Geographic Variation in Opisthonema oglinum (Lesueur, 1818) in the Southeastern Brazilian Bight Inferred from Otolith Shape and Chemical Signatures
Abstract
1. Introduction
2. Materials and Methods
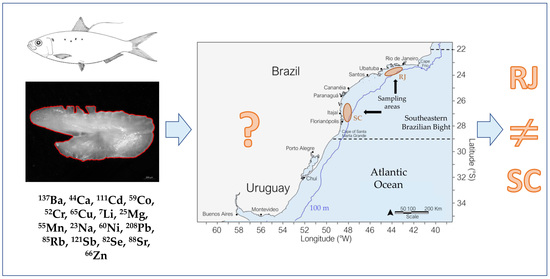
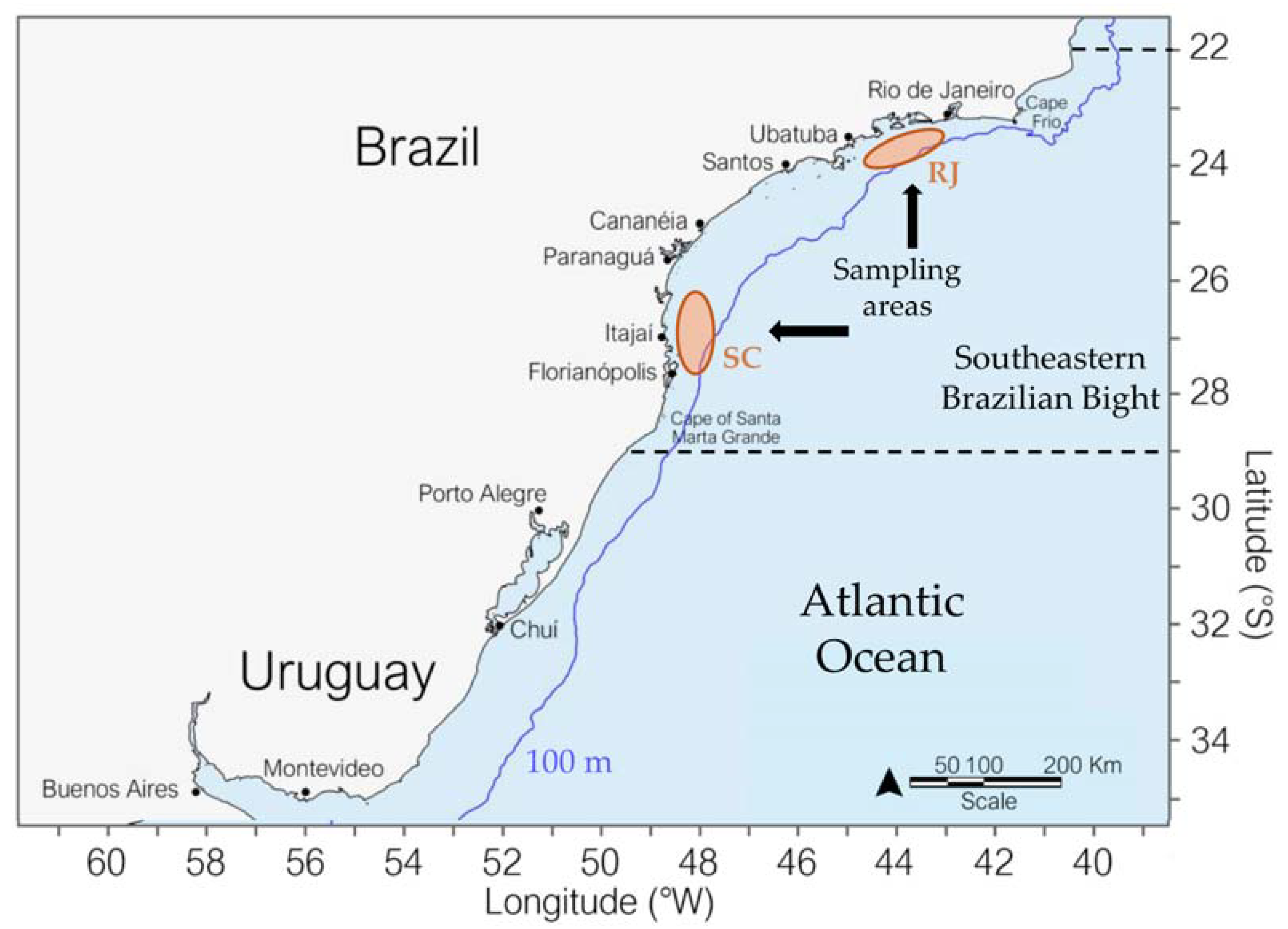
2.1. Study Area
2.2. Biological Sampling
2.3. Otolith Shape Analysis
2.4. Otolith Chemical Analysis
2.5. Statistical Analysis
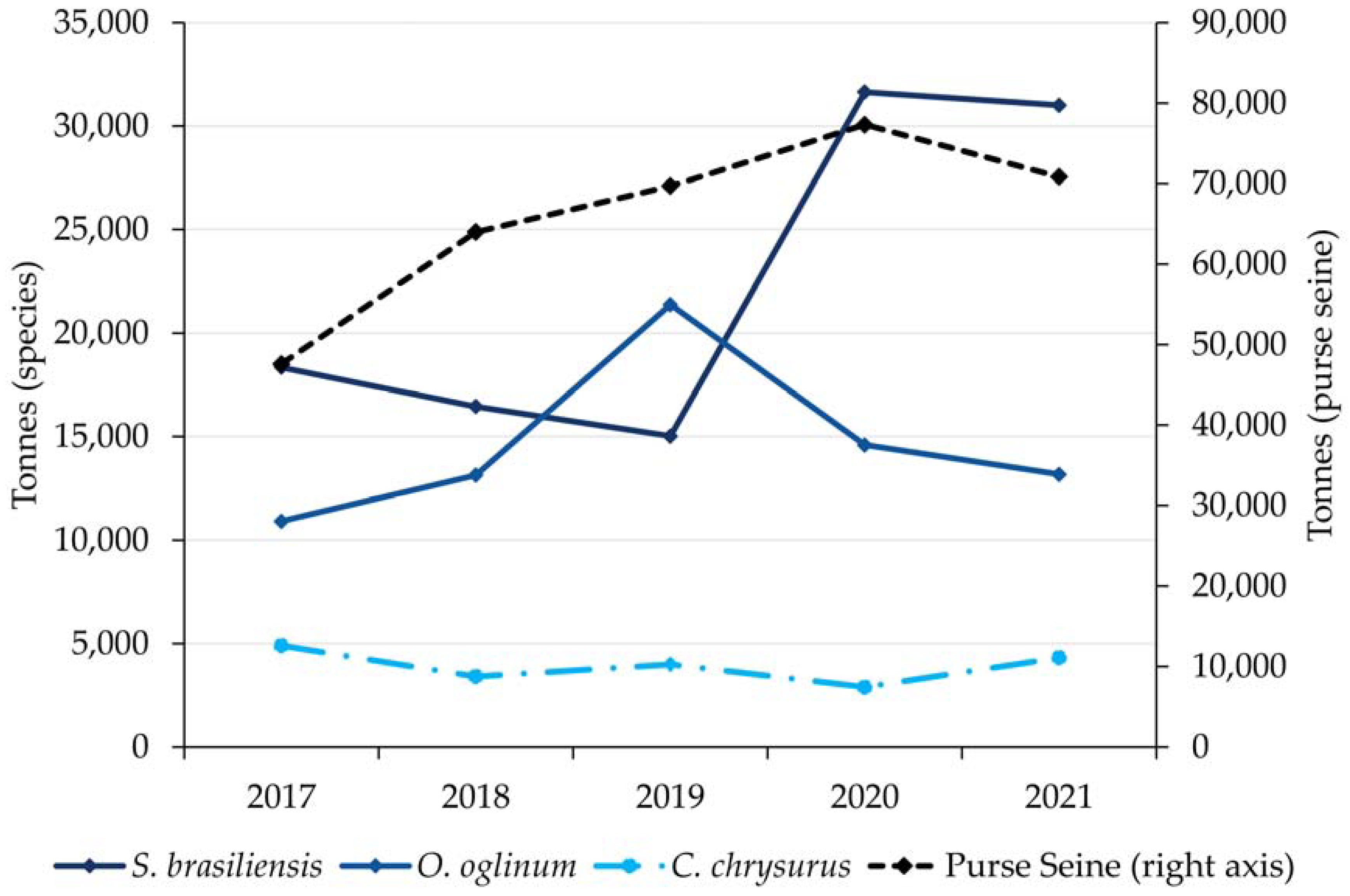
3. Results
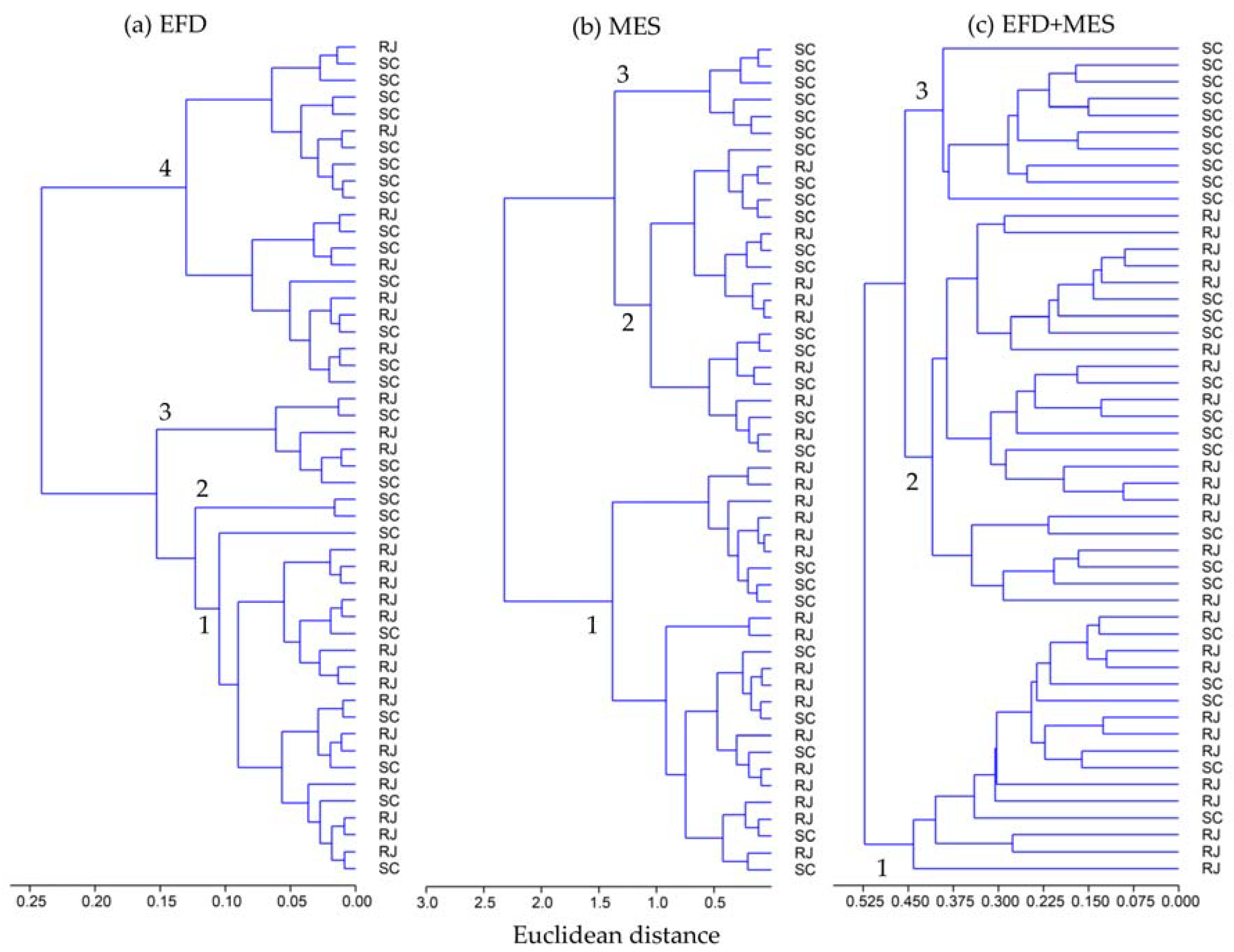
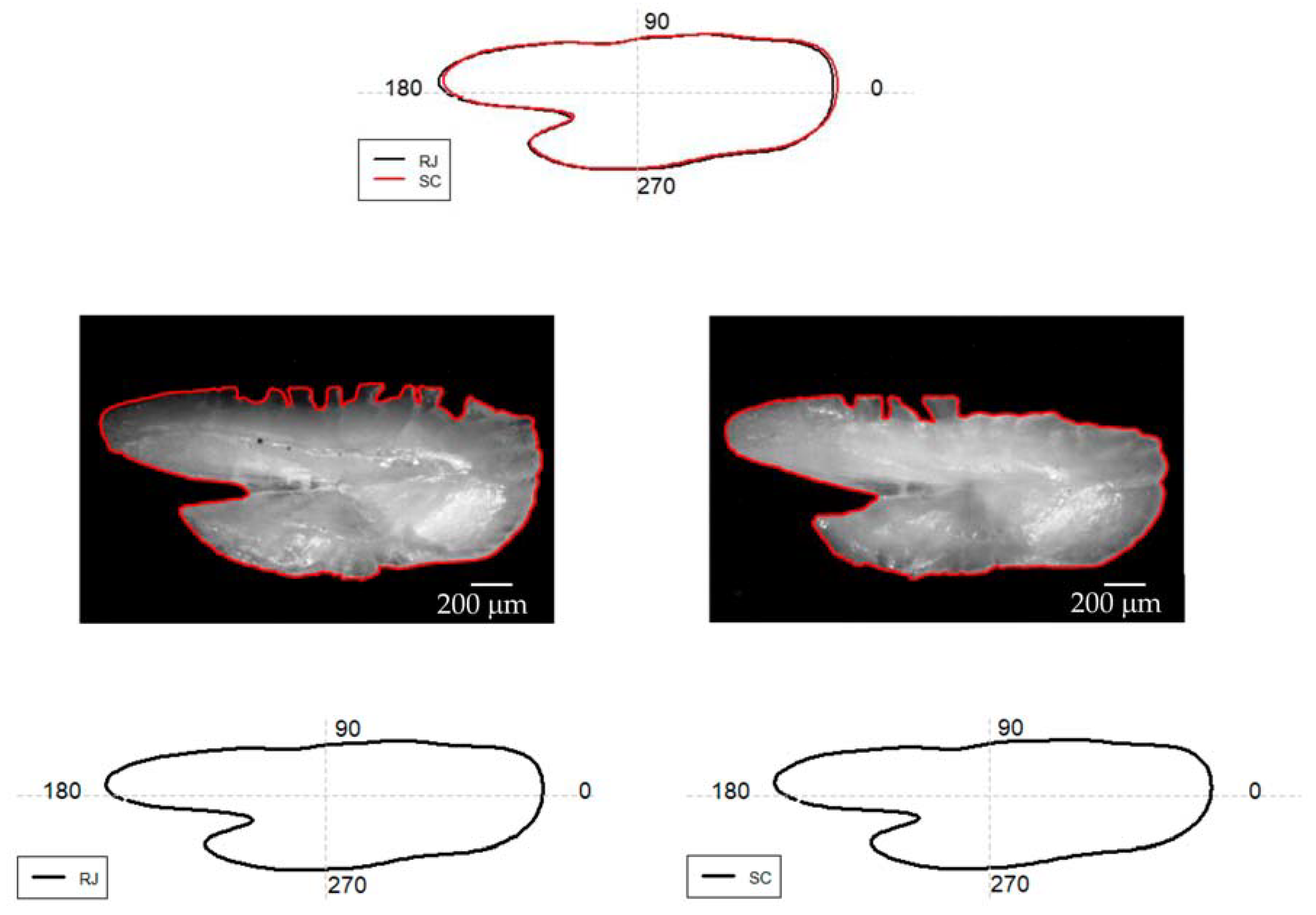
3.1. Otolith Shape Analysis
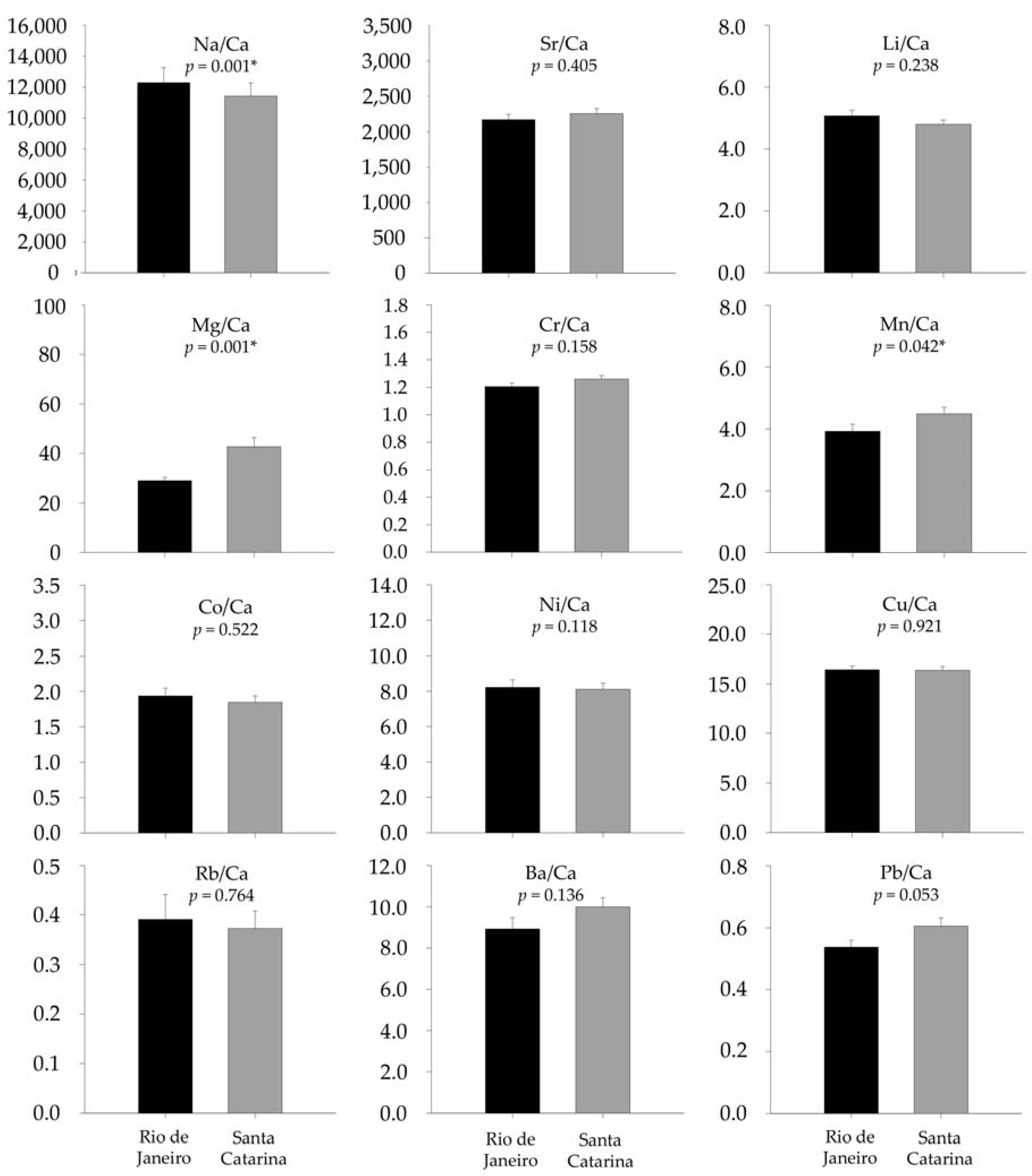
3.2. Otolith Chemical Analysis
3.3. Otolith Shape and Chemical Analyses Combined
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). Fishery and Aquaculture Statistics 2019/Statistiques des Pêches et de L’aquaculture 2019/FAO Anuario. Estadísticas de Pesca y Acuicultura 2019; FAO: Rome, Italy, 2021; 82p. [Google Scholar]
- UN (United Nations). Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 19 March 2023).
- Haddon, M. Modelling and Quantitative Methods in Fisheries, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; 433p. [Google Scholar]
- Aoki, I.; Yamakawa, T.; Takasuka, A. (Eds.) Fish Population Dynamics, Monitoring, and Management: Sustainable Fisheries in the Eternal Ocean; Springer: Tokyo, Japan, 2018; 245p. [Google Scholar]
- Crispo, E.; Chapman, L.J. Geographic variation in phenotypic plasticity in response to dissolved oxygen in an African cichlid fish. J. Evol. Biol. 2010, 23, 2091–2103. [Google Scholar] [CrossRef]
- Planque, B.; Loots, C.; Petitgas, P.; Lindstrøm, U.L.F.; Vaz., S. Understanding what controls the spatial distribution of fish populations using a multi-model approach. Fish. Oceanogr. 2011, 20, 1–17. [Google Scholar] [CrossRef]
- Caselle, J.E.; Hamilton, S.L.; Schroeder, D.M.; Love, M.S.; Standish, J.D.; Rosales-Casian, J.A.; Sosa-Nishizaki, O. Geographic variation in density, demography, and life history traits of a harvested, sex-changing, temperate reef fish. Can. J. Fish. Aquat. Sci. 2011, 68, 288–303. [Google Scholar] [CrossRef]
- Semmar, N.; Vaz-dos-Santos, A.M. Highlighting growth regulation processes in fish populations by a simplex simulation approach: Application to Merluccius hubbsi stocks in the Southwestern Atlantic. ICES J. Mar. Sci. 2020, 77, 1401–1413. [Google Scholar] [CrossRef]
- Cadrin, S.X.; Kerr, L.A.; Mariani, S. Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; 566p. [Google Scholar]
- Kerr, L.A.; Hintzen, N.T.; Cadrin, S.X.; Clausen, L.; Dickey-Collas, M.W.; Goethel, D.R.; Hatfield, E.M.C.; Kritzer, J.P.; Nash, R.D.M. Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J. Mar. Sci. 2017, 74, 1708–1722. [Google Scholar] [CrossRef]
- Secor, D.H. The unit stock concept: Bounded fish and fisheries. In Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Cadrin, S.X., Kerr, L.A., Mariani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 17–28. [Google Scholar]
- Tanner, S.E.; Reis-Santos, P.; Cabral, H.N. Otolith chemistry in stock delineation: A brief overview, current challenges and future prospects. Fish. Res. 2016, 173, 206–213. [Google Scholar] [CrossRef]
- Soeth, M.; Daros, F.A.; Correia, A.T.; Fabré, N.N.; Medeiros, R.; Feitosa, C.V.; Duarte, O.S.; Lenz, T.M.; Spach, H.L. Otolith phenotypic variation as an indicator of stock structure of Scomberomorus brasiliensis from the southwestern Atlantic Ocean. Fish. Res. 2022, 252, 106357. [Google Scholar] [CrossRef]
- Popper, A.N.; Lu, Z. Structure-function relationships in fish otolith organs. Fish. Res. 2000, 46, 15–25. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Vaz-dos-Santos, A.M. (Eds.) Generalidades de los otolitos. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 21–28. [Google Scholar]
- Adelir-Alves, J.; Daros, F.A.; Spach, H.L.; Soeth, M.; Correia, A.T. Otoliths as a tool to study reef fish population structure from coastal islands of South Brazil. Mar. Biol. Res. 2018, 14, 973–988. [Google Scholar] [CrossRef]
- Ferreira, I.; Santos, D.; Moreira, C.; Feijó, D.; Rocha, A.; Correia, A.T. Population structure of Chelidonichthys lucerna in Portugal mainland using otolith shape and elemental signatures. Mar. Biol. Res. 2019, 15, 500–512. [Google Scholar] [CrossRef]
- Moura, A.; Muniz, A.A.; Mullis, E.; Wilson, J.M.; Vieira, R.P.; Almeida, A.A.; Pinto, E.; Brummer, G.J.A.; Gaever, P.V.; Gonçalves, J.M.S.; et al. Population structure and dynamics of the Atlantic mackerel (Scomber scombrus) in the North Atlantic inferred from otolith chemical and shape signatures. Fish Res. 2020, 230, 105621. [Google Scholar] [CrossRef]
- Assis, C.A. The lagenar otoliths of teleosts: Their morphology and its application in species identification, phylogeny and systematics. J. Fish Biol. 2003, 62, 1268–1295. [Google Scholar] [CrossRef]
- Assis, C.A. The utricular otoliths, lapilli, of teleosts: Their morphology and relevance for species identification and systematics studies. Sci. Mar. 2005, 69, 259–273. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Plath, M. All good things come in three—Species delimitation through shape analysis of saccular, lagenar and utricular otoliths. Mar. Freshw. Res. 2012, 63, 934. [Google Scholar] [CrossRef]
- Curcio, N.; Tombari, A.; Capitanio, F. Otolith morphology and feeding ecology of an Antarctic nototheniid, Lepidonotothen larseni. Antarct. Sci. 2018, 26, 124–132. [Google Scholar] [CrossRef]
- D’Iglio, C.; Famulari, S.; Albano, M.; Carnevale, A.; Di Fresco, D.; Constanzo, M.; Lanteri, G.; Spanò, N.; Savoca, S.; Capillo, G. Intraspecific variability of the saccular and utricular otoliths of the hatchetfish Argyropelecus hemigymnus (Cocco, 1829) from the Strait of Messina (Central Mediterranean Sea). PLoS ONE 2023, 18, e0281621. [Google Scholar] [CrossRef]
- Checkley, D.; Alheit, J.; Oozeki, Y.; Roy, C. Climate Change and Small Pelagic Fish; University Press: Cambridge, UK, 2009; 372p. [Google Scholar]
- Petitgas, P. Life cycle spatial patterns of small pelagic fish in the Northeast Atlantic. ICES Coop. Res. Rep. 2010, 306, 5408. [Google Scholar] [CrossRef]
- Brochier, T.; Auger, P.A.; Pecquerie, L.; Machu, E.; Capet, X.; Thiaw, M.; Mbaye, B.C.; Braham, C.B.; Ettahiri, O.; Charouki, N.; et al. Complex small pelagic fish population patterns arising from individual behavioral responses to their environment. Progr. Oceanogr 2018, 164, 12–27. [Google Scholar] [CrossRef]
- Takasuka, A. Biological mechanisms underlying climate impacts on population dynamics of small pelagic fish. In Fish Population Dynamics, Monitoring, and Management: Sustainable Fisheries in the Eternal Ocean; Aoki, I., Yamakawa, T., Takasuka, A., Eds.; Springer: Tokyo, Japan, 2018; pp. 19–50. [Google Scholar]
- González-Cabellos, L.W.; Mengual-Izquierdo, A. Age and growth of the Atlantic thread herring, Opisthonema oglinum (Le Sueur, 1818) (Teleostei: Clupeidae), of Margarita Island, Venezuela. Cienc. Mar. 1995, 21, 387–399. [Google Scholar] [CrossRef]
- Lessa, R.P.T.; Duarte-Neto, P.J.; Morize, E.; Maciel, R. Otolith microstructure analysis with OTC validation confirms age overestimation in Atlantic thread herring Opisthonema oglinum from north-eastern Brazil. J. Fish Biol. 2008, 73, 1690–1700. [Google Scholar] [CrossRef]
- Rautenberg, K.A.; Correia, A.T.; Schwingel, P.R.; Vaz-dos-Santos, A.M. Changing growth pattern in small pelagic species targets of purse seine fisheries in Southwestern Atlantic. Front. Mar. Sci. 2019, 6, 00021. [Google Scholar] [CrossRef]
- Payan-Alejo, J.; Jacob-Cervantes, M.L.; Rodríguez-Domínguez, G. Age and growth of thread herring Opisthonema libertate, in the southern Gulf of California. Lat. Am. J. Aquat. Res. 2020, 48, 15–22. [Google Scholar] [CrossRef]
- García-Abad, M.D.L.C.; Yáñez-Arancibia, A.; Sánchez-Gil, P.; Tapia-García, M. Distribución, abundancia y reproducción de Opisthonema oglinum (Pisces: Clupeidae) en la plataforma continental del sur del Golfo de México. Rev. Biol. Trop. 1998, 46, 257–266. [Google Scholar] [CrossRef]
- Petermann, A.; Schwingel, P.R. Overlap of the reproductive cycle and recruitment of the four main species caught by the purse seine fleet in Brazil. Lat. Am. J. Aquat. Res. 2016, 44, 1069–1079. [Google Scholar] [CrossRef]
- Furtado-Ogawa, E. Alimentação da sardinha-bandeira, Opisthonema oglinum (LE SUEUR), no Estado do Ceará. Arq. Cienc. Mar. 1970, 10, 201–202. [Google Scholar]
- Finucane, J.H.; Grimes, C.B.; Naughton, S.P. Diets of young King and Spanish Mackerel Off the Southeast United States. Northeast Gulf Sci. 1990, 11, 145–153. [Google Scholar]
- Andrade, G.D.Q.; Bispo, E.D.S.; Druzian, J.I. Avaliação da qualidade nutricional em espécies de pescado mais produzidas no Estado da Bahia. Food Sci. Technol. 2009, 29, 721–726. [Google Scholar] [CrossRef]
- Marques, C.O.; Seabra, L.M.J.; Damasceno, K.S.F.S.C. Qualidade microbiológica de produtos à base de sardinha (Opisthonema oglinum). Hig. Aliment. 2009, 23, 9389. [Google Scholar]
- Finucane, J.H.; Shaffer, R.N. Species profile of Atlantic thread herring, Opisthonema oglinum (Lesueur 1818). NOAA Tech. Memo. 1986, 182, 1–30. [Google Scholar]
- Wenner, C.A.; Sedberry, G.R. Species composition, distribution, and relative abundance of fishes in the coastal habitat off the southeastern United States. NOAA Tech. Rep. NMFS 1989, 79, 1–49. [Google Scholar]
- Smith, J.W. Atlantic Thread Herring, Opisthonema oglinum along the North Carolina Coast. Mar. Fish. Rev. 1994, 56, 1–7. [Google Scholar]
- Houde, E.D. Abundance and potential yield of the Atlantic thread herring, Opisthonema oglinum, and aspects of its early life history in the eastern Gulf of Mexico. Fish. Bull. 1977, 75, 493–512. [Google Scholar]
- Vega-Cendejas, M.E.; Mexicano-Cíntora, G.; Arce, A.M. Biology of the thread herring Opisthonema oglinum (Pisces: Clupeidae) from a beach seine fishery of the Campeche Bank, Mexico. Fish. Res. 1997, 30, 117–126. [Google Scholar] [CrossRef]
- Páramo, J.E.; Viaña, J.E. Evaluación hidroacústica del machuelo (Opisthonema oglinum) y la sardina (Sardinella aurita), en la zona norte del Caribe colombiano, durante julio-agosto y diciembre de 1997. Bol. Inv. Mar. Costeras 2002, 31, 33–52. [Google Scholar] [CrossRef]
- Manjarrés-Martínez, L.M.; Cuello, F. Una línea de referencia histórica acerca de la distribución espacial del machuelo (Opisthonema oglinum) en el área de afloramiento del Mar Caribe de Colombia. Intropica 2019, 14, 148–159. [Google Scholar] [CrossRef]
- Feltrim, M.C.; Schwingel, P.R. Opisthonema oglinum (Lesueur, 1818). In Análise das Principais Pescarias Comerciais da Região Sudeste-Sul do Brasil: Dinâmica Populacional das Espécies em Explotação; Cergole, M.C., Ávila-da-Silva, A.O., Rossi-Wongtschowski, C.L.D.B., Eds.; Instituto Oceanográfico—USP: São Paulo, Brasil, 2005; pp. 112–115. [Google Scholar]
- Nóbrega, M.F.; Lessa, R.P.T.; Santana, F.M. Peixes Marinhos da região Nordeste do Brasil; Martins & Cordeiro: Fortaleza, Brasil, 2009; 205p. [Google Scholar]
- MMA (Ministério do Meio Ambiente). Programa REVIZEE: Avaliação do Potencial Sustentável de Recursos Vivos da Zona Econômica Exclusiva do Brasil—Relatório Executivo; MMA: Brasilia, Brasil, 2006; 280p. [Google Scholar]
- Brandini, F.P.; Tura, P.M.; Santos, P.P. Ecosystem responses to biogeochemical fronts in the South Brazil Bight. Progr. Oceanogr. 2018, 164, 52–62. [Google Scholar] [CrossRef]
- FIPERJ (Estatística Pesqueira do Estado do Rio de Janeiro). Projeto de Monitoramento da Atividade Pesqueira no Estado do Rio de Janeiro. Fundação Instituto de Pesca do Estado do Rio de Janeiro (FIPERJ). Available online: http://pescarj.fundepag.br/ (accessed on 23 March 2023).
- IP/APTA/SAA/SP (Estatística Pesqueira Marinha e Estuarina do Estado de São Paulo). Programa de Monitoramento da Atividade Pesqueira Marinha e Estuarina do Estado de São Paulo. Instituto de Pesca (IP), Agência Paulista de Tecnologia dos Agronegócios (APTA), Secretaria de Agricultura e Abastecimento do Estado de São Paulo (SAA/SP). Available online: http://www.propesq.pesca.sp.gov.br/ (accessed on 23 March 2023).
- UNIVALI/EMCT/LEMA (Estatística Pesqueira de Santa Catarina). Projeto de Monitoramento da Atividade Pesqueira do Estado de Santa Catarina. Laboratório de Estudos Marinhos Aplicados (LEMA), da Escola do Mar, Ciência e Tecnologia (EMCT) da Universidade do Vale do Itajaí (UNIVALI). 2022. Available online: http://pmap-sc.acad.univali.br/ (accessed on 23 March 2023).
- Paiva, M.P.; Motta, P.C.S. Capturas da sardinha-verdadeira, Sardinella brasiliensis (Steindachner) (Osteichthyes: Clupeidae) e da fauna acompanhante, no Estado do Rio de Janeiro (Brasil). Arq. Cienc. Mar. 1999, 32, 85–88. [Google Scholar] [CrossRef]
- Occhialini, D.S.; Schwingel, P.R. Composição e variação espaço-temporal da captura da frota de traineiras entre 1997 e 1999 no Porto de Itajaí, SC. Braz. J. Aquat. Sci. Tech. 2003, 7, 11–22. [Google Scholar] [CrossRef]
- Cergole, M.C.; Dias Neto, J. Plano de Gestão para o Uso Sustentável de Sardinha-Verdadeira Sardinella brasiliensis no Brasil; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Brasilia, Brazil, 2011; 90p. [Google Scholar]
- Ruas, L.C.; Vaz-dos-Santos, A.M. Age structure and growth of the rough scad, Trachurus lathami (Teleostei: Carangidae), in the Southeastern Brazilian Bight. Zoologia 2017, 34, e20475. [Google Scholar] [CrossRef]
- Queiroz, C.; Sampaio, M.I.C.S.; Schneider, H. Population analysis in Atlantic thread herring from the Brazilian Coast. RECEI 2020, 6, 31–40. [Google Scholar] [CrossRef]
- Moreira, C.; Correia, A.T.; Vaz-Pires, P.; Froufe, E. Genetic diversity and population structure of the blue jack mackerel Trachurus picturatus across its western distribution. J. Fish Biol. 2019, 94, 725–731. [Google Scholar] [CrossRef]
- Castro, B.M.; Lorenzetti, J.A.; Silveira, I.C.A.; Miranda, L.B. Estrutura termohalina e circulação na região entre o Cabo de São Tomé (RJ) e o Chuí (RS). In O Ambiente Oceanográfico da Plataforma Continental e do Talude na Região Sudeste-Sul do Brasil; Rossi-Wongtschowski, C.L.D.B., Madureira, L.S.P., Eds.; EDUSP: São Paulo, Brazil; pp. 11–120.
- Piola, A.R.; Palma, E.D.; Bianchi, A.A.; Castro, B.M.; Dottori, M.; Guerrero, R.A.; Marrari, M.; Matano, R.P.; Möller, O.O.; Saraceno, M. Physical oceanography of the SW Atlantic shelf: A review. In Plankton Ecology of the Southwestern Atlantic; Hoffmeyer, M., Sabatini, M., Brandini, F.P., Calliari, D., Santinelli, N., Eds.; Springer: Basel, Switzerland, 2018; pp. 37–56. [Google Scholar] [CrossRef]
- de Souza, R.B.; Robinson, I.S. Lagrangian and satellite observations of the Brazilian Coastal Current. Cont. Shelf Res. 2004, 24, 241–262. [Google Scholar] [CrossRef]
- Fries, A.S.; Coimbra, J.P.; Nemazie, D.A.; Summers, R.M.; Azevedo, J.P.S.; Filoso, S.; Newton, M.; Gelli, G.; Oliveira, R.C.N.; Pessoa, M.A.R.; et al. Guanabara Bay ecosystem health report card: Science, management, and governance implications. Reg. Stud. Mar. Sci. 2019, 25, 100474. [Google Scholar] [CrossRef]
- Costa, M.R.; Monteiro-Neto, C.; Tubino, R.A.; Angelini, R. Pesca e Sustentabilidade: Passado, Presente e Futuro; Alexandre Honorato Duarte Ferreira: Rio de Janeiro, Brazil, 2021; 175p. [Google Scholar]
- Rovai, A.S.; Coelho-Jr, C.; de Almeida, R.; Cunha-Lignon, M.; Menghini, R.P.; Twilley, R.R.; Cintrón-Molero, G.; Schaeffer-Novelli, Y. Ecosystem-level carbon stocks and sequestration rates in mangroves in the Cananéia-Iguape lagoon estuarine system, southeastern Brazil. For. Ecol. Manag. 2021, 479, 118553. [Google Scholar] [CrossRef]
- Lana, P.C.; Bernardino, A.F. Brazilian Estuaries, a Benthic Perspective; Springer: Basel, Switzerland, 2018; 212p. [Google Scholar]
- Gaeta, S.A.; Brandini, F.P. Produção primária do fitoplâncton na região entre o Cabo de São Tomé (RJ) e o Chuí (RS). In O Ambiente Oceanográfico da Plataforma Continental e do Talude na Região Sudeste-Sul do Brasil; Rossi-Wongtschowski, C.L.D.B., Madureira, L.S.P., Eds.; EDUSP: São Paulo, Brazil; pp. 219–264.
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Frechet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Correia, A.T.; Moura, A.; Triay-Portella, R.; Santos, P.T.; Pinto, E.; Almeida, A.A.; Sial, A.N.; Muniz, A.A. Population structure of the chub mackerel (Scomber colias) in the NE Atlantic inferred from otolith elemental and isotopic signatures. Fish Res. 2021, 234, 105785. [Google Scholar] [CrossRef]
- Santos, L.; Vaz-dos-Santos, A.M. Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic. Fishes 2023, 8, 1002. [Google Scholar] [CrossRef]
- Rautenberg, K.A. Padrões de Crescimento e Estrutura Etária de Opisthonema oglinum e Chloroscombrus chrysurus na Região Sudeste—Sul do Brasil. Master’s Thesis, Federal University of Paraná, Brazil, 2021. [Google Scholar]
- Libungan, L.A.; Pálsson, S. ShapeR: An R Package to Study Otolith Shape Variation among Fish Populations. PLoS ONE 2015, 10, e0121102. [Google Scholar] [CrossRef]
- Rooker, J.R.; Zdanowicz, V.S.; Secor, D.H. Chemistry of tuna otoliths: Assessment of base composition and postmortem handling effects. Mar. Biol. 2001, 139, 35–43. [Google Scholar] [CrossRef]
- Patterson, H.M.; Thorrold, S.R.; Shenker, J.M. Analysis of otolith chemistry in Nassau grouper (Epinephelus striatus) from the Bahamas and Belize using solution-based ICP-MS. Coral. Reefs. 1999, 18, 171–178. [Google Scholar] [CrossRef]
- Higgins, R.; Isidro, E.; Menezes, G.; Correia, A.T. Otolith elemental signatures indicate population separation in deep-sea rockfish, Helicolenus dactylopterus and Pontinus kuhlii, from the Azores. J. Sea Res. 2013, 83, 202–208. [Google Scholar] [CrossRef]
- Doornik, J.A.; Hansen, H. An omnibus test for univariate and multivariate normality. Oxf. Bull. Econ. Stat. 2008, 70, 927–939. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists; Elsevier: New York, NY, USA, 2004. [Google Scholar]
- Legendre, P.; Legendre, L.F.J. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 990p. [Google Scholar]
- Härdle, W.K.; Simar, L. Applied Multivariate Statistical Analysis, 4th ed.; Springer: Heidelberg, Germany, 2015; 580p. [Google Scholar]
- Khan, U.; Bal, H.; Battal, Z.S.; Seyhan, K. Using otolith and body shape to discriminate between stocks of European anchovy (Engraulidae: Engraulis encrasicolus) from the Aegean, Marmara and Black Seas. J. Fish Biol. 2022, 101, 1452–1465. [Google Scholar] [CrossRef]
- Xuan, W.; Xie, Y.; Zhu, K.; Zhu, W.; Li, P.; Zhao, Y. Discriminant analysis of two Branchiostegus species in the East China Sea based on otoliths and shape morphology. J. Appl. Ecol. 2023, 34, 527–534. [Google Scholar] [CrossRef]
- Stransky, C. 2014. Morphometric outlines. In Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Cadrin, S.X., Kerr, L.A., Mariani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 129–140. [Google Scholar]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A.T. Otolith shape analysis as a tool to infer the population structure of the blue jack mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
- Ferguson, G.J.; Ward, T.M.; Gillanders, B.M. Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish. Res. 2011, 110, 75–83. [Google Scholar] [CrossRef]
- Wudji, A.; Kim, H.J.; Oh, C.W. Population Structure of Indian Mackerel (Rastrelliger kanagurta) in Java and Bali Island, Indonesia Inferred from Otolith Shape. Sains Malays. 2022, 51, 39–50. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Correia, A.T. Population structure of the Brazilian sardine (Sardinella brasiliensis) in the South-West Atlantic inferred from body morphology and otolith shape signatures. Hydrobiologia 2022, 849, 1367–1381. [Google Scholar] [CrossRef]
- Hoff, N.T.; Dias, J.F.; Pinto, E.; Almeida, A.; Schroeder, R.; Correia, A.T. Past and contemporaneous otolith fingerprints reveal potential anthropogenic interferences and allows refinement of the population structure of Isopisthus parvipinnis in the South Brazil Bight. Biology 2022, 11, 1005. [Google Scholar] [CrossRef]
- Vaz-dos-Santos, A.M.; Santos-Cruz, N.N.; Souza, D.; Giombelli-da-Silva, A.; Gris, B.; Rossi-Wongtschowski, C.L.D.B. Otoliths sagittae of Merluccius hubbsi: An efficient tool for the differentiation of stocks in the Southwestern Atlantic. Braz. J. Oceanogr. 2017, 65, 520–525. [Google Scholar] [CrossRef]
- Mapp, J.; Hunter, E.; Van-Der-Kooij, J.; Songer, S.; Fisher, M. Otolith shape and size: The importance of age when determining indices for fish-stock separation. Fish. Res. 2017, 190, 43–52. [Google Scholar] [CrossRef]
- Siliprandi, C.C.; Brenha-Nunes, M.R.; Rossi- Wongtschowski, C.L.D.B.; Santificetur, C.; Conversani, V.R.M. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil part III: Clupeiformes (Clupeidae, Engraulidae, Pristigasteridae). Braz. J. Oceanogr. 2016, 64, 1–22. [Google Scholar] [CrossRef]
- Abaunza, P.; Murta, A.; Campbell, N.; Cimmaruta, R.; Comesaña, A.; Dahle, G.; García Santamaría, M.T.; Gordo, L.S.; Iversen, S.A.; Mackenzie, K.; et al. Stock identity of horse mackerel (Trachurus trachurus) in the Northeast Atlantic and Mediterranean Sea: Integrating the results from different stock identification approaches. Fish. Res. 2008, 89, 196–209. [Google Scholar] [CrossRef]
- Smoliński, S.; Schade, F.M.; Berg, F. Assessing the performance of statistical classifiers to discriminate fish stocks using Fourier analysis of otolith shape. Can. J. Fish. Aquat. Sci. 2019, 77, 674–683. [Google Scholar] [CrossRef]
- Muniz, A.A.; Moura, A.; Triay-Portella, R.; Moreira, C.; Santos, P.T.; Correia, A.T. Population structure of the chub mackerel (Scomber colias) in the North-East Atlantic inferred from otolith shape and body morphometrics. Mar. Fresh. Res. 2020, 72, 341–352. [Google Scholar] [CrossRef]
- Neves, J.; Veríssimo, A.; Múrias Santos, A.; Garrido, S. Comparing otolith shape descriptors for population structure inferences in a small pelagic fish, the European sardine Sardina pilchardus (Walbaum, 1792). J. Fish Biol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Swearer, S.E. Otolith Biochemistry—A Review. Rev. Fish. Sci. Aquac. 2019, 27, 458–489. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; EIMF; Johnson, R.C.; Waring, C.P.; Trueman, C.N. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 6, 806–816. [Google Scholar] [CrossRef]
- Lombarte, A.; Tuset, V.M. Morfometría de otolitos. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; Volpedo, A.V., Vaz-dos-Santos, A.M., Eds.; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 269–303. [Google Scholar]
- Albuquerque, C.Q. Los otolitos como indicadores del ciclo de vida, de patrones de poblacionales, y del uso y características ambientales de los ecosistemas. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; Volpedo, A.V., Vaz-dos-Santos, A.M., Eds.; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 139–166. [Google Scholar]
- Sturrock, A.M.; Trueman, C.N.; Darnaude, A.M.; Hunter, E. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? J. Fish. Biol. 2012, 81, 766–795. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Sial, A.N.; Caeiro, A.; Vaz-Pires, P.; Correia, A.T. Population structure of the blue jack mackerel (Trachurus picturatus) in the NE Atlantic inferred from otolith microchemistry. Fish. Res. 2018, 197, 113–122. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Triay-Portella, R.; Méndez, A.; Castro, J.P.; Correia, A.T. Unravelling the spatial-temporal population structure of Trachurus picturatus across the North-East Atlantic using otolith fingerprinting. Estuar. Coast. Shelf Sci. 2022, 272, 107860. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.A.; Pisonero, J.; Correia, A.T. Use of otolith elemental signatures to unravel lifetime movements patterns of Atlantic spadefish, Chaetodipterus faber, in the Southwest Atlantic Ocean. J. Sea Res. 2020, 158, 101873. [Google Scholar] [CrossRef]
- Hicks, A.S.; Closs, G.P.; Swearer, S.E. Otolith microchemistry of two amphidromous galaxiids 563 across an experimental salinity gradient: A multi-element approach for tracking diadromous 564 migrations. J. Exp. Mar. Biol. Ecol. 2010, 394, 86–97. [Google Scholar] [CrossRef]
- Lea, D.W.; Shen, G.T.; Boyle, E.A. Coralline barium records temporal variability in equatorial Pacific upwelling. Nature 1989, 340, 373–376. [Google Scholar] [CrossRef]
- Limburg, K.E.; Wuenschel, M.J.; Hüssy, K.; Heimbrand, Y. Making the otolith magnesium chemical calendar-clock tick: Plausible mechanism and empirical evidence. Rev. Fish. Sci. Aquact. 2018, 26, 479–493. [Google Scholar] [CrossRef]
- Macdonald, J.I.; Drysdale, R.N.; Witt, R.; Cságoly, Z.; Marteinsdóttir, G. Isolating the influence of ontogeny helps predict island-wide variability in fish otolith chemistry. Rev. Fish Biol. Fish. 2020, 30, 173–202. [Google Scholar] [CrossRef]
- Chaves, P.T.C.; Vendel, A.L. Análise comparativa da alimentação de peixes (Teleostei) entre ambientes de marisma e de manguezal num estuário do sul do Brasil (Baía de Guaratuba, Paraná). Rev. Bras. Zool. 2008, 25, 10–15. [Google Scholar] [CrossRef]
- Laugier, F.; Feunteun, E.; Pecheyran, C.; Carpentier, A. Life history of the small Sandeel, Ammodytes tobianus, inferred from otolith microchemistry. A methodological approach. Estuar. Coast. Shelf Sci. 2015, 165, 237–246. [Google Scholar] [CrossRef]
- Aschenbrenner, A.; Ferreira, B.P.; Rooker, J.R. Spatial and temporal variability in the otolith chemistry of the Brazilian snapper Lutjanus alexandrei from estuarine and coastal environments. J. Fish Biol. 2016, 89, 753–769. [Google Scholar] [CrossRef]
- Limburg, K.E.; Olson, C.; Walther, Y.; Dale, D.; Slomp, C.P.; Høie, H. Tracking Baltic hypoxia and cod migration over millennia with natural tags. Proc. Natl. Acad. Sci. USA 2011, 108, E177–E182. [Google Scholar] [CrossRef] [PubMed]
- Hüssy, K.; Limburg, K.E.; de Pontual, H.; Thomas, O.R.B.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Anna, M.; Sturrock, A.M. Trace element patterns in otoliths: The role of biomineralization. Rev. Fish. Sci. Aquac. 2021, 29, 445–477. [Google Scholar] [CrossRef]
- Proctor, C.H.; Thresher, R.E. Effects of specimen handling and otolith preparation on concentration of elements in fish otoliths. Mar. Biol. 1998, 131, 681–694. [Google Scholar] [CrossRef]
- Halden, N.M.; Friedrich, L.A. Trace-element distributions in fish otoliths: Natural markers of life histories, environmental conditions and exposure to tailings effluence. Mineral. Mag. 2008, 72, 593–605. [Google Scholar] [CrossRef]
- Daros, F.A.; Condini, M.V.; Altafin, J.P.; de Oliveira Ferreira, F.; Hostim-Silva, M. Fish otolith microchemistry as a biomarker of the world’s largest mining disaster. Sci. Total Environ. 2022, 807, 151780. [Google Scholar] [CrossRef]
- Meniconi, M.F.G.; Silva, T.A.; Fonseca, M.L.; Lima, S.O.F.; Lima, E.F.A.; Lavrado, H.P.; Figueiredo, A.G., Jr. Baía de Guanabara: Síntese do Conhecimento Ambiental—v. 2. Biodiversidade; Petrobrás: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Stransky, C.; Garbe-Schonberg, C.D.; Günther, D. Geographic variation and juvenile migration in Atlantic redfish inferred from otolith microchemistry. Mar. Fresh. Res. 2005, 56, 677–691. [Google Scholar] [CrossRef]
- Swan, S.; Geffen, A.; Morales-Nin, B.; Gordon, J.; Shimmield, T.; Sawyer, T.; Massuti, E. Otolith chemistry: An aid to stock separation of Helicolenus dactylopterus (bluemouth) and Merluccius merluccius (European hake) in the Northeast Atlantic and Mediterranean. ICES J. Mar. Sci. 2006, 63, 504–513. [Google Scholar] [CrossRef]
- Svedäng, H.; André, C.; Jonsson, P.; Elfman, M.; Limburg, K.E. Migratory behaviour and otolith chemistry suggest fine-scale sub-population structure within a genetically homogenous Atlantic Cod population. Environ. Biol. Fishes 2010, 89, 383–397. [Google Scholar] [CrossRef]
- Hansson, S.V.; Desforges, J.P.; van Beest, F.M.; Bach, L.; Halden, N.M.; Sonne, C.; Mosbech, A.; Søndergaard, J. Bioaccumulation of mining derived metals in blood, liver, muscle and otoliths of two Arctic predatory fish species (Gadus ogac and Myoxocephalus scorpius). Environ. Res. 2020, 183, 109194. [Google Scholar] [CrossRef]
- Forrester, G.E.; Swearer, S.E. Trace elements in otoliths indicate the use of open-coast versus bay nursery habitats by juvenile California halibut. Mar. Ecol. Prog. Ser. 2002, 241, 201–213. [Google Scholar] [CrossRef]
- Spilsbury, F.; McDonald, B.; Rankenburg, K.; Evans, N.J.; Grice, K.; Gagnon, M.M. Multivariate analysis of otolith microchemistry can discriminate the source of oil contamination in exposed fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109253. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Pinto, E.; Almeida, A.; Correia, A.T. Stock structure of the Brazilian sardine Sardinella brasiliensis from Southwest Atlantic Ocean inferred from otolith elemental signatures. Fish. Res. 2022, 248, 106192. [Google Scholar] [CrossRef]
- Santos, C.; Schwarz-Jr, R.; Oliveira-Neto, J.F.; Spach, H.L. A ictiofauna em duas planícies de maré do setor euhalino da baía de Paranaguá, PR. Bolm. Inst. Pesca 2002, 28, 49–60. [Google Scholar]
- Contente, R.F.; Stefanoni, M.F.; Spach, H.L. Fish assemblage structure in an estuary of the Atlantic Forest biodiversity hotspot (southern Brazil). Ichthyol. Res. 2011, 58, 38–50. [Google Scholar] [CrossRef]
- Barbanti, B.; Caires, R.; Marceniuk, A.P. A ictiofauna do Canal de Bertioga, São Paulo, Brasil. Biota Neotrop. 2013, 13, 276–291. [Google Scholar] [CrossRef]
- Vaz-dos-Santos, A.M.; Costa, M.R.; Cruvinel, C.M. Analysis of the ichthyofauna caught in a stationary uncovered pound net in the Toque Toque Pequeno Beach, northern coast of São Paulo State, Brazil. BioScience 2013, 2, 7–16. [Google Scholar]
- Cattani, A.P.; Jorge, F.G.D.; Ribeiro, G.C.; Wedekin, L.L.; Lopes, P.C.A.S.; Rupil, G.M.; Spach, H.L. Fish assemblages in a coastal bay adjacent to a network of marine protected areas in southern Brazil. Braz. J. Oceanogr. 2016, 64, 295–308. [Google Scholar] [CrossRef]
- Dias, J.F.; da Rocha, M.L.F.; Schmidt, T.C.S.; Villamarin, B.C.; Morais, D.B. Ichthyofauna as an environmental quality indicator of the Bertioga channel, São Paulo (Brazil). Braz. J. Oceanogr. 2017, 65, 29–43. [Google Scholar] [CrossRef]
- Santos, S.R.; Galvao, K.P.; Adler, G.H.; Andrade-Tubino, M.F.; Vianna, M. Spatiotemporal distribution and population biology aspects of Cetengraulis edentulus (Actinopterygii: Clupeiformes: Engraulidae) in a South-western Atlantic estuary, with notes on the local Clupeiformes community: Conservation implications. Acta Ichthyol. Piscat. 2020, 50, 139–150. [Google Scholar] [CrossRef]
- Bonecker, A.C.T.; Katsuragawa, M.; de Castro, M.S.; Gomes, E.D.A.P.; Namiki, C.A.P.; Zani-Teixeira, M.L. Larval fish of the Campos Basin, southeastern Brazil. Check List 2012, 8, 1280–1291. [Google Scholar] [CrossRef]
- Madureira, L.S.P.; Rossi-Wongtschowski, C.L.D.B. Prospecção de Recursos Pesqueiros Pelágicos na Zona Econômica Exclusiva da Região Sudeste-Sul do Brasil: Hidroacústica e Biomassas; Instituto Oceanográfico (USP): São Paulo, Brazil, 2005; 144p. [Google Scholar]
- FURG (Fundação Universidade Federal do Rio Grande). Mapeamento e Estimativa de Biomassa na Área de Ocorrência da Sardinha-verdadeira (Sardinella brasiliensis) através de Metodologia Hidroacústica (Cruzeiro ECOSAR IV); FURG: Rio Grande, Brazil, 2008; 24p. [Google Scholar]
- FURG (Fundação Universidade Federal do Rio Grande). Mapeamento e Estimativa de Biomassa na Área de Ocorrência da Sardinha-verdadeira (Sardinella brasiliensis) através de Metodologia Hidroacústica (Cruzeiro ECOSAR V); FURG: Rio Grande, Brasil, 2009; 35p. [Google Scholar]
- FURG (Fundação Universidade Federal do Rio Grande). Mapeamento e Estimativa de Biomassa na Área de Ocorrência da Sardinha-verdadeira (Sardinella brasiliensis) através de Metodologia Hidroacústica (Cruzeiro ECOSAR VI); FURG: Rio Grande, Brasil, 2010; 30p. [Google Scholar]
- FURG (Fundação Universidade Federal do Rio Grande). Mapeamento e Estimativa de Biomassa na Área de Ocorrência da Sardinha-verdadeira (Sardinella brasiliensis) através de Metodologia Hidroacústica (Cruzeiro ECOSAR VII); FURG: Rio Grande, Brasil, 2010; 32p. [Google Scholar]
- Dahle, G.; Johansen, T.; Westgaard, J.I.; Aglen, A.; Glover, K.A. Genetic management of mixed-stock fisheries “real-time”: The case of the largest remaining cod fishery operating in the Atlantic in 2007–2017. Fish. Res. 2018, 205, 77–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz-dos-Santos, A.M.; Rautenberg, K.A.; Augusto, C.G.; Ballester, E.L.C.; Schwingel, P.R.; Pinto, E.; Almeida, A.; Correia, A.T. Geographic Variation in Opisthonema oglinum (Lesueur, 1818) in the Southeastern Brazilian Bight Inferred from Otolith Shape and Chemical Signatures. Fishes 2023, 8, 234. https://doi.org/10.3390/fishes8050234
Vaz-dos-Santos AM, Rautenberg KA, Augusto CG, Ballester ELC, Schwingel PR, Pinto E, Almeida A, Correia AT. Geographic Variation in Opisthonema oglinum (Lesueur, 1818) in the Southeastern Brazilian Bight Inferred from Otolith Shape and Chemical Signatures. Fishes. 2023; 8(5):234. https://doi.org/10.3390/fishes8050234
Chicago/Turabian StyleVaz-dos-Santos, André Martins, Kathleen Angélica Rautenberg, Cristiane Gallego Augusto, Eduardo Luis Cupertino Ballester, Paulo Ricardo Schwingel, Edgar Pinto, Agostinho Almeida, and Alberto Teodorico Correia. 2023. "Geographic Variation in Opisthonema oglinum (Lesueur, 1818) in the Southeastern Brazilian Bight Inferred from Otolith Shape and Chemical Signatures" Fishes 8, no. 5: 234. https://doi.org/10.3390/fishes8050234
APA StyleVaz-dos-Santos, A. M., Rautenberg, K. A., Augusto, C. G., Ballester, E. L. C., Schwingel, P. R., Pinto, E., Almeida, A., & Correia, A. T. (2023). Geographic Variation in Opisthonema oglinum (Lesueur, 1818) in the Southeastern Brazilian Bight Inferred from Otolith Shape and Chemical Signatures. Fishes, 8(5), 234. https://doi.org/10.3390/fishes8050234