De Novo Transcriptome Analysis of the Early Hybrid Triploid Loach (Misgurnus anguillicaudatus) Provides Novel Insights into Fertility Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Review Board Statement
2.2. Artificially Induced Spawning and Insemination
2.3. Ploidy Identification
2.4. Sample Collection
2.5. Histological Observation of Early Gonads of Hybrid Triploid Loaches
2.6. RNA Sequencing and Establishing the cDNA Library
2.7. De Novo Assembly and Functional Annotation
2.8. Identification and Analysis of Differentially Expressed Genes
2.9. Quantitative PCR Verification
3. Results
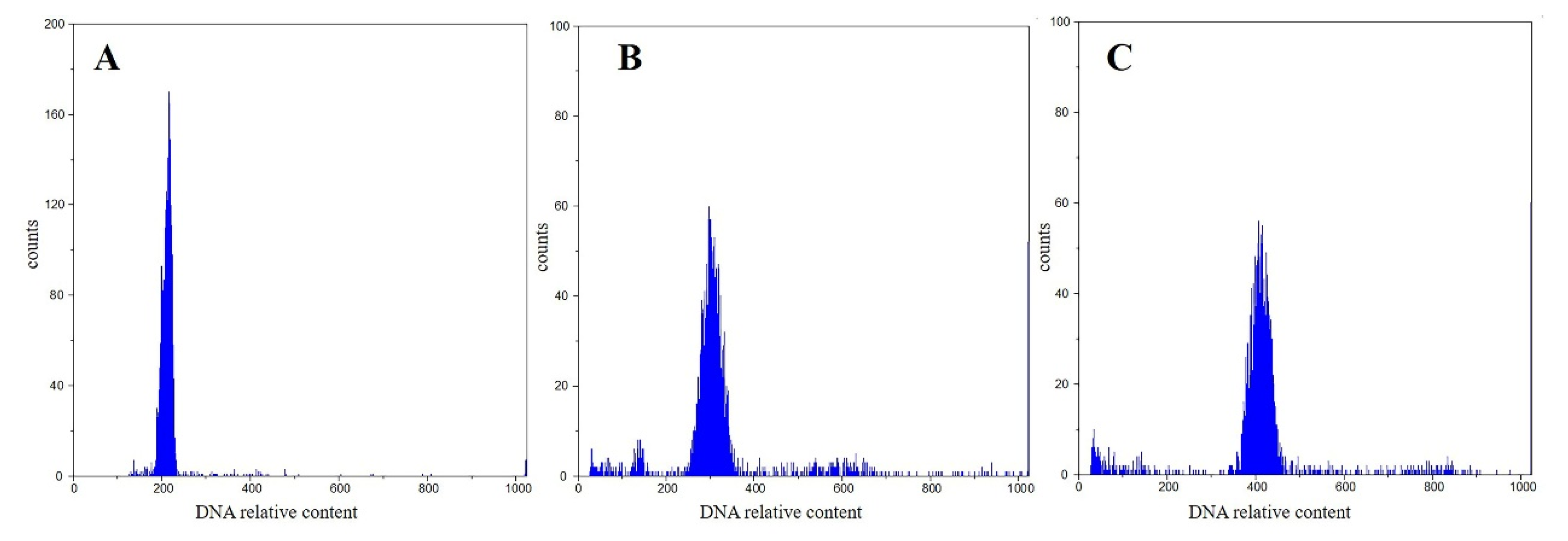
3.1. Ploidy Tests
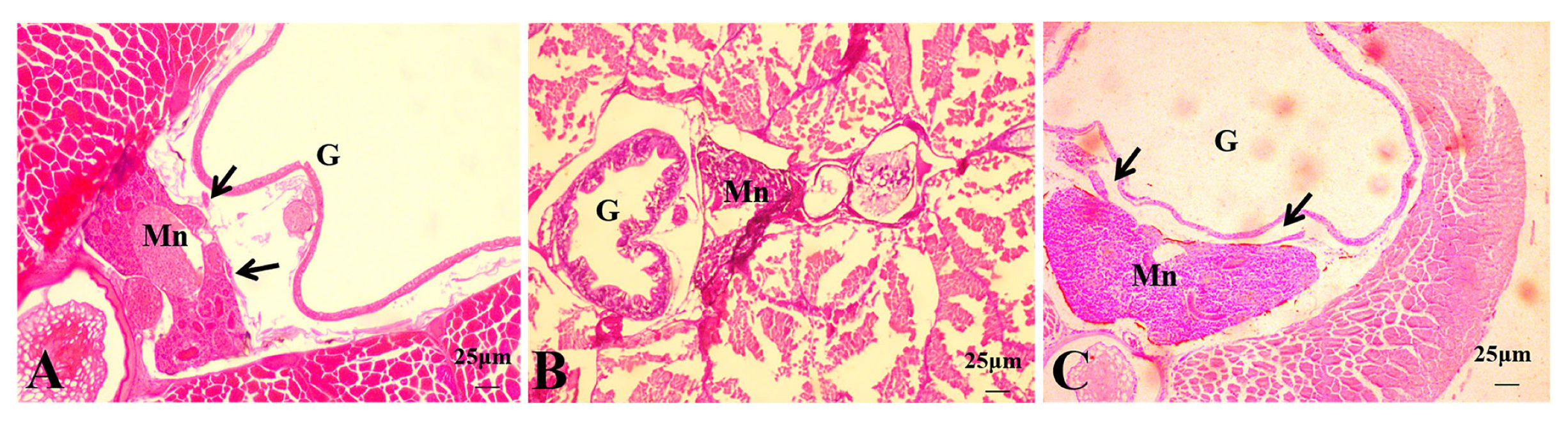
3.2. Histological Observations of Hybrid Triploid Loaches
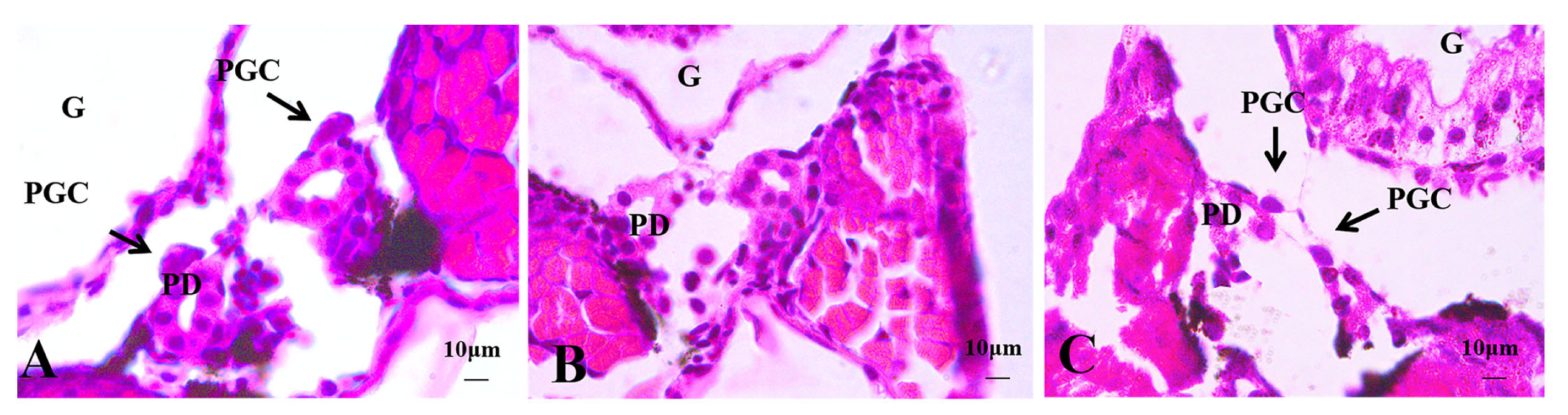
3.2.1. Histological Observations of 4 dph Hybrid Triploid Loaches
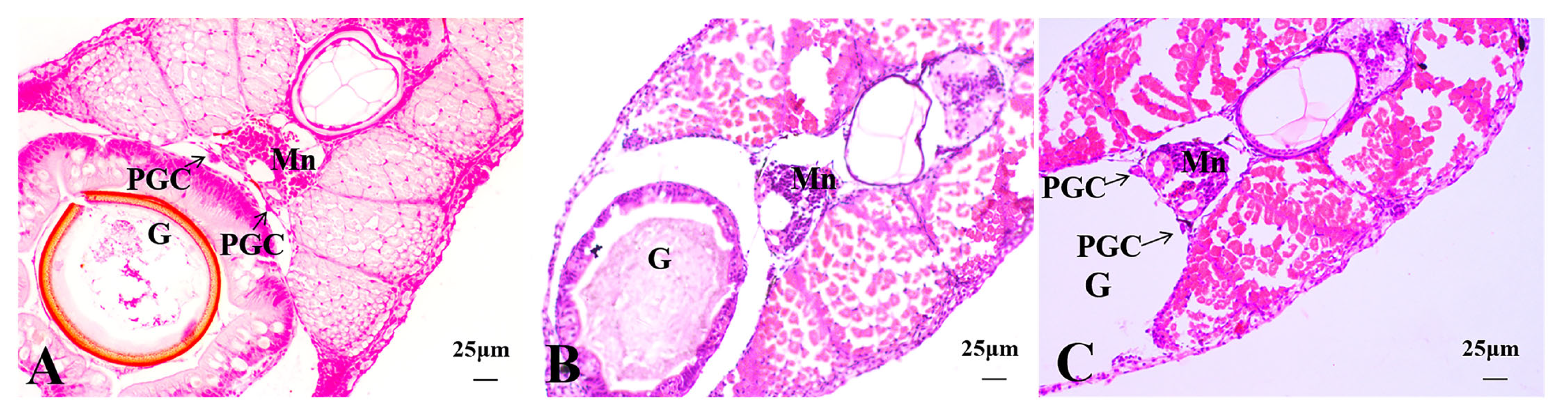
3.2.2. Histological Observations of 15 dph Hybrid Triploid Loaches
3.2.3. Histological Observations of 22 dph Hybrid Triploid Loaches
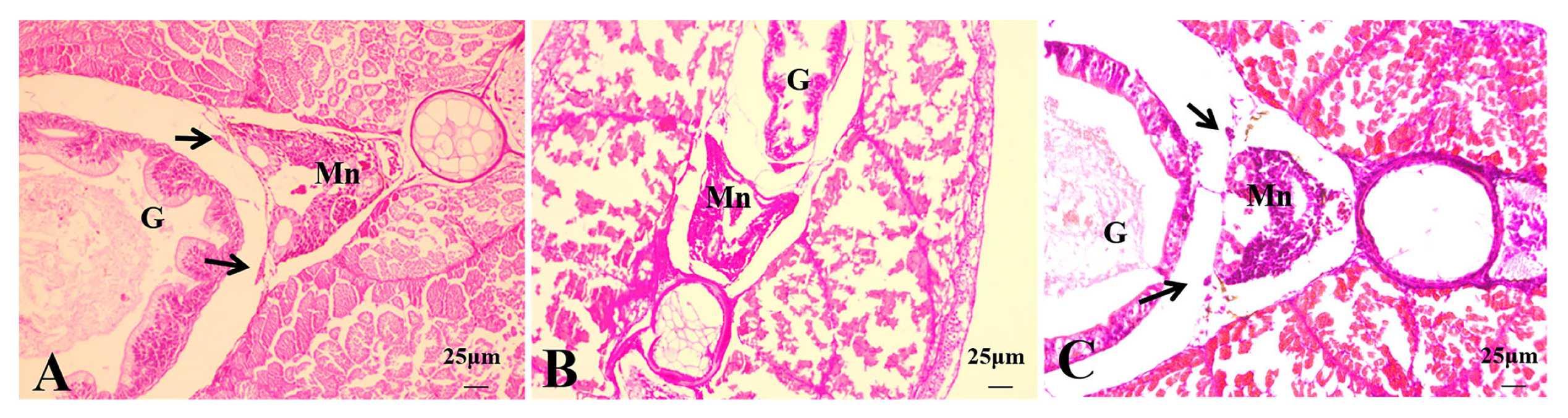
3.2.4. Histological Observations of 50 dph Hybrid Triploid Loaches
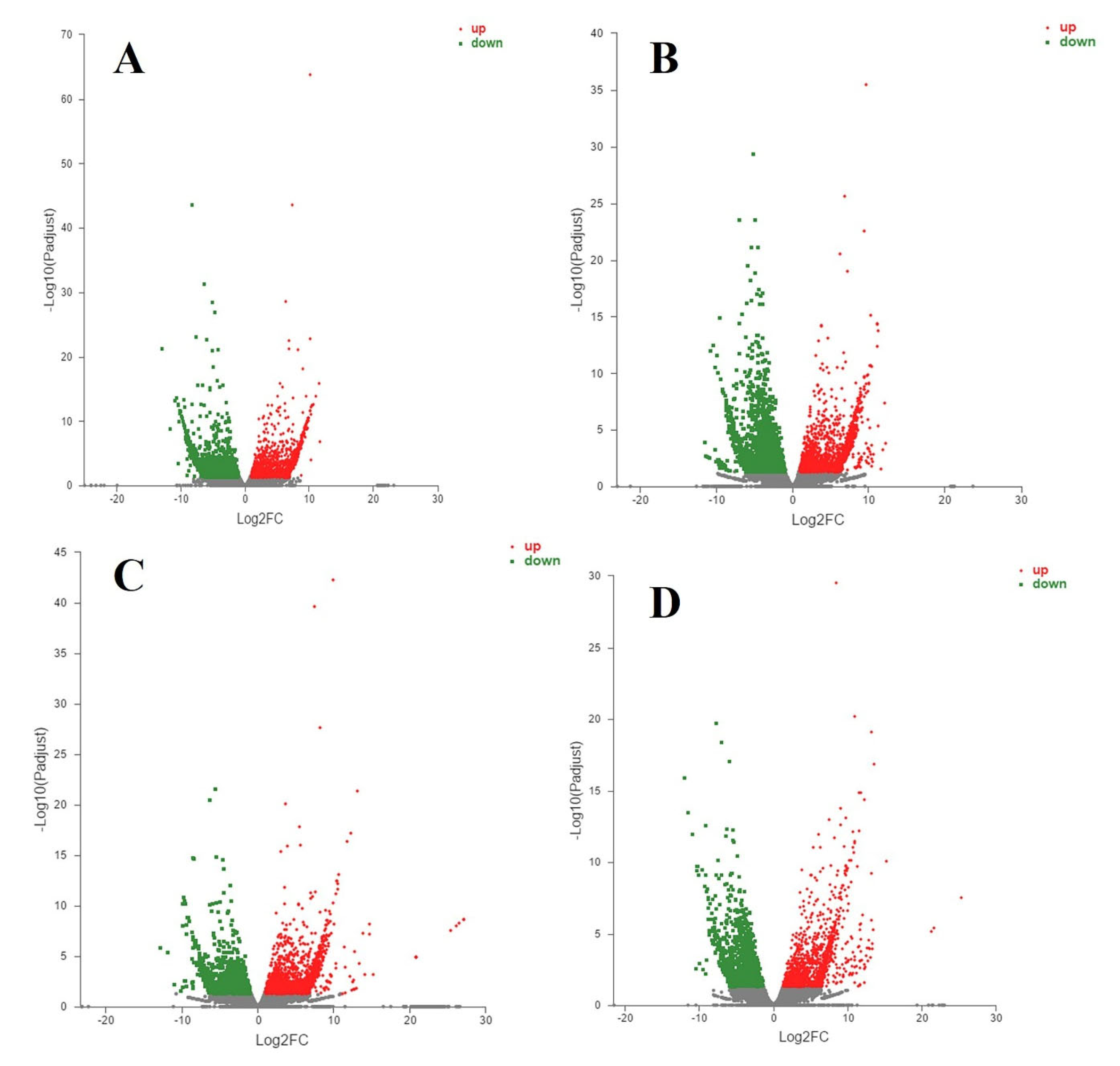
3.3. RNA-Sequencing Analysis
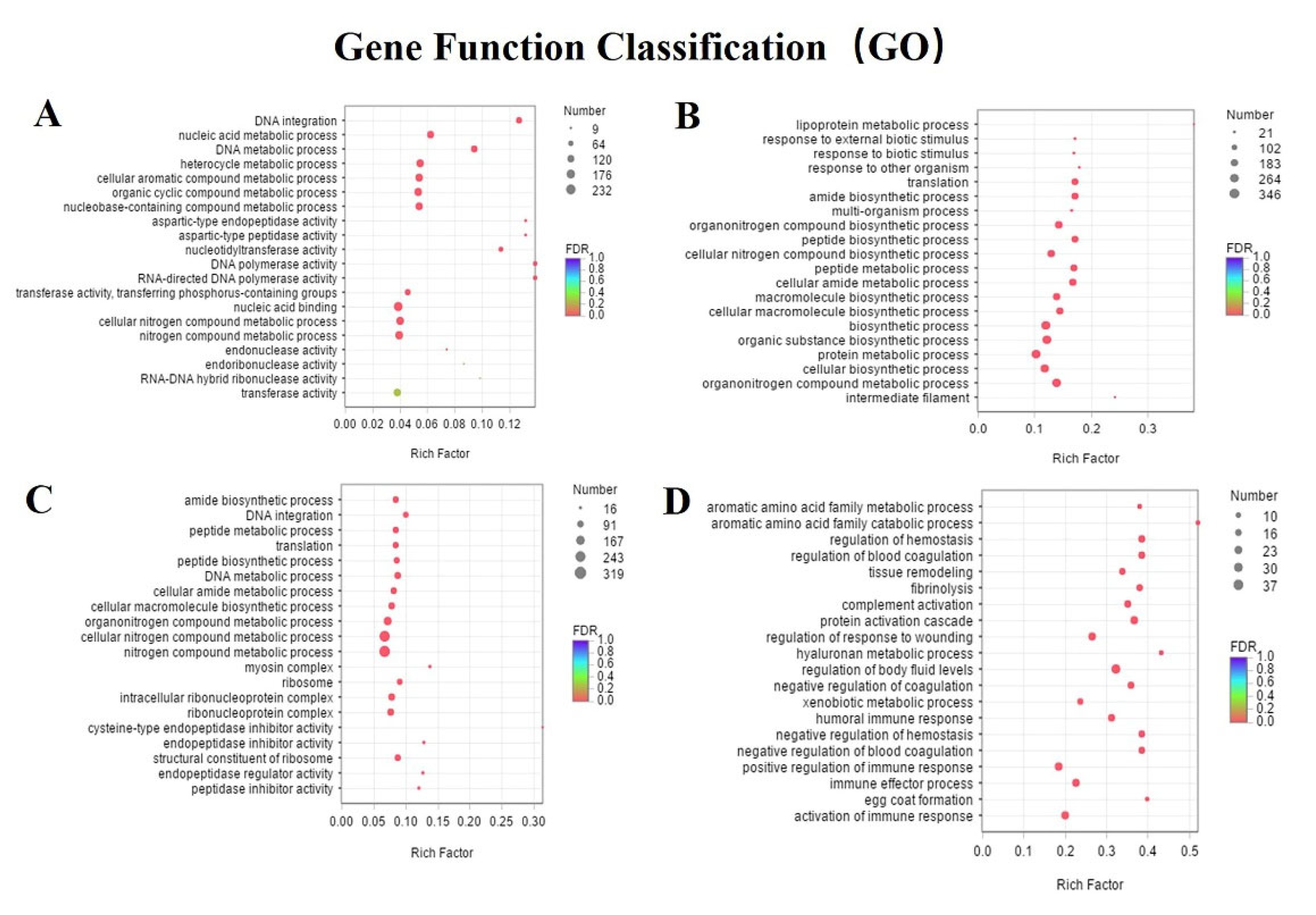
3.4. Differentially Expressed Genes and Enrichment Analyses
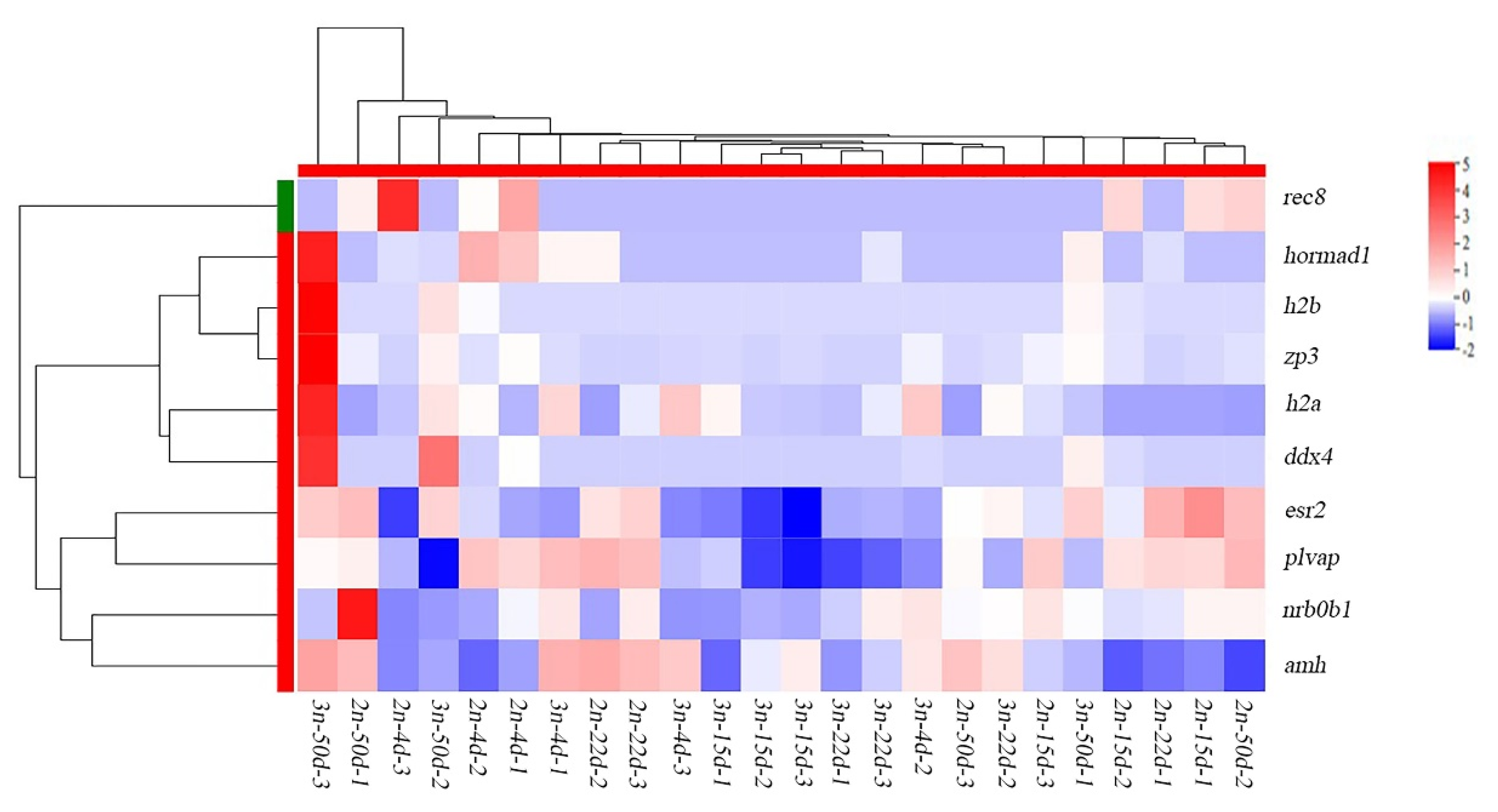
3.5. Identification of Differentially Expressed Genes Associated with Gonadal Development
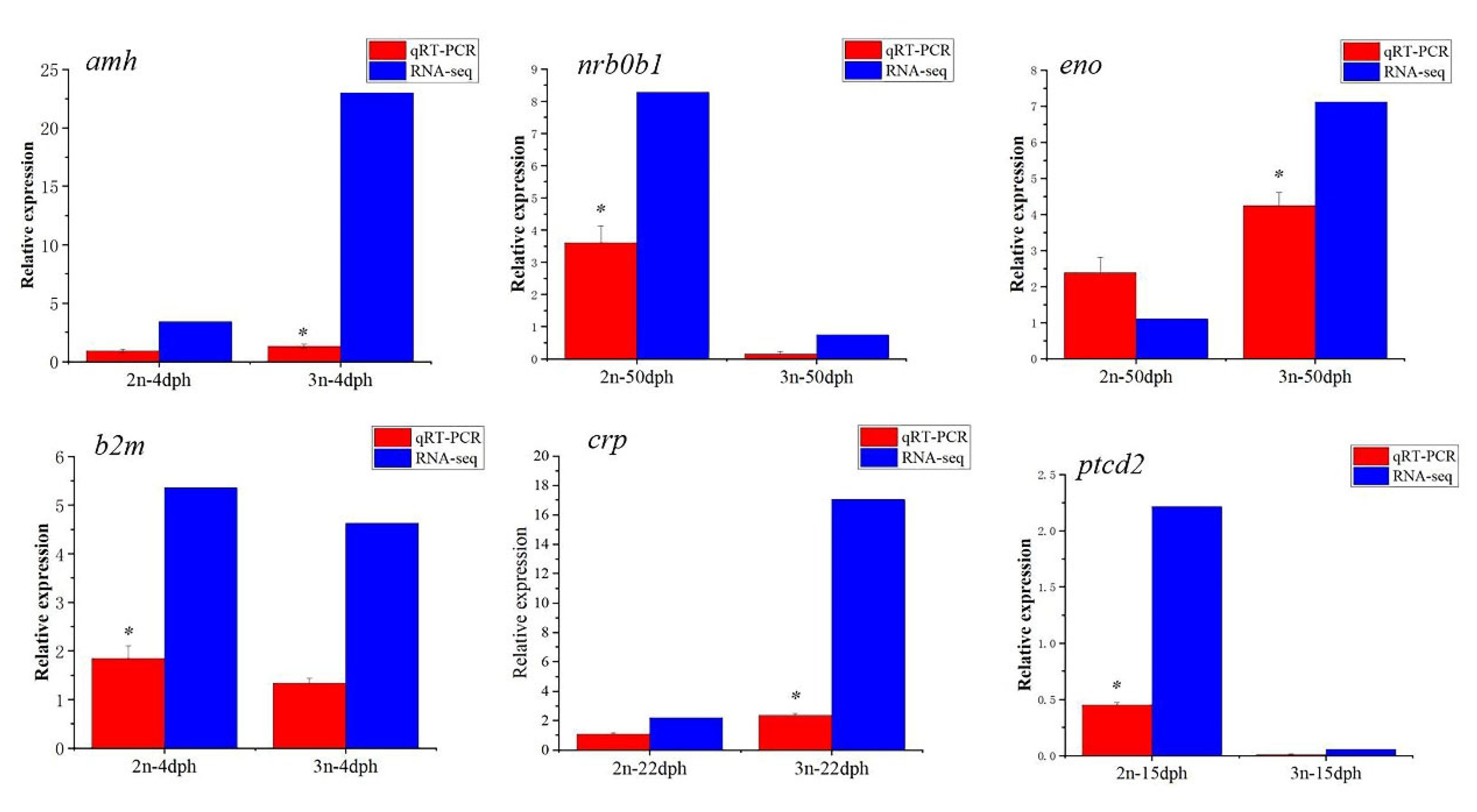
3.6. Verification of Differentially Expressed Genes by qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, X. The edible value and medicinal value of loach. Technol. Mark. 2009, 16, 116. [Google Scholar]
- Milton, J.; Paray, B.A.; Rather, I.A. A review on the biology and physiology of loach Misgurnus anguillicaudatus in China. Indian J. Mar. Sci. 2018, 47, 759–765. [Google Scholar]
- Li, Y.J.; Tian, P.P.; Li, Y.; Yin, J.; Arai, K. Comparison of karyotypes and morphological characteristics in oriental weatherfish with different ploidy from Honghu lake. J. Dalian Ocean Univ. 2009, 24, 236–241. [Google Scholar]
- Iqbal, A.; Nafath-ul-Arab; Ahmad, I.; Shah, T.H.; Asimi, O.A.; Yousuf, Z.; Bhat, B.A.; Baba, S.H.; Fatima, A.; Razak, N. A review on induction of triploidy in fish using heat, pressure and cold shock treatments. J. Entomol. Zool. Stud. 2020, 8, 381–385. [Google Scholar]
- Tiwary, B.K.; Kirubagaran, R.; Ray, A.K. The biology of triploid fish. Rev. Fish Biol. Fish. 2004, 14, 391–402. [Google Scholar] [CrossRef]
- Zhou, L.; Gui, J. Natural and artificial polyploids in aquaculture. Aquacult. Fish. 2017, 2, 103–111. [Google Scholar] [CrossRef]
- Ojima, Y.; Takai, A. The occurrence of spontaneous polyploid in the Japanese common loach, Misgurnus anguicaudatus. Proc. Jpn. Acad. Ser. B 1979, 55, 487–491. [Google Scholar] [CrossRef]
- Feng, B.; Yi, S.V.; Li, R.; Zhou, X. Comparison of age and growth performance of diploid and tetraploid loach Misgurnus anguillicaudatus in the Yangtze River basin, China. Environ. Biol. Fish. 2017, 100, 815–828. [Google Scholar] [CrossRef]
- Abbas, K.; Li, M.Y.; Wang, W.M.; Zhou, X.Y. First record of the natural occurrence of hexaploid loach Misgurnus anguillicaudatus in Hubei Province, China. J. Fish. Biol. 2010, 75, 435–441. [Google Scholar] [CrossRef]
- Cui, L.; Abbas, K.; Yu, Y.; Wang, W.; Zhou, L.; Zhou, X. First record of the natural occurrence of pentaploid loach, Misgurnus anguillicaudatus in Hubei Province, China. Folia Zool. 2013, 62, 14–18. [Google Scholar] [CrossRef]
- Li, Y.J.; Yi, T.; Zhang, M.Z.; Tian, P.P.; Zhuo, Y.; Abe, S.; Arai, K. Chromosome banding and FISH with an rDNA probe in the diploid and tetraploid Misgurnus anguillicaudatus. Ichthyol. Res. 2010, 57, 358–366. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, Z.; Zhang, M.Z.; Qian, C.; Abe, S.; Arai, K. The origin of natural tetraploid Misgurnus anguillicaudatus (Teleostei:Cobitidae) inferred from meiotic chromosome configurations. Genetica 2011, 139, 805. [Google Scholar] [CrossRef]
- Li, Y.J.; Gao, Y.C.; Zhou, H.; Ma, H.Y.; Lin, Z.Q.; Ma, T.Y.; Sui, Y.; Arai, K. Aneuploid progenies of triploid hybrids between diploid and tetraploid Misgurnus anguillicaudatus in China. Genetica 2016, 14, 601–609. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, T.Y.; Zhang, R.; Xu, Q.Z.; Shen, F.; Qin, Y.J.; Xu, W.; Wang, Y.; Li, Y.J. Analysis of Different Ploidy and Parent–Offspring Genomic DNA Methylation in the Misgurnus anguillicaudatus. Int. J. Mol. Sci. 2016, 17, 1299. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, M.Z.; Qian, C.; Gao, M.; Arai, K. Fertility and ploidy of gametes of diploid, triploid and tetraploid loaches, Misgurnus anguillicaudatus, in China. J. Appl. Ichthyol. 2012, 28, 900–905. [Google Scholar] [CrossRef]
- Zhang, Q.; Arai, K.; Yamashita, M. Cytogenetic mechanisms for triploid and haploid egg formation in the triploid loach Misgurnus anguillicaudatus. J. Exp. Zool. 1998, 281, 608–619. [Google Scholar] [CrossRef]
- Fujimoto, T.; Yasui, G.S.; Hayakawa, M.; Sakao, S.; Yamaha, E.; Arai, K. Reproductive capacity of neo-tetraploid loaches produced using diploid spermatozoa from a natural tetraploid male. Aquaculture 2010, 308, S133–S139. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2016, 37, 163–176. [Google Scholar] [CrossRef]
- Ma, H.H.; Zhou, X.J. How to improve the technical level of tissue sectioning. Chin. J. Clin. Exp. Pathol. 2014, 30, 1279–1281. [Google Scholar]
- He, J.L.; Bao, G.; Wang, T.Q.; Sun, C.Y.; Li, N.; Sun, D.M. Preparation of paraffin-embedded sections of zebrafish larv. Chin. J. Comp. Med. 2020, 30, 35–41. [Google Scholar]
- Benfey, T.J. Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Rev. Aquac. 2015, 8, 264–282. [Google Scholar] [CrossRef]
- Murray, D.S.; Kainz, M.J.; Hebberecht, L.; Sales, K.R.; Hindar, K.; Gage, M.J. Comparisons of reproductive function and fatty acid fillet quality between triploid and diploid farm Atlantic salmon (Salmo salar). Roy. Soc. Open Sci. 2018, 5, 180493. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Hershberger, W.K. Early growth and survival of heat-shocked and tetraploid-derived triploid rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 96, 97–107. [Google Scholar] [CrossRef]
- Hamasaki, M.; Takeuchi, Y.; Miyaki, K.; Yoshizaki, G. Gonadal development and fertility of triploid grass puffer Takifugu niphobles induced by cold shock treatment. Mar. Biotechnol. 2013, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, R.F. Sexual maturation in triploid male plaice (Plenronectes platessa) and plaice x flounder (Platichthy sflesus) hybrids. J. Fish Biol. 2006, 19, 415–426. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, G.R.; Wei, K.J. Genetic structure of sympatric diploid and tetraploid loach (Misgurnus anguillicaudatus) populations. J. Huazhong Agric. Univ. 2011, 30, 624–630. [Google Scholar] [CrossRef]
- Oshima, K.; Morishima, K.; Yamaha, E.; Arai, K. Reproductive capacity of triploid loaches obtained from Hokkaido Island, Japan. Ichthyol. Res. 2005, 52, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, Q.Z.; Zhang, R.; Zhuang, Z.X.; Ma, Y.Q.; Wang, W.; Ma, T.Y.; Sui, Y.; Liu, Y.; Cao, X.J. Gonadal transcriptome analysis of hybrid triploid loaches (Misgurnus anguillicaudatus) and their diploid and tetraploid parents. PLoS ONE 2018, 13, e0198179. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, D.J.; You, Y.L. Gonad differentiation in loach (Misgurnus anguillicaudatus) and the temperature effects. J. Fish. Sci. China 2007, 1, 74–82. [Google Scholar]
- Sun, Y.Q.; Chen, Q.; Zhen, X.Y.; Xu, Q.Z.; Zhuang, Z.X.; Wang, W.; Cao, X.J.; Zhou, H. Histological observation of early gondal differentiation and experssion of gonadal development-related genes in hybrid triploid Misgurnus anguillicaudatus. J. Huazhong Agric. Univ. 2020, 39, 82–88. [Google Scholar]
- Coumailleau, P.; Pellegrini, E.; Adrio, F.; Diotel, N.; Cano-Nicolau, J.; Nasri, A.; Vaillant, C.; Kah, O. Aromatase, estrogen receptors and brain development in fish and amphibians. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 152–162. [Google Scholar] [CrossRef]
- Hawkins, M.B.; Thornton, J.W.; Crews, D.; Skipper, J.K.; Dotte, A.; Thomas, P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc. Natl. Acad. Sci. USA 2000, 97, 10751–10756. [Google Scholar] [CrossRef]
- Chauvigné, F.; Parhi, J.; Ollé, J.; Cerdà, J. Dual estrogenic regulation of the nuclear progestin receptor and spermatogonial renewal during gilthead seabream (Sparus aurata) spermatogenesis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 206, 36–46. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, A.Y. A review on sex steroid hormone estrogen receptors in mammals and fish. Int. J. Endocrinol. 2020, 2020, 5386193. [Google Scholar] [CrossRef]
- Kawahara, T.; Yamashita, I. Estrogen-independent ovary formation in the medaka fish, Oryzias latipes. Zool. Sci. 2000, 17, 65–68. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, H.; Han, C.; Huang, J.; Zhu, Q.; Liu, D.; Han, L.; Li, S.; Li, G.; Lin, H.; et al. Estrogen receptor-related receptors in mandarin fish (Siniperca chuatsi): Molecular cloning, characterization, and estrogen responsiveness. Aquac. Rep. 2022, 24, 101137. [Google Scholar] [CrossRef]
- Sasaki, K.; Takaoka, S.; Obata, Y. Oocyte-specific gene knockdown by intronic artificial microRNAs driven by Zp3 transcription in mice. J. Reprod. Dev. 2021, 67, 229–234. [Google Scholar] [CrossRef]
- Kassouri, S.; Boukenaoui, N.; Charallah, S.; Moudilou, E.; Chakhma, A.; Exbrayat, J.M.; Amirat, Z.; Khammar, F. Atretic Ovarian Follicles Morphology and Immunolocalization of Active Caspase-3 in Algerian Bedouin Goat (Capra hircus) Ovaries. Kafkas Univ. Vet. Fak. 2018, 25, 147–156. [Google Scholar]
- Lv, C.; Huang, H.L.; Yi, D.J.; Peng, T.L.; Tan, H.J.; Quan, R.P.; Deng, H.W.; Xiao, H.M. Mutant ZP1 impedes incorporation of ZP3 and ZP4 in the zona pellucida, resulting in zona absence and female infertility in rats. Biol. Reprod. 2021, 104, 1262–1270. [Google Scholar] [CrossRef]
- Spies, I.; Drinan, D.; Petrou, E.; Spurr, R.; Hauser, L. Evidence for divergent selection and spatial differentiation in a putative zona pellucida gene is indicative of local adaptation in Pacific cod. Authorea. [Preprint]. 2021. Available online: https://doi.org/10.22541/au.161831368.86121137/v1 (accessed on 5 June 2022).
- He, W.X.; Wu, M.; Liu, Z.; Li, Z.; Wang, Y.; Zhou, J.; Yu, P.; Zhang, X.J.; Zhou, L.; Gui, J.F. Oocyte-specific maternal Slbp2 is required for replication-dependent histone storage and early nuclear cleavage in zebrafish oogenesis and embryogenesis. RNA 2018, 24, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Pravrutha, R.; Rominger, M.C.; Young, J.M.; Antoine, M.; Toshio, T.; Malik, H.S. Novel classes and evolutionary turnover of histone H2B variants in the mammalian germline. Mol. Biol. Evol. 2022, 2, 2. [Google Scholar]
- He, X.Y.; Fang, X.; Luo, B.Y.; Qiu, G. Identification and characterization of a new germline-specific marker vasa gene and its promoter in the giant freshwater prawn Macrobrachium rosenbergii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 259, 110716. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.Y.; Song, P.; Chen, Y.G.; Peng, M.Y.; Gui, J.F. Expression of gene ddx4 during gonadal natural sex reversal of ricefield eel, Monopterus albus. Curr. Zool. 2005, 3, 469–475. [Google Scholar]
- Olsen, L.C.; Aasland, R.; Fjose, A. A vasa-like gene in zebrafish identifies putative primordial germ cells. Mech. Dev. 1997, 66, 95–105. [Google Scholar] [CrossRef]
- Abu-Rekaiba, R.; Razuki, W.M.; Al-Anbari, E.H. Polimorphic Explore of Esr1, Esr2 and Foxl2 Genes and Interaction Effect of Esri and Foxl2 with Productive Traits of Brown Local Iraqi Chickens. IOP Conf. Ser. Earth Environ. Sci. 2021, 910, 012004. [Google Scholar] [CrossRef]
- Miura, T.; Miura, C.; Konda, Y.; Yamauchi, K. Spermatogenesis-preventing substance in Japaneseeel. Development 2002, 129, 2689–2697. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, B.; Chen, W.; Ge, W. Anti-Müllerian hormone (Amh/amh) plays dual roles in maintaining gonadal homeostasis and gametogenesis in zebrafish. Mol. Cell Endocrinol. 2020, 517, 110963. [Google Scholar] [CrossRef]
- Morinaga, C.; Saito, D.; Nakamura, S.; Sasaki, T.; Asakawa, S.; Shimizu, N.; Mitani, H.; Furutani, M.; Tanaka, M. The hotei mutation of medaka in the anti-Müllerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc. Natl. Acad. Sci. USA 2007, 104, 9691–9696. [Google Scholar] [CrossRef]
- Halm, S.; Rocha, A.; Miura, T.; Prat, F.; Zanuy, S. Anti-Müllerian hormone (AMH/AMH) in the European sea bass: Its gene structure, regulatory elements, and the expression of alternatively-spliced isoforms. Gene 2007, 388, 148–158. [Google Scholar] [CrossRef]
- Fukuda, T.; Daniel, K.; Wojtasz, L.; Toth, A.; Höög, C. A novel mammalian HORMA domain-containing protein, HORMAD1, preferentially associates with unsynapsed meiotic chromosomes. Exp. Cell Res. 2010, 316, 158–171. [Google Scholar] [CrossRef]
- Pangas, S.A.; Yan, W.; Matzuk, M.M.; Rajkovic, A. Restricted germ cell expression of a gene encoding a novel mammalian HORMA domain-containing protein. Gene Expr. Patterns. 2004, 5, 257–263. [Google Scholar] [CrossRef]
- Shin, Y.H.; Choi, Y.; Erdin, S.U.; Yatsenko, S.A.; Kloc, M.; Yang, F.; Wang, P.J.; Meistrich, M.L.; Rajkovic, A. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet. 2010, 6, e1001190. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer (F)/Reverse Primer ® (5’–3’) | Amplicon Size (bp) | Efficiency (%) | R2 | |
|---|---|---|---|---|---|
| nrb0b1 | F | CTGAAGGGCTTGGATGTA ACTGTTGGTGCTCGGGAT | 286 | 95.2 | 0.9932 |
| R | |||||
| b2m | F | AGATTACTCGCAGGATTT CACGAATGACTGTGGGTT | 231 | 94.8 | 0.9951 |
| R | |||||
| crp | F | AATAGGCCCTAAGGAAGC ACAGACCCGACAAGAGTG | 237 | 102.8 | 0.9927 |
| R | |||||
| eno | F | AATGGACTGAACTGGGTA AAGATCATCATCGGAATG | 169 | 97.1 | 0.9923 |
| R | |||||
| ptcd2 | F | ATTCAGACGCTAAGGAGG GGACTGGCTTGATGTTGT | 204 | 100.7 | 0.9916 |
| R | |||||
| amh | F | ATCCATTCACTATCCCTC TCATCTCCTTTGCCTCCT | 295 | 96.6 | 0.9904 |
| R | |||||
| β-actin | F | CTCAATCCCAAAGCCAACAG GGAAGAGCATAACCCTCGTAGA | 300 | 100.5 | 0.9986 |
| R | |||||
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|---|
| N2_4d_1 | 43,504,388 | 6,569,162,588 | 42,980,754 | 6,194,571,365 | 0.0251 | 98.02 | 93.95 | 41.62 |
| N2_4d_2 | 51,249,740 | 7,738,710,740 | 50,304,140 | 7,195,894,523 | 0.0251 | 98.01 | 94.01 | 44.27 |
| N2_4d_3 | 41,854,824 | 6,320,078,424 | 41,335,714 | 5,942,308,333 | 0.0252 | 98.02 | 93.9 | 43.17 |
| N3_4d_1 | 42,804,690 | 6,463,508,190 | 42,321,508 | 6,100,440,038 | 0.0249 | 98.13 | 94.2 | 41.33 |
| N3_4d_2 | 48,765,686 | 7,363,618,586 | 48,076,332 | 6,825,251,255 | 0.0248 | 98.14 | 94.32 | 45.37 |
| N3_4d_3 | 42,968,870 | 6,488,299,370 | 42,577,432 | 6,176,175,856 | 0.0251 | 98.04 | 94.03 | 45.03 |
| N2_15d_1 | 41412,970 | 6,253,358,470 | 41,068,528 | 6,044,516,182 | 0.0247 | 98.19 | 94.39 | 45.3 |
| N2_15d_2 | 41,047,032 | 6,198,101,832 | 40,632,264 | 5,893,917,626 | 0.0247 | 98.17 | 94.34 | 45.94 |
| N2_15d_3 | 41,202,814 | 6,221,624,914 | 40,749,538 | 6,048,037,172 | 0.0249 | 98.11 | 94.18 | 46.73 |
| N3_15d_1 | 43,718,254 | 6,601,456,354 | 43,223,404 | 6,251,258,113 | 0.0247 | 98.18 | 94.33 | 47.74 |
| N3_15d_2 | 42,451,088 | 6,410,114,288 | 42,014,502 | 6,072,319,846 | 0.0245 | 98.27 | 94.59 | 47.7 |
| N3_15d_3 | 44,231,926 | 6,679,020,826 | 43,873,216 | 6,286,529,530 | 0.0257 | 97.84 | 93.33 | 48.92 |
| N2_22d_1 | 42,771,784 | 6,458,539,384 | 42,375,152 | 6,198,131,254 | 0.0246 | 98.24 | 94.47 | 45.78 |
| N2_22d_2 | 42,539,684 | 6,423,492,284 | 42,208,434 | 6,225,066,614 | 0.0245 | 98.27 | 94.54 | 45.4 |
| N2_22d_3 | 41,868,322 | 6,322,116,622 | 41,542,220 | 6,087,783,844 | 0.0247 | 98.19 | 94.37 | 45.86 |
| N3_22d_1 | 46,701,188 | 7,051,879,388 | 46,269,630 | 6,701,892,134 | 0.0246 | 98.21 | 94.47 | 48.29 |
| N3_22d_2 | 44,174,544 | 6,670,356,144 | 43,678,314 | 6,313,610,315 | 0.0246 | 98.24 | 94.51 | 45.88 |
| N3_22d_3 | 43,001,286 | 6,493,194,186 | 42,489,286 | 6,104,613,377 | 0.0247 | 98.22 | 94.4 | 44.58 |
| N2_50d_1 | 42,615,574 | 6,434,951,674 | 42,215,848 | 6,228,367,646 | 0.025 | 98.07 | 94.08 | 45.38 |
| N2_50d_2 | 42,871,908 | 6,473,658,108 | 42,493,250 | 6,316,701,059 | 0.0247 | 98.21 | 94.4 | 46.18 |
| N2_50d_3 | 45,091,340 | 6,808,792,340 | 44,718,858 | 6,516,081,264 | 0.0246 | 98.24 | 94.49 | 47.78 |
| N3_50d_1 | 49,468,546 | 7,469,750,446 | 49,037,504 | 7,116,102,829 | 0.0249 | 98.11 | 94.15 | 47.48 |
| N3_50d_2 | 44,556,980 | 6,728,103,980 | 44,014,614 | 6,373,127,533 | 0.0242 | 98.38 | 94.87 | 47.92 |
| N3_50d_3 | 44,772,444 | 6,760,639,044 | 44,368,620 | 6,406,016,822 | 0.0246 | 98.25 | 94.5 | 46.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Shan, G.; Li, X.; Guo, W.; Ma, K.; Yang, Y.; Li, Y.; Zhang, Y.; Zhou, H.; Cao, X. De Novo Transcriptome Analysis of the Early Hybrid Triploid Loach (Misgurnus anguillicaudatus) Provides Novel Insights into Fertility Mechanism. Fishes 2023, 8, 70. https://doi.org/10.3390/fishes8020070
Zhou Z, Shan G, Li X, Guo W, Ma K, Yang Y, Li Y, Zhang Y, Zhou H, Cao X. De Novo Transcriptome Analysis of the Early Hybrid Triploid Loach (Misgurnus anguillicaudatus) Provides Novel Insights into Fertility Mechanism. Fishes. 2023; 8(2):70. https://doi.org/10.3390/fishes8020070
Chicago/Turabian StyleZhou, Ziyu, Gu Shan, Xin Li, Wenxuan Guo, Kexin Ma, Yueyao Yang, Yifan Li, Yunbang Zhang, He Zhou, and Xiaojuan Cao. 2023. "De Novo Transcriptome Analysis of the Early Hybrid Triploid Loach (Misgurnus anguillicaudatus) Provides Novel Insights into Fertility Mechanism" Fishes 8, no. 2: 70. https://doi.org/10.3390/fishes8020070
APA StyleZhou, Z., Shan, G., Li, X., Guo, W., Ma, K., Yang, Y., Li, Y., Zhang, Y., Zhou, H., & Cao, X. (2023). De Novo Transcriptome Analysis of the Early Hybrid Triploid Loach (Misgurnus anguillicaudatus) Provides Novel Insights into Fertility Mechanism. Fishes, 8(2), 70. https://doi.org/10.3390/fishes8020070






