, crude fat, crude fiber, crude ash, moisture, and nitrogen-free extracts (digestible carbohydrates). More information can be found at https://www.nutricontrol.nl/en-gb/analysis/nutritional-value/weende-analysis/ (accessed on 30 March 2023). Ingredients and proximate analysis are reported in Table 1. The gross energy was calculated based on the formula: Gross energy = (23.9 PC + 39.8 CFatC + 20.1 CFibreC + 17.5 NFE):100; where PC = feed protein content (%), CFatC = feed crude fat content (%), CFibreC = feed crude fibre content (%), and NFE = nitrogen free extract in feed (%), 23.9; 39.8; 20.1; 17.5—calorimetric multipliers.

Table 1.
Ingredients and proximate analysis of the basal diet.
The experimental diets were prepared as follows: (1) basal (control) diet (control group); (2) SYN1 diet (5 × 106 CFU g−1 L. reuteri + 5 × 106 CFU g−1 B. clausii + 0.25 mg g−1 OLE) (SYN1 group); (3) SYN2 diet (1 × 107 CFU g−1 L. reuteri + 1 × 107 CFU g−1 B. clausii + 0.25 mg g−1 OLE) (SYN2 group). The dose of OLE (0.25 mg g−1) in the diet was established based on the results of the in vitro effect of the prebiotic agent (OLE) on L. reuteri and B. clausii (see above). The dose of probiotics was based on crustacean studies available in the literature [59]. The bacterial cells of the two probiotics were grown (under the conditions reported in the Section 2.4) until they reached the population density corresponding to 5 × 106 and 1 × 107 CFU mL−1. The cell suspension was centrifuged at 3000 rpm at 4 °C for 15 min (Centrifuge 5804 R Eppendorf, Milan, Italy). Finally, powdered ingredients were mixed with the cell pellet and OLE. Water was added to form a dough that was pressure pelleted with a meat grinder in order to achieve the pellets. The pellets were oven dried at 35 °C and then stored in plastic bags at 4 °C.
2.7. Crayfish and Sample Collection
The feeding trial with 54 healthy A. astacus crayfish (1.35 ± 0.04 g) was carried out at the Institute of Veterinary Medicine and Animal Sciences of the Estonian University of Life Sciences (Tartu, Estonia). Crayfish originated from the laboratory of the same institute and were stocked at a density of six individuals per 112-L−1 tank (0.32 m2), three tanks (i.e., 18 animals) to each treatment (9 tanks in total) in a recirculating aquaculture system (RAS). Each tank contained 18 plastic tubes (2 cm diameter and 10 cm length) (three times the number of crayfish for each tank) as hiding places for the crayfish. During the feeding trial, the water temperature was maintained at 22.5 °C. DO (7.90 ± 0.35 mg L−1), pH (8.19 ± 0.10), hardness (183.48 ± 8.95 mg L−1 CaCO3), NO2− (0 mg L−1), and NO3− (<10 mg L−1) contents were measured every week. DO was measured by Marvet Junior oxygen meter, pH was measured by XC PC7 pH meter, and hardness, NO2−, and NO3− were measured by JBL EasyTest 6in1. Animals were held under L:D 12:12 h. Each diet was randomly assigned to a tank. Crayfish were fed 2% body weight once a day (2:00 p.m.) for 84 days. During this period, crayfish molted an average of 1.5 times. Biometry was carried out at the onset and the end of the feeding trial.
2.8. Evaluation of Growth Performance
After 84 days, each specimen was singularly weighed (±0.01) on an electronic scale (KERN, Balingen, Germany) for estimation of growth. The growth parameters and the survival rate were measured as follows:
where BL = body length (cm), W = weight (g), Wi = initial weight, Wf = final weight, and t = time (days). All parameters were corrected throughout the feeding trial based on the amount of ingested feed.
Weight gain (WG, %) = Wf − Wi/Wi × 100
Specific growth rate (SGR, % day−1) = ln(Wf) − ln(Wi)/t × 100
Survival Rate (SR, %) = final individual numbers/initial individual numbers × 100
Feed Conversion Ratio (FCR) = Feed consumed/WG
Foulton’s Condition Factor = W/BL3 × 100
2.9. Microbiota Intestinal Isolation and Analysis by Culture-Dependent Methods
After 84 days, 3 specimens per tank (total 9 per treatment) were sampled at random for microbiological analysis. The samples were prepared for microbiota analysis according to [60]. Briefly, the gastrointestinal tract was aseptically removed from crayfish specimens, preserving its integrity. It was homogenized with Buffered Peptone Water (Oxoid, Waltham, MA, USA) and shaken by a vortex, avoiding sample overheating, until the sample was dispersed in the diluent. 100 µL of serially diluted samples were spread onto Luria Bertani (LB) Agar (CONDA), MacConkey Agar (CONDA), Cetrimide Agar Base (CONDA), Bacillus ChromoSelect Agar (BCA), Tryptose Sulfite Cycloserine (TSC) (CONDA), and Sabouraud Agar (CONDA) for determination of total aerobic mesophilic bacteria, Enterobacteriaceae, Pseudomonas spp., Bacillus spp., and total anaerobic bacteria and yeasts, respectively. The plates were incubated for 24–96 h at the appropriate conditions to allow for microbial growth. Plates containing 30–300 colonies were selected for colony-forming units (CFU) g−1. AnaeroJar anaerobic jar (Bio-Class) and bags for Atmosphere Generation System CampyGenTM (Thermo Fisher Scientific) were employed to recreate anaerobiosis. Pure cultures representing microbial isolates of each group were stored at −80 °C in broth media added with 10% glycerol (v/v) (Carlo Erba Reagents, Waltham, MA, USA). Microbial counts were carried out in triplicates and expressed as Log CFU g−1 ± SD.
2.10. Statistical Analysis
Data were presented as mean values ± standard deviation (SD). The data expressed as percentages were transformed using the arcsine method. Brown–Forsythe and Kolmogorov–Smirnov tests were used to respectively confirm homogeneity of variance and data normality [61]. One-way ANOVA followed by Dunnett post hoc test (p < 0.05) were applied to compare dietary treatments. Two-way ANOVA followed by Bonferroni post hoc test (p < 0.05) were adopted for the microbiological analysis performed by culture-dependent methods. Data were calculated with GraphPad Prism software, version 8.0.2 (GraphPad, Inc., San Diego, CA, USA).
3. Results
3.1. Characterization of the OLE
The total phenolic content in OLE was 202.13 ± 4.77 mg GAE g−1. Figure 1 reports the chromatogram of OLE analyzed by HPLC at a wavelength of 280 nm. The most abundant polyphenols were hydroxytyrosol and oleuropein.
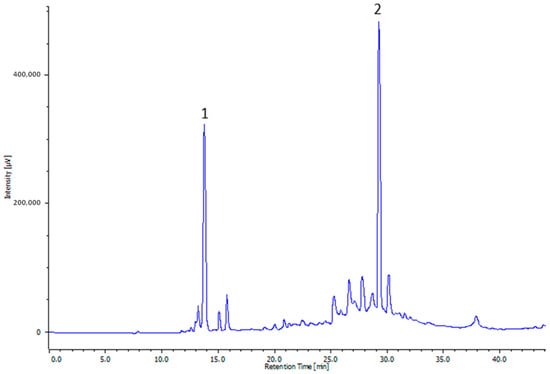
Figure 1.
Representative HPLC profile of olive leaf extract (OLE). The numbers indicate the following molecules: hydroxytyrosol (1) and oleuropein (2).
3.2. In Vitro Effect of the Prebiotic Agent (OLE) on the Probiotic Agent (L. reuteri and B. clausii) Growth
According to the “agar well diffusion” assay, OLE at a concentration of 1 and 2 mg well−1 showed no inhibitory activity against L. reuteri and B. clausii, while OLE at a 4 mg well−1 exhibited a weak inhibitory activity only against B. clausii. AMX, used as a positive control, showed antibacterial activity against both L. reuteri and B. clausii, while no effects were observed for the hydroalcoholic buffer used as a negative control. The mean diameter inhibition zones (MDIZ) are reported in Table 2.

Table 2.
Mean diameter inhibition zones (MDIZ) of olive leaves extracts (OLE) determined with the “agar well diffusion” assay. Data are expressed as mean ± SD of triplicates. AMX = amoxicillin.
The results of the prebiotic effect of 0, 50, 100, 400, and 1000 μg mL−1 of OLE on L. reuteri and B. clausii growth and survival are presented in Figure 2. The greatest prebiotic effect was recorded during the first 48 h of incubation. In particular, OLE at 1000 μg mL−1 caused a significant increase in growth of L. reuteri at 48 h (Figure 2A,B), while the prebiotic effect on B. clausii was not statistically significant (Figure 2C,D).
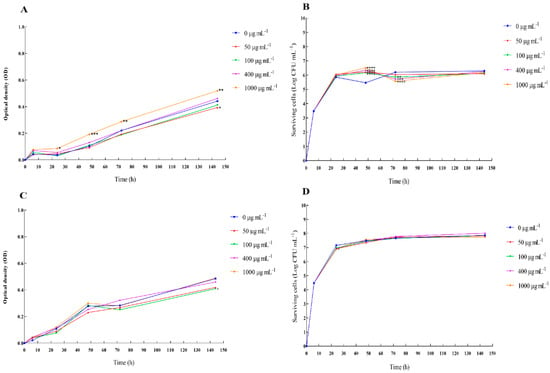
Figure 2.
Prebiotic effect of OLE on the growth and survival (fitness assay) of Lactobacillus reuteri and Bacillus clausii. The growth and survival of L. reuteri cells (A,B) and B. clausii cells (C,D) in the absence and presence of 0, 50, 100, 400, and 1000 μg mL−1 of OLE during a 144 h-observation period. The experiment was carried out in triplicate. A two-way ANOVA test followed by Bonferroni correction was applied for multiple comparisons against the control. Results are reported as means ± SD. Asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001) report the statistical significance of cultures treated against control (bacterial cultures without extracts).
The results of the prebiotic effect of 0, 20, and 40 mg mL−1 OLE on L. reuteri and B. clausii growth are presented in Figure 3. OLE at 40 mg mL−1 showed prebiotic activity on L. reuteri and B. clausii at 24, 48, and 72 h. OLE at 20 mg mL−1 showed prebiotic activity at 72 h on L. reuteri and 48 and 72 h on B. clausii.
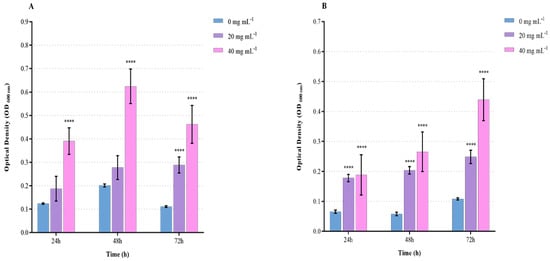
Figure 3.
Prebiotic effect of OLE on Lactobacillus reuteri and Bacillus clausii growth (macroplate growth assay). L. reuteri (A) and B. clausii (B) growth after 24, 48, and 72 h of incubation in the absence and presence of OLE (0, 20, 40 mg mL−1). Data are reported as means ± SD. Asterisks (**** p < 0.0001) report the statistical significance of cultures treated against control (bacterial cultures without extracts).
Growth performances and survival rates of freshwater crayfish fed with synbiotics for 84 days are shown in Table 3. The administration of synbiotics significantly improved (p < 0.05) final weight, specific growth rate, feed conversion ratio, body condition, and survival rate in adult crayfish with respect to control specimens.

Table 3.
Growth performances and survival rates of freshwater crayfish fed with synbiotics for 84 days. Data are reported as mean ± SD (n = 3).
In particular, crayfish fed the SYN1 diet achieved the highest final weight (2.47 g), weight gain (80.20%), specific growth rate (0.70% body weight day−1), and the lowest feed conversion ratio (2.91). The survival rate of crayfish fed the SYN1 diet was significantly (p < 0.05) higher compared to the control. SYN2 diet significantly enhanced (p < 0.05) specific growth rate (0.63% body weight day−1) and feed conversion ratio (2.98) compared to the control, while no significant improvement in terms of WG and survival rate were observed. Finally, the non-lethal morphometric index (Fulton’s condition factor, FCF), used to evaluate the body condition [62], significantly increased in crayfish fed SYN1 diet compared with the control group.
3.3. Microbiological Analysis
Figure 4 shows the profiles of the gastrointestinal microbiota of crayfish on a basal diet and crayfish on synbiotic-supplemented diets. The number of total microorganisms and total aerobic mesophilic bacteria significantly increased in synbiotic groups, particularly in the SYN2 group. The Enterobacteriaceae, not detected in the control group, increased significantly in the two groups subjected to synbiotic diets. The number of Bacillus spp. and anaerobic bacteria significantly escalated in the SYN1 and SYN2 groups with respect to the control. Finally, the yeasts significantly increased in the SYN1 group.
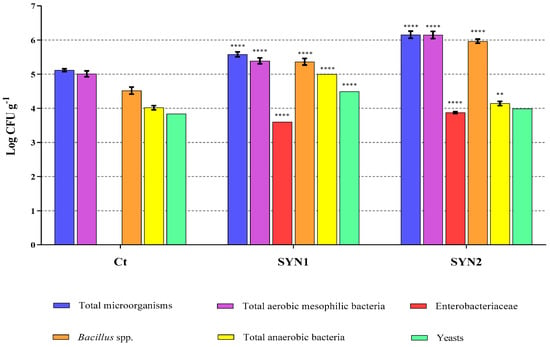
Figure 4.
Gastrointestinal microbiota analysis by culture-dependent method in crayfish fed the control diet and synbiotic supplemented diets. Data are reported as mean ± SD and expressed as Log CFU g−1. Asterisks (** p < 0.01; **** p < 0.0001) report the statistical significance of groups fed the synbiotic supplemented diets with respect to the control group.
4. Discussion
In this study, we report the evidence of prebiotic attributes of OLE rich in polyphenols to develop, in combination with probiotic microorganisms, synbiotics to be used in crayfish nutrition. Synbiotics, the combination of prebiotic and probiotic, currently represent one of the emerging food strategies for the improvement of the growth performances, health status, and well-being of shellfish [63,64,65,66,67] and finfish species [68,69,70,71,72]. Currently, much attention is paid to synbiotics containing polyphenols in place of the traditional carbohydrates as prebiotics agents [10]. In this study, the compatibility of OLE with two different probiotic strains, L. reuteri and B. clausii, was tested. The aim was to manufacture a synbiotic product suitable for ameliorating growth performances and intestinal microbiota of the farmed crayfish A. astacus. olive leaf extract possesses prebiotic characteristics, reported in several studies on farmed animals, such as pigs [73,74], ruminants [75], fish [76,77,78], and Crustaceans [11].
The olive leaf extract contains phenolic compounds in variable amounts. This is due to the interaction of various factors, such as type of cultivar, age of the plant, geographic production area, climate, and harvesting time. The latter, in particular, are particularly important since the maximum peak in phenolic content is found in leaves harvested in the cold months, particularly in December [79,80,81]. In our study, the total phenolic amount in the hydroalcoholic dry OLE resulted to be 202.13 ± 4.77 mg GAE g−1. This is in agreement with a previous study, in which olive leaf extract showed a total phenolic amount ranging from 72.27 to 249.81 mg GAE g−1 [82]. Hydroxytyrosol and oleuropein resulted to be the most abundant polyphenols of OLE, as confirmed by data in the literature [22,83,84].
Initially, the in vitro approaches were necessary to detect the growth and survival of the probiotic strains exposed to different concentrations of OLE, to select optimal synergistic combinations in the synbiotic formulation to be tested in in vivo experiments. The “agar well diffusion” assay, microbiological screening used to qualitatively highlight the microbial growth, indicates the absence of significant inhibitory effects of OLE against the probiotic bacteria. The result of the fitness assay showed a significant increment in the growth rate of L. reuteri, in the presence of OLE, with an evident prebiotic effect of OLE at a concentration of 1000 μg mL−1. No statistically significant effect of OLE at a concentration of 1000 μg mL−1 was detected in B. clausii. The microplate growth assay carried out with higher concentrations of OLE (20 and 40 mg mL−1) allowed for the confirmation of the prebiotic effect on L. reuteri and B. clausii. The increase in the growth rate of the probiotics following the addition of OLE could be due to the presence of oleuropesides (oleuropein and verbascoside), substituted phenols (tyrosol and hydroxytyrosol), and flavones (luteolin, apigenin) [82,85]. The outcome of our study is in accordance with several studies in the literature. For example, [86] reported that olive leaf extract increased the growth of Lactobacillus acidophilus and Bifidobacterium infantis in milk. These data are also confirmed by a study of [87] in which different concentrations of olive leaf extract enhanced the rise of two probiotic strains, L. acidophilus and Bifidobacterium bifidum.
The obtained results confirmed that dietary supplementation with synbiotics improved the growth performance of crayfish. Crayfish fed with SYN1 showed improved performance (final weight, WG, SGR, and survival rate), Fulton’s condition factor, and lower FCR than SYN2 diet, indicating that the amount of probiotic administered is relevant. In the literature, the dose of probiotics for crustaceans ranges between 106 and 109 per gram of diet [59]. In this study, the dose established for L. reuteri is in accordance with the literature data regarding the administration of probiotics belonging to the Lactobacillus genus in crustaceans [88,89,90,91]. Furthermore, for B. clausii, the doses selected are in agreement with previous studies conducted using probiotics of the Bacillus genus in finfish and shellfish diets [7,90,92,93]. It is worth noting that the presence of OLE may have exerted a double action. On the one hand, OLE may have sustained and ameliorated the survival of the probiotics administered with the diet, and on the other hand, it may have exerted growth promoting effects, as already reported in Pontastacus leptodactylus [11].
In this study, the growth rate was in agreement with a study conducted by [94] on juvenile A. astacus of similar weight, reared at the same water temperature used here for a period of 3 months (64% increase in our study compared to 67% of the [94] study). However, the molt frequency and shed intervals were higher than those found here. Since temperature is known to positively influence molt frequency [94], our results require an explanation. A possible hypothesis may lie in the fact that since crayfish usually eat their shells, numbers may have been overlooked in this study where crayfish shared the same tank, making it difficult to estimate the exact number of molts. On the contrary, in [94], A. astacus were individually reared, which surely made the assessment of shell number more accurate. It is worth emphasizing that the focus of the present study was not the evaluation of the relationship between the temperature and the molt frequency but instead the evaluation of growth parameters improvement as a consequence of dietary administration of synbiotics. The present results are in agreement with previous findings on P. leptodactylus fed with Enterococcus faecalis + XOS [64]; Litopenaeus vannamei fed with Bacillus spp. (Bacillus sp. D2.2) + sweet potato extract [66]; Cherax quadricarinatus fed with Micrococcus spp. + alginate [95]; Macrobrachium rosenbergii fed with Pediococcus acidilactici + Saccharomyces cerevisiae + β-glucan [96]; L. vannamei fed with Lactobacillus plantarum + cacao pod husk pectin [67]; Eriocheir sinensis fed with probiotics (Lactobacillus acidophilus, Bacillus subtilis and Saccharomyces cerevisiae) + FOS [90]; L. vannamei fed with Pediococcus pentosaceus + FOS [97]. Growth enhancement in shellfish and finfish species could be the consequence of digestive enzyme increased activities, improvement in the production of some metabolites (vitamins and short-chain fatty acids), hydrolysis of non-digestible substrate, enhancement of voluntary feed intake, and adaptive responses of digestive tract morphology [57,65,68,98,99,100].
Recently, the relationship between the intestinal microbiota and some physiological functions of the host, such as metabolism, development, and health status, has been fully ascertained [101]. Several factors could shape the composition of the complex microbial community [102]. Among them, diet is the one in which is easier, more immediate, and more effective to intervene. The health-promoting effect deriving from the synergistic and contemporary action of prebiotics and probiotics in synbiotics is an increasingly applied strategy to control the growth of harmful intestinal bacteria [103]. In fact, prebiotic compounds constitute the substrate for the selective growth of probiotic strains and the production of secondary metabolites (e.g., SCFAs) conferring gut health benefits [104]. In the present study, synbiotics administration modulated crayfish gastrointestinal microbiota, causing a general increase in the bacteria load, in agreement with a study reporting the increase in the total microbial load in specimens of giant crayfish (Macrobrachium rosenbergii) after the use of Bacillus licheniformis as a probiotic [105]. In the present study, Enterobacteriaceae were found only in synbiotic-fed crayfish. This is in agreement with a study [106] in which the administration of seaweeds (Ulva lactuca) as a feed additive promoted the growth of Enterobacteriaceae in specimens of white shrimp (Penaeus vannamei). These microorganisms resulted to be of crucial importance for the correct development and functioning of intestinal microflora, being involved in a series of processes, including digestion and food absorption, production of advantageous metabolites, and protection against pathogens [107,108]. Lately, it has been reported that the microencapsulation of Enterobacter spp. exerts a protective effect against bacterial cold-water disease in rainbow trout, Oncorhynchus mykiss [109]. The beneficial increase in Bacillus spp. levels in crayfish supplemented with synbiotics is sustained by the use of some Bacillus strains as feeding additives to stimulate growth, immune response [110], and phagocytic, anti-peroxidase, and lysozyme activities in aquatic species [111]. Regarding the presence of anaerobic bacteria, they significantly increased in the groups treated with synbiotics (facultative anaerobics), particularly in the group fed the SYN1 diet. These data are consistent with various studies. Particularly, in a recently published study carried out in smooth marron (Cherax cainii), the addition of different Lactobacilli in the diet generated an augmented community of beneficial anaerobic bacteria, including oxygen-tolerant anaerobes belonging to Lactobacillus genus and strict anaerobes belonging to Bacteroidetes and Fusobacteria groups, associated with improved health and immune status [112]. Finally, there was a significant increase in yeasts in specimens fed the SYN1 diet, while no significant changes were observed in the SYN2 group. At present, the knowledge of the eukaryotic component present in the crayfish intestine is still limited. However, previously conducted studies have established the presence of microorganisms of the Alternaria, Tuber, Cladosporium, and Saccharomyces genus as components of the intestinal mycobiota of whiteleg shrimp, Litopenaeus vannamei [113]. Research is currently implementing the use of eukaryotic microorganisms, including yeasts of the genus Saccharomyces, as substitutes or dietary additives commonly used in aquaculture, due to their affirmative impact on the intestinal microbiota, growth performance, and immune response of aquatic species [114,115].
5. Conclusions
In conclusion, OLE demonstrated to be a good substrate for in vitro growth of probiotic bacteria. The OLE promoted microbial fitness, with the prebiotic effect particularly evident on Lactobacillus reuteri and Bacillus clausii in the first 72 h of incubation. In addition, the lowest probiotic-containing synbiotics positively influenced the core intestinal microbiota, causing a balanced increase of intestinal microbial communities. OLE-based synbiotics promoted the crayfish gut resident probiotics belonging to the Enterobacteriaceae family, which includes strains with proven beneficial activities for intestinal health and general animal welfare. The results of the study could be useful for functional food development in crayfish farming.
Author Contributions
C.P. and M.P. Conceptualization, main manuscript text writing, reviewing, and editing; D.S., S.F., G.F. and G.A.D.C. Investigation and data curation; K.K. Investigation, funding acquisition, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Maritime and Fisheries Fund (EMFF 2014–2020 implementation plan, Estonian Agricultural Registers and Information Board project ID 821017780002).
Institutional Review Board Statement
The experiment described in this study is not subject to ethics committee approval and consent procedures. Animal welfare was ensured in the laboratory experiment.
Data Availability Statement
Original data are available at request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, A.; Sheikh Abdullah, S.R.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Manage, M.M. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Mohd-Aris, A.; Muhamad-Sofie, M.H.N.; Zamri-Saad, M.; Daud, H.M.; Ina-Salwany, M.Y. Live vaccines against bacterial fish diseases: A review. Vet. World. 2019, 12, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Synbiotic Effects of Aspergillus oryzae and β-Glucan on Growth and Oxidative and Immune Responses of Nile Tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2020, 12, 172–183. [Google Scholar] [CrossRef]
- Hasyimi, W.; Widanarni, W.; Yuhana, M. Growth Performance and Intestinal Microbiota Diversity in Pacific White Shrimp Litopenaeus vannamei Fed with a Probiotic Bacterium, Honey Prebiotic, and Synbiotic. Curr. Microbiol. 2020, 77, 2982–2990. [Google Scholar] [CrossRef]
- Yao, W.; Li, X.; Zhang, C.; Wang, J.; Cai, Y.; Leng, X. Effects of dietary synbiotics supplementation methods on growth, intestinal health, non-specific immunity and disease resistance of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 112, 46–55. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant-polyphenols based second-generation synbiotics: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef]
- Parrillo, L.; Coccia, E.; Volpe, M.G.; Siano, F.; Pagliarulo, C.; Scioscia, E.; Varricchio, E.; Safari, O.; Eroldogan, T.; Paolucci, M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 2017, 473, 161–168. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Shakouri, M.; Yousefi, S.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Mazandarani, M.; Mozanzadeh, M.T.; Tulino, M.G.; Faggio, C. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 2020, 100, 171–178. [Google Scholar] [CrossRef]
- Jahazi, A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Ahmadi, A.; Bagheri, D.; Hoseinifar, S.H.; Morshedi, V.; Paolucci, M. Beneficial role of polyphenols as feed additives on growth performances, immune response and antioxidant status of Lates calcarifer (Bloch, 1790) juveniles. Aquaculture 2022, 552, 737955. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal Biomedicines: A New Opportunity for Aquaculture Industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Lujan, R.J.; Rodriguez, J.; Castro, M. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Mohamed, R.; Pineda, M.; Aguilar, M. Antioxidant Capacity of Extracts from Wild and Crop Plants of the Mediterranean Region. J. Food Sci. 2007, 72, S059–S063. [Google Scholar] [CrossRef]
- Gholamhosseini, A.; Kheirandish, M.R.; Shiry, N.; Akhlaghi, M.; Soltanian, S.; Roshanpour, H.; Banaee, M. Use of a methanolic olive leaf extract (Olea europaea) against white spot virus syndrome in Penaeus vannamei: Comparing the biochemical, hematological and immunological changes. Aquaculture 2020, 528, 735556. [Google Scholar] [CrossRef]
- Di Meo, M.C.; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.P.; Giaquinto, D.; Palladino, A.; Zotti, T.; Vito, P.; Paolucci, M.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In Vitro Antioxidant Properties. ACS Food Sci. Technol. 2022, 2, 31–40. [Google Scholar] [CrossRef]
- Martínez, C.P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. ISRN Microbiol. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. Updating the importance of lactic acid bacteria in fish farming: Natural occurrence and probiotic treatments. J. Mol. Microbiol. Biotechnol. 2007, 14, 107–114. [Google Scholar] [CrossRef]
- Ringø, E.; Francois-Joel, G. Lactic acid bacteria in fish: A review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Bhogoju, S.; Khwatenge, C.N.; Taylor-Bowden, T.; Akerele, G.; Kimathi, B.M.; Donkor, J.; Nahashon, S.N. Effects of Lactobacillus reuteri and Streptomyces coelicolor on growth performance of broiler chickens. Microorganisms 2021, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Q.; Zeng, X.; Yang, F.; Zhang, J.; Liu, H.; Ma, X.; Qiao, S. Complete Genome Sequence of Lactobacillus reuteri I5007, a Probiotic Strain Isolated from Healthy Piglet. J. Biotechnol. 2014, 179, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic Isolates from Unconventional Sources: A Review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Ibrahim, S.A.; Kim, C.; Shahbazi, A. Significance of Bile Salt Tolerant Lactobacillus reuteri. In Proceedings of the 2007 National Conference on Environmental Science and Technology; Nzewi, E., Reddy, G., Luster-Teasley, S., Kabadi, V., Chang, S.-Y., Schimmel, K., Uzochukwu, G., Eds.; Springer: New York, NY, USA, 2009; pp. 17–23. [Google Scholar] [CrossRef]
- Hayek, S.A.; Shahbazi, A.; Worku, M.; Ibrahim, S.A. Enzymatic activity of Lactobacillus grown in a sweet potato base medium. Br. Microbiol. Res. J. 2014, 4, 509–522. [Google Scholar] [CrossRef]
- Santos, F.; Wegkamp, A.; de Vos, W.M.; Smid, E.J.; Hugenholtz, J. High-Level Folate Production in Fermented Foods by the B12 Producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 2008, 74, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.C.R.; Seney, S.L.; Summers, K.L.; Nomizo, A.; Martinis, E.C.P.D.; Reid, G. Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol. Immunol. 2009, 53, 487–495. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Höltzel, A.; Walter, J.; Jung, G.; Hammes, W.P. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 2000, 66, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Toh, H.; Fukuda, S.; Horikawa, H.; Oshima, K.; Suzuki, T.; Murakami, M.; Hisamatsu, S.; Kato, Y.; Takizawa, T.; et al. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008, 15, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Talarico, T.L.; Dobrogosz, W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989, 33, 674–679. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.; Cutting, S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Olmos, J.; Acosta, M.; Mendoza, G.; Pitones, V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch. Microbiol. 2020, 202, 427–435. [Google Scholar] [CrossRef]
- Padmavathi, P.; Sunitha, K.; Veeraiah, K. Efficacy of probiotics in improving water quality and bacterial flora in fish ponds. Afr. J. Microbiol. Res. 2012, 6, 7471–7478. [Google Scholar] [CrossRef]
- Holdich, D.M. Biology of Freshwater Crayfish, 1st ed.; Blackwell Science: Oxford, UK, 2001; pp. 1–720. [Google Scholar]
- Holdich, D.M. Distribution of crayfish in Europe and some adjoining countries. Bull. Fr. Peche Piscicult. 2002, 367, 611–650. [Google Scholar] [CrossRef]
- Edgerton, B.F.; Evans, L.H.; Stephens, F.J.; Overstreet, R.M. Synopsis of freshwater crayfish diseases and commensal organisms. Aquaculture 2002, 206, 57–135. [Google Scholar] [CrossRef]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2010, 11, 394–395. [Google Scholar] [CrossRef]
- Kaldre, K.; Paaver, T.; Hurt, M.; Gross, R. Continuing expansion of non-indigenous crayfish species in Northern Europe: First established spiny-cheek crayfish Faxonius limosus (Refinesque, 1817) population in Estonia. BioInvasions Rec. 2020, 9, 127–132. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and cultivar-dependent traits of Prunus avium and Prunus cerasus polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Tenth Edition; CLSI Document M07-A10; Clinical and Laboratory Standard Institute: Waine, PA, USA, 2015; Available online: https://clsi.org/media/1632/m07a10_sample.pdf (accessed on 10 January 2021).
- Varaldo, P.E. Antimicrobial resistance and susceptibility testing: An evergreen topic. J. Antimicrob. Chemother. 2002, 50, 1–4. [Google Scholar] [CrossRef]
- Akinyele, T.A.; Igbinosa, E.O.; Akinpelu, D.A.; Okoh, A.I. In vitro assessment of the synergism between extracts of Cocos nucifera husk and some standard antibiotics. Asian Pac. J. Trop. Biomed. 2017, 7, 306–313. [Google Scholar] [CrossRef]
- Ackefors, H.; Castell, J.D.; Boston, L.D.; Räty, P.; Svensson, M. Standard experimental diets for crustacean nutrition research. II. Growth and survival of juvenile crayfish Astacus astacus (Linné) fed diets containing various amounts of protein, carbohydrate and lipid. Aquaculture 1992, 104, 341–356. [Google Scholar] [CrossRef]
- Safari, O.; Shahsavani, D.; Paolucci, M.; Atash, M.M.S. Single or combined effects of fructo- and mannan oligosaccharide supplements on the growth performance, nutrient digestibility, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquaculture 2014, 432, 192–203. [Google Scholar] [CrossRef]
- Safari, O.; Atash, M.M.S.; Paolucci, M. Effects of dietary L-carnitine level on growth performance, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquaculture 2015, 439, 20–28. [Google Scholar] [CrossRef]
- Tran, N.T.; Li, S. Potential role of prebiotics and probiotics in conferring health benefits in economically important crabs. Fish Shellfish Immunol. Rep. 2022, 3, 100041. [Google Scholar] [CrossRef] [PubMed]
- Orso, G.; Solovyev, M.M.; Facchiano, S.; Tyrikova, E.; Sateriale, D.; Kashinskaya, E.; Pagliarulo, C.; Hoseinifar, H.S.; Simonov, E.; Varricchio, E.; et al. Chestnut Shell Tannins: Effects on Intestinal Inflammation and Dysbiosis in Zebrafish. Animals 2021, 11, 1538. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall: Hoboken, NJ, USA, 2007; pp. 1–255. [Google Scholar]
- Robinson, M.L.; Gomez-Raya, L.; Rauw, W.M.; Peacock, M.M. Fulton’s body condition factor K correlates with survival time in a thermal challenge experiment in juvenile Lahontan cutthroat trout (Oncorhynchus clarki henshawi). J. Therm. Biol. 2008, 33, 363–368. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M. Modulation of growth performance, immunity, and disease resistance in narrow-clawed crayfish, Astacus leptodactylus leptodactylus (Eschscholtz, 1823) upon synbiotic feeding. Aquaculture 2017, 479, 333–341. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M. Effect of in vitro selected synbiotics (galactooligosaccharide and mannanoligosaccharide with or without Enterococcus faecalis) on growth performance, immune responses and intestinal microbiota of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquac. Nutr. 2018, 24, 247–259. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M.; Motlagh, H.A. Effects of synbiotics on immunity and disease resistance of narrow-clawed crayfish, Astacus leptodactylus leptodactylus (Eschscholtz, 1823). Fish Shellfish Immunol. 2017, 64, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Harpeni, E.; Santoso, L.; Supono, S.; Wardiyanto, W.; Widodo, A.; Yolanda, L. Effects of dietary probiotic Bacillus sp. D2.2 and prebiotic sweet potato extract on growth performance and resistance to Vibrio harveyi in pacific white shrimp, Litopenaeus vannamei. Aquac. Indones. 2018, 18, 56–61. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chang, C.C.; Cheng, W. Synbiotic combination of prebiotic, cacao pod husk pectin and probiotic, Lactobacillus plantarum, improve the immunocompetence and growth of Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 118, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Mirvaghefi, A.R.; Amoozegar, M.A.; Sharifian, M.; Esteban, M.Á. Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon synbiotic feeding. Fish Shellfish Immunol. 2015, 45, 27–32. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Talebi, M.; Yousefi, M.; Van Doan, H.; Rufchaei, R.; Paolucci, M. Combined and Singular Effects of Ethanolic Extract of Persian Shallot (Allium hirtifolium Boiss) and Synbiotic Biomin ®IMBO on Growth Performance, Serum- and Mucus-Immune Parameters and Antioxidant Defense in Zebrafish (Danio rerio). Animals 2021, 11, 2995. [Google Scholar] [CrossRef]
- Safari, O.; Sarkheil, M.; Shahsavani, D.; Paolucci, M. Effects of Single or Combined Administration of Dietary Synbiotic and Sodium Propionate on Humoral Immunity and Oxidative Defense, Digestive Enzymes and Growth Performances of African Cichlid (Labidochromis lividus) Challenged with Aeromonas hydrophila. Fishes 2021, 6, 63. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Naraballobh, W.; Paolucci, M.; Wongmaneeprateep, S.; Charoenwattanasak, S.; Dawood, M.A.O.; Abdel-Tawwab, M. Dietary inclusion of watermelon rind powder and Lactobacillus plantarum: Effects on Nile tilapia’s growth, skin mucus and serum immunities, and disease resistance. Fish Shellfish Immunol. 2021, 116, 107–114. [Google Scholar] [CrossRef]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Van Doan, H.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef]
- Leskovec, J.; Rezar, V.; Svete, A.N.; Salobir, J.; Levart, A. Antioxidative Effects of Olive Polyphenols Compared to Vitamin E in Piglets Fed a Diet Rich in N-3 PUFA. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of Olive By-Products in Livestock with Emphasis on Small Ruminants: Implications on Rumen Function, Growth Performance, Milk and Meat Quality. Animals 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Sicuro, B.; Barbera, S.; Daprà, F.; Gai, F.; Gasco, L.; Paglialonga, G.; Palmegiano, G.B.; Vilella, S. The olive oil by-product in ’Rainbow trout Onchorynchus mykyss (Walbaum)’ farming: Productive results and quality of the product. Aquac. Res. 2010, 41, e475–e486. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef]
- Arsyad, M.A.; Akazawa, T.; Nozaki, C.; Yoshida, M.; Oyama, K.; Mukai, T.; Ogawa, M. Effects of olive leaf powder supplemented to fish feed on muscle protein of red sea bream. Fish Physiol. Biochem. 2018, 44, 1299–1308. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernandez-Gutiérrez, A.; Segura-Carretero, A. Chemometric Analysis for the Evaluation of Phenolic Patterns in Olive Leaves from Six Cultivars at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Malik, N.S.A.; Perez, J.L.; Brockington, J.E. Seasonal changes of individual phenolic compounds in leaves of twenty olive cultivars grown in Texas. J. Agric. Sci. Technol. 2012, 2, 242–247. [Google Scholar]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2009, 87, 369–376. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, C.; González, L.; Gámbaro, A.; Valdés, A.; Ibañez, E.; Vieitez, I. Characterization and incorporation of extracts from olive leaves obtained through maceration and supercritical extraction in Canola oil: Oxidative stability evaluation. LWT 2022, 160, 113274. [Google Scholar] [CrossRef]
- Haddadin, M.S.Y. Effect of Olive Leaf Extracts on the Growth and Metabolism of Two Probiotic Bacteria of Intestinal Origin. Pak. J. Nutr. 2010, 9, 787–793. [Google Scholar] [CrossRef]
- Marhamatizadeh, M.H.; Ehsandoost, E.; Gholami, P.; Mohaghegh, M.D. Effect of Olive Leaf Extract on Growth and Viability of Lactobacillus acidophilus and Bifidobacterium bifidum for Production of Probiotic Milk and Yoghurt. Int. J. Farming Allied Sci. 2013, 2, 572–578. [Google Scholar]
- Roomiani, L.; Ahmadi, S.; Ghaeni, M. Immune response and disease resistance in the white shrimp, Litopenaeus vannamei induced by potential probiotic Lactobacillus bulgaricus. Vet. Fak. Derg. 2018, 65, 323–329. [Google Scholar] [CrossRef]
- Valipour, A.; Nedaei, S.; Noori, A.; Khanipour, A.A.; Hoseinifar, S.H. Dietary Lactobacillus plantarum affected on some immune parameters, air-exposure stress response, intestinal microbiota, digestive enzyme activity and performance of narrow clawed crayfish (Astacus leptodactylus, Eschscholtz). Aquaculture 2019, 504, 121–130. [Google Scholar] [CrossRef]
- Wan, J.-J.; Pan, J.-L.; Shen, M.-F.; Xue, H.; Sun, M.-L.; Zhang, M.-Q.; Zhu, X.-H.; Ma, X.-K. Changes in the growth performance, antioxidant enzymes and stress resistance caused by dietary administration of synbiotic (fructooligosaccharide and probiotics) in juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquacult. Int. 2022, 30, 467–481. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Bae, J.; Jang, W.J.; Lee, S.; Bai, S.C. Evaluation of Potential Probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms 2020, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, M.; Feng, J.; Chen, Y.; Li, M.; Chang, X. Effects of dietary Bacillus licheniformis on growth performance, intestinal morphology, intestinal microbiome, and disease resistance in common carp (Cyprinus carpio L.). Aquacult. Int. 2021, 29, 1343–1358. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhu, G.; Zhao, K.; Jiang, W.; Li, H.; Wang, W.; Kumar, V.; Dong, S.; Zhu, W.; et al. Mechanism of the Potential Therapeutic Candidate Bacillus subtilis BSXE-1601 Against Shrimp Pathogenic Vibrios and Multifunctional Metabolites Biosynthetic Capability of the Strain as Predicted by Genome Analysis. Front. Microbiol. 2020, 11, 581802. [Google Scholar] [CrossRef]
- Kouba, A.; Kanta, J.; Buřič, M.; Policar, T.; Kozák, P. The effect of water temperature on the number of moults and growth of juvenile noble crayfish, Astacus astacus (Linnaeus). Freshw. Crayfish 2010, 17, 37–41. [Google Scholar]
- Amrullah, A.; Wahidah, W. Immune response and growth performance of crayfish Cherax quadricarinatus fed with supplementary diet of synbiotic. J. Akuakultur Indones. 2019, 18, 33. [Google Scholar] [CrossRef]
- Miao, S.; Han, B.; Zhao, C.; Hu, J.; Zhu, J.; Zhang, X.; Sun, L. Effects of dietary Pediococcus acidilactici GY2 single or combined with Saccharomyces cerevisiae or/and β-glucan on the growth, innate immunity response and disease resistance of Macrobrachium rosenbergii. Fish Shellfish Immunol. 2020, 98, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.T.X.; Linh, N.T.H.; Baruah, K.; Thuy, D.T.B.; Phuoc, N.N. The Combined Use of Pediococcus pentosaceus and Fructooligosaccharide Improves Growth Performance, Immune Response, and Resistance of Whiteleg Shrimp Litopenaeus vannamei Against Vibrio parahaemolyticus. Front. Microbiol. 2022, 13, 826151. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Ye, J.-D.D.; Wang, K.; Li, F.-D.D.; Sun, Y.-Z.Z. Single or combined effects of fructo- and mannan oligosaccharide supplements and Bacillus clausii on the growth, feed utilization, body composition, digestive enzyme activity, innate immune response and lipid metabolism of the Japanese flounder Paralichthys olivaceus. Aquac. Nutr. 2011, 17, e902–e911. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Mirvaghefi, A.; Amoozegar, M.A.; Merrifield, D.L.; Ringø, E. In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Nutr. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Chen, X.; Yi, H.; Liu, S.; Zhang, Y.; Su, Y.; Liu, X.; Bi, S.; Lai, H.; Zeng, Z.; Li, G. Probiotics Improve Eating Disorders in Mandarin Fish (Siniperca chuatsi) Induced by a Pellet Feed Diet via Stimulating Immunity and Regulating Gut Microbiota. Microorganisms 2021, 9, 1288. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef]
- Peng, M.; Patel, P.; Nagarajan, V.; Bernhardt, C.; Carrion, M.; Biswas, D. Feasible Options to Control Colonization of Enteric Pathogens with Designed Synbiotics. In Dietary Interventions in Gastrointestinal Diseases; Watson, R., Preedy, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–149. [Google Scholar] [CrossRef]
- Tran, N.T.; Tang, Y.; Li, Z.; Zhang, M.; Wen, X.; Ma, H.; Li, S. Galactooligosaccharides and Resistant Starch Altered Microbiota and Short-Chain Fatty Acids in an in vitro Fermentation Study Using Gut Contents of Mud Crab (Scylla paramamosain). Front. Microbiol. 2020, 11, 1352. [Google Scholar] [CrossRef]
- Ranjit, K.N.; Raman, R.P.; Jadhao, S.B.; Brahmchari, R.K.; Kumar, K.; Dash, G. Effect of dietary supplementation of Bacillus licheniformis on gut microbiota, growth and immune response in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquac. Int. 2012, 21, 387–403. [Google Scholar] [CrossRef]
- Elizondo-González, R.; Quiroz-Guzmán, E.; Howe, A.; Yang, F.; Flater, J.; Gemin, M.; Palacios, E.; Peña-Rodríguez, A. Changes on the intestinal bacterial community of white shrimp Penaeus vannamei fed with green seaweeds. J. Appl. Phycol. 2020, 32, 2061–2070. [Google Scholar] [CrossRef]
- Parsa, A.; Bahramian, S.; Sharifpour, I. Effect of oral consumption of Aloe vera gel on intestinal microflora and liver tissue of rainbow trout. Iran. J. Fish Sci. 2016, 15, 590–596. [Google Scholar]
- Chen, H.; Liu, F.; Ouyang, M.; Zhou, H.; Lou, B. Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond. Fishes 2022, 7, 241. [Google Scholar] [CrossRef]
- Ghosh, B.; Cain, K.D.; Nowak, B.F.; Bridle, A.R. Microencapsulation of a putative probiotic Enterobacter species, C6-6, to protect rainbow trout, Oncorhynchus mykiss (Walbaum), against bacterial coldwater disease. J. Fish Dis. 2014, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Wu, Z.Q.; Li, D.L.; Yu, X.B.; Wang, G.X.; Ling, F. Effects of Dietary Administration of Shewanella xiamenensis A-1, Aeromonas veronii A-7, and Bacillus subtilis, Single or Combined, on the Grass Carp (Ctenopharyngodon idella) Intestinal Microbiota. Probiotics Antimicrob. Proteins 2017, 9, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Foysal, M.J.; Fotedar, R.; Siddik, M.A.B.; Tay, A. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 2020, 10, 5916. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Li, L.; Zhang, X.; Chen, J. The Effect of Disease and Season to Hepatopancreas and Intestinal Mycobiota of Litopenaeus vannamei. Front. Microbiol. 2019, 10, 889. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Guo, X.; Liang, Y.; Wang, W. Yeast culture dietary supplementation modulates gut microbiota, growth and biochemical parameters of grass carp. Microb. Biotechnol. 2018, 11, 551–565. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Fotedar, R.; Chaklader, M.R.; Foysal, M.J.; Nahar, A.; Howieson, J. Fermented Animal Source Protein as Substitution of Fishmeal on Intestinal Microbiota, Immune-Related Cytokines and Resistance to Vibrio mimicus in Freshwater Crayfish (Cherax cainii). Front. Physiol. 2020, 10, 1635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).