Abstract
Fish represent one of the most perishable food groups. Therefore, it is important to find viable alternatives that contribute to the preservation of quality and increase the shelf life of fishery products, and one alternative is to use natural extracts with antimicrobial activity. The objective of this study was to determine the effect of a natural extract prepared with garlic (NGE) on the quality and shelf life of tilapia fillets stored on ice for 18 days. For this purpose, NGE was prepared by homogenizing peeled garlic cloves with distilled water, which were then centrifuged to obtain the extract (NGE); then, the fish fillets were immersed in the extract and were coated in NGE. The fillets with NGE were packed in high-density polyethylene bags and stored in crushed ice for 18 days. The adenosine 5′-triphosphate (ATP) and degradation products, K-value, color, texture, water holding capacity, pH, total mesophilic count, and total volatile bases (TVB-N) were monitored during storage. The ATP content, K-value, pH, total microbial count, and TVB-N changed with respect to ice storage time, and the results between fillets with NGE and control fillets differed. In conclusion, the application of NGE increased the shelf life of fillets stored on ice by 6 days, obtaining a shelf life of 18 days on ice, which shows its potential to improve the utilization of the species.
Key Contribution:
One of the main problems involving seafood is its short shelf life during ice storage. For this reason, food technologists are studying different ways to increase its shelf life. In order to solve this problem, this study looks at the effect of a natural extract prepared with garlic on the quality and shelf life of tilapia fillets stored on ice for 18 days.
1. Introduction
Fisheries and aquaculture represent an important source of income and employment, and their food products are greatly appreciated by consumers worldwide because of their health benefits, flavor, texture, protein content, types, quality of fatty acids (polyunsaturated), and the variety of vitamins and minerals they provide. However, these products represent one of the most perishable foods [1], and thus preservation methods should be applied to avoid economic losses and possible negative effects on consumers’ health [2,3,4,5].
Some authors indicate that the freshness and quality of fishery products decrease immediately after capture and death due to the development of a series of post mortem biochemical changes. These changes can be endogenous or exogenous; the former involves food enzymes, while the latter involves the response to microorganisms’ growth and metabolism, as well as storage and handling conditions [6,7].
To reduce spoilage and preserve the shelf life of fishery products, various technologies have been used. Currently, in the fishing industry, several traditional and emerging technologies are being used to preserve fishery products. Traditional technologies include refrigeration, freezing, the use of modified and controlled atmospheres, canning, drying, dehydrating, salting, and smoking [7,8,9]. Moreover, in the case of new technologies, high pressures, irradiation, bio-preservation, active and intelligent packaging [3,10], and antimicrobial compounds [11], among others, can be used.
Nowadays, there is a growing demand from consumers to purchase fishery products with greater freshness and quality. Thus, several technologies have been developed. For example, the use of natural compounds from plants, animals, and microorganisms are some of the most innovative [12] practices, involving natural extracts, essential oils, or derived compounds [13]. These natural compounds may contain secondary metabolites that slow down or inhibit the development of bacteria, yeasts, and molds, and they contain aldehydes, aliphatic alcohols, phenolic compounds, acids, terpenes, ketones, and isoflavonoids, as well as their precursors, including thymol, carvacrol, eugenol, geraniol, and citral, among others. Until now, antimicrobial compounds have been obtained from rosemary, oregano, onion, green tea, tomato plants, and garlic; however, very few studies have evaluated their usefulness in preserving the quality and extending the shelf life of fish products [8,11,14].
Garlic (Allium sativum) is a vegetable with medicinal properties that belongs to the Liliaceae family, and it has biological activity due to its anticarcinogenic effect. Garlic stimulates the immune system, reduces cardiovascular diseases, and has antioxidant and antimicrobial properties [14]. Regarding its antimicrobial effect, garlic extract has been reported to have broad antibacterial activity on Staphylococcus, Klebsiella, Clostridium, Escherichia, Proteus, Salmonella, Mycobacterium, and Helicobacter [15]. In garlic, the compound that provides the antimicrobial activity is allicin, which is a volatile sulfur-containing molecule with a distinctive odor [16]. Additionally, allicin has been found to act as an antiparasitic, antiviral, and antifungal agent. The literature indicates that the antimicrobial activity of garlic extract is also caused by the presence of hydrophobic compounds such as diallyl polysulfide, ajoene, and vinyldithiin [17]. In the case of tilapia, which is one of the main species cultivated worldwide, to our knowledge no reports such as the one proposed in this study are available. Therefore, the objective of this study is to evaluate the effect of a natural extract made from garlic on the freshness, quality, and shelf life of tilapia (Oreochromis niloticus) fillets on ice over 18 days of storage.
2. Materials and Methods
2.1. Obtaining Tilapia Specimens
The Oreochromis niloticus fillets used in this study were purchased commercially from a fishery product retailer in Hermosillo, Sonora, Mexico (average weight and size of 101.87 ± 14.66 g and 16.0 ± 0.41 cm, respectively). The tilapia fillets were shipped to the Food Research Laboratory at the Universidad de Sonora (UNISON) in Hermosillo, where they were washed with distilled water and ice.
2.2. Preparation and Application of the Natural Garlic Extract (NGE)
The natural garlic extract (NGE) preparation process was carried out as follows: 200 g of peeled garlic was homogenized with 800 mL of distilled water in a Molimex (CDMX, México) blender for 2 min. Subsequently, the NGE obtained was centrifuged in a refrigerated centrifuge (SIGMA, Pasadena, CA, USA) at 9000× g and 4 °C for 30 min. The supernatant was then collected and cooled to refrigeration temperature (2 °C) prior to its use as a preserving agent. The NGE concentration was of 87.5 g of dry garlic/L of water, and 20 mL were used per kg of fillets (1.75 g of dry garlic per kg of fillets). Its application was carried out as follows: first, the tilapia fillets were washed, drained, and immersed in the NGE for 2 min. Then, they were drained for 5 min, packed in high-density polyethylene bags, and stored in crushed ice for 18 days. At the same time, the tilapia fillets (without NGE) were washed with distilled water, drained, and packed directly into the bags as described above to be used as the control group.
2.3. Ice Storage Study
The fillets—with and without NGE—packed in polyethylene bags were subjected to an 18-day ice storage study that consisted of storing the fillets described above in an airtight cooler in alternating beds of ice–fillet–ice. During this time, the determinations of ATP and degradation products, K-value, color, pH, texture, WHC, as well as the total mesophilic count and TVB-N were evaluated at 0, 3, 6, 9, 12, 15, and 18 days of storage. It is important to note that ice was replaced daily and four fillets per sampling day were analyzed for the NGE and control batches, respectively. The analyses performed are described below.
2.4. Quality Determinations
2.4.1. Adenosine 5′-Triphosphate (ATP) and Degradation Products
The determination of the ATP and related compound concentration was carried out using high performance liquid chromatography (HPLC) according to the technique described by Ryder [18] that consisted of preparing a homogenate with 3 g of tilapia muscle with 15 mL of 0.6 M perchloric acid at 0 °C. Then, the homogenate was centrifuged for 1 min at 18,000 rpm in an Ultra-Turrax T18 Basic (IKA Works Inc., Wilmington, NC, USA) and centrifuged at 0 °C in a refrigerated Thermo Electron Model IEC-MULTI RF centrifuge (Thermo Fisher Scientific, Asheville, NC, USA) at 5500× g for 10 min. Subsequently, 7 mL of the supernatant was adjusted to pH 6.5–6. 8 with 1 M KOH, and allowed to stand for 30 min at 0 °C to precipitate the potassium perchlorate and remove it by filtration using Whatman No. 4 paper. Finally, the quantity of the supernatant was increased to 15 mL using distilled water and stored frozen (−80 °C) until quantification.
To determine ATP concentration and related products using HPLC, 20 μL of the neutralized and diluted supernatant was injected into an Agilent 1200 series chromatograph (Agilent, Ratingen, Germany) using a 4.6 mm inner diameter × 250 mm long C18 reverse phase column (ODS Ultrasphere, Beckman Instruments, Inc. Fullerton, CA, USA). A buffer of 0.04 M KH2PO4 and 0.06 M K2HPO4 was used as the mobile phase at a flow rate of 1 mL/min. Nucleotides, nucleosides and nitrogenous base were detected at 254 nm with a diode array detector (Agilent 1200 Series Infinity, Agilent Scientific Instruments, Santa Clara, CA, USA).
2.4.2. K-Value (Freshness Index)
The concentrations of adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), inosine 5′-monophosphate (IMP), inosine (HxR), and hypoxanthine (Hx) were used to calculate the K-value. For this purpose, the equation reported by Sagedhal et al. [19] was used, as follows:
K-index (%) = ((HxR + Hx)/(ATP + ADP + AMP + IMP + HxR + Hx)) × 100.
2.4.3. pH
The measurement of the muscle pH was performed using the methodology proposed by Woyewoda et al. [20]. Two grams of tilapia muscle were homogenized with 18 mL of distilled water in an Ultra-Turrax T18 Basic homogenizer (Wilmington, NC, USA) for 1 min at 18,000 rpm. To measure the pH, the electrode of a potentiometer (Orion 420 A, Thermo Electron Corporation, Waltham, MA, USA) was introduced into the homogenate and calibrated daily.
2.4.4. Color
The color determination of tilapia fillets from the control lot and with NGE was carried out using tristimulus colorimetry using the CIE L*a*b* color space. For this purpose, a colorimeter (HunterLab MiniScan, Resto, VA, USA) was used, where L*, a*, and b* corresponded to lightness, red-green hue, and yellow-blue hue, respectively. The colorimeter was set in reflectance mode with a reading port opening of 0.5 cm and calibrated according to the manufacturer’s specifications.
2.4.5. Texture
A texturometer (Shimadzu Model EZ-S, Tokyo, Japan) was used to determine muscle texture. We used a Warner–Bratzler shear cell for this purpose. The maximum shear force (N) required to cut the samples used (dimensions 10 × 10 × 20 mm) was recorded. Thus, the force applied was transverse to the muscle fibers, using a 50 kg compression cell with a head speed of 20 cm/min for the measurement.
2.4.6. Water Holding Capacity (WHC)
The WHC of tilapia fillets with NGE and the control was calculated using the technique reported by Cheng et al. [21]. Two grams of fillet was taken and placed in 50 mL centrifuge tubes. The sample was centrifuged for 60 min at 7500× g at 4 °C in a refrigerated centrifuge (Thermo Fisher Scientific, Asheville, NC, USA). The WHC was calculated as the amount of water loss with relation to the initial content of the sample. The following equation was used for this determination:
where Wi = initial weight, and Wf = final weight after decanting the water from the tube and drying the surface of the muscle.
WHC (%) = 100 − ((Wi − Wf)/Wi × 100)
2.4.7. Total Volatile Bases (TVB-N)
The determination of TVB-N was carried out according to the methodology described by Woyewoda et al. [20]. An aliquot of 10 g of each fillet muscle with NGE and the control was transferred to a 1 L ball flask, to which 2 g of magnesium oxide (MgO) and 30 mL of distilled H2O were added. The mixture was homogenized for 1 min in an Ultra-Turrax (Wilmington, NC, USA). Next, 20 drops of 1-2-3® brand edible vegetable oil were added for defoaming purposes. The TVB-N were distilled for 25 min and collected in 15 mL of 2% boric acid to be later titrated with 0.03 N H2SO4; similarly, a blank was distilled. Finally, the TVB-N obtained were reported as mg N/100 g of the sample.
2.4.8. Aerobic Mesophilic Count
The evaluation of aerobic mesophiles present in tilapia fillets was carried out using the technique described in the Mexican Official Standard “aerobic microorganism plate count” [22]. Ten grams of each fillet with NGE and the control were taken and mixed with 90 mL of 1% (w/v) sterile peptonized water using a previously sterilized conventional blender. Subsequently, five serial dilutions were prepared with 1 mL of the largest dilution and mixed with 9 mL of sterile 1% (w/v) peptone water. Subsequently, 1 mL of each dilution was manually shaken and placed in a sterile Petri dish, and 20 mL of agar plate count (APC) was poured into the dish. The agar and inoculum were homogenized using the technique described by NOM-092-SSA1-1994 [22]. The plates were incubated in an inverted position at 35 ± 2 °C for 48 h. Each of the dilutions was inoculated in triplicate following the same methodology. Finally, between 25 and 250 colony forming units (CFUs) were counted on the dilution. The results were reported as log CFU/g of muscle.
2.4.9. Statistical Analysis
The data obtained were analyzed with classical statistical tools such as the mean and standard deviation. Likewise, an analysis of variance and Tukey’s multiple range test were performed. For this purpose, the statistical program NCSS (Version 6.0.22) (Kaysville, UT, USA) [23] was used. For each determination, four replicates were carried out, except for the microbiological determinations, where only two replicates were used. A significance level of 5% was used to analyze the data.
3. Results
3.1. Effect of NGE on the Post Mortem Biochemistry of Tilapia Muscle
3.1.1. Nucleotide Catabolism
In the muscle of aquatic organisms, ATP degradation represents an important post mortem biochemical change in freshness and quality, where its degradation to Hx has been widely used to monitor freshness in fishery products [24]. Figure 1 shows the average values of ATP concentration and related compounds in tilapia fillets treated with NGE and the control stored for 18 days on ice. The initial ATP values (0.840 ± 0.957 and 1.156 ± 0.794 µmol/g) shown in Figure 1 for the fillet with NGE (a) and the control (b) were slightly higher than the 0.26, 0.15, and 0.10 µmol/g of fillet reported by Montoya-Camacho et al. [25], Ocaño-Higuera et al. [26], and Ocaño-Higuera et al. [27] for tilapia (Oreochromis niloticus), cazon fish (Mustelus lunulatus), and ray fish (Dayastis brevis), respectively. However, they were lower than those found in fillets from freshly slaughtered fish (6–12 µmol/g), and this can be attributed to the energy expenditure of the organism during collection and slaughter, as well as the time elapsed between slaughter, filleting, and sampling for analysis. Likewise, Figure 1 shows that IMP was the nucleotide with the highest concentration in tilapia muscle, showing initial values of 4.992 ± 0.762 and 4.914 ± 0.627 µmol/g for fillets with NGE and the control, respectively. However, the IMP values obtained in this study were lower than the concentrations of 7.0 and 11.3 µmol/g obtained in red tail shad (Brycon cephalu) and sierra (Scomberomorus sierra) muscles by Batista et al. [28] and Castillo-Yáñez et al. [29], respectively. In the case of nucleotides (ADP and AMP), nucleosides (HxR), and the nitrogenous base (Hx), the initial values were less than 1 µmol/g for both the fillets treated with NGE and the control.
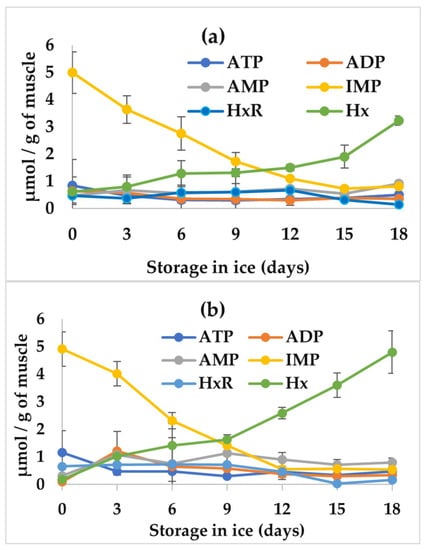
Figure 1.
Concentration of ATP, ADP, AMP, IMP, HxR, and Hx in fillets of tilapia (Oreochromis niloticus) with (a) natural garlic extract (NGE) and (b) the control, stored for 18 days on ice. The values are the average of n = 4 ± SD (standard deviation). Significant differences (p < 0.05) between NGE and the control were found only in Hx.
With respect to storage time, only IMP and Hx concentrations showed significant changes (p < 0.05), where IMP was reduced to 0.811 ± 0.119 and 0.175 ± 0.172 µmol/g for fillets with NGE and the control, respectively, at day 18 of ice storage, while Hx increased (p < 0.05) from 0.612 ± 0.173 and 0.548 ± 0.0730 to values of 3.229 ± 0.173 and 4.802 ± 0.768 µmol/g for fillets with NGE and the control, respectively, also at day 18 of ice storage. This behavior is what normally occurs in fish species during ice storage. The literature indicates that AMP and IMP are the compounds responsible for a sweet taste, which is considered a characteristic of fresh fish [24,30], while the formation and accumulation of HxR and Hx are related to a bitter taste, and consequently to a loss of freshness [31]. In this study, Hx production in tilapia fillets was indicative of the onset of autolytic and bacterial spoilage [32]. In this regard, and with respect to the taste and odor of tilapia fillets with NGE, it is important to highlight that an evaluation carried out by the members of the working group, and not by a sensory panel, of fresh and cooked fillets with NGE detected a slight garlic odor and flavor, which rather than generating negative effects on the perception and acceptance of consumers, provided a better odor and flavor. Therefore, it is considered that NGE has potential for use without affecting consumer acceptance.
Regarding the comparison between NGE and the control, significant differences (p < 0.05) were detected in Hx concentrations during the 18 days of ice storage, when the concentration of this nucleoside was lower at the end of storage in fillets with NGE compared to the control. As described above, this result can be related to the onset of microbial growth, and this was further corroborated in the determinations of the K-value, TVB-N, and aerobic mesophilic count.
3.1.2. K-Value
The K-value or freshness index is important to determine the freshness and quality of fishery products. This value is defined as the percentage of the sum of the concentrations of non-phosphorylated ATP degradation products (HxR and Hx) over the total sum of ATP concentrations and degradation products up to Hx [33].
Figure 2 shows the behavior of the K-value in tilapia fillets with NGE and the control stored for 18 days on ice, with initial values of 13.42 and 11.35% for the NGE and control tilapia fillets, respectively. These initial values are similar to those described by Liu et al. [34] and Khalid [35], who reported K-values of 12.00 and 13.80% for tilapia (Oreochromis niloticus) and Pacific salmon (Onchrhynchus nerka) muscles, respectively. However, the values are lower than those reported by Castillo-Yáñez et al. [29], who obtained an initial value of 21.23% for sierra (Scomberomorus sierra) muscle.
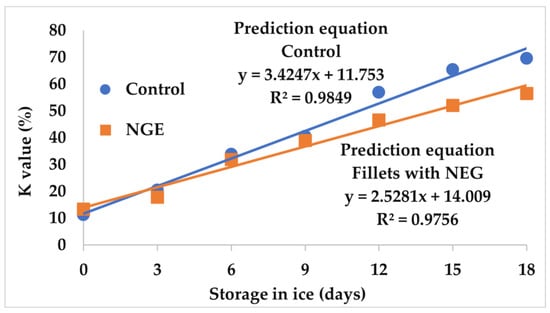
Figure 2.
Behavior of the K-value in tilapia fillets with natural garlic extract (NGE) and the control stored on ice for 18 days. Values are the mean of n = 4 ± SD (standard deviation).
With respect to tilapia fillet ice storage, at day 18 a linear and significant increase (p < 0.05) was observed in the determination of the K-value, reaching final values of 56.51 and 69.61% for tilapia fillets treated with NGE and the control, respectively. These values are higher than 39.5 ± 2.6% found by Montoya-Camacho et al. [25] in tilapia fillets stored for 20 days at 0 °C. This difference may be due to the fact that the fillets in this study were acquired commercially, while those used by Montoya-Camacho et al. [25] were acquired freshly slaughtered directly from an aquaculture company. Moreover, the K-values of this study are similar to the 58.9% described by Ocaño-Higuera et al. [26] in cazon fish (Mustelus lunulatus) stored for 18 days on ice. Furthermore, the values obtained are lower than those found by Özoğul et al. [36] and Castillo-Yáñez [29], who reported a final value of 90.0 and 80.6% for turbot (Scophthalmus maximus) and sierra (Scomberomorus sierra) fillets, respectively. Finally, a significant effect (p < 0.05) was found between tilapia fillets with NGE and the control, which showed that its application extends the shelf life of tilapia fillets, and this may be a consequence of the antimicrobial and antioxidant properties of garlic [37].
Saito et al. [38] reported a quality classification based on the K-value, for which they indicated that fishery products with values below 20% were very fresh, values below 50% were moderately fresh, and values above 70% were not fresh and should not be consumed. Based on this classification, tilapia fillets from the control at day 18 were almost in the not fresh category (69.60%), while those with NGE showed a lower edible quality than those moderately fresh. However, it should be emphasized that to use the aforementioned classification, it is necessary to take into account that the K-index is a parameter that depends on the species. Thus, it should be calculated for each fish species.
3.2. Effect of the NGE on Tilapia Muscle Physicochemical Quality
3.2.1. pH
In general, the pH of live fish immediately after slaughter is close to 7.0, but this value decreases rapidly immediately after death, which occurs as glycogen is converted to lactic acid and fish go through rigor mortis. Most fish species reach post mortem pH values from 6.0 to 6.8 [39]. This measurement is of great importance when judging the freshness and quality of fishery products [40]. Figure 3 shows the behavior of the pH in the fillets stored for 18 days on ice, where the initial pH values of 7.04 ± 0.143 and 7.24 ± 0.028 can be observed for the fillets with NGE and the control, respectively. These initial values are higher than those reported by Tomé et al. [41] and Montoya Camacho et al. [25], who obtained initial pH values of 6.48 and 6.77, respectively, in tilapia (Oreochromis spp.) muscle stored on ice. These differences may be due to species, diet, muscle type, season of the year, and activity level, as well as to stress during capture [26,27,28,41,42].

Figure 3.
pH values obtained in tilapia fillets (Oreochromis niloticus) with NGE and the control stored on ice for 18 days. Values are the mean of n = 4 ± SD (standard deviation).
Furthermore, according to Figure 3, the pH increased significantly (p < 0.05) only in the control fillets, finding a value of 7.8 ± 0.12 at day 18 of ice storage. These values are higher than those described by Khalafalla et al. [39], Attouchi and Sadok [43], and Tomé et al. [41], who reported final pH values of 7.2, 6.80, and 6.73 for the muscles of Oreochromis niloticus, Spaurus aurata, and Oreochromis spp., respectively. The increase in pH observed in the control fillets during storage can be related mainly to the generation of volatile compounds such as amines produced through autolytic means (tyramine, putrescine, histamine, and cadaverine) and ammonia [44], as well as to the degradation of free amino acids by bacterial action [34]. Moreover, a significant increase (p < 0.05) in pH was observed in the tilapia fillets with NGE; however, in the control fillets, the pH did not increase (p > 0.05) during storage on ice. This result could be caused by the presence of antimicrobial compounds in garlic, in which some organosulfur compounds are found, such as allyl sulfides, ajoene, and allicin [45].
Regarding the effect of NGE application on the pH of tilapia fillets, significant differences (p < 0.05) were observed in this parameter between fillets treated with the extract and the control. Fillets with NGE showed a lower pH than the control fillet. This result may be due to the presence of some organic acids found in garlic bulbs, including pyruvic, malic, and citric acids [46].
3.2.2. Color
Color is a parameter used to evaluate the quality of fishery products, and it especially influences consumer acceptance for these products [26]. Figure 4 shows the values obtained for the parameters L*, a*, and b* in the tilapia fillets with NGE and the control stored for 18 days on ice. For the L* parameter, initial values of 62.02 ± 1.78 and 60.04 ± 3.10 were observed for fillets treated with the extract and the control, respectively. The initial values are similar to the 62.3 ± 1.5 reported by Montoya-Camacho et al. [25] for tilapia fillets at the beginning of storage. With respect to storage, only the control fillets showed a slight significant increase (p < 0.05), reaching a value of 65.40 ± 4.74 at 18 days. This increase could be caused by the loss of fluids through exudation that occurs in the fillet; this generates a watery appearance on the fillet surface and, thus, an increase in brightness [32]. Regarding the effect of NGE, no differences were found (p > 0.05) compared with the control.
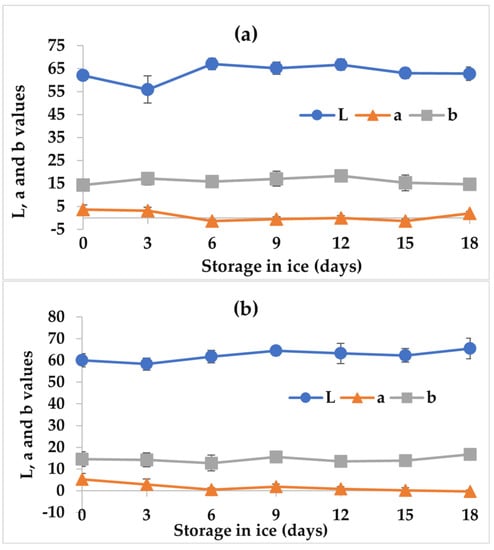
Figure 4.
Change of color (L*, a*, and b* values) in tilapia fillets (Oreochromis niloticus) with (a) NGE and (b) the control stored for 18 days on ice. The values are the average of n = 4 ± SD (standard deviation).
Moreover, in parameter a*, tilapia fillets with NGE and the control showed a slightly significant reduction (p < 0.05) with respect to storage time, obtaining values of 1.94 ± 0.77 and −0.36 ± 0.64 at day 18 of ice storage, respectively. This decrease could be attributed to the fact that some sarcoplasmic proteins, such as myoglobin and hemoglobin, are solubilized in the water lost by the fillet during storage, causing the red color to decrease [32]. In the b* parameter, the values did not change significantly (p > 0.05) with respect to storage time in ice nor with the NGE application. Consequently, the color of tilapia fillets with the extract and the control showed colorations towards orange-yellow in the chromatic sphere, recording hue values of 82.46 and 91.23, respectively.
3.2.3. Texture and Water Holding Capacity (WHC)
Texture evaluation is an important parameter to assess quality in fresh products [47,48]. Muscle smoothness is modified by several factors such as the degradation of Z-discs, the destruction of connectin, the dissociation of the actin–myosin complex, and by collagen denaturation. Texture is also affected by endogenous proteases that degrade myofibrillar proteins. The latter seems to be the main cause of muscle softening, since some of them are fully active at a post mortem pH between 5.5 and 6.5 [49].
Figure 5a shows texture behavior evaluated by the sheer force technique in tilapia fillets with NGE and the control during 18 days of storage on ice. This figure shows that the initial values were 5.80 ± 1.01 and 6.54 ± 0.48 N for the fillets treated with the extract and the control, respectively. These values are similar to the initial value of 6.52 ± 0.22 N reported by Montoya-Camacho et al. [25] for tilapia (Oreochromis niloticus) fillets. This quality parameter was not affected by either ice storage time or NGE application (p > 0.05). Therefore, since no significant differences (p > 0.05) were observed in this determination of shear force between storage time and treatments, and the results suggest that the autolytic enzymes responsible for muscle fiber degradation did not affect the muscle integrity of the fillets.
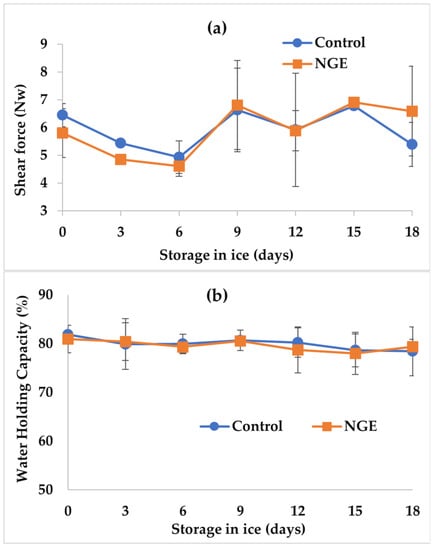
Figure 5.
Changes in texture (a) and water holding capacity (b) in tilapia fillets with natural garlic extract (NGE) and the control stored on ice for 18 days. Values are the mean of n = 4 ± SD (standard deviation).
The WHC is the ability of meat to retain its own water during the application of external forces [50]. A loss in its value is mainly due to the denaturation (hydrolysis and/or aggregation) that occurs in the myofibrillar protein of meat during storage in ice [26], which is related to the loss of muscle texture [51,52].
Figure 5b illustrates the behavior of WHC in tilapia fillets treated with NGE and the control during 18 days of ice storage. The figure shows that the initial WHC values were 80.92 ± 2.81 and 81.84 ± 0.35% for fillets with the extract and the control, respectively. These initial values are lower than those described by Montoya-Camacho et al. [25], who obtained initial WHC values of 95.3 ± 0.4% in tilapia (Oreochromis spp.) fillets stored on ice. These differences can be attributed to diet, stress during capture, species, season of the year, or type of muscle studied, as well as activity level [26,27,28,41,42]. With respect to ice storage, no significant changes (p > 0.05) were observed in the fillets with NGE and the control, reaching values of 79.36 ± 0.52 and 78.43 ± 5.00%, respectively. These values are lower than those described by Coronado and Moreno ([32], who reported a final value of 88.5% for tilapia (Oreochromis niloticus) muscle. Likewise, no significant differences (p > 0.05) were found between the control and the fillets with NGE.
3.2.4. Total Volatile Bases (TVB-N)
The determination of TVB-N is widely used to evaluate the quality of fishery products [53]. Figure 6a shows the behavior of TVB-N in fillets treated with NGE and the control stored for 18 days on ice. The figure shows that the initial values were 22.13 ± 1.29 and 22.82 ± 1.70 mg N/100 g of muscle for the fillets with the extract and the control, respectively. These values are similar to the 26.2 ± 1.6 mg N/100 g reported by Montoya-Camacho et al. [25] and are higher than the 6.5 and 10.2 mg N/100 g for tilapia (Oreochromis niloticus) muscle described by Liu et al. [34] and Pankyamma et al. [54], respectively. Regarding storage time, a significant increase (p < 0.05) in TVB-N values was observed, reaching up to 34.76 ± 10.60 and 60.21 ± 10.56 mg N/100 g in the tilapia fillets with NGE and the control at day 18, respectively. In addition, significant differences (p < 0.05) were found between the control and the fillets with the extract. In the case of fishery products, a maximum allowable value of 30 mg N/100 g of muscle is used for a product fit for human consumption [55]. This value was reached at 12 days in the control fillet and at 18 days in the fillet with NGE. It is important to note that for these days of storage, the pH and microbial load values also increased. Therefore, the TVB-N content increased due to the growth of spoilage microorganisms that are capable of generating volatile compounds characteristic of spoilage. This result indicated that NGE increased the shelf life of tilapia fillets by six days compared to the control batch.

Figure 6.
Changes in TVB-N (a) and the bacterial viable counts (b) in tilapia fillets with natural garlic extract (NGE) and the control stored on ice for 18 days. Values are the mean of n = 4 ± SD (standard deviation).
3.3. Effect on the Microbiological Quality
Currently, the fishing industry is suffering from great economic losses due to the rapid deterioration of this group of foods, which is a consequence of the microbiota they present, where they mostly possess Gram-negative spoilage bacteria [44]. In general, the microbial load of fishery products is closely related to the initial level of bacterial contamination and the conditions of the aquatic environment (salinity, light availability, oxygen levels, density, pH, etc.), as well as to the preservation method applied (cold, heat, reduction in moisture content, biopreservation, etc.) that influences the growth and survival of bacteria [56].
Figure 6b shows the behavior of the mesophilic microbial load in tilapia fillets treated with NGE and the control during 18 days of storage on ice, where values of 4.43 and 4.49 log CFU/g were obtained for the fillets with the extract and the control, respectively. This value is higher than that reported by Coronado and Moreno [52], who found an initial value of 3.2 log CFU/g for tilapia fillets (Oreochromis niloticus). Moreover, a significant increase (p < 0.05) in the microbial count was observed with respect to storage time, where values of 5.92 and 7.15 log CFU/g were obtained for the fillets with the extract and the control, respectively. Likewise, significant differences (p < 0.05) were observed between the fillets with NGE and the control, and this showed the antimicrobial power of the latter. Similar studies have shown the antimicrobial effect of garlic on tilapia fillets. For example, Tawfik et al. [57] and Kirrella et al. [58] used essential oils from this vegetable. Studies report that the capacity of garlic or the extracts obtained from it is mainly due to allicin, ajoene, and various aliphatic sulfides [45]. Unlike these studies, the present work has the advantage that a simple garlic extract was used and it was easy to apply to fish fillets.
The Mexican Official Standard (NOM-027-SSA1-1993) [59] establishes 7 log CFU/g or 10,000,000 CFU/g of microorganisms as the maximum permitted value for fresh and/or refrigerated fish fit for human consumption. Based on the results obtained in this determination, the control fillets had an edible quality up to day 12 of storage, while the fillets with the extract continued to be of edible quality even at day 18 of storage on ice. These results agree with those described above for the K-index, TVB-N, and the increase in hypoxanthine content at day 18 of storage.
4. Conclusions
The determinations of the K-index, total mesophilic count, total volatile bases, and pH showed their usefulness in evaluating the antibacterial effect of NGE, the application of which, by immersion, in commercial tilapia fillets increased the shelf life of the fillets by six days compared to the control batch. In general, the results of this study showed the potential of NGE to extend the shelf life of tilapia fillets, which could consequently lead to a greater utilization of this species, and to less waste. In addition, this extract may be applied to other fishery species to extend the shelf life and reduce post-harvest losses or wastage.
Author Contributions
V.M.O.-H., S.V.-H. and E.I.J.-R. designed the study. J.A.C.-G., C.B.O.-L., D.F.C.-R. and C.J.P.-M. performed formal analyses of the manuscript. V.M.O.-H., S.R.-C., A.M.G.-G., H.J.B.-C. and M.T.S.-M. wrote the original draft. All authors contributed to writing, reviewing, and editing the manuscript. All authors contributed to the research and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was J.A.C.G.’s undergraduate thesis and was supported by the University of Sonora (No research project). Some members of the Red Temática de Bioproductos y Bioprocesos participated in this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, T.; Li, T.; Li, J. Preparation of coaxial polylactic acid-propyl gallate electrospun fibers and the effect of their coating on salmon slices during chilled storage. ACS Appl. Mater. Interfaces 2019, 11, 6463–6474. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria-Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Hong, H.; Zhang, L.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2020, 20, 252–288. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Abd El-Hay, M.M. Processing and preparation of fish. In Postharvest and Postmortem Processing of Raw Food Materials; Jafari, S.M., Ed.; Woodhead Publishing: New Delhi, India, 2022; pp. 315–342. [Google Scholar] [CrossRef]
- Ramírez, G.H.; Castillo, Y.F.; Montaño, C.E.; Ruíz-Cruz, S.; Márquez, R.E.; Canizales, R.D.; Torres-Arreola, W.; Montoya, C.N.; Ocaño-Higuera, V.M. Protective effect of an edible tomato plant extract/chitosan coating on the quality and shelf life of sierra fish fillets. J. Chem. 2018, 2018, 2436045. [Google Scholar] [CrossRef]
- Aponte, M.; Anastasio, A.; Marrone, R.; Mercogliano, R.; Peruzy, M.F.; Murru, N. Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT 2018, 93, 412–419. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef]
- Cortesi, M.L.; Panebianco, A.; Giuffrida, A.; Anastasio, A. Innovations in seafood preservation and storage. Vet. Res. Commun. 2009, 33, S15–S23. [Google Scholar] [CrossRef]
- Anastasio, A.; Marrone, R.; Chirollo, C.; Smaldone, G.; Attouchi, M.; Adamo, P.; Sadok, S.; Pepe, T. Swordfish steaks vacuum-packed with Rosmarinus officinalis. Ital. J. Food Saf. 2014, 26, 390–397. [Google Scholar]
- Garba, I.; Umar, A.I.; Abdulrahman, A.B.; Tijjani, M.B.; Aliyu, M.S.; Zango, U.U.; Muhammad, A. Phytochemical and antibacterial properties of garlic extracts. Bayero J. Pure Appl. 2013, 6, 45–48. [Google Scholar] [CrossRef]
- Liaqat, A.; Zahoor, T.; Atif Randhawa, M.; Shahid, M. Characterization and antimicrobial potential of bioactive components of sonicated extract from garlic (Allium sativum) against foodborne pathogens. J. Food Process. Preserv. 2019, 43, e13936. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Moya-Salvador, A. Compuestos Organosulfurados Presentes en Aliáceas y sus Propiedades Saludables. Master’s Thesis, Facultad de Farmacia, Universidad de Sevilla, Sevilla, Spain, 2020; p. 59. [Google Scholar]
- Ryder, J.M. Determination of adenosine triphosphate and its breakdown products in fish muscle by High Performance Liquid Chromatography. J. Agric. Food Chem. 1985, 33, 678–680. [Google Scholar] [CrossRef]
- Sagedhal, A.; Busalmen, J.P.; Roldán, H.A.; Paredi, M.E.; Crupkin, M. Post-mortem changes in adenosine triphosphate and related compounds in mantle of squid (Illex argentinus) at different stages of sexual maturation. J. Aquat. Food Prod. Technol. 1997, 6, 43–56. [Google Scholar] [CrossRef]
- Woyewoda, A.D.; Shaw, S.J.; Ke, P.J.; Burns, B.G. Recommended Laboratory Methods for Assessment of Fish Quality; Canadian Technical Report of Fisheries and Aquatic Sciences; Department of Fisheries and Oceans: Halifax, NS, Canada, 1986; p. 143. [Google Scholar]
- Cheng, C.S.; Hamann, D.D.; Webb, N.B.; Sidwell, V. Effects of species and storage time on minced fish gel texture. J. Food Sci. 1979, 44, 1087–1092. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. NOM-092-SSA1-1994. Método para la Cuenta de Bacterias Aerobias en Placa; Diario Oficial de la Federación. Secretaria de Gobernación, México. 1995, p. 4. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=4886029&fecha=12/12/1995#gsc.tab=0 (accessed on 9 May 2023).
- Hintze, J.L. NCSS 2000 Statistical System for Windows-User’s Guide; Number Cruncher Statistical Systems: Kaysville, UT, USA, 1998. [Google Scholar]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1792. [Google Scholar] [CrossRef]
- Montoya-Camacho, N.; Márquez-Ríos, E.; Castillo-Yáñez, F.J.; Ruíz-Cruz, S.; Cárdenas-López, J.L.; Arvizu-Flores, A.A.; Torres-Arreola, W.; Ocaño-Higuera, V.M. Efecto de la temperatura de almacenamiento sobre el rigor mortis del músculo de tilapia (Oreochromis niloticus). Biotecnia 2020, 22, 88–93. [Google Scholar] [CrossRef]
- Ocaño-Higuera, V.M.; Márquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938. [Google Scholar] [CrossRef]
- Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Márquez-Ríos, E.; Canizales-Rodríguez, D.F.; Castillo-Yáñez, F.J.; Ruíz-Bustos, E.; Graciano-Verdugo, A.Z.; Plascencia-Jatomea, M. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011, 125, 49–54. [Google Scholar] [CrossRef]
- Batista, M.G.; Lessi, E.; Kodaira, M.; Falcao, P.D.T. Alterações bioquímicas post-mortem de matrinxã Brycon cephalus (Günther, 1869) procedente da piscicultura, mantido em gelo. Cienc. Tecnol. Aliment. 2004, 24, 573–581. [Google Scholar] [CrossRef]
- Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Márquez-Ríos, E.; Lugo-Sánchez, M.E.; Lozano-Taylor, J. Freshness loss in sierra fish (Scomberomorus sierra) muscle stored in ice as affected by poscapture handling practices. J. Food Biochem. 2007, 31, 56–67. [Google Scholar] [CrossRef]
- Howgate, P. Kinetics of degradation of adenosine triphosphate in chill-stored rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. 2005, 40, 579–588. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, Y.; Choi, H.; Lee, K.G. ATP degradation products as freshness indicator of flatfish during storage. Food Sci. Biotechnol. 2019, 28, 1891–1897. [Google Scholar] [CrossRef]
- Montoya-Camacho, N. Efecto de la Temperatura de Almacenamiento Sobre el Rigor Mortis y la Calidad del Músculo de Tilapia (Oreochromis niloticus). Master’s Thesis, Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Hermosillo, Sonora, México, 2013. [Google Scholar]
- Ehira, S.; Uchiyama, H. Determination of fish freshness using the K value and comments on some other biochemical changes in relation to freshness. In Seafood Quality Determination; Kramer, D.E., Liston, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 185–193. [Google Scholar]
- Liu, S.; Fan, W.; Zhong, S.; Ma, C.; Li, P.; Zhou, K.; Zhu, M. Quality evaluation of tray-packed tilapia fillets stored at 0°C based on sensory, microbiological, biochemical and physical attributes. Afr. J. Biotechnol. 2010, 9, 692–701. [Google Scholar] [CrossRef]
- Khalid, I.S. Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem. 2007, 101, 592–600. [Google Scholar]
- Özoğul, Y.; Özoğul, F.; Kuley, E.; Ozkutuk, A.S.; Gökbulut, C.; Köse, S. Biochemical, sensory and microbiological attributes of wild turbot (Scophthalmus maximus), from the black sea, during chilled storage. Food Chem. 2006, 99, 752–758. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT-Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Saito, T.; Arai, K.; Matruyoshi, M. A new method for estimating the freshness of fish. Bull. Jpn. Soc. Sci. Fish. 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Khalafalla, F.A.; Ali, F.H.; Hassan, A.H. Quality improvement and shelf-life extension of refrigerated Nile tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 33–40. [Google Scholar] [CrossRef]
- Copes, J.; Pellicer, K.; del Hoyo, G.; Brocado, S.; Gianuzzi, L. Estudio del comportamiento del pH en filetes de pejerrey de laguna (Odhontheses bonariensis) conservados con y sin vacío, utilizando distintos tratamientos y almacenados a temperaturas de 4, 0 y −5 °C. Analecta Vet. 2008, 28, 21–25. [Google Scholar]
- Tomé, E.; Iglesias, M.; Kodaira, M.; González, A. Efecto de la temperatura de almacenamiento en el rigor mortis y en la estabilidad de la tilapia (Oreochromis spp.) cultivada. Rev. Científica FCV-LUZ 2000, 11, 373–378. [Google Scholar]
- Durán, A.; Erdemli, U.; Karakaya, M.; Tyilmaz, M. Effects of slaughter methods on physical, biochemical and microbiological quality of rainbow trout (Oncorynchus mykiss) and mirror carp (Cyprinus carpio) filleted in pre-, in- or post-rigor periods. Fish. Sci. 2008, 74, 1146–1156. [Google Scholar] [CrossRef]
- Attouchi, M.; Sadok, S. The effect of powdered thyme sprinkling on quality changes of wild and farmed gilthead sea bream fillets stored in ice. Food Chem. 2010, 119, 1527–1534. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; Documento Técnico de Pesca N° 348; FAO: Rome, Italy, 1995; p. 202. [Google Scholar]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Mujica, H.; Pérez, C.M. Características físicas y químicas de ajo cosechado en dos estados de madurez y almacenado en condiciones ambientales. Bioagro 2006, 18, 171–175. [Google Scholar]
- Nunak, N.; Scheining, G. Instrumental textural changes in raw white shrimp during iced storage. J. Aquat. Food Prod. Technol. 2011, 20, 350–360. [Google Scholar] [CrossRef]
- Pornrat, S.; Sumate, T.; Rommance, S.; Sumolaya, K.; Kerr, W.L. Changes in the ultrastructure and texture of prawn muscle (Macrobrachuim rosenbergii) during cold storage. LWT-Food Sci. Technol. 2007, 40, 1747–1754. [Google Scholar] [CrossRef]
- Ashie, I.N.; Smith, J.P.; Simpson, B.K. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef] [PubMed]
- Haileslassie, W.; Gebrehiwot, M.; Balcha, E.; Hagos, Y.; Kidane, W. Determination of pH and water holding capacity of beef from selected butcher shops of Mekelle, Ethiopia. J. Vet. Med. Anim. Health 2018, 10, 159–164. [Google Scholar]
- Chan, S.S.; Roth, B.; Jessen, F.; Jakobsen, A.N.; Lerfall, J. Water holding properties of Atlantic salmon. Compr. Rev. Food Sci. Food Saf. 2022, 21, 477–498. [Google Scholar] [CrossRef]
- Coronado, B.A.L.; Moreno, V.M.J. Evaluación de la Calidad Física, Química y Microbiológica de Filete de Tilapia (Oreochromis niloticus) Adicionado con Antimicrobianos Naturales Durante su Almacenamiento en Hielo. Bachelor’s Thesis, Universidad de Sonora, Sonora, México, 2011. [Google Scholar]
- Moosavi-Nasab, M.; Khoshnoudi-Nia, S.; Azimifar, Z.; Kamyab, S. Evaluation of the total volatile basic nitrogen (TVB-N) content in fish fillets using hyperspectral imaging coupled with deep learning neural network and meta-analysis. Sci. Rep. 2021, 11, 5094. [Google Scholar] [CrossRef]
- Pankyamma, S.V.; Rani, K.S.; Binsi, P.K. Gravading process of Nile tilapia (Oreochromis niloticus) and evaluation of its biochemical and sensory changes during refrigerated storage. J. Food Process. Preserv. 2020, 44, e14631. [Google Scholar] [CrossRef]
- Gökodlu, N.; Özden, Ö.; Erkan, N. Physical, chemical, and sensory analyses of freshly harvested sardines (Sardina pilchardus) stored at 4 °C. J. Aquat. Food Prod. Technol. 1998, 7, 5–15. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Arlino, S.; Boehme, K.; de Miguel, T.; Pascoal, A.; Barros-Velázquez, J. Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food Bioproc. Tech. 2008, 1, 43–63. [Google Scholar] [CrossRef]
- Tawfik, T.E.A.E.; Farghaly, R.M.; Gad-Elrab, H.M.; Abdel-Aziz, N.M. Effect of some essential oils against Aeromonas hydrophila artificially inoculated into raw Nile Tilapia fish fillets during refrigeration storage. SVU-Int. J. Vet. Sci. 2022, 5, 88–102. [Google Scholar] [CrossRef]
- Kirrella, G.; Moustafa, N.; Kishk, D.; Abdallah, R. Impact of using some essential oils on sensory acceptability and Aeromonas hydrophila contamination of Nile tilapia fish fillet during refrigeration storage. Kafrelsheikh Vet. Med. J. 2021, 19, 9–13. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. 1993. NOM-027-SSA1-1993 “Productos de la pesca. Pescados Frescos-Refrigerados y Congelados. Especificaciones Sanitarias”. Diario Oficial de la Federación. Secretaria de Gobernación, México. 1995, p. 8. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=4870248&fecha=03/03/1995&print=true (accessed on 9 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).