1. Introduction
Marine resources are declining, primarily as a result of human consumption [
1,
2,
3]. Commercial fishing has been considered as the main sector responsible for this decline, while recreational fishing was thought to have a negligible effect on total yields [
4]. However, recreational fishing is a widespread coastal activity, and its popularity is increasing globally [
5] with largely unknown consequences to the marine environment [
6]. Estimating recreational fishing activities is a challenging task due to the highly diverse fishing activities which are heterogeneous, both temporally and spatially [
5]. From individual anglers on the coast to deep sea fishing trips, the boundaries of these activities are broad, possibly overlapping, and may conflict with commercial fisheries [
7,
8]. This great variation in fishing activity makes it very difficult to determine annual yields, fishing pressure, and impacts on the marine ecosystem [
9].
Recent evaluations have suggested that global marine annual recreational yields are around 900,000 tons, representing a little less than 1% of total marine catch [
10]. However, recreational fishers rarely report their activities, and catch evaluations are considered to be underestimated [
6]. Furthermore, catch may vary greatly between countries and regions, and thus evaluations may overlook areas where yields are higher than average. In some cases, recreational yields may even exceed commercial catch [
10]. Moreover, apart from biomass reduction, recreational fishing activities may impact size distribution and change species composition [
6,
11]. Therefore, there is an urgent need to improve our knowledge of recreational fishing, which acquires detailed species-level data specific to fishing methods and locations [
12,
13].
In the Mediterranean Sea, the popularity of recreational fishing is increasing together with improved and more efficient fishing techniques [
5]. Awareness of the importance of evaluating the magnitude and characteristics of this sector has increased, with several studies evaluating the ecological impacts and contributions of recreational fishing [
7,
12,
14,
15,
16,
17,
18,
19,
20]. Some studies analyze the fisher profile and motivation [
19], while others describe catch composition and ecological effect [
20,
21]. These studies emphasize that recreational fishing may substantially affect vulnerable species catch size and may impact the reproductive potential of fish.
These new insights point to the substantial part recreational fishing holds in terms of yields [
7], which have been increasing together with the number of fishers [
22]. In the Mediterranean, the catch size of recreational fishing is generally estimated to be between 10–50% of the total catch size [
23]. However, some studies estimate much smaller contributions of recreational fishers. For example, for some common species, recreational catch only corresponded to 0.8% of the commercial catch [
24].
Due to the large spatiotemporal variation in recreational fishing activity, sampling measures are often more complex than the surveys used for evaluating commercial fishing. New evolving methods include data collected from social media [
25]. Other studies use satellite data to evaluate the number of fishing vessels [
8]. Nevertheless, regardless of the method used, some parts of the Mediterranean Sea remain poorly investigated.
The Eastern Mediterranean (Levantine) basin is located on the front-line of an extensive invasion via the Suez Canal. The Levant is also the hottest and saltiest region of the Mediterranean Sea [
26,
27,
28]. These conditions benefit thermophilic non-indigenous species of Red Sea origin [
29,
30,
31], turning the Levantine basin into a unique blend of native and non-indigenous species. In Israel, even though commercial fishing is declining, recreational fishing has increased in popularity. For example, according to the Israel Nature and Parks Authority, only 1000 fishers hold a commercial fishing license, while over 6000 fishers hold a recreational fishing license (excluding angling from the shore), and the number exceeds 70,000 when including angling [
32]. However, there is currently no data on recreational fisher activity patterns, species composition of the catch, or annual yields. Information on the magnitude and spatiotemporal characteristics of recreational fishing in the extreme climate of the Eastern Mediterranean Sea is rare (Michailidis et al., 2020).
In this study, we surveyed the Israeli coast and quantified recreational fishing techniques, fishing preferences, catch biomass, and target species composition using a diversity of methods. These include coastal and aerial surveys, phone interviews, and shore interviews. We also compared the catch composition and length distribution of recreational fishing to that of the commercial fishing industry. Our study is the first to comprehensively characterize the recreational fishing sector activities along the Israeli coastline. We thus provide an important estimate of the contribution and impact of this fishing sector in a region suffering from extreme climatic conditions and severe invasion.
2. Methods
2.1. Data Collection
We used three different methods to monitor recreational fishing: (1) coastal surveys including interviews, (2) phone surveys, and (3) aerial surveys from a helicopter. All surveys were conducted between 2016 and 2018. Additionally, we obtained complementary data regarding the number of registered fishers and fishers holding licenses from the Israeli Ministry of Agriculture and the Israel Nature and Parks Authority.
2.2. Coastal Surveys
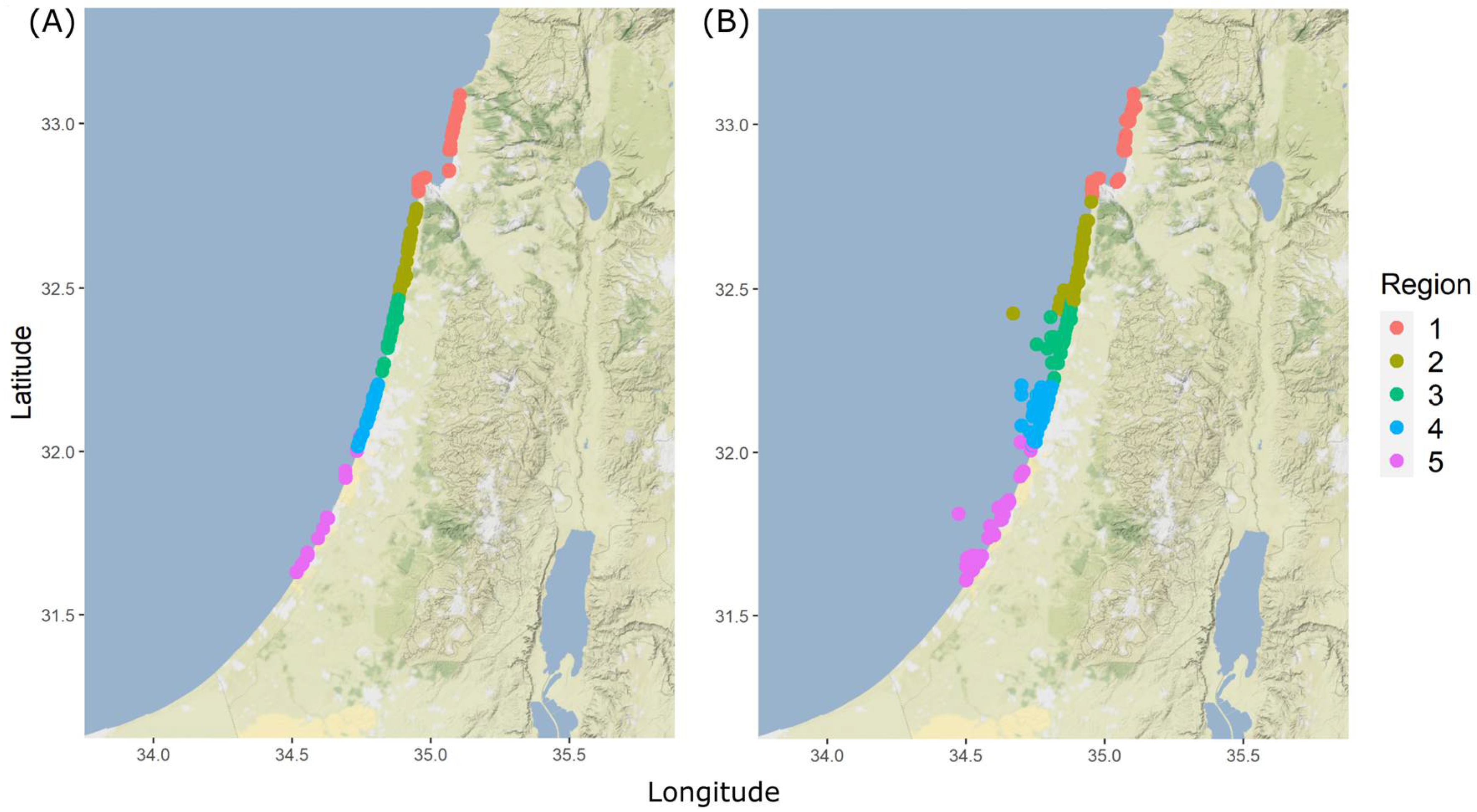
The coastal surveys covered a substantial part of the Israeli coast (120 km out of 145 km available for fishing, excluding areas that were not accessible by foot such as ports or military areas). The total Israeli Mediterranean coastline was divided into five regions (
Figure 1). Each region was then split into different sites with a mean coastline length of 3 km. Different sites within each region were surveyed once every fortnight during a period of two years by a team of 15 surveyors. Weekends and weekdays were given equal sampling effort (although recreational fishing peaks on weekends). The time of the survey varied in order to capture dial variation, but surveys were not conducted at night, due to security reasons, and because this is when most recreational fishing is minimal.
Each coastal survey was composed of two steps. First, surveyors walked along a segment of coast, and documented the number of fishers and the number of fishing poles. Second, on the return, surveyors interviewed fishers and documented their catch. With the permission of the fisher, the surveyors measured and documented all the fish caught. When possible, fish total length (TL) was documented to the nearest millimeter, and a picture was taken for identification. In addition, surveyors questioned the fishers, and the following parameters were recorded: (1) Fishing duration (hours): the time measured since the fisher’s arrival; (2) time of the interview; and (3) fisher experience: number of years fishing. In addition, the surveyors recorded at the site level: (1) month (1 to 12 calendar months); (2) sea condition: quiet/wave/storm; (3) MPA (protected/non-protected): within or outside a marine protected area where all fishing methods are forbidden, except angling from the coast; and (4) hotspot (yes/no): these were defined as artificial sites less than 1 km long, such as ports and marinas. At these sites, the mean number of fishers was much higher than in the open coast (10.2 fishers compared to 4.5).
During 2017–2018, 274 surveys were conducted in 43 different sites along the Israeli coast, 1254 fishers were interviewed, and 3350 individual fishes representing 38 species were documented (
Tables S1 and S2 ).
Biomass was calculated using a species-specific, Mediterranean-based length-weight relationship (
W =
aLb) gathered from FishBase [
33], choosing values estimated as close as possible to the Israeli coast.
2.3. Phone Interviews
The time that fishers spend on the shore during angling from boats or kayaks, or during spear fishing, is brief and hence under-sampled from shore surveys. Thus, we contacted by phone fishers who use these methods of fishing in the open sea. We then spoke with each fisher at least once a month asking them to report their fishing activities. The same questionnaire used for the shore surveys was applied here. The phone interviews took place between August 2017 and January 2018. We managed to recruit 34 fishers that were willing to be regularly interviewed, and who were distributed along the entire Israeli coastline (
Figure 1B). The fishers were divided into three groups: 13 fishers angling from a boat (78 interviews), 12 angling from kayaks (46 interviews), and 9 spear fishers (96 interviews).
2.4. Aerial Surveys
We used helicopter surveys to document the number of fishers more accurately along the entire Israeli coast. There were four helicopter flights during the time of the survey, each covering half the Israeli Mediterranean coast, so that all together, the entire coast was surveyed twice. On each flight, two surveyors documented fishing activities. One of the surveyors focused on the number of anglers from the shore, and the other on fishing activities at the open sea (fishing from kayaks, fishing from boats, or spear fishing). The surveys were conducted on Friday mornings, in January 2017, January 2018, November 2016, and March 2017.
2.5. Commercial Fishing Data Collection
We compared the length distributions and species composition from recreational fishing to other commercial fishing sectors: trawl fishery [
34] and artisanal fishery (set nets and long-lines [
35,
36]). For details on catch estimates and the species composition of these methods see
File S1 and Tables S3 and S4.
2.6. Statistical Analysis
Coastal Surveys: Annual Catch
The annual catch extracted by anglers fishing from the shore was calculated using two separate models, one that calculated the extracted biomass per fisher per hour (kg/hour/fisher) (“biomass model”) and the other, the number of fishers per kilometer (“fisher model”). The results of these two models were combined to estimate the total annual catch along the Israeli coast. The two models were constructed using GAMs (General Additive Models) which are suitable for describing nonlinear relationships between variables, and capable of dealing with cyclic data [
37,
38]. All GAMs were constructed using the
‘mgcv’ R package.
For the
biomass model, we used
‘catch (kg)’ per hour as the response variable. The response variable was zero-inflated and thus modeled with a Tweedie distribution (
p = 1.5) [
39,
40,
41] Candidate predictors included:
(1) time of the interview (morning/noon/evening),
(2) hotspot (yes/no),
(3) substrate (sand/rock/artificial),
(4) sea condition (quiet/wave/storm),
(5) MPA (protected/non-protected) as fixed effects,
(6) Fishing duration (hours), which represented the time each fisher was fishing until the time of the interview. This was modeled using a univariate adaptive smoother, appropriate when there is a large variation (“wiggleness”) in the variable.
(7) Month was modeled using a cyclic cubic regression spline smoother with the knot number (k) defined to be 12.
(8) Fisher experience (years) was modeled using the cubic regression smoother. Finally,
site was used as a random effect.
For the fisher model, we estimated the number of fishers per kilometer. We used a negative binomial distribution to deal with zero inflation in the count data. The candidate predictors were (1) hotspot (yes/no), (2) time of the interview (morning/noon/evening), (3) MPA (protected/non-protected), (4) sea condition (quiet/wave/storm), and (5) day of the week (weekday/weekend) as fixed effects. As in the biomass model, month was calculated with a cyclic cubic regression spline smoother, and site was modeled as a random effect.
In both cases, we used AICc to exclude less-supported variables and we chose the best supported explanatory variables while avoiding over fitting. When the difference between models in AICc was small, we preferred models that had common variables between the fisher and biomass models to facilitate the calculation of the summed catch.
2.7. Predictions
To predict the annual catch for the Israeli coast, we needed to multiply, per each site and each month, the number of fishers (number/km) and biomass (kg per fisher/hour) estimated by the GAMs, by (1) the number of available fishing days a month, (2) the number of fishing hours per day, and (3) the number of kilometers available for fishing, to obtain annual estimates. These were then summed across months and sites to obtain the total annual catch.
The number of days suitable for fishing in a month is dependent on weather conditions, and can change from one year to another. Thus, we examined different scenarios for each fishing technique (angling from the coast, fishing from a boat or a kayak, and spear fishing) according to estimations made by 7 marine rangers of the INPA (Israeli Nature and Parks Authority) who spend their day in the sea and encounter fishers regularly. We used the mean estimation, but presented also the min and max estimations. As we only examined fishing activity during the day, the number of fishing hours per day ranged between 12 and 16, according to the seasonal variation. Finally, as the coastal surveys covered almost all of the Israeli coast available for fishing, we could sum the biomass predicted at each site to receive annual yields with high accuracy. To account for the 25 km that were available for fishing but were not surveyed, we calculated the mean biomass and the number of fishers per km for a non-hotspot site, and added it to the calculation.
The final calculation of biomass was based on predicting the biomass caught for one hour, at each one of the sites, during the different months of the year, for a fisher with 20 years of experience (experience significantly affected biomass and 20 years of experience was both the mean and median fisher experience). We then multiplied the catch per hour by 12 h of fishing, and by the number of available fishing days (according to the two scenarios explained above, 70%, 78%, and 85% of days available for fishing). This was then multiplied by the fisher density estimates (at each site and across months, for both weekdays and weekends as we observed 1.7 more fishers on weekends):
These values were summed across sites and months to derive Israel’s annual catch.
In addition to the calculation of the annual biomass fished along the Israel coast, which included fish reported to be released, we compiled, following the same protocol, an estimation of the kept annual biomass based on the fishers’ reports.
2.8. Fishing at Sea
Annual yields of angling from a boat, angling from a kayak, and spear fishing were calculated based on phone and aerial surveys. The biomass extracted was estimated using two different methods:
2.8.1. Method 1: The Number of Fishers Based on Registered Fishing Licenses
In the first method, we used the product of biomass per fishing day, mean fishing days per month (both estimated from the phone surveys), number of months per year (12), and the number of active fishers.
The number of active fishers was estimated using the number of fishing licenses registered in the Israeli Ministry of Agriculture and the Israel Nature and Park Authority. The number of licensed fishers may be misleading, as not all fishers are regularly active. Thus, we calculated three different scenarios for the number of active fishers, according to estimations made by 5 marine rangers of the INPA. Presented are mean (82% are active), maximum (90% are active), and minimum (75% are active) estimations. Fishers angling from a boat, or a kayak hold the same license. Since we could not separate the biomass of each technique, we calculated the weighted total biomass for these two methods assuming the relative number of fishers from the boat and kayak is similar to their relative number in the interviews.
We repeated this calculation for the reported kept biomass to assess the magnitude of catch and release practice.
2.8.2. Method 2: The Number of Fishers Based on Aerial Observations
For the second method, we used the number of fishers observed from the aerial surveys. We calculated the mean biomass per fishing day as reported on the phone surveys and multiplied it by the number of fishers observed from the aerial survey, the number of available fishing days a month, the number of months a year, and turnover rate. Turnover rate means the number of times new fishers arrive and leave during one day. We set the turnover rate as three, using the assumption that new fishers arrive and leave three times a day: early morning, morning, and afternoon. As all aerial surveys were conducted on weekends, to estimate the number of fishers on weekdays, we divided the number of fishers observed by 1.7, which is similar to the ratio observed for angling from the shore (1.7 on weekends compared to weekdays). The total number of fishers was estimated by calculating the mean density across all surveys, multiplied by the number of available kilometers for fishing. Again, we used estimations of 7 marine rangers of the INPA for the number of available fishing days. We show the mean (285), maximum (310), and minimum (255) estimations. These scenarios are more restrictive than the ones used to assess the available fishing day for anglers, as the fishing at sea methods are more sensitive to sea conditions (visibility, waves, etc.):
We repeated this calculation for the kept biomass only.
2.9. Comparing among Fishing Methods
We made comparisons among the different fishing methods by calculating the predicted annual catch for each species for each fishing method, separated into size bins. We compared the absolute catch values of anglers from the coast and recreational fishing at sea, and compared these to commercial fishing from trawling, set nets, and long lines.
The Calculations Involved Several Steps
- (1)
The annual yields of commercial methods were based on the most recent estimations made in 2017 by the Israeli Fisheries Department in the Ministry of Agriculture (unpublished data). In total, the Israeli fishing fleet extract about 1500 tons a year. The trawl fisheries extract about 775 tons, and the nets and long lines about 638 tons.
- (2)
We estimated the relative proportion of each size bin of each species from the total catch of each fishing method. Here, we assumed that the proportion of the species and size binds from each survey represents their proportion in the annual catch. Data sources used for the relative proportion of each species are described in
File S1.
- (3)
To convert biomass to the number of individuals, we divided the absolute biomass by the mean weight of fish within each size bin.
4. Discussion
In this study, we document recreational fishing at an entire country level for two years, and we use different strategies to provide the most comprehensive estimate to date of recreational fishing in Israel. We find that recreational fishing composes 18–45% of total annual biomass extracted from the sea, which is similar to estimates from other Mediterranean countries [
42,
43]. We find differences between the species targeted by each of the fishing methods. However, some highly commercial species are caught by several methods, showing that these methods likely compete over catch. We also show that in this area of the Mediterranean, non-indigenous species have become an important part of the catch in all fishing methods. This result is compatible with other studies conducted in the eastern Mediterranean Sea, which showed that the two most important species in the catch were invasive rabbitfishes (
Siganus luridus and
Siganus rivulatus) [
42]. We recommend that recreational fishing must be considered non-negligible, and should be monitored and managed.
4.1. Total Catch
We find that recreational fishing composes a substantial portion, of between 18–45%, of total annual biomass extracted from the sea. Our results of total annual biomass extracted fall within the range estimated in other Mediterranean countries. For example, a review of studies from Spain, France, Italy, and Turkey found that total catch of recreational fishing ranges from 10% up to 50% of the total commercial fishing catch (Font & Lloret, 2014). We also fall into the range of similar results as those obtained in former, less detailed evaluations made in Israel [
32]. A recent study examining the recreational catch in Cyprus found that the marine recreational catch exceeds the coastal commercial catch by 46% [
42]. Studies conducted along the Turkish coast and in Malta show that the recreational fishing catch constitutes about 30% of commercial fishing [
7,
44] and in Tunisia, recreational fishers’ catch represents 20–40% of commercial fishing catch [
43]. Furthermore, for certain species, a greater proportion of recreational fishing was found. For example, in Portugal, the recreational catch of
Diplodus saragus was estimated to constitute 65% of the commercial landings [
45]. Taken together, recreational catch estimates compared to commercial catch in Israel appear to be within the range found in other Mediterranean countries.
We found that the catch of recreational angling from shore represents 15–18% of the annual biomass extracted yearly across all fishing methods. Recreational fishing at sea represents a more variable estimate, with the higher end estimated values exceeding those of angling from the coast (4–27%). The reason for such high estimates for recreational fishing at sea (up to 27%) compared to shore could be explained by each species’ method targets [
6,
10,
46]. For example, anglers from the coast may target smaller species with lower biomass than fishers at sea who target large species, and therefore may reach higher catch [
19]. The wide variability in estimating recreational catch at sea is mainly due to the challenge of documenting these activities compared to the anglers fishing from the coast [
45]. This variation in estimated yields illustrates the importance of documenting fishing activities to the lowest resolution, in terms of gear and species, possible in order to fully understand the fishery impact [
4], especially where there is a variety of recreational fishing methods [
2,
19]. Without detailed documentation of the catch of specific fishing methods and species, evaluations can be partial and potentially misleading.
4.2. Catch Composition
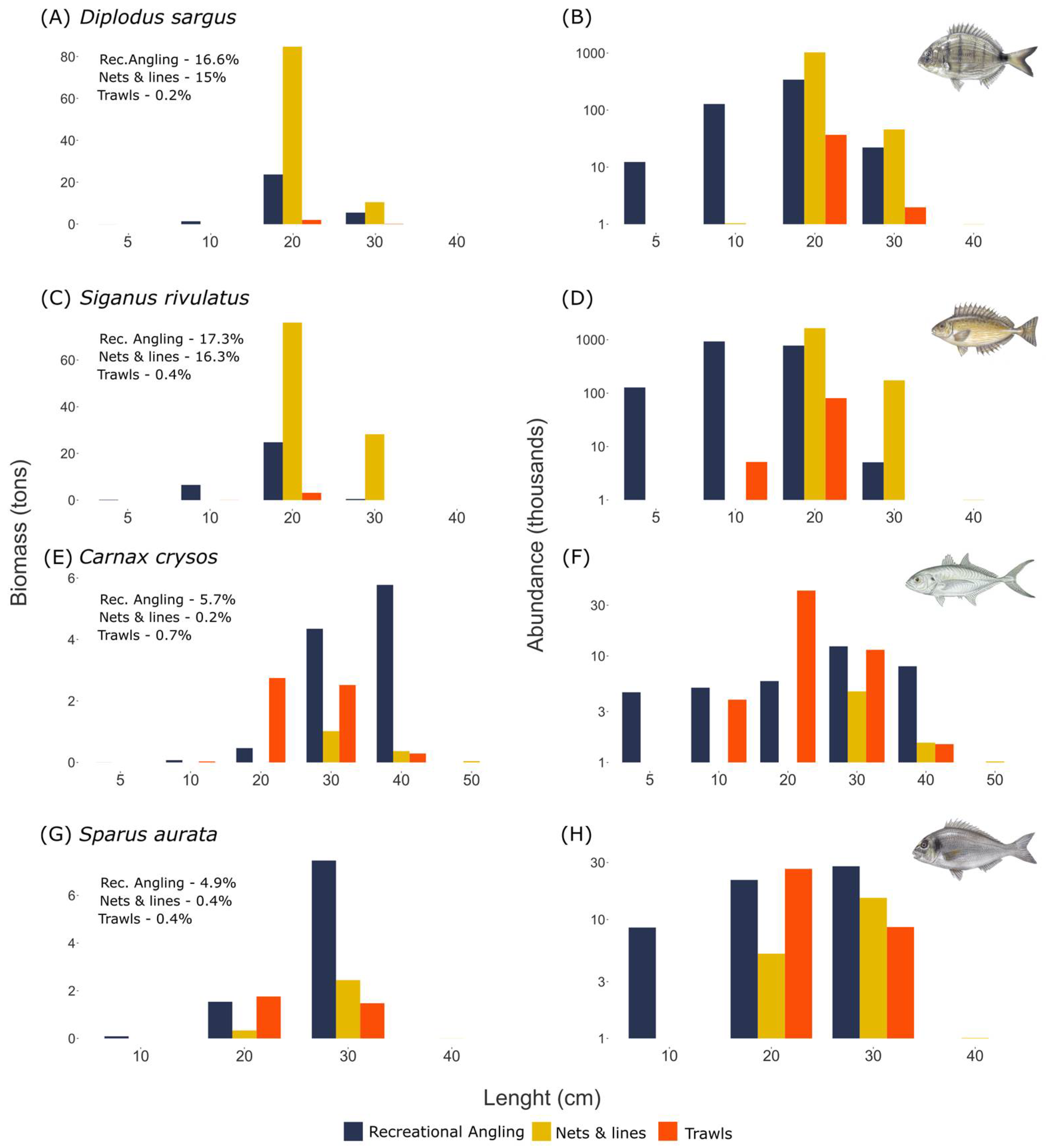
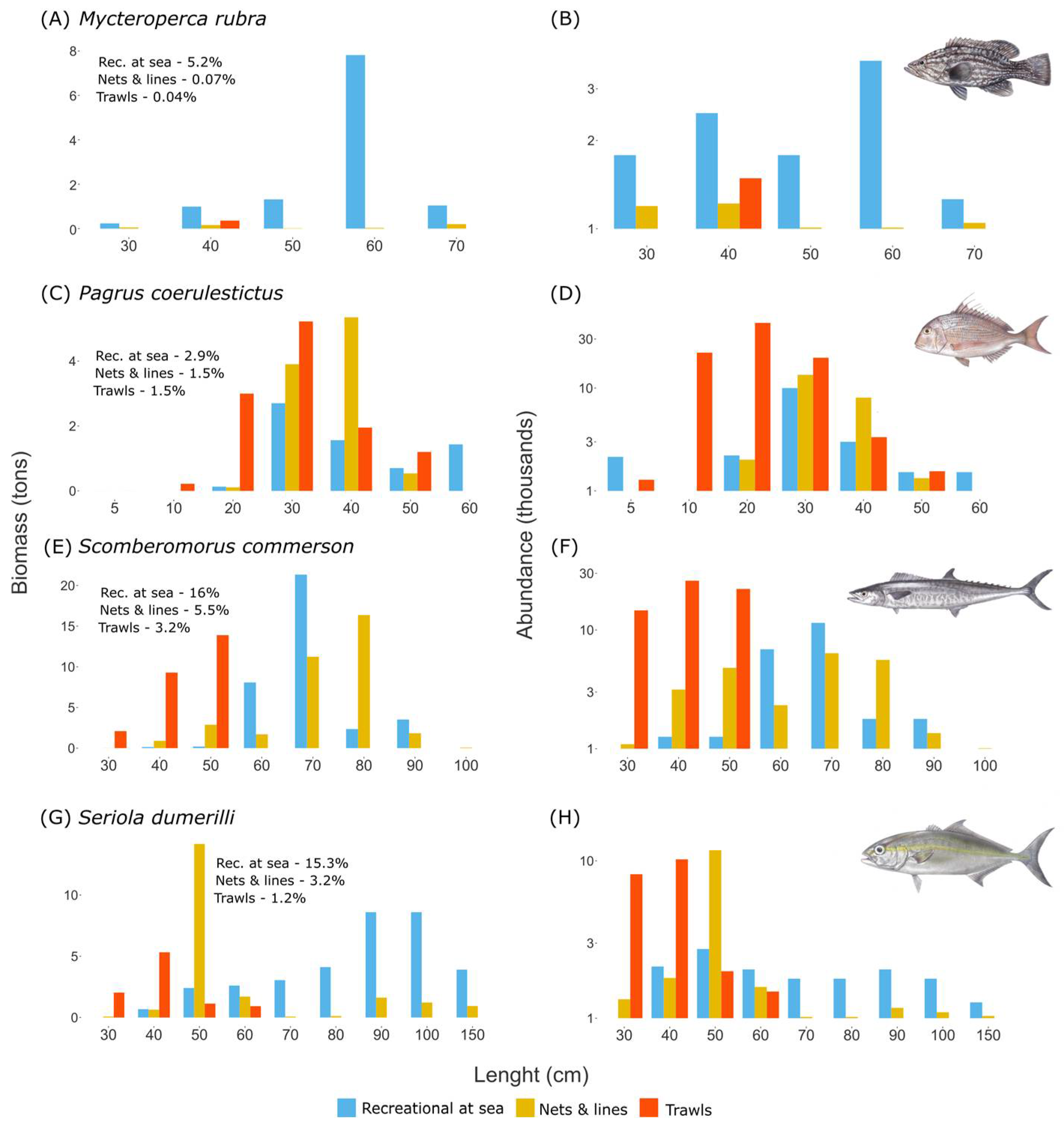
The variety of the recreational fishing sector and the multispecies character of the Mediterranean Sea lead to a large variety of caught species. We find that recreational fishing from the coast targets mostly small reef-associated species such as Diplodus sargus, Lithognathus mormyrus, and species from the Mugilidae family. Recreational fishing at sea targets larger species such as the grouper Mycteroperca rubra, the Sparide Pagrus coeruleostictus, and pelagic species such as Seriola dumerili.
Interestingly, non-indigenous species have become an important part of the catch within all recreational methods examined. Some species such as
Scomberomorus commerson,
Siganus rivulatus, and
Nemipterus randelli are heavily fished both in recreational and commercial fishing. The presence of non-indigenous species in the catch had already been shown to have a positive effect on the trawl industry in Israel [
31]. However, some of the non-indigenous species such as
Siganus rivulatus are known to have additional disastrous ecological impacts by removing algae cover from reef meadows [
27]. Other species such as
Lagocephalus sceleratus (the most abundant species in the recreational fishing at sea) are less favored by fishers as they are poisonous and cannot be consumed or sold. This species also causes damage to fishing gears of both recreational and commercial set nets and long lines, by cutting nets and lines, and by eating fish already caught in the fishing gear [
47]. Overall, non-indigenous species have become an integral part of recreational fishing. This study thus provides a baseline against which to compare future changes to local fish biodiversity.
4.3. Comparing Recreational and Commercial Fishing
We find that species composition of recreational fishing at sea overlaps with set nets and long lines. However, recreational fishing at sea targets a wider size range with a focus on larger sizes compared to set nets and long lines. Thus, the total annual biomass of species such as
Seriola dumerili and the non-indigenous
Scomberomorus commerson, may be equal, or even exceed the catch of the commercial methods. In addition, the catch of large high-quality spawners from species such as
Seriola dumerili and
Mycteroperca rubra through recreational fishing at sea can also damage the overall stock of the species [
48]. For example, the individuals
Seriola dumerili, that are larger than 100 cm (length at first maturity of this species is at 99.5 cm), represent 17% of all the individuals caught in recreational fishing at sea. However, individuals of the same size represent only 1% of all the individuals caught in the set nets and long line fishery. We also show that recreational fishing at sea and set nets and long lines have a common seasonal peak at the end of spring and the beginning of summer (May–June), that results in the catch of common seasonal species such as
Seriola dumerili and
Scomberomorus commerson. Similar seasonality increases the potential conflict between recreational fishing at sea, and set nets and long lines.
Trawl fishery has a low overlap in terms of species composition with both angling from the coast and recreational fishing at sea. However, for some species, such as Caranx crysos and Sparus aurata, that represent only a small fraction of the trawl catch (0.4% and 0.7%, respectively), the total biomass caught by recreation fishing and commercial trawling are comparable (annual catch of 11 tons in recreational angling and 5.6 tons in trawls for Cranx crysos, 9 tons in recreational angling compared to 3.2 tons in trawls for Sparus aurata). Additionally, the trawl industry targets many young individuals of species caught also by recreational fishing at sea, such as Scomberomorus commerson or Pagrus coeruleostictus. For example, we estimate that annually 38,600 individuals of the species Scomberomorus commerson are caught at sizes smaller than the size at first maturity (50 cm) by the trawl industry compared to only 260 individuals caught annually at the same size by the recreational fishing at sea.
Similar conflicts between fishing sectors were found in other Mediterranean countries [
8,
16,
21,
43,
48]. Some of these conflicts stem from recreational fishing targeting high trophic level predators, leading to a large effect on the food web as a whole [
15]. Besides the ecological aspect, this conflict between small-scale commercial fishing and recreational fishers manifests itself economically, as recreational fishers often illegally sell their catch. According to Ben Lamine et al. 2018, up to 47.91% of non-professional fishers admitted to selling their catch. Our study shows that in Israel, most potential ecological conflict is between recreational fishing at sea, and set nets and long lines. This overlap supports the necessity to include recreational data in stock assessments and ecosystem management of the coastal areas [
21]. On the other hand, we find that angling from the coast, although it may extract substantial annual biomass, has low overlap with commercial fishing methods. Overall, this study illustrates the importance of estimating species-level catch for all fishing methods in order to evaluate and compare fishing impacts.
4.4. Caveats
The coastal surveys were effective for documenting angling activity from the shore. During the two years of surveys, we managed to cover a significant part of the Israeli coastline: 120 km out of the 145 km available for fishing. The amount of data collected enabled us to perform advanced statistical models to predict the annual catch with a high confidence. In contrast, tracking the catch of active recreational fishers at sea (i.e., angling from a boat or a kayak and spear fishing) is almost impossible from the coast, mainly due to these fishing techniques being sporadic and the fishers’ individualistic character. Thus, in this study, we combined several different methods to evaluate the number and catch of recreational fishers at sea: (1) aerial survey, (2) registered fishing licenses, and (3) phone surveys. Each one of the methods has assumptions and limitations.
To estimate catch composition, we used phone surveys. There are a few potential problems with this method of data collection. First, as opposed to the coastal surveys where the data was collected by surveyors in the field, phone surveys are exposed to a reporting bias where the fishers may not be accurately describing their catch in terms of species and biomass [
8,
10,
17]. Additionally, we reached fishers via personal communications, meaning one fisher led to another. This strategy may result in a bias caused by interviewing a particular group of fishers with similar expertise, and may not necessarily be a representative of the catch by fishers with different experience and background. We also could not obtain data regarding illegal recreational fishing (professional recreational fishers who sell their catch), which may include a substantial part of this sector [
43]. However, despite some potential bias, this method is commonly used in recreational fishing surveys [
8,
10,
17].
Aerial survey is an expensive but useful method quantifying recreational activity [
45]. When comparing the predictions made using the number of licensed fishers to the ones made by the aerial survey, we see that the estimated annual yields are considerably lower for the aerial surveys. Even though in Israel recreational fishing license and registration is mandatory, not all registered fishers are necessarily active, and evaluating the number of active fishers per day is a challenging task. The aerial surveys provide a real snapshot of the active fishers at the present time, and thus may more accurately describe the actual activity. However, fishing activities may occur during the entire day and even at night; thus, there may be a higher turnover rate than we estimated, which is hard to quantify.
4.5. Monitoring Fishing Activities in Israel
Our study provides information on recreational fisher habits, which can assist in monitoring and coastal spatial planning. For example, we found that anglers prefer to fish on artificial substrates in hot spots over marinas, fishing ports, and wave breakers where the number of fishers per survey was the highest. Fishers do not occur in high densities in MPAs, as, in many cases, it is more difficult to approach comfortable fishing locations.
Monitoring should be extended to include regularly collected long term data. This can reveal changes in catch composition and length distribution of the catch, and expose changes associated with climate change or environmental extremes. An update in the Israeli fishing regulations occurred in 2016, and since 2018 these regulations are more regularly enforced by the Israel Nature and Parks Authority (INPA). A team of 15 marine rangers are responsible for implementing fishing regulations, such as a 5 kg/two big fish bag limits, and seasonal restrictions. This may induce long term changes on the impacts of recreational fishers.
Additional monitoring could be based on ranger documentation of fishing activities at the open sea. As opposed to other studies on recreational fishing that are made from land, rangers are regularly present at the open sea. Thus, they can document recreational fisher activity patterns, and approach fishing boats and kayaks and document their catch. For recreational fishing to be managed correctly, a systematic sampling plan should be made for rangers to collect data for monitoring.