Comparing the Size at Onset of Sexual Maturity of Edible Crab (Cancer pagurus, Cancridae) in Berwickshire and Northumberland
Abstract
1. Introduction
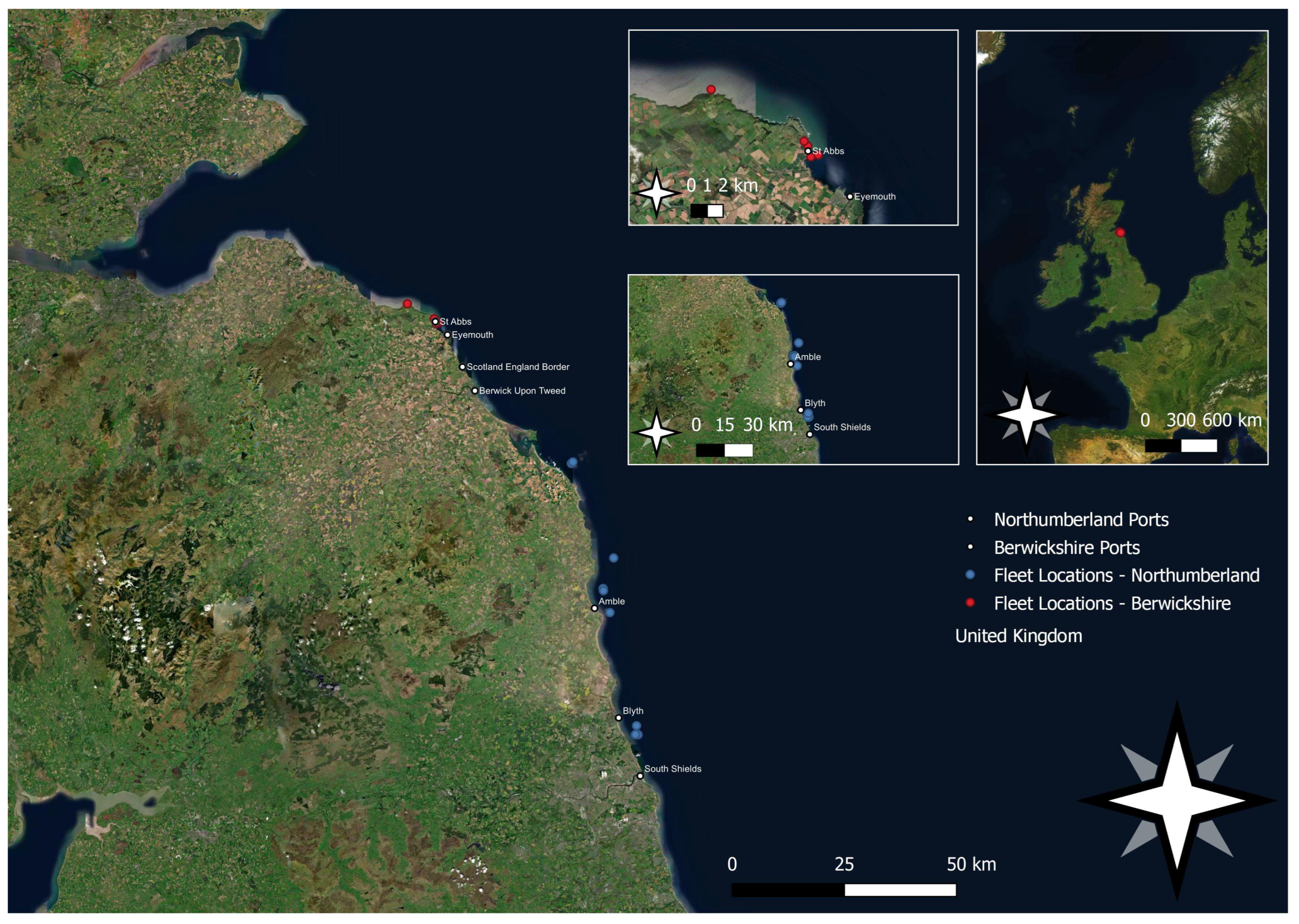
2. Materials and Methods
2.1. Statistical Analysis
2.2. Morphometric Maturity
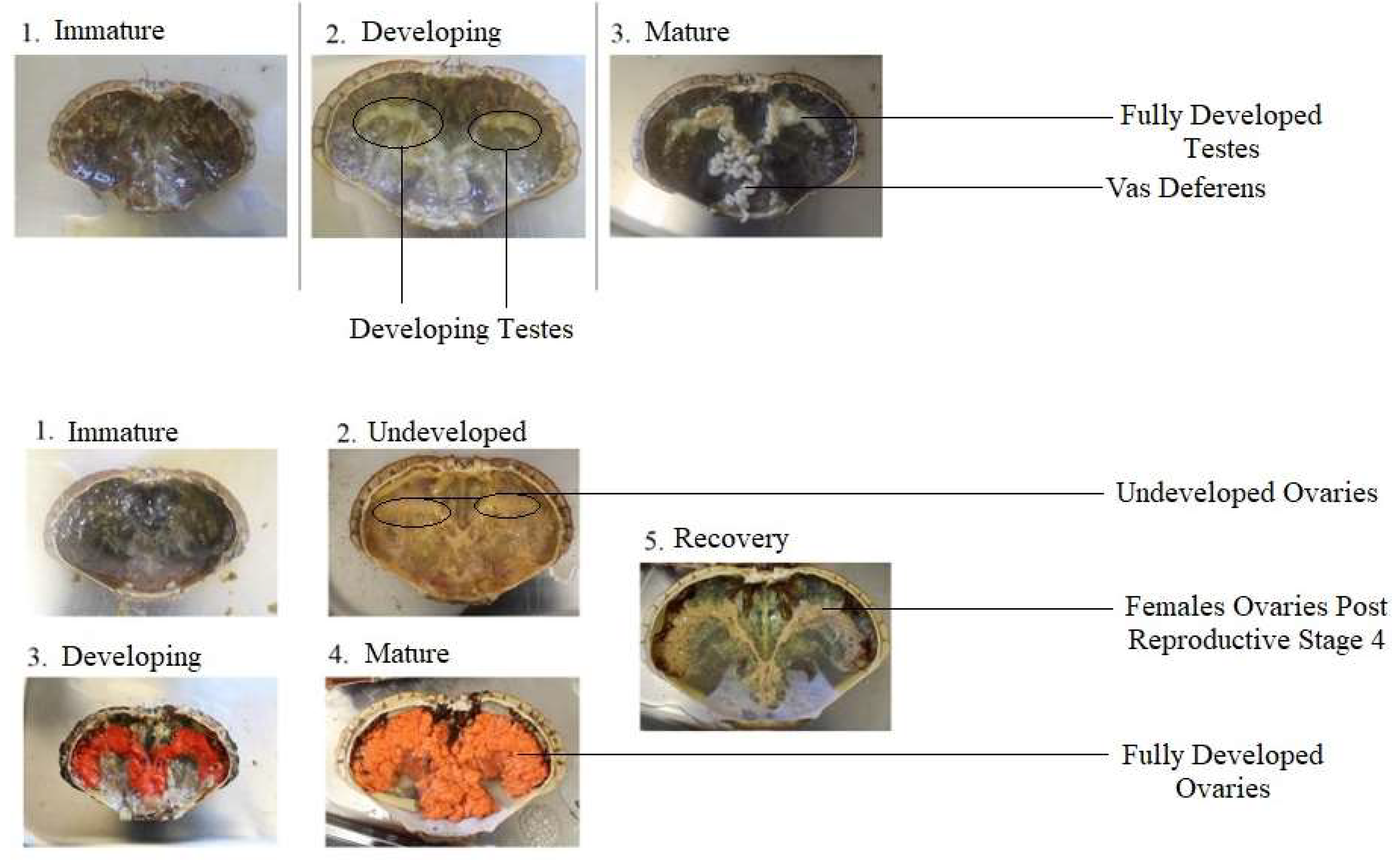
2.3. Physiological Maturity
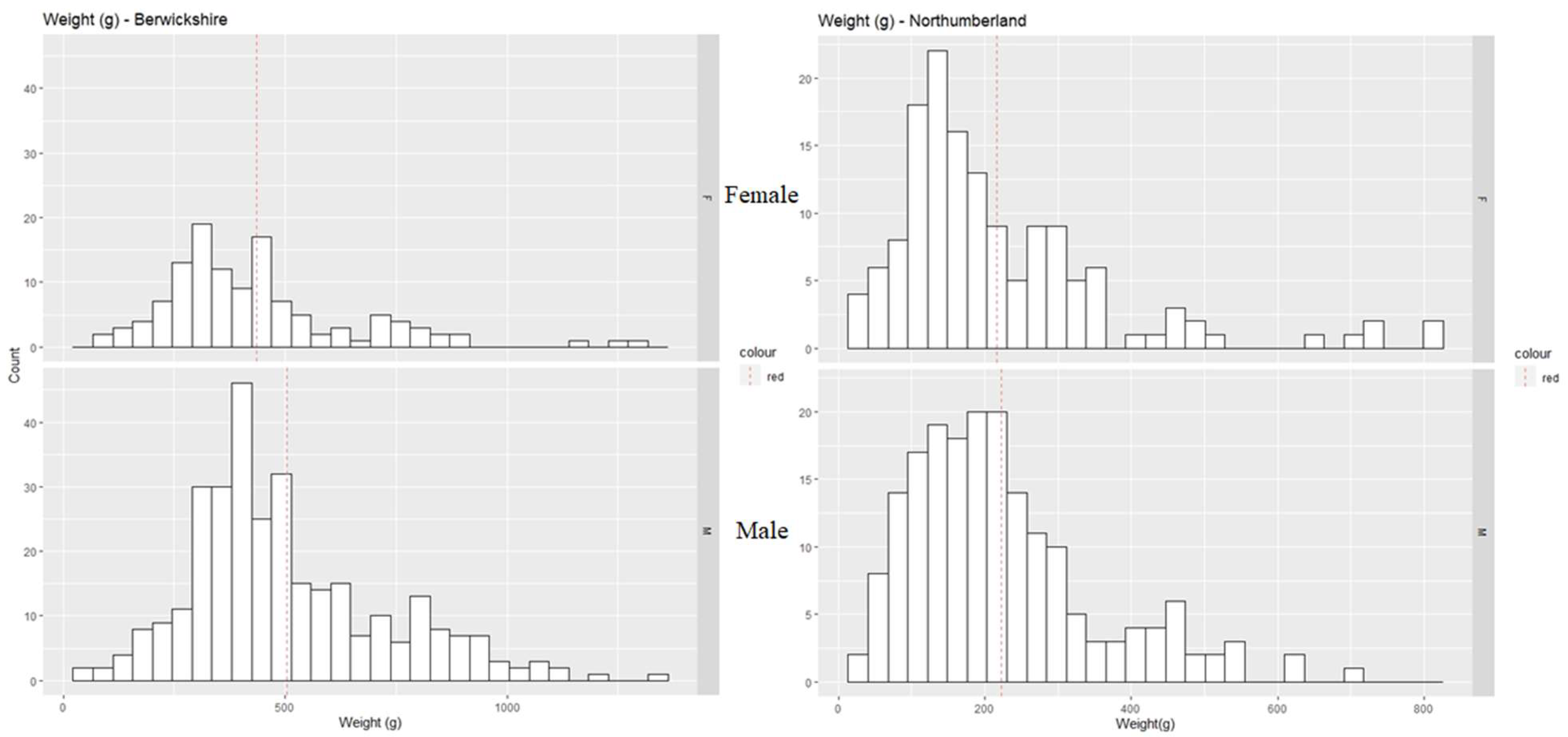
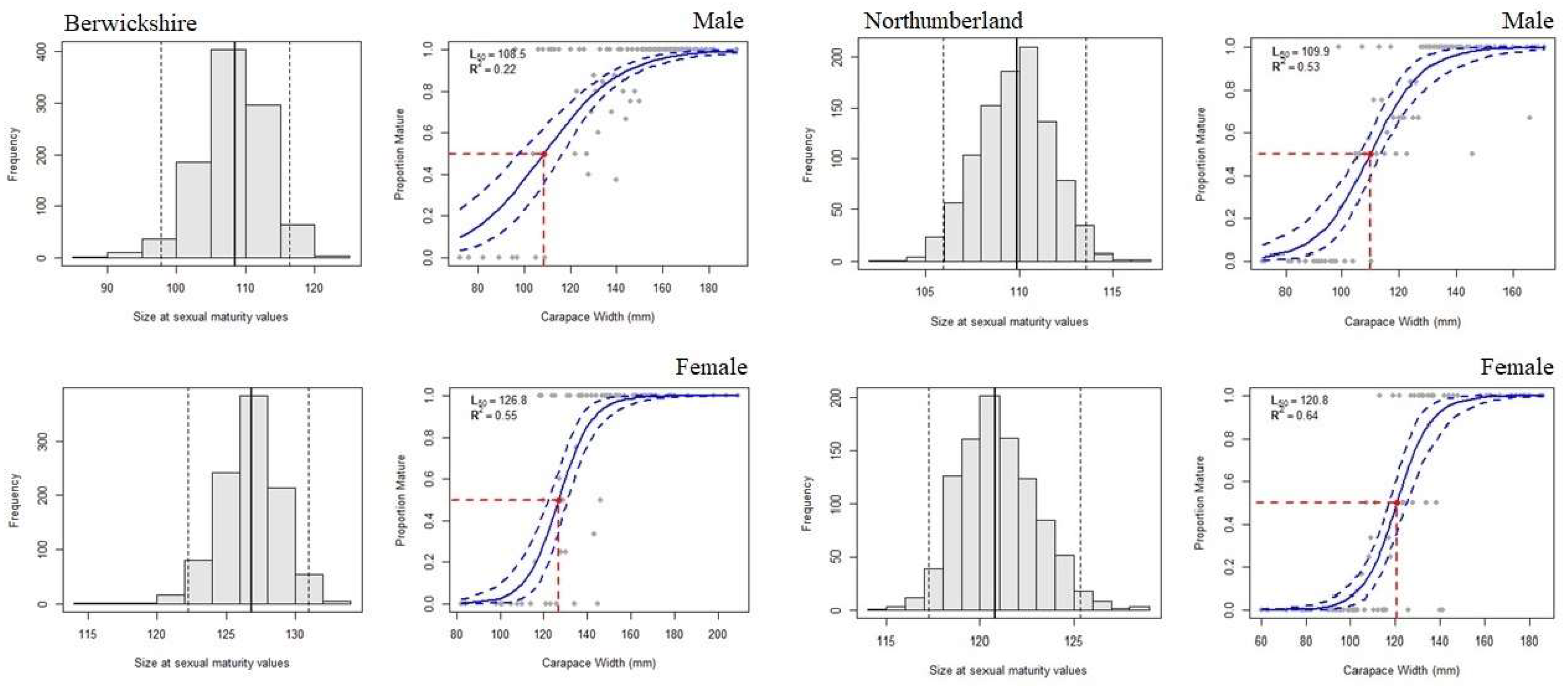
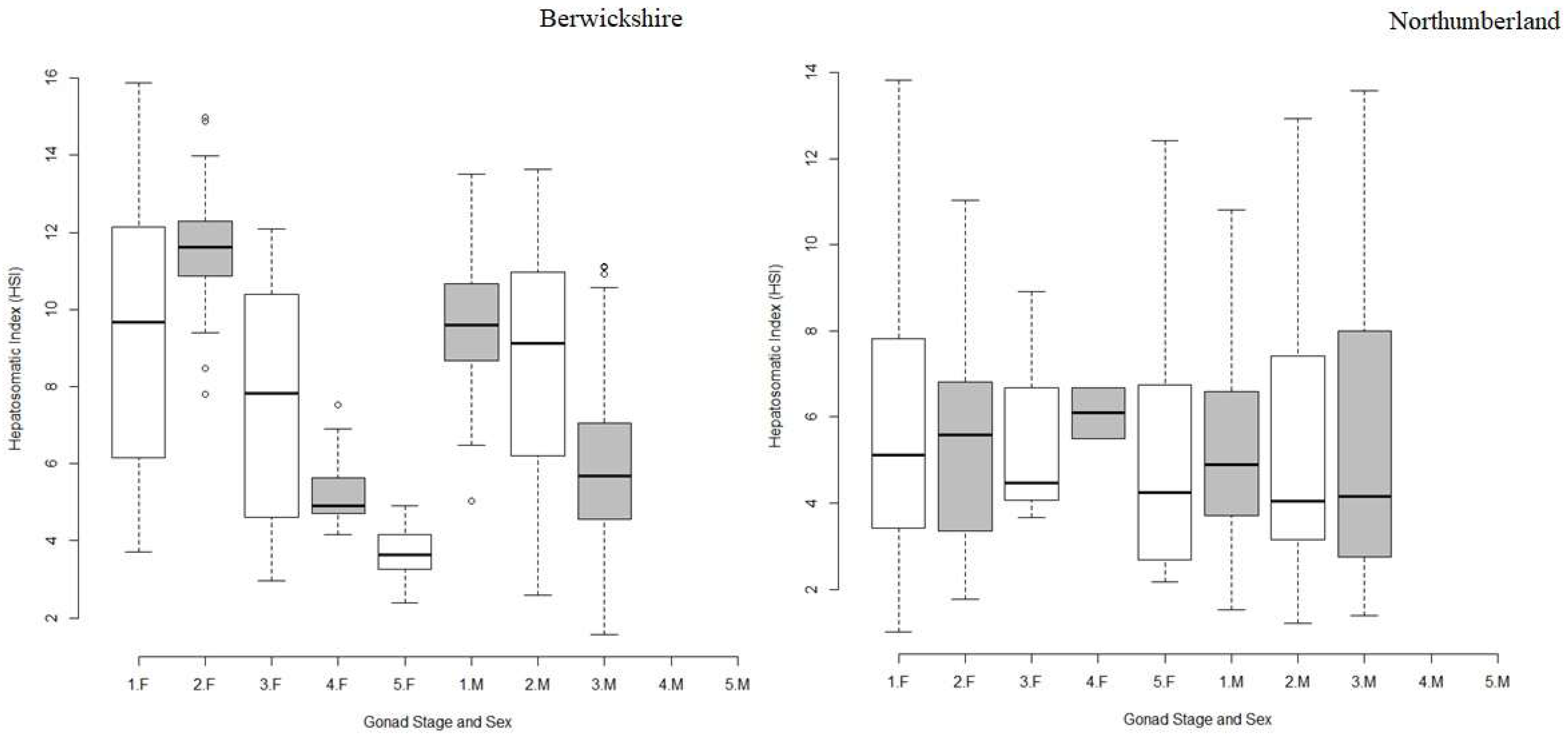
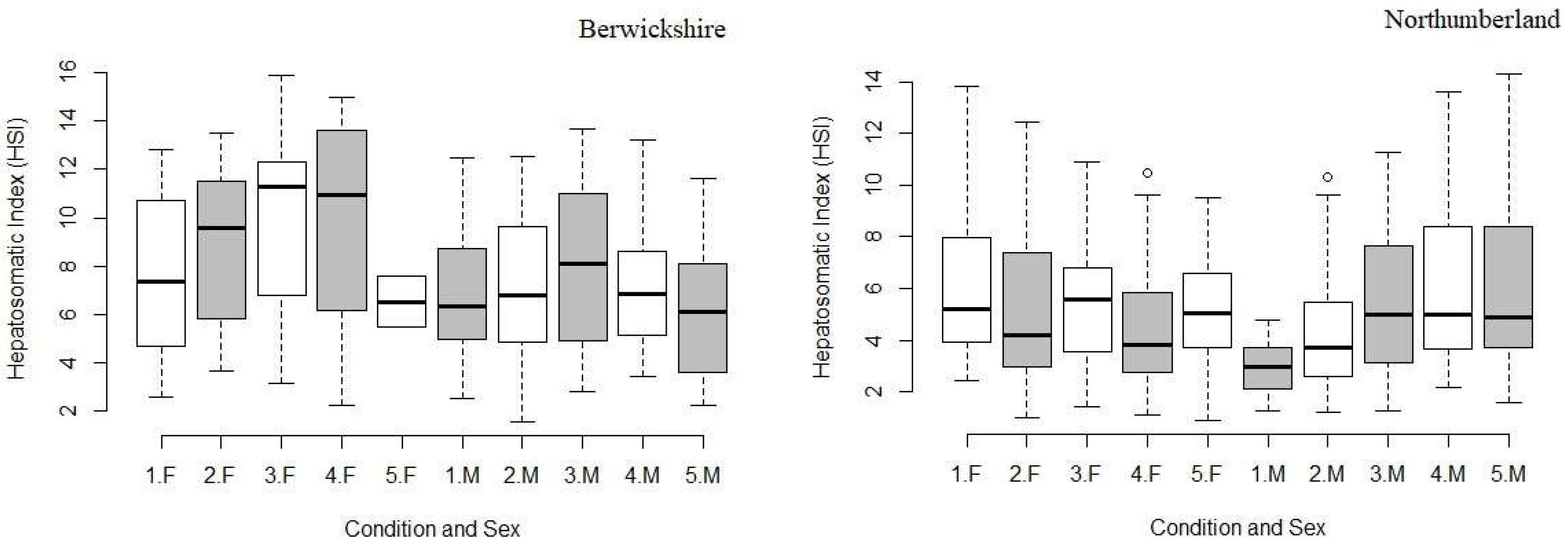
3. Results
3.1. Physiological Maturity
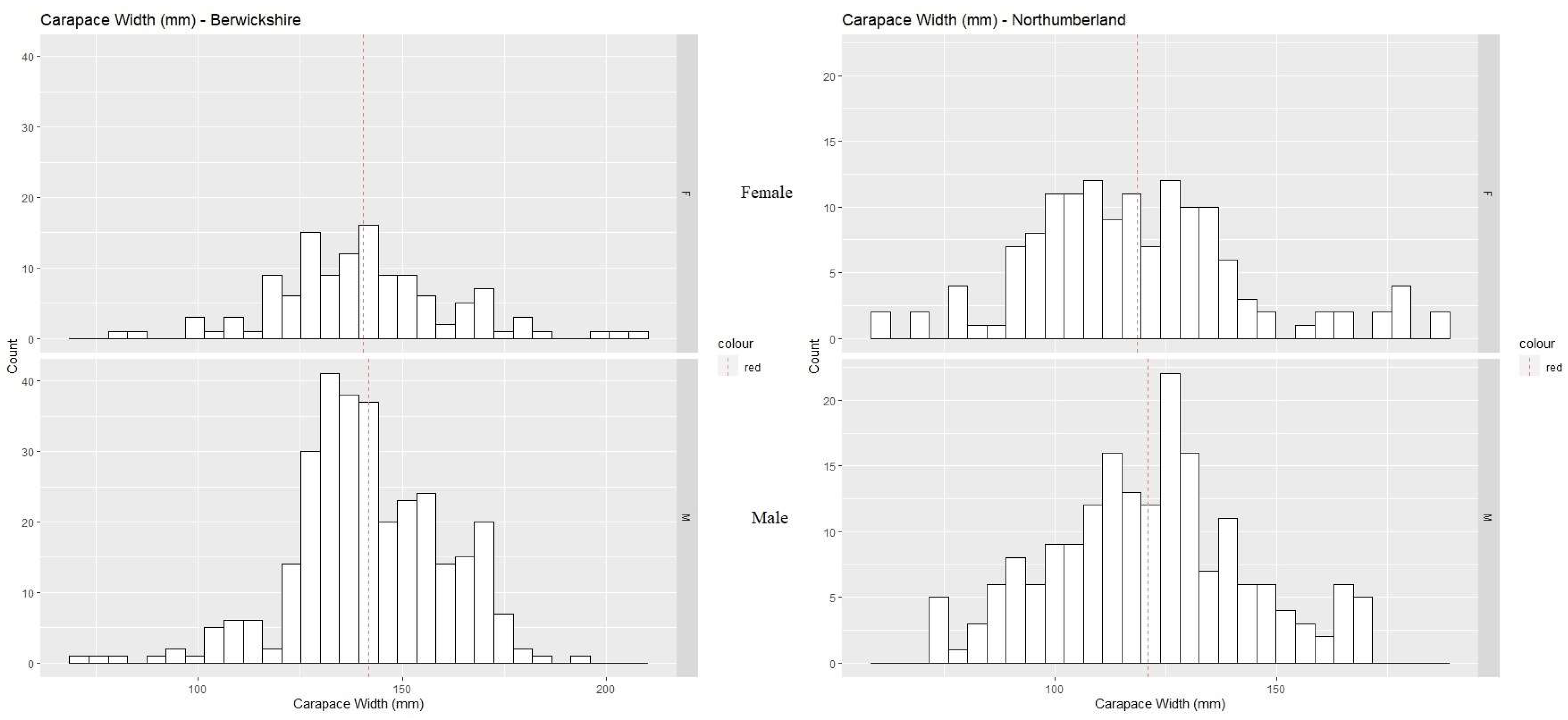
3.2. Morphometric Maturity
3.3. Hepatopancreas Weight and Hepatosomatic Index (HSI)
3.4. Gonad Weight and Gonadosomatic Index (GSI)
4. Discussion
4.1. Physiological Maturity
4.2. Morphometric Maturity
4.3. Hepatopancreas Weight and Hepatopancreas Index (HSI)
4.4. Gonad Weight and Gonadosomatic Index (GSI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seafish. Fleet Enquiry Tool. Available online: www.seafish.org (accessed on 25 September 2020).
- M. Management Organisation. UK Sea Fisheries Statistics 2019. 2019. Available online: https://www.gov.uk/government/statistics/uk-sea-fisheries-annual-statistics-report-2019 (accessed on 25 September 2020).
- The Scottish Government. The Specified Crustaceans (Prohibition on Landing, Sale and Carriage) (Scotland) Order 2017. Scotland. 2018. Available online: https://www.legislation.gov.uk/ssi/2017/455/contents/made (accessed on 25 September 2020).
- Sea Fisheries, England Sea Fisheries, Northern Ireland Conservation of Sea Fish The Undersized Edible Crabs Order 2000. 2000. Available online: https://www.legislation.gov.uk/uksi/2000/2029?view=plain (accessed on 25 September 2020).
- North Eastern Inshore Fisheries and Conservation Authority (NEIFCA). Minimum Conservation Reference Sizes. 2022. Available online: https://www.ne-ifca.gov.uk/minimum-conservation-reference-sizes (accessed on 6 May 2022).
- The Scottish Government. Scottish Sea Fisheries Statistics 2020. 2020. Available online: https://www.gov.scot/publications/scottish-sea-fisheries-statistics-2020/ (accessed on 25 September 2020).
- Haig, J.A.; Rayner, G.; Akritopoulou, E.; Kaiser, M.J. Fecundity of Cancer pagurus in Welsh waters; a comparison with published literature. Fish. Conserv. Sci. Rep. 2015, 24. [Google Scholar]
- Mesquita, C.; Dobby, H.; Sweeting, S.; Jones, C.S.; Pierce, G.J. Size-at-maturity of Brown Crab (Cancer pagurus) in Scottish waters based on gonadal and morphometric traits. Fish. Res. 2020, 229, 105610. [Google Scholar] [CrossRef]
- Bennett, D.B. Factors in the life history of the edible crab (Cancer pagurus L.) that influence modelling and management. ICES Mar. Sci. Symp. 1995, 199, 89–98. [Google Scholar]
- Edwards, E. Edible Crab and Its Fishery in British Waters; Fishing News Books: Farnham, UK, 1979. [Google Scholar]
- Tallack, S.M.L. The Biology and Exploitation of Three Crab Species in the Shetland Islands, Scotland: Cancer pagurus, Necora puber and Carcinus maenas. Open University: Maidenhead, UK, 2002. [Google Scholar]
- Latrouite, D.; Noel, P. Observations sur la Maturite Sexuelle et la Ponte du Tourteau Cancer Pagllrlls en Manche. 1993. Available online: https://archimer.ifremer.fr/doc/00000/1420/ (accessed on 25 September 2020).
- Mesquita, C.; Dobby, H.; Pierce, G.J.; Jones, C.S.; Fernandes, P.G. Abundance and spatial distribution of brown crab (Cancer pagurus) from fishery-independent dredge and trawl surveys in the North Sea. ICES J. Mar. Sci. 2020, 78, 597–610. [Google Scholar] [CrossRef]
- Tallack, S.M.L. The reproductive cycle and size at maturity observed in Cancer pagurus in the Shetland Islands, Scotland. J. Mar. Biol. Assoc. United Kingd. 2007, 87, 1181–1189. [Google Scholar] [CrossRef]
- Andersens, H. Ices Scrab Report 2005 Ices Living Reources Committee Ices cm 2005/g:10 ref. d Report of the Study Group on the Biology and Life History of Crabs (Sgcrab) International Council for the Exploration of the Sea Conseil International Pour l’Exploration de la Mer. 2005. Available online: www.ices.dk (accessed on 19 September 2020).
- Smith, M. Development of a Multiple Indicator Framework Macro-Crustacean Fishery Assessment and Management. 2010. Available online: https://secure.toolkitfiles.co.uk/clients/25364/sitedata/Redesign/Key_Species/Edible-Crab-Species-Profile.pdf?c (accessed on 19 September 2020).
- Tully, O.; Robinson, M.; Cosgrove, R.; O’keeffe, E.; Doyle, O.; Lehane, B. The Brown Crab (Cancer pagurus L.) Fishery: Analysis of the Resource in 2004–2005. 2006. Available online: https://www.researchgate.net/publication/237612664_The_Brown_Crab_Cancer_pagurus_L_Fishery_Analysis_of_the_resource_in_2004-2005 (accessed on 15 April 2023).
- Öndes, F.; Kaiser, M.J.; Murray, L.G. Relative growth and size at onset of sexual maturity of the brown crab, Cancer pagurus in the Isle of Man, Irish Sea. Mar. Biol. Res. 2017, 13, 237–245. [Google Scholar] [CrossRef]
- Ungfors, A. Sexual maturity of the edible crab (Cancer pagurus) in the Skagerrak and the Kattegat, based on reproductive and morphometric characters. ICES J. Mar. Sci. 2007, 64, 318–327. [Google Scholar] [CrossRef]
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Understanding the effects of electromagnetic field emissions from Marine Renewable Energy Devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Mar. Pollut. Bull. 2018, 131, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Haig, J.A.; Pantin, J.R.; Salomonsen, H.; Kaiser, M.J. Size at Maturity of the Edible Crab (Cancer pagurus) in Welsh Waters; Fisheries & Conservation Science report; Bangor University: Bangor, Wales, 2015; p. 26. [Google Scholar]
- RStudio. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 13 May 2021).
- Torrejon-Magallanes, J. sizeMat: An R Package to Estimate Size at Sexual Maturity. 2020. Available online: https://cran.r-project.org/web/packages/sizeMat/vignettes/sizeMat.html#references (accessed on 13 May 2021).
- Somerton, D.A. A Computer Technique for Estimating the Size of Sexual Maturity in Crabs. Canadian J. Fish. Aquat. Sci. 1980, 37, 1488–1494. [Google Scholar] [CrossRef]
- Roa, R.; Ernst, B.; Tapia, F. Estimation of Size at Sexual Maturity: An Evaluation of Analytical and Resampling Procedures. 1999. Available online: https://spo.nmfs.noaa.gov/sites/default/files/13roafis.pdf (accessed on 6 May 2022).
- Haig, J.A.; Bakke, S.; Bell, M.C.; Bloor, I.S.; Cohen, M.; Coleman, M.; Dignan, S.; Kaiser, M.J.; Pantin, J.R.; Roach, M.; et al. Reproductive traits and factors affecting the size at maturity of Cancer pagurus across Northern Europe. ICES J. Mar. Sci. 2016, 73, 2572–2585. [Google Scholar] [CrossRef]
- Small, J.; Smith, C. Edible/Brown Crab (Cancer pagurus); Southern Inshore Fisheries and Conservation Authority: Poole, UK, 2021.
- Öndes, F.; Kaiser, M.J.; Murray, L.G. Torres, G. Reproductive Ecology, Fecundity, and Elemental Composition of Eggs in Brown Crab Cancer pagurus in the Isle of Man. J. Shellfish. Res. 2016, 35, 539–547. [Google Scholar] [CrossRef]
- Boon, A. Stock Status Assessment for the Cancer pagurus Fishery of the Northumberland Coast in 2019. 2019. Available online: https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=0CAIQw7AJahcKEwjwpdfdk_D-AhUAAAAAHQAAAAAQAw&url=https%3A%2F%2Fnifca.gov.uk%2Fwp-content%2Fuploads%2F2022%2F02%2FFlag-Brown-Crab-Report-Final.docx&psig=AOvVaw20oS1v-d2m7Vma96TZj_Hu&ust=1683993752900926 (accessed on 25 September 2020).
- Ungfors, A. Fisheries Biology of the Edible Crab (Cancer pagurus) in the Kattegat and the Skagerrak: Implications for Sustainable Management. Ph.D. Thesis, Göteborg University, Gothenburg, Sweden, 2008. [Google Scholar]
- Le Foll, A. Contribution a l’etude de la biologie du crabe-tourteau Cancer pagurus sur les cotes de Bretagne sud. Revue des Travaux de l’Institut des Pêches Maritimes 1984, 48, 5–22. [Google Scholar]
- Hartnoll, R.G. Growth, Sexual Maturity and Reproductive Output. In Factors in Adult Growth, 1st ed.; Wenner, A.M., Ed.; Routledge: Oxfordshire, UK, 1985. [Google Scholar] [CrossRef]
- Bakke, S.; Larssen, W.E.; Woll, A.K.; Søvik, G.; Gundersen, A.C.; Hvingel, C.; Nilssen, E.M. Size at maturity and molting probability across latitude in female Cancer pagurus. Fish. Res. 2018, 205, 43–51. [Google Scholar] [CrossRef]
- Shields, J.D. The reproductive ecology and fecundity of Cancer crabs. In Crustacean Egg Production; CRC Press: Boca Raton, FL, USA, 1991; pp. 193–213. [Google Scholar]
- Hines, A.H. Fecundity and Reproductive Output in Nine Species of Cancer crabs (Crustacea, Brachyura, Cancridae). Can. J. Fish. Aquat. Sci. 1991, 48, 267–275. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Mating in the Brachyura. Crustaceana 1969, 16, 161–181. [Google Scholar] [CrossRef]
- Hancock, D.A.; Edwards, E. Estimation of Annual Growth in the Edible Crab (Cancer pagurus L.). ICES J. Mar. Sci. 1967, 31, 246–264. [Google Scholar] [CrossRef]
- Woll, A.K.; Berge, G.M. Feeding and management practices affect quality improvement in wild-caught edible crab (Cancer pagurus). Aquaculture 2007, 269, 328–338. [Google Scholar] [CrossRef]
- Flores, A.; Wiff, R.; Ganias, K.; Marshall, C.T. Accuracy of gonadosomatic index in maturity classification and estimation of maturity ogive. Fish. Res. 2019, 210, 50–62. [Google Scholar] [CrossRef]
- Barrento, S.; Marques, A.; Teixeira, B.; Anacleto, P.; Vaz-Pires, P.; Nunes, M.L. Effect of Season on the Chemical Composition and Nutritional Quality of the Edible Crab Cancer pagurus. J. Agric. Food Chem. 2009, 57, 10814–10824. [Google Scholar] [CrossRef] [PubMed]
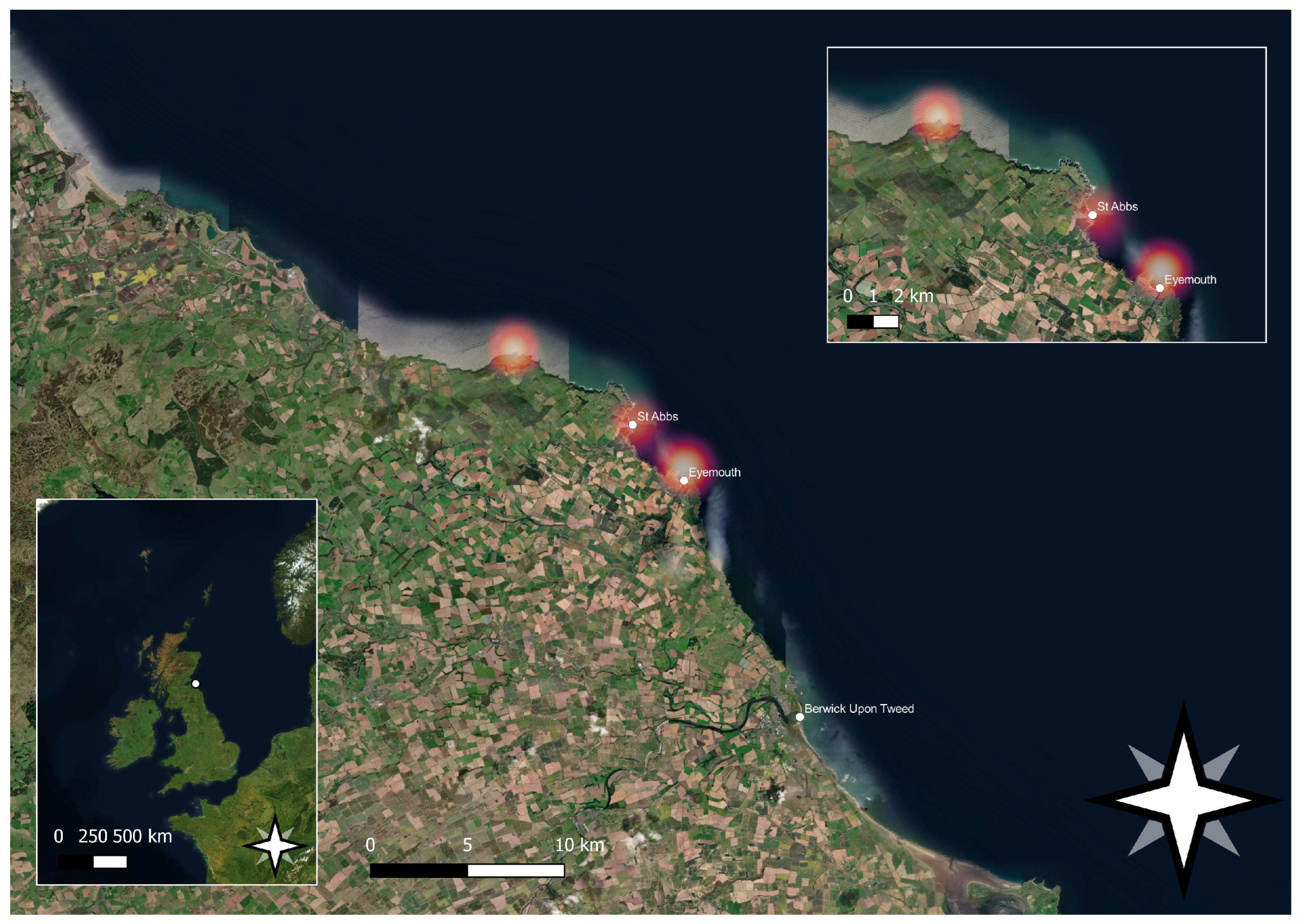
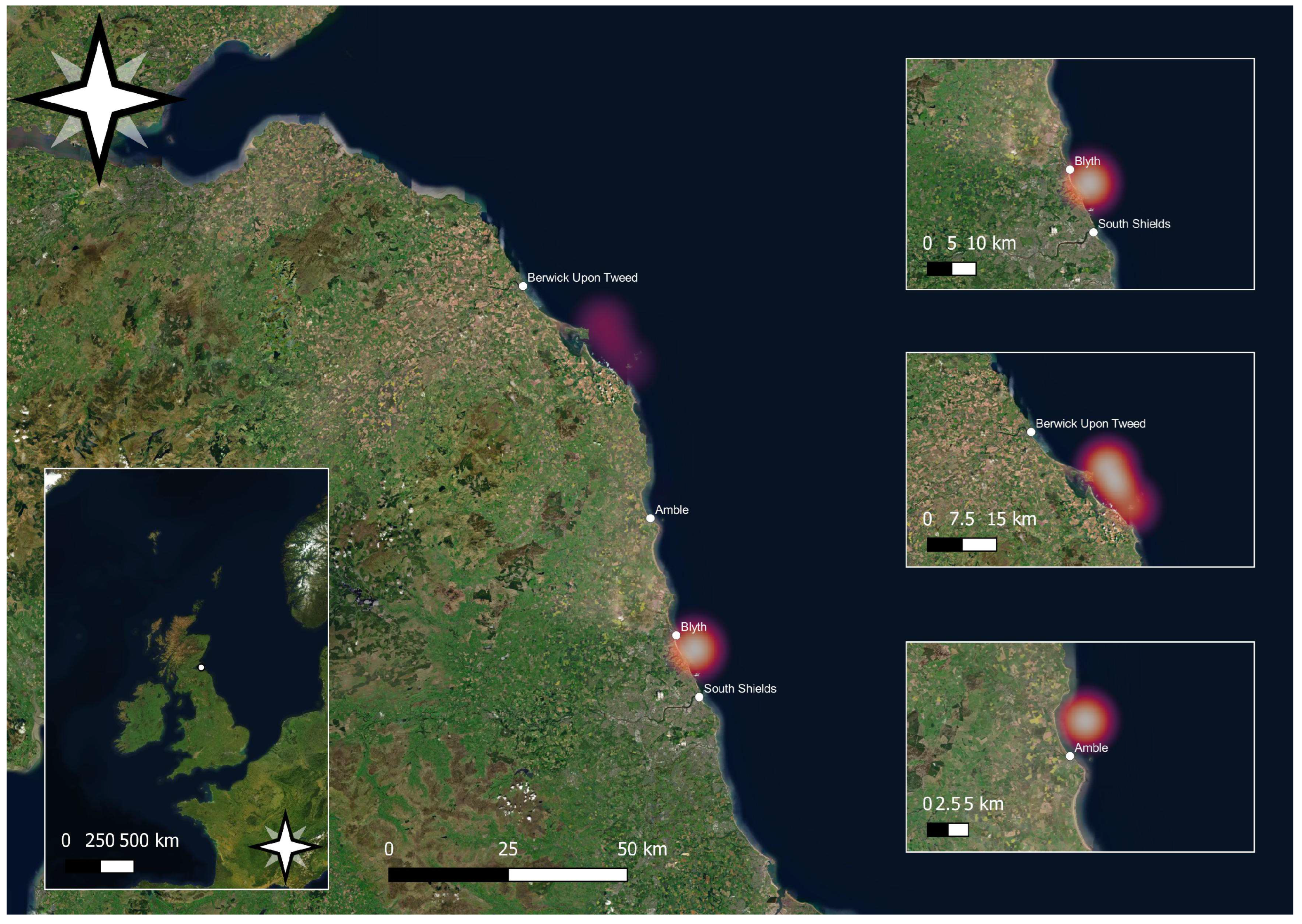
| Region | Landings and Value Per Annum (Tonne)/(£’000) | ||
|---|---|---|---|
| 2017 | 2018 | 2019 | |
| Berwickshire | 530/820 | 498/1014 | 463/1081 |
| Northumberland | 1057/1375 | 971/1598 | 949/2140 |
| Region | Sex | Maturity Metric | Size at 50% Maturity (mm) | Reference |
|---|---|---|---|---|
| North Wales | M | Gonad development | 56–94 | [7] |
| North Wales | F | Gonad development | 86–105 | [7] |
| South Wales | M | Gonad development | 56–94 | [7] |
| South Wales | F | Gonad development | 101–115 | [7] |
| Norfolk England | M | 105 | [9] | |
| Norfolk England | F | 110 | [9] | |
| Selsey England | M | 115 | [9] | |
| Selsey England | F | 125 | [9] | |
| E Coast England | F | Sperm plugs | 116 | [10] |
| SW Ireland | F | Mature gonads | 127–139 | [10] |
| England | M | Chelae | 110 | [10] |
| Shetland Scotland | M | Averaged all | 116 | [11] |
| Shetland Scotland | F | Averaged all | 128 | [11] |
| Shetland Scotland | F | Sperm plugs | 123 | [11] |
| Shetland Scotland | M | Mature gonads | 125 | [11] |
| Shetland Scotland | F | Mature gonads | 133.5 | [11] |
| Shetland Scotland | F | Hatched | 144 | [11] |
| Shetland Scotland | M | Averaged functional | 125 | [11] |
| Shetland Scotland | F | Averaged functional | 139 | [11] |
| Western Channel England | F | Mature gonads | 137–147 | [12] |
| Scotland East and West | M | Mature gonads | 101–106 | [13] |
| Scotland East and West | F | Mature gonads | 127–128 | [13] |
| Scotland | F | Sperm plug | 122.9 | [14] |
| Scotland | F | Ovigerous | 143.7 | [14] |
| Ireland | F | Mature gonads | 132–138 | [15] |
| Eastern Channel England | M | Mature gonads | 105 | [16] |
| Eastern Channel England | F | Mature gonads | 126 | [16] |
| Western Channel England | M | Mature gonads | 90 | [16] |
| Western Channel England | F | Mature gonads | 112 | [16] |
| North Sea | M | Mature gonads | 89 | [16] |
| North Sea | F | Mature gonads | 109 | [16] |
| Ireland | F | Gonad development | 120 | [17] |
| Isle of Man, Irish Sea | M | Gonad maturity | 89 | [18] |
| Isle of Man, Irish Sea | F | Gonad maturity | 108 | [18] |
| Condition Index | Condition Description |
|---|---|
| 1 | High standard of health. Chelipeds present. Chelae present. No black spot lesions. No damage. |
| 2 | Good standard of health. One to two chelipeds are missing. Limited black spot lesions. |
| 3 | Average standard of health. More than two chelipeds missing. Black spot lesions present. |
| 4 | Bad standard of health. One or both chelae missing. Black spot lesions covering around 50% of the carapace. Limited damage to the body. |
| 5 | Poor standard of health. One or both chelae missing and chelipeds missing. Large surface area of black spot lesions on the body. Damaged carapace. |
| Moult Stage | Description |
|---|---|
| Early Post Moult | Soft, white, no biofouling on carapace, very sharp toes. |
| Recent Moult | No biofouling on carapace, sharp toes, carapace not fully hardened. |
| Inter-Moult | Carapace covered with biofouling usually in the form of biofilm attached to the hairs of the chelipeds, toes are worn to a smooth rounded shape. |
| Degraded | Carapace shows signs of great biofouling in the form of tubeworms, barnacles, large surface area coverage of biofilms especially attached to the hairs of the chelipeds, damage to carapace (holes, indentations), distinguishable smell to the individual. |
| Female | |||||
|---|---|---|---|---|---|
| Stage | 1-Immature | 2-Undeveloped | 3-Developing | 4-Mature | 5-Recovery |
| Details | No egg cells present | Pre-vitellogenesis | Early secondary vitellogenesis | Late secondary vitellogenesis | Post Reproductive |
| Visual | Gonad is transparent and thin in structure. | Gonad lobes are visible with a light pink/grey coloration. | Gonad has more noticeable pink colour. Covers less than 50% of the cavity. | Ovaries are very large, covers over 50% of the cavity with a prominent orange/red colour. | Remnant eggs are visible with the ovary exhibiting a loose structure and white appearance. |
| Male | |||||
| Stage | 1-Immature | 2-Developing | 3-Mature | ||
| Details | Spermatids | Spermatozoa | Spermatophore | ||
| Visual | Small testes that are transparent or undetectable. | White and obvious testes. | Both swollen testes and vas deferens. | ||
| Region | Sex | CW (mm) | WW (g) | CI | MS | CL (mm) | CD (mm) | CH (mm) | HW(g) | GS |
|---|---|---|---|---|---|---|---|---|---|---|
| Berwickshire | M | 141.76 ± 1.05 | 503.52 ± 12.60 | 2.14 ± 0.06 | 3.16 ± 0.03 | 34.51 ± 0.39 | 23.22 ± 0.29 | 37.18 ± 0.43 | 33.44 ± 0.77 | 2.45 ± 0.04 |
| F | 140.48 ± 1.99 | 436.33 ± 19.93 | 2.21 ± 0.10 | 3.11 ± 0.07 | 27.57 ± 0.46 | 18.18 ± 0.29 | 29.50 ± 0.46 | 69.55 ± 34.72 | 2.29 ± 0.11 | |
| Northumberland | M | 121.03 ± 1.63 | 222.89 ± 9.40 | 3.17 ± 0.09 | 2.89 ± 0.05 | 26.75 ± 0.55 | 17.55 ± 0.40 | 29.04 ± 0.63 | 11.65 ± 0.70 | 1.99 ± 0.06 |
| F | 118.56 ± 2.07 | 216.34 ± 12.65 | 2.78 ± 0.11 | 2.70 ± 0.06 | 23.20 ± 0.46 | 14.77 ± 0.30 | 24.83 ± 0.48 | 11.59 ± 0.90 | 1.93 ± 0.12 |
| Region | Measurement (mm) | Sex | N | Segmented Regression | Linear Regression | ||||
|---|---|---|---|---|---|---|---|---|---|
| Slopes | BP (mm) | R2 | R2 | BP (mm) | CI | ||||
| Berwickshire | CH | M | 308 | 0.187, 0.233 | 137.99 ± 9.77 | 0.85 | 0.7 | 141.1 | 139.4–143 |
| CD | M | 308 | 0.108, 0.125 | 83.99 ± 7.33 | 0.76 | 0.83 | 142.3 | 140.9–143.7 | |
| CL | M | 308 | 0.166, 0.225 | 119.06 ± 12.37 | 0.86 | 0.86 | 153.3 | 151.8–154.9 | |
| CH | F | 0.89 | 134.7 | 132.8–136.8 | |||||
| Northumberland | CH | M | 170 | 0.178, 0.266 | 129 ± 4.77 | 0.92 | 0.84 | 130.1 | 128–132.5 |
| CD | M | 170 | 0.105, 0.155 | 119.67 ± 5.68 | 0.89 | 0.92 | 121.8 | 120.4–123.3 | |
| CL | M | 170 | 0.012, 0.015 | 129 ± 6.72 | 0.89 | 0.93 | 121.2 | 119.9–122.5 | |
| CH | F | 0.64 | 120.8 | 117.2–125.3 |
| Sex | Condition Index | Gonadosomatic Index (GSI) | |
|---|---|---|---|
| Berwickshire | Northumberland | ||
| M | 1 | 2.32 ± 0.09 | 1.60 ± 0.51 |
| 2 | 2.15 ± 0.10 | 1.28 ± 0.16 | |
| 3 | 1.95 ± 0.21 | 2.07 ± 0.37 | |
| 4 | 2.80 ± 0.21 | 2.61 ± 0.31 | |
| 5 | 2.87 ± 0.19 | 2.65 ± 0.30 | |
| F | 1 | 4.86 ± 0.87 | 5.11 ± 1.86 |
| 2 | 7.39 ± 1.29 | 6.03 ± 3.05 | |
| 3 | 1.37 ± 0.41 | 2.34 | |
| 4 | 4.06 ± 0.83 | NA | |
| 5 | 4.40 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Easton, B.A.A.; Boon, A.; Richards, J.; Scott, K. Comparing the Size at Onset of Sexual Maturity of Edible Crab (Cancer pagurus, Cancridae) in Berwickshire and Northumberland. Fishes 2023, 8, 260. https://doi.org/10.3390/fishes8050260
Easton BAA, Boon A, Richards J, Scott K. Comparing the Size at Onset of Sexual Maturity of Edible Crab (Cancer pagurus, Cancridae) in Berwickshire and Northumberland. Fishes. 2023; 8(5):260. https://doi.org/10.3390/fishes8050260
Chicago/Turabian StyleEaston, Blair Alexander Andrew, Andrew Boon, Joe Richards, and Kevin Scott. 2023. "Comparing the Size at Onset of Sexual Maturity of Edible Crab (Cancer pagurus, Cancridae) in Berwickshire and Northumberland" Fishes 8, no. 5: 260. https://doi.org/10.3390/fishes8050260
APA StyleEaston, B. A. A., Boon, A., Richards, J., & Scott, K. (2023). Comparing the Size at Onset of Sexual Maturity of Edible Crab (Cancer pagurus, Cancridae) in Berwickshire and Northumberland. Fishes, 8(5), 260. https://doi.org/10.3390/fishes8050260