Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina
Abstract
1. Introduction
2. Material and Methods
2.1. Tested Compounds
2.2. Fish Rearing Conditions
2.3. Experimental Design and Diets
2.4. Growth Efficiency
2.5. Sampling
2.6. Estimation of Health-Related Indicators
2.6.1. Hematological Examination
2.6.2. Hepatorenal Function Indices
2.6.3. Stress Indicators
2.6.4. Evaluation of Oxidant/Antioxidant Status
2.6.5. Evaluation of Nonspecific Immunological Indicators
2.6.6. Neuro-Stress and DNA Damage Assays
2.7. Transcriptional Analysis of Stress and Immune-Related Genes
2.8. Parasitic Challenge Test
2.9. Statistical Analysis
3. Results
3.1. Survival Percent and Growth Performance
3.2. Hematological Indices
3.3. Protein Profile and Hepatorenal Function
3.4. Immunological Response Parameters
3.5. Activity of Antioxidant Enzymes
3.6. Stress, DNA Damage Markers, and AChE Activity
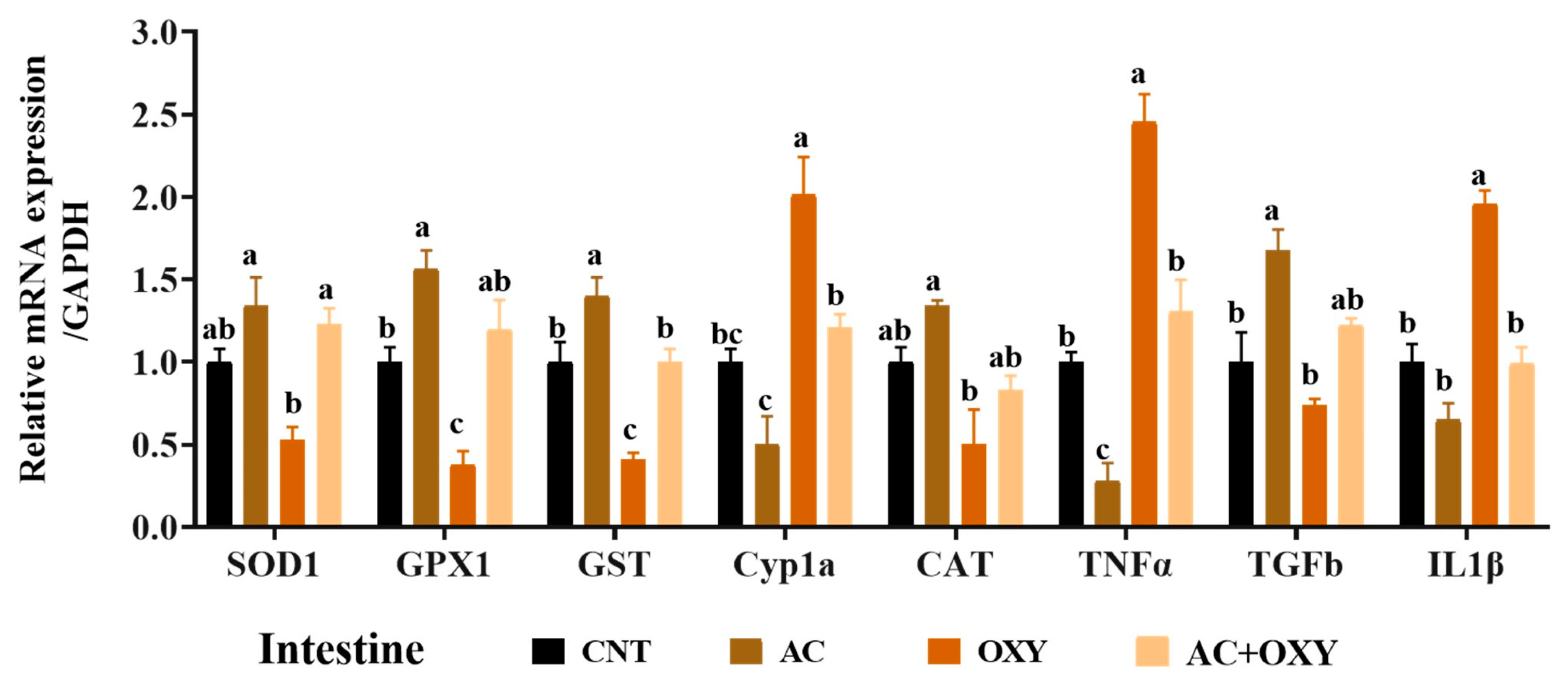
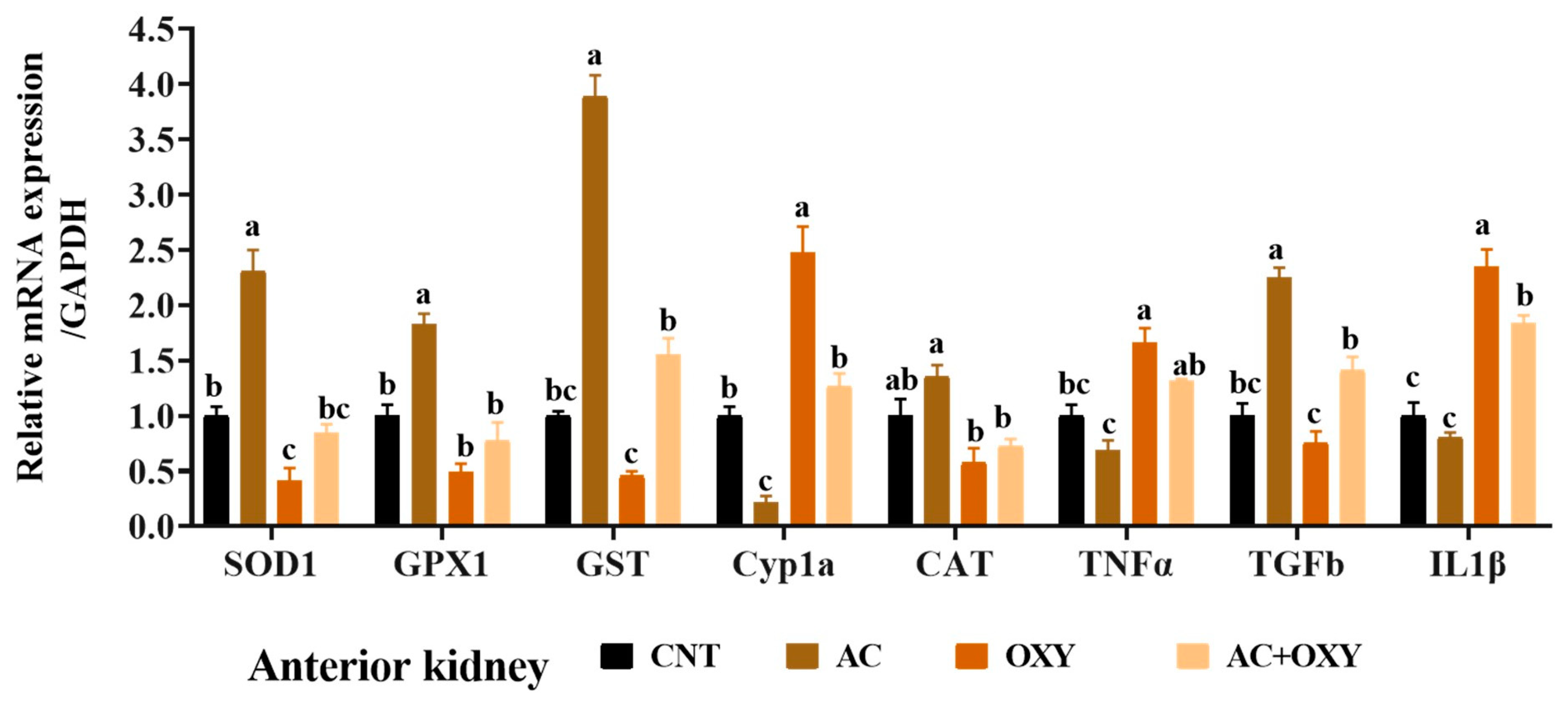
3.7. Relative Expression of Immune and Antioxidant-Related Genes
3.8. Quadriacanthus Aegypticus Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adewolu, M.A.; Adeniji, C.A.; Adejobi, A.B. Feed utilization, growth and survival of Clarias gariepinus (Burchell 1822) fingerlings cultured under different photoperiods. Aquaculture 2008, 283, 64–67. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Abd El-Hakim, Y.M.; Metwally, M.M.; Ghfar, S.S.A.; Khalil, A.A. The single or combined Silybum marianum and co-enzyme Q10 role in alleviating fluoride-induced impaired growth, immune suppression, oxidative stress, histological alterations, and reduced resistance to Aeromonas sobria in African catfish (Clarias gariepinus). Aquaculture 2022, 548, 737693. [Google Scholar]
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory Effect of Papaya Extract against Chlorpyrifos-Induced Oxidative Stress, Immune Suppression, Endocrine Disruption, and DNA Damage in Female Clarias gariepinus. Int. J. Environ. Res. Public 2022, 19, 4640. [Google Scholar] [CrossRef] [PubMed]
- Ikpi, G.; Offem, B. Bacterial infection of mudfish Clarias gariepinus (Siluriformes: Clariidae) fingerlings in tropical nursery ponds. Rev. Biol. Trop. 2011, 59, 751–759. [Google Scholar] [CrossRef]
- Mbokane, E.M.; Moyo, N.A.G. Effect of dietary Artemisia afra on growth, some innate immunological parameters in Clarias gariepinus challenged with Aeromonas hydrophila. Aquac. Int. 2020, 28, 539–553. [Google Scholar] [CrossRef]
- Moustafa, G.G.; Shaaban, F.; Hadeed, A.A.; Elhady, W.M. Immunotoxicological, biochemical, and histopathological studies on Roundup and Stomp herbicides in Nile catfish (Clarias gariepinus). Vet. World 2016, 9, 638. [Google Scholar] [CrossRef]
- Abd El-Rahman, G.I.; Ahmed, S.A.; Khalil, A.A.; Abd-Elhakim, Y.M. Assessment of hematological, hepato-renal, antioxidant, and hormonal responses of Clarias gariepinus exposed to sub-lethal concentrations of oxyfluorfen. Aquat. Toxicol. 2019, 217, 105329. [Google Scholar] [CrossRef]
- Stagg, N.J.; LeBaron, M.J.; Eisenbrandt, D.L.; Gollapudi, B.B.; Klaunig, J.E. Assessment of possible carcinogenicity of oxyfluorfen to humans using mode of action analysis of rodent liver effects. Toxicol. Sci. 2012, 128, 334–345. [Google Scholar] [CrossRef]
- Mantzos, N.; Karakitsou, A.; Hela, D.; Patakioutas, G.; Leneti, E.; Konstantinou, I. Persistence of oxyfluorfen in soil, runoff water, sediment and plants of a sunflower cultivation. Sci. Total Environ. 2014, 472, 767–777. [Google Scholar] [CrossRef]
- Hall, K.E.; Ray, C.; Ki, S.J.; Spokas, K.A.; Koskinen, W.C. Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manag. 2015, 159, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Sayed, D.A. Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) snails. Environ. Sci. Pollut. Res. 2019, 26, 7960–7968. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.B.; Keese, R.J.; Camper, N.D.; Whitwell, T.; Wilson, P.C. Pendimethalin and oxyfluorfen residues in pond water and sediment from container plant nurseries. Weed Technol. 1994, 8, 299–303. [Google Scholar] [CrossRef]
- Nilsen, E.; Zaugg, S.; Alvarez, D.; Morace, J.; Waite, I.; Counihan, T.; Hardiman, J.; Torres, L.; Patiño, R.; Mesa, M. Contaminants of legacy and emerging concern in largescale suckers (Catostomus macrocheilus) and the foodweb in the lower Columbia River, Oregon and Washington, USA. Sci. Total Environ. 2014, 484, 344–352. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; AbdelMageed, M.; Abd-Elhakim, Y.M.; Abdel-Warith, A.-W.A.; Younis, E.M.; Abd-Allah, N.A.; Davies, S.J.; El-Kholy, M.S.; Ahmed, S.A. The effect of dietary Crataegus Sinaica on the growth performance, immune responses, hemato-biochemical and oxidative stress indices, tissues architecture, and resistance to Aeromonas sobria infection of acrylamide-exposed Clarias gariepinus. Aquac. Rep. 2023, 30, 101576. [Google Scholar] [CrossRef]
- Cheung, C.; Zheng, G.J.; Li, A.M.Y.; Richardson, B.J.; Lam, P.K.S. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat. Toxicol. 2001, 52, 189–203. [Google Scholar] [CrossRef]
- El-Bouhy, Z.M.; Mohamed, F.A.; Elashhab, M.W.; El-Houseiny, W. Toxicity bioassay and sub-lethal effects of profenofos-based insecticide on behavior, biochemical, hematological, and histopathological responses in Grass carp (Ctenopharyngodon idella). Ecotoxicology 2023, 32, 196–210. [Google Scholar] [CrossRef]
- Khalil, A.A.; Abd-Elhakim, Y.M.; Said, E.N.; Moselhy, A.A.; Abu-Elsaoud, A.M.; El-Houseiny, W. Milk thistle and co-enzyme Q10 fortified diets lessen the nickel chloride-induced neurotoxic and neurobehavioral impairments in Oreochromis niloticus via regulating the oxidative stress response, acetylcholinesterase activity, and brain nickel content. Aquaculture 2022, 553, 738102. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Mohamed, W.A.; El-Houseiny, W.; Ibrahim, R.E.; Abd-Elhakim, Y.M. Palliative effects of zinc sulfate against the immunosuppressive, hepato- and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 504, 227–238. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Moustafa, A.A.; Mohamed, A.A.R. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Laing, K.J.; Wang, T.; Zou, J.; Holland, J.; Hong, S.; Bols, N.; Hirono, I.; Aoki, T.; Secombes, C.J. Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor-α. Eur. J. Biochem. 2001, 268, 1315–1322. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Arisha, A.H.; Moselhy, A.A.; Dahshan, H.; Saber, T.; Saber, T.M.; Ahmed, M.M. Alleviative effects of dietary Silybum marianum and co-enzyme Q10 on waterborne nickel-induced impaired growth, immunosuppression, tissue damage, immune-related genes dysregulation, and reduced resistance to Pseudomonas aeruginosa in Oreochromis niloticus. Aquac. Rep. 2022, 26, 101308. [Google Scholar] [CrossRef]
- Xia, X.; Lu, C.; Li, M.; Wang, P.; Dong, H.; Gao, Y.; Chang, Z. Toxic effects of oxyfluorfen on Paramisgurnus dabryanus. J. Henan Agric. Sci. 2016, 45, 122–126. [Google Scholar]
- Powe, D.K.; Dasmahapatra, A.K.; Russell, J.L.; Tchounwou, P.B. Toxicity implications for early life stage Japanese medaka (Oryzias latipes) exposed to oxyfluorfen. Environ. Toxicol. 2018, 33, 555–568. [Google Scholar] [CrossRef]
- Hassanein, H.M. Toxicological effects of the herbicide oxyfluorfen on acetylcholinesterase in two fish species: Oreochromis niloticus and Gambusia affinis. J. Environ. Sci. Health Part A 2002, 37, 521–527. [Google Scholar] [CrossRef]
- Hassanein, H.; Banhawy, M.; Soliman, F.; Abdel-Rehim, S.; Müller, W.; Schröder, H. Induction of hsp70 by the herbicide oxyfluorfen (Goal) in the Egyptian Nile fish, Oreochromis niloticus. Arch. Environ. Contam. Toxicol. 1999, 37, 78–84. [Google Scholar]
- Mansour, A.T.; Amen, R.M.; Mahboub, H.H.; Shawky, S.M.; Orabi, S.H.; Ramah, A.; Hamed, H.S. Exposure to oxyfluorfen-induced hematobiochemical alterations, oxidative stress, genotoxicity, and disruption of sex hormones in male African catfish and the potential to confront by Chlorella vulgaris. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 267, 109583. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Ghamry, H.I.; Althobaiti, S.A.; Almalki, D.A.; Shakweer, M.S.; Hassan, M.A.; Khamis, T.; Abdel-Ghany, H.M.; Ahmed, S.A. Moringa oleifera and Azadirachta indica Leaves enriched diets mitigate chronic oxyfluorfen toxicity induced immunosuppression through disruption of pro/anti-inflammatory gene pathways, alteration of antioxidant gene expression, and histopathological Alteration in Oreochromis niloticus. Fishes 2022, 8, 15. [Google Scholar]
- Almarri, S.H.; Khalil, A.A.; Mansour, A.T.; El-Houseiny, W. Antioxidant, Immunostimulant, and Growth-Promoting Effects of Dietary Annona squamosa Leaf Extract on Nile Tilapia, Oreochromis niloticus, and Its Tolerance to Thermal Stress and Aeromonas sobria Infection. Animals 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; Algharib, S.A.; Mohamed, E.A.; Metwally, M.M.; Mahmoud, Y.K.; Alghamdi, Y.S.; Soliman, M.M.; Abd-Elhakim, Y.M.; El-Murr, A.E. Dietary parsley seed mitigates methomyl-induced impaired growth performance, hemato-immune suppression, oxidative stress, hepato-renal damage, and Pseudomonas aeruginosa susceptibility in Oreochromis niloticus. Antioxidants 2022, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.T.; Mahboub, H.H.; Elshopakey, G.E.; Aziz, E.K.; Alhajji, A.H.; Rayan, G.; Ghazzawy, H.S.; El-Houseiny, W. Physiological Performance, Antioxidant and Immune Status, Columnaris Resistance, and Growth of Nile Tilapia That Received Alchemilla vulgaris-Supplemented Diets. Antioxidants 2022, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef]
- Sakipova, Z.; Giorno, T.B.S.; Bekezhanova, T.; Siu Hai Wong, N.; Shukirbekova, A.; Fernandes, P.D.; Boylan, F. Pharmacological evaluation of Artemisia cina crude CO2 subcritical extract after the removal of santonin by means of high speed countercurrent chromatography. Molecules 2020, 25, 2728. [Google Scholar] [CrossRef]
- Tan, R.X.; Zheng, W.; Tang, H. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef]
- Miraldi, E.; Ferri, S.; Franchi, G.G. Santonin: A new method of extraction from, and quantitative determination in Artemisia caerulescens ssp. cretacea (fiori) br.-catt. & gubell. by high-performance liquid chromatography. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 1998, 9, 296–298. [Google Scholar]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Saleh, O.A.; Sakr, S.F.; Abdelhadi, Y.M. Effect of wormseed plants; Artemisia cina L. and chamomile; Matricaria chamomilla L. on non specific immune response of Clarias gariepinus (African catfish). Abbassa Int. J. Aqua. 2010, 3, 246–259. [Google Scholar]
- Ramadan, R.; Doaa, A. Use of Artemisia cina against Gyrodactylus rysavyi Infecting Cyprinus carpio in Comparison with Praziquntel. J. Arab. Aquac. Soc. 2012, 7, 239–258. [Google Scholar]
- Canadian Council on Animal Care. Canadian Council on Animal Care Guidelines on: The Care and Use of Fish in Research, Teaching and Testing; CCAC: Ottawa, ON, Canada, 2005; p. 84. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Feldman, B.V.; Zinkl, J.G.; Jain, N.C.; Schalm, O.W. Schalm’s Veterinary Hematology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Bain, B.J.; Bates, I.; Laffan, M.A. Dacie and Lewis Practical Haematology E-Book; Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Henry, R.J. Clinical Chemistry, Principles and Technics; Hoeber Medical Division, Harper & Row: New York, NY, USA, 1964. [Google Scholar]
- Reinhold, R. Determination of serum albumin. Clin. Chem. 1953, 21, 1370–1372. [Google Scholar]
- Coles, E. Veterinary Clinical Pathology; WB Saunders Company: Philadelphia, PA, USA; London, UK, 1986. [Google Scholar]
- Adeyemi, O.T.; Osilesi, O.; Adebawo, O.O.; Onajobi, F.D.; Oyedemi, S.O.; Afolayan, A. Alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in selected tissues of rats fed on processed atlantic horse mackerel (Trachurus trachurus). Adv. Biosci. Biotechnol. 2015, 6, 139. [Google Scholar] [CrossRef]
- Ajeniyi, S.; Solomon, R. Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat. Sci. 2014, 12, 124–138. [Google Scholar]
- Kaplan, A. Glucose. In Clinical Chemistry; The CV Mosby Co.: St. Louis, MO, USA; Toronto, ON, Canada; Princeton, NJ, USA, 1984; 418p. [Google Scholar]
- Trinder, P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969, 22, 246. [Google Scholar] [CrossRef]
- Tunn, S.; Möllmann, H.; Barth, J.; Derendorf, H.; Krieg, M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin. Chem. 1992, 38, 1491–1494. [Google Scholar] [CrossRef]
- Naito, H. Cholesterol. In Clinical Chemistry; The CV Mosby Co.: St. Louis, MO, USA; Toronto, ON, Canada; Princeton, NJ, USA, 1984. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Sakai, M.; Kobayashi, M.; Yoshida, T. Activation of rainbow trout, Oncorhynchus mykiss, phagocytic cells by administration of bovine lactoferrin. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 755–759. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, Y.; Husodo, A.H.; Astuti, I. Detection of urinary 8-hydroxydeoxyguanosine (8-OHdG) levels as a biomarker of oxidative DNA damage among home industry workers exposed to chromium. Procedia Environ. Sci. 2015, 23, 290–296. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, Z.; Zhou, J.; Zhang, J.; Zhong, H.; Fu, G.; Zhong, L. The Protective Effect of Taurine on Oxidized Fish-Oil-Induced Liver Oxidative Stress and Intestinal Barrier-Function Impairment in Juvenile Ictalurus punctatus. Antioxidants 2021, 10, 1690. [Google Scholar] [CrossRef]
- Ibor, O.R.; Eni, G.; Andem, A.B.; Bassey, I.U.; Arong, G.A.; Asor, J.; Regoli, F.; Arukwe, A. Biotransformation and oxidative stress responses in relation to tissue contaminant burden in Clarias gariepinus exposed to simulated leachate from a solid waste dumpsite in Calabar, Nigeria. Chemosphere 2020, 253, 126630. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Paperna, I. Studies on monogenetic trematodes in Israel. 3. Monogenetic trematodes of the Cyprinidae and Claridae of the Lake of Galilee. Bamidgeh 1961, 13, 14–29. [Google Scholar]
- El-Naggar, M.M.; Serag, H.M. Quadriacanthus aegypticus n. sp., a monogenean gill parasite from the Egyptian teleost Clarias lazera. Syst. Parasitol. 1986, 8, 129–140. [Google Scholar] [CrossRef]
- Stoskopf, M.K. Biology and management of laboratory fishes. In Laboratory Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1063–1086. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Huang, L.; Jia, K.; Xiong, H.; Tian, G.; Xu, J.; Yuan, W.; Lu, C.; Xiao, X.; Lu, H. Oxyfluorfen exposure can cause acute kidney injury by promoting ROS-induced oxidative stress and inflammation in zebrafish. J. Hazard. Mater. 2022, 440, 129823. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Zheng, Z.; Tan, J.Y.; Liu, H.; Zhou, X.; Xiang, X.; Wang, K. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Mbokane, E.M.; Moyo, N.A.G. Effects of dietary levels of essential oil extracts from Moringa oleifera and Artemisia afra on kidney histology, haemato-immunological parameters and disease resistance in Clarias gariepinus. Aquac. Res. 2020, 51, 410–425. [Google Scholar] [CrossRef]
- Michael, P.O. Toxicity effect of atrazine on histology, haematology and biochemical indices of Clarias gariepinus. Int. J. Fish. Aquat. Stud. 2018, 6, 87–92. [Google Scholar]
- Lutnicka, H.; Bojarski, B.; Witeska, M.; Chmurska-Gąsowska, M.; Trybus, W.; Trybus, E.; Kopacz-Bednarska, A.; Lis, M. Effects of MCPA herbicide on hematological parameters and ultrastructure of hematopoietic tissues of common carp (Cyprinus carpio L.). Folia Biol. 2018, 66, 1–11. [Google Scholar] [CrossRef]
- Lutnicka, H.; Bojarski, B.; Król, T.; Trybus, W.; Trybus, E.; Kopacz-Bednarska, A.; Witeska, M.; Pankiewicz, L.; Pawlak, K. Hematological parameters and ultrastructure of hematopoietic tissues in common carp (Cyprinus carpio L.) exposed to sublethal concentration of pendimethalin. Folia Biol. 2018, 66, 121–132. [Google Scholar] [CrossRef]
- Rio, B.; Parent-Massin, D.; Lautraite, S.; Hoellinger, H. Effects of a diphenyl-ether herbicide, oxyfluorfen, on human BFU-E/CFU-E development and haemoglobin synthesis. Hum. Exp. Toxicol. 1997, 16, 115–122. [Google Scholar] [CrossRef]
- Soares, M.P.; Cardoso, I.L.; Ishikawa, M.M.; de Oliveira, A.d.S.S.; Sartoratto, A.; Jonsson, C.M.; de Queiroz, S.C.d.N.; Duarte, M.C.T.; Rantin, F.T.; Sampaio, F.G. Effects of Artemisia annua alcohol extract on physiological and innate immunity of Nile tilapia (Oreochromis niloticus) to improve health status. Fish Shellfish Immunol. 2020, 105, 369–377. [Google Scholar] [CrossRef]
- Pulsford, A.; Lemaire-Gony, S.; Tomlinson, M.; Collingwood, N.; Glynn, P. Effects of acute stress on the immune system of the dab, Limanda limanda. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1994, 109, 129–139. [Google Scholar] [CrossRef]
- Krijt, J.; Stranska, P.; Maruna, P.; Vokurka, M.; Sanitrak, J. Herbicide-induced experimental Variegate prophyria in mice: Tissue porphyrinogen accumulation and response to porphyrogenic drugs. Can. J. Physiol. Pharmacol. 1997, 75, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Jia, K.; Zheng, Z.; Chen, X.; Bai, Z.; Yang, Y.; Chen, B.; Yuan, W.; Chen, W. Oxyfluorfen induces hepatotoxicity through lipo-sugar accumulation and inflammation in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 230, 113140. [Google Scholar] [CrossRef] [PubMed]
- Mbokane, E.; Moyo, N. A preliminary investigation into the potential effect of Artemisia afra on growth and disease resistance in sub-adults of Oreochromis mossambicus. Aquaculture 2018, 482, 197–202. [Google Scholar] [CrossRef]
- Mahassni, S.H.; Khudauardi, E.R. A pilot study: The effects of an aqueous extract of Lepidium sativum seeds on levels of immune cells and body and organs weights in Mice. J. Ayurvedic. Herb. Med. 2017, 3, 27–32. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, N.; Zhao, X.; Sang, X.; Yang, N.; Feng, Y.; Chen, R.; Chen, Q. Dihydroartemisinin regulates the immune system by promotion of CD8+ T lymphocytes and suppression of B cell responses. Sci. China Life Sci. 2020, 63, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Hassim, H.A.; Yusoff, M.S.M.; Bakar, S.N.A.; Srisapoome, P. Short-and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus). Fish Shellfish Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 97, 540–553. [Google Scholar] [CrossRef]
- Ladurner, A.; Schachner, D.; Schueller, K.; Pignitter, M.; Heiss, E.H.; Somoza, V.; Dirsch, V.M. Impact of trans-resveratrol-sulfates and-glucuronides on endothelial nitric oxide synthase activity, nitric oxide release and intracellular reactive oxygen species. Molecules 2014, 19, 16724–16736. [Google Scholar] [CrossRef]
- Schwartz, J.I.; Dallob, A.L.; Larson, P.J.; Laterza, O.F.; Miller, J.; Royalty, J.; Snyder, K.M.; Chappell, D.L.; Hilliard, D.A.; Flynn, M.E. Comparative inhibitory activity of etoricoxib, celecoxib, and diclofenac on COX-2 versus COX-1 in healthy subjects. J. Clin. Pharmacology 2008, 48, 745–754. [Google Scholar] [CrossRef]
- Soltani, T.; Safahieh, A.; Zolgharnien, H.; Matroodi, S. Interactions of oxidative DNA damage and CYP1A gene expression with the liver enzymes in Klunzinger’s mullet exposed to benzo [a] pyrene. Toxicol. Rep. 2019, 6, 1097–1103. [Google Scholar] [CrossRef]
- Saxena, R.B. Entirely gone out useful plant—Artemisia cina. Indo Am. J. Pharm. Sci. 2015, 3, 2015. [Google Scholar]
- Alak, G.; Yeltekin, A.Ç.; Tas, I.H.; Ucar, A.; Parlak, V.; Topal, A.; Kocaman, E.M.; Atamanalp, M. Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defence system in liver tissues of rainbow trout exposed to eprinomectin. Fish Shellfish Immunol. 2017, 65, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Anjana Vaman, V.; Tinu, S.; Geetha, C.; Lissy, K.; Mohanan, P. Effect of fibrin glue on antioxidant defense mechanism, oxidative DNA damage and chromosomal aberrations. Toxicol. Mech. Methods 2013, 23, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Teuber, M. Veterinary use and antibiotic resistance. Curr. Opin. Microbiol. 2001, 4, 493–499. [Google Scholar] [CrossRef]

| Ingredients (g/100 g) | Experimental Diets | |
|---|---|---|
| Control | 5% Artemisia cina | |
| Fish meal 66% | 18 | 18 |
| Ground corn | 25 | 20 |
| Soybean meal 44% | 35 | 35 |
| Corn oil | 3 | 3 |
| Wheat bran | 10 | 10 |
| Cod liver oil | 2 | 2 |
| starch | 4 | 4 |
| Artemisia cina powder | 0 | 5 |
| Vitamin premix 1 | 1 | 1 |
| Mineral premix 2 | 2 | 2 |
| Total | 100 | 100 |
| Chemical analysis | ||
| Crude protein (N × 6.25) | 32.10 | 32.05 |
| Crude lipids | 11.27 | 11.34 |
| Crude fiber | 4.45 | 4.25 |
| Ash | 7.60 | 7.75 |
| Nitrogen free extract 3 | 44.58 | 44.61 |
| Gross energy (kcal/kg) 4 | 470.56 | 471.59 |
| Gene Name | Primer Sequences | NCBI Accession No. | Reference | |
|---|---|---|---|---|
| GAPDH | F | TGTCCGTTTGGAGAAGCCT | NM_001201199.1 | [65] |
| R | ATCAGGTCACAGACACGGTTG | |||
| CAT | F | CTGGGACCTGACAGGCAATA | KF977829 | [66] |
| R | CTCCAGAAGTCCCACACCAT | |||
| SOD1 | F | ACCATGAAAGCTGTTTGCGT | NM_001200992 | [66] |
| R | TGGACATGAAAGCCATGCAG | |||
| GPX1 | F | ACCTGACCGCTGACATAGAG | NM_001200741 | [66] |
| R | ACATCAGACAGCCCTTCACA | |||
| GST | F | ATCACCAGAAAGGCATTCGC | GU588174 | [66] |
| R | TCCAGGTCATTCTGATGGCA | |||
| CYP1A | F | CCAGCACGAGCATGAAGAAA | KP336485 | [66] |
| R | ATGCTCTTTGACCAGCCTCT | |||
| TNF-α | F | CGCCAGCGGTAAACACG | XM_017464718.1 | [65] |
| R | CCGTTGAATGTCCGAAAGG | |||
| IL-1β | F | CTGAAGGGTGGAAACAAGGAT | AJ586102.1 | [65] |
| R | GGAGTCACCAGTGCCGTTT | |||
| TGF-β1 | F | GGAACGGCTGAGTGGGTCT | XM_017483625.1 | [65] |
| R | TGCTTACTGAGGCGGCTATG | |||
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | AC | OXY | AC+OXY | |
| Initial body weight (g) | 70.00 a ± 1.154 | 72.00 a ± 1.154 | 70.33 a ± 1.452 | 71.16 a ± 1.166 |
| Final body weight (g) | 120.00 b ± 1.732 | 136.33 a ± 2.027 | 101.33 c ± 1.763 | 113.00 b ± 2.081 |
| Weight gain (g) | 50.00 b ± 0.577 | 64.33 a ± 0.881 | 31.00 d ± 0.577 | 41.83 c ± 1.092 |
| Specific growth rate (%) | 0.89 b ± 0.003 | 1.06 a ± 0.002 | 0.60 d ± 0.010 | 0.77 c ± 0.010 |
| Feed intake (g) | 75.00 b ± 0.577 | 82.00 a ± 1.154 | 59.00 c ± 1.154 | 71.00 b ± 1.154 |
| Feed conversion ratio | 1.50 c ± 0.005 | 1.27 d ± 0.005 | 1.90 a ± 0.034 | 1.69 b ± 0.029 |
| Mortality (%) | 0.00 b ± 0.000 | 0.00 b ± 0.000 | 25.00 a ± 2.886 | 3.33 b ± 3.333 |
| Survival rate | 100.00 a ± 0.000 | 100.00 a ± 0.000 | 75.00 b ± 2.886 | 96.66 a ± 3.333 |
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | AC | OXY | AC+OXY | |
| Erythrogram | ||||
| Red blood cells (106/mm3) | 2.68 a ± 0.090 | 2.88 a ± 0.091 | 1.29 c ± 0.087 | 2.20 b ± 0.063 |
| Hemoglobin (gm/dl) | 10.31 b ± 0.087 | 10.87 a ± 0.053 | 7.54 d ± 0.061 | 8.95 c ± 0.057 |
| Hematocrit (%) | 30.93 b ± 0.261 | 32.63 a ± 0.160 | 22.64 d ± 0.183 | 26.85 c ± 0.173 |
| Mean corpuscular volume (fl) | 115.60 b ± 2.928 | 113.57 b ± 4.286 | 176.89 a ± 10.363 | 121.82 b ± 2.715 |
| Mean corpuscular hemoglobin (%) | 38.53 b ± 0.978 | 37.85 b ± 1.427 | 58.96 a ± 3.456 | 40.60 b ± 0.907 |
| Leukogram | ||||
| White blood cells (103/mm3) | 7.61 a ± 0.138 | 7.95 a ± 0.030 | 5.17 c ± 0.024 | 7.09 b ± 0.049 |
| Lymphocytes (103/mm3) | 3.71 a ± 0.070 | 3.82 ab ± 0.024 | 2.59 c ± 0.012 | 3.39 b ± 0.024 |
| Heterophils (103/mm3) | 2.55 b ± 0.043 | 2.67 a ± 0.012 | 1.67 c ± 0.005 | 2.45 b ± 0.008 |
| Eosinophils (103/mm3) | 0.45 ab ± 0.018 | 0.47 a ± 0.008 | 0.29 c ± 0.008 | 0.40 b ± 0.008 |
| Monocytes (103/mm3) | 0.89 b ± 0.018 | 0.97 a ± 0.008 | 0.62 d ± 0.005 | 0.83 c ± 0.008 |
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | AC | OXY | AC+OXY | |
| Total protein (g/dL) | 6.10 a ± 0.21 | 6.18 a ± 0.22 | 3.60 c ± 0.12 | 5.18 b ± 0.14 |
| Albumin (g/dL) | 2.72 a ± 0.15 | 2.71 a ± 0.15 | 1.38 c ± 0.04 | 2.13 b ± 0.06 |
| Globulin (g/dL) | 3.37 a ± 0.06 | 3.46 a ± 0.07 | 2.21 b ± 0.15 | 3.05 a ± 0.08 |
| ALT (U/L) | 16.03 b ± 0.84 | 16.01 b ± 0.89 | 50.23 a ± 1.36 | 19.73 b ± 0.82 |
| AST (U/L) | 31.83 b ± 1.48 | 31.83 b ± 1.01 | 60.33 a ± 1.45 | 36.83 b ± 1.01 |
| ALP (IU/L) | 37.10 b ± 0.40 | 36.66 b ± 0.24 | 52.36 a ± 1.36 | 39.60 b ± 1.06 |
| Total bilirubin (mg/dL) | 0.24 bc ± 0.01 | 0.23 d ± 0.02 | 1.01 a ± 0.03 | 0.32 b ± 0.01 |
| Urea (mg/dL) | 2.77 b ± 0.06 | 2.60 b ± 0.06 | 7.50 a ± 0.29 | 3.13 b ± 0.19 |
| Creatine (mg/dL) | 0.60b c ± 0.01 | 0.56 c ± 0.01 | 1.01 a ± 0.02 | 0.67 b ± 0.014 |
| Lysozyme activity (units/L) | 16.82 b ± 0.31 | 20.28 a ± 0.92 | 9.90 c ± 0.25 | 14.43 b ± 0.54 |
| Complement 3 (µg/mL) | 74.06 a ± 1.38 | 1.35 ± 79.96 a | 49.63 c ± 1.67 | 65.66 b ± 1.11 |
| Nitric oxide (µmol/L) | 39.16 b ± 1.17 | 46.83 a ± 1.48 | 21.16 c ± 1.30 | 34.00 b ± 1.15 |
| Phagocytic activity (%) | 26.73 b ± 0.43 | 29.45 a ± 0.47 | 15.83 d ± 0.52 | 23.95 c ± 0.24 |
| Experimental Groups | ||||
|---|---|---|---|---|
| Control | AC | OXY | AC+OXY | |
| Glutathione peroxidase (U/L) | 123.33 b ± 1.17 | 130.72 a ± 1.29 | 87.76 d ± 0.75 | 114.73 c ± 1.51 |
| Superoxide dimutase (U/mL) | 4.76 a ± 0.11 | 5.08 a ± 0.06 | 2.92 c ± 0.06 | 4.05 b ± 0.08 |
| Catalase (U/L) | 190.90 a ± 3.41 | 198.38 a ± 2.31 | 144.96 c ± 2.63 | 175.63 b ± 2.86 |
| Malondialdehyde (nmol/mL) | 13.55 b ± 0.18 | 11.58 c ± 0.24 | 24.66 a ± 0.73 | 14.90 b ± 0.06 |
| Glutathione dehydrogenase (μmol/mL) | 2.16 a ± 0.02 | 2.23 a ± 0.01 | 1.34 c ± 0.03 | 1.99 b ± 0.03 |
| (mg/dL) Cortisol | 60.98 c ± 1.79 | 60.13 c ± 1.81 | 90.93 a ± 2.37 | 73.80 b ± 1.37 |
| Glucose (mg/dL) | 79.00 bc ± 1.15 | 76.66 c ± 1.76 | 107.66 a ± 1.45 | 85.66 b ± 1.763 |
| Cholesterol (mg/dL) | 90.50 b ± 1.32 | 80.00 c ± 2.89 | 122.83 a ± 1.59 | 97.33 b ± 1.45 |
| 8-OHdG (ng/mL) | 19.61 c ± 0.62 | 18.66 c ± 0.57 | 77.01 a ± 0.73 | 26.26 b ± 0.72 |
| AChE (U/L) | 56.28 a ± 0.40 | 55.43 a ± 0.54 | 17.43 c ± 0.69 | 51.10 b ± 0.78 |
| Experimental Groups | |||||
|---|---|---|---|---|---|
| Control | AC | OXY | AC+OXY | ||
| Survived fish | number | 21 | 25 | 15 | 20 |
| Survival rate | percent | 84 | 100 | 60 | 80 |
| Infested fish | number | 20 | 3 | 5 | 2 |
| Prevalence | percent | 95.23 | 12 | 33.33 | 10 |
| Mean intensity | - | 87.66 a ± 1.45 | 17.0 bc ± 1.15 | 20.0 b ± 1.15 | 11.66 c ± 0.88 |
| Signs of asphyxia | number | 16/21 | 2/25 | 14/15 | 5/20 |
| score | +++ | + | ++++ | ++ | |
| Postmortem change in gills | number | 13/20 | 0/3 | 5/5 | 1/2 |
| score | +++ | - | ++++ | ++ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Houseiny, W.; Anter, R.G.A.; Arisha, A.H.; Mansour, A.T.; Safhi, F.A.; Alwutayd, K.M.; Elshopakey, G.E.; Abd El-Hakim, Y.M.; Mohamed, E.M.M. Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina. Fishes 2023, 8, 297. https://doi.org/10.3390/fishes8060297
El-Houseiny W, Anter RGA, Arisha AH, Mansour AT, Safhi FA, Alwutayd KM, Elshopakey GE, Abd El-Hakim YM, Mohamed EMM. Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina. Fishes. 2023; 8(6):297. https://doi.org/10.3390/fishes8060297
Chicago/Turabian StyleEl-Houseiny, Walaa, Reham G. A. Anter, Ahmed H. Arisha, Abdallah Tageldein Mansour, Fatmah Ahmed Safhi, Khairiah Mubarak Alwutayd, Gehad E. Elshopakey, Yasmina M. Abd El-Hakim, and Engy M. M. Mohamed. 2023. "Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina" Fishes 8, no. 6: 297. https://doi.org/10.3390/fishes8060297
APA StyleEl-Houseiny, W., Anter, R. G. A., Arisha, A. H., Mansour, A. T., Safhi, F. A., Alwutayd, K. M., Elshopakey, G. E., Abd El-Hakim, Y. M., & Mohamed, E. M. M. (2023). Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina. Fishes, 8(6), 297. https://doi.org/10.3390/fishes8060297









