The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Threshold Panel Data Model
3. Results
3.1. Preliminary Analysis
3.2. Summary Descriptive Statistics
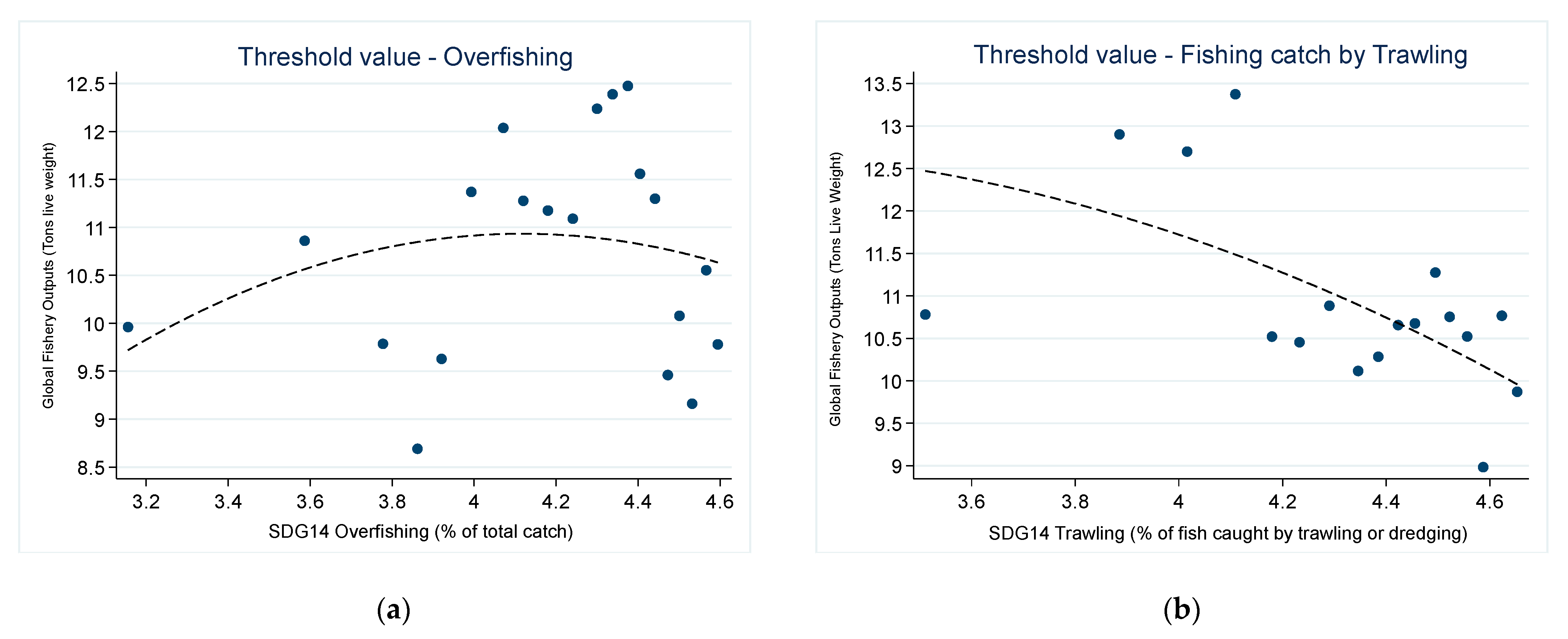
3.3. The Threshold Effect of Overfishing on Global Fishery Outputs
3.4. Additional Analysis by Controling for the Country GDP Growth and Property Rate
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Definition of Variables
| Indicators | Definition | Measurement unit | Sources |
|---|---|---|---|
| Dependent variable | |||
| GFO | Global fishing outputs is the cultivation of aquatic organisms such as fish, mollusks, aquatic plants, and crabs. Farming requires specific rearing process interventions to improve production, such as frequent stocking, feeding, predator protection, etc., which pertain specifically to aquaculture products meant for final harvest for consumption. | Tons live weight | Food and Agriculture Organization of the United Nations (FAO) https://www.fao.org/fishery/en/statistics, accessed on 17 August 2022 |
| Threshold variables | |||
| SDG14 Overfishing | Fish grabbed from overfishing or tumbled stocks | % of total catch | Sustainable development reporting https://dashboards.sdgindex.org/rankings, accessed on 17 August 2022 |
| SDG14 Trawling | Fish grabbed by trawling or dredging | % of fish caught by trawling | |
| Countries control variables | |||
| NFV | The global number of fishing vessels is the number of boats or ships used to catch fish in the sea, on a lake, etc. | Number of motorized vessels propelled by engines | Food and Agriculture Organization of the United Nations (FAO) https://www.fao.org/fishery/en/statistics, accessed on 17 August 2022 |
| NF | Global number of fishers is the total number of people who work full-time in the commercial fishing industry in freshwater, brackish water, and marine habitats to capture and land all aquatic creatures and plants. | Number of fishers | |
| HIC | High income | Dummy variable | |
| LMIC | Lower-middle income | Dummy variable | |
| UMIC | Upper-middle income | Dummy variable | |
| Technological progress (Time trend) | Time takes values from 1 to 20 for years between 2001 and 2020: 2001 = 1, 2002 = 2, … 2020 = 20. | Number | |
| GDP growth rate | Gross domestic product annual growth rate | Annual percentage | World Development Indicators, The World Bank https://databank.worldbank.org/source/world-development-indicators, accessed on 17 August 2022 |
| Poverty rate | Proportion of poverty headcount at national poverty lines | Percentage of population | |
Appendix B. The Groups of Species of Global Fishery Outputs by Continent (Measurement Unit: Tons Live Weight)
| Continent | ASFIS Species | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | Aquatic Animals NEI | - | - | - | - | - | 1 | 1 | 1 | 1 | 1 |
| Africa | Aquatic Plants | 83,592 | 117,188 | 105,561 | 79,492 | 81,158 | 88,530 | 96,267 | 119,311 | 114,132 | 138,329 |
| Africa | Crustaceans | 32 | 32 | 15 | 10 | 12 | 7 | 8 | 12 | 12 | 4 |
| Africa | Crustaceans | 5824 | 8411 | 8570 | 7884 | 11,919 | 10,587 | 9644 | 9242 | 3968 | 5701 |
| Africa | Demersal Marine Fish | 100,753 | 118,054 | 142,292 | 139,912 | 168,590 | 234,809 | 256,915 | 223,014 | 227,051 | 164,650 |
| Africa | Freshwater and Diadromous Fish | 297,489 | 324,675 | 366,077 | 408,994 | 463,477 | 506,358 | 550,954 | 706,978 | 754,869 | 1,112,276 |
| Africa | Marine Fish NEI | 43 | 17 | 16 | 15 | 23 | 17 | 1 | 11 | 35 | 18 |
| Africa | Molluscs excl. Cephalopods | 1533 | 1696 | 1921 | 2049 | 2074 | 2332 | 2662 | 2812 | 2775 | 3034 |
| Africa | Pelagic Marine Fish | 1 | 9 | 152 | 91 | 366 | 604 | 517 | 613 | 380 | 373 |
| Americas | Aquatic Animals NEI | 693 | 735 | 728 | 728 | 797 | 820 | 746 | 949 | 968 | 871 |
| Americas | Aquatic Plants | 65,608 | 71,829 | 40,104 | 20,283 | 15,512 | 38,234 | 26,402 | 28,038 | 88,733 | 12,932 |
| Americas | Crustaceans | 206,581 | 272,388 | 340,109 | 367,022 | 422,576 | 499,748 | 503,061 | 530,940 | 525,049 | 560,834 |
| Americas | Demersal Marine Fish | 2244 | 2287 | 656 | 1805 | 2223 | 2214 | 2609 | 2197 | 2323 | 1967 |
| Americas | Freshwater and Diadromous Fish | 1,250,587 | 1,286,977 | 1,311,579 | 1,375,873 | 1,422,785 | 1,476,098 | 1,441,335 | 1,500,145 | 1,516,139 | 1,420,627 |
| Americas | Marine Fish NEI | 25 | 24 | 5 | 9 | - | - | 1 | 5 | 8 | 2 |
| Americas | Molluscs excl. Cephalopods | 239,211 | 238,282 | 282,194 | 393,052 | 323,835 | 384,666 | 408,607 | 431,474 | 417,776 | 527,056 |
| Americas | Pelagic Marine Fish | 532 | 541 | 570 | 4261 | 4640 | 5004 | 3752 | 4095 | 3898 | 3269 |
| Asia | Aquatic Animals NEI | 182,666 | 186,754 | 331,465 | 375,808 | 425,463 | 464,650 | 505,831 | 614,948 | 720,963 | 790,435 |
| Asia | Aquatic Plants | 10,781,141 | 11,691,332 | 12,464,506 | 13,773,982 | 14,726,157 | 15,513,891 | 16,212,167 | 17,109,776 | 18,444,553 | 20,008,197 |
| Asia | Crustaceans | 1,759,554 | 1,929,317 | 2,648,350 | 3,007,946 | 3,337,173 | 3,833,752 | 4,279,664 | 4,470,283 | 4,756,460 | 4,905,196 |
| Asia | Demersal Marine Fish | 182,352 | 211,239 | 529,909 | 572,340 | 634,916 | 666,357 | 704,565 | 726,825 | 742,121 | 734,873 |
| Asia | Freshwater and Diadromous Fish | 18,314,993 | 19,453,023 | 19,987,314 | 21,873,479 | 23,318,699 | 24,817,123 | 26,360,428 | 28,573,708 | 29,870,489 | 31,586,011 |
| Asia | Marine Fish NEI | 477,758 | 541,287 | 219,605 | 238,503 | 279,932 | 330,340 | 323,394 | 540,757 | 436,285 | 460,565 |
| Asia | Molluscs excl. Cephalopods | 9,219,565 | 9,866,930 | 10,261,153 | 10,640,295 | 10,994,804 | 11,441,253 | 11,804,560 | 11,831,314 | 12,200,074 | 12,477,154 |
| Asia | Pelagic Marine Fish | 163,604 | 172,228 | 193,159 | 187,420 | 198,576 | 227,291 | 244,940 | 243,986 | 279,708 | 278,688 |
| Asia | Others | 35 | 32 | 2924 | 13,046 | 16,407 | 21,267 | 21,203 | 24,275 | 19,223 | 61,115 |
| Europe | Aquatic Animals NEI | 5 | - | - | 4 | - | 25 | 25 | 55 | 148 | 478 |
| Europe | Aquatic Plants | 542 | 181 | 104 | 253 | 290 | 851 | 363 | 1326 | 1870 | 2058 |
| Europe | Cephalopods | 16 | 14 | 8 | 12 | 16 | 11 | 27 | 30 | 15 | 10 |
| Europe | Crustaceans | 2725 | 2538 | 3236 | 3244 | 3278 | 3331 | 3252 | 3266 | 3379 | 3285 |
| Europe | Demersal Marine Fish | 116,212 | 108,978 | 132,664 | 121,215 | 141,221 | 155,366 | 172,614 | 182,780 | 191,260 | 193,266 |
| Europe | Freshwater and Diadromous Fish | 1,226,100 | 1,263,484 | 1,313,460 | 1,347,136 | 1,307,027 | 1,330,771 | 1,488,822 | 1,518,792 | 1,658,491 | 1,716,060 |
| Europe | Marine Fish NEI | 1991 | 1593 | 3891 | 2973 | 4012 | 4879 | 5897 | 5460 | 5892 | 5443 |
| Europe | Molluscs excl. Cephalopods | 746,304 | 666,992 | 709,370 | 697,961 | 679,485 | 696,196 | 694,292 | 615,097 | 657,376 | 604,396 |
| Europe | Pelagic Marine Fish | 1260 | 1917 | 1803 | 10,698 | 8616 | 16,751 | 17,776 | 12,543 | 10,221 | 10,231 |
| Oceania | Aquatic Animals NEI | 480 | 342 | 1134 | 941 | 2065 | 2273 | 2137 | 1892 | 1550 | 1852 |
| Oceania | Aquatic Plants | 12,514 | 5104 | 4604 | 6095 | 8139 | 11,381 | 2628 | 3202 | 6557 | 12,809 |
| Oceania | Crustaceans | 5217 | 5993 | 5712 | 6304 | 6089 | 6190 | 5544 | 5498 | 6160 | 6770 |
| Oceania | Demersal Marine Fish | 7 | 7 | 1 | 2 | 1 | 2 | - | - | - | - |
| Oceania | Freshwater and Diadromous Fish | 25,211 | 25,780 | 23,042 | 24,496 | 27,622 | 32,859 | 39,360 | 40,310 | 48,096 | 51,486 |
| Oceania | Marine Fish NEI | 190 | 159 | 1532 | 1410 | 2508 | 2462 | 3253 | 3966 | 4947 | 5649 |
| Oceania | Molluscs excl. Cephalopods | 81,096 | 92,149 | 93,267 | 102,669 | 113,025 | 115,647 | 120,658 | 120,741 | 110,933 | 116,610 |
| Oceania | Pelagic Marine Fish | 3889 | 4008 | 2373 | 4554 | 2231 | 3611 | 2139 | 4532 | 8786 | 7284 |
| Oceania | Others | 877 | 1285 | 1954 | 1849 | 2900 | 2420 | 2210 | 1903 | 1866 | 2145 |
| Africa | Aquatic Animals NEI | 1 | 1 | 1 | 1 | 25 | 37 | 101 | 63 | 63 | 60 |
| Africa | Aquatic Plants | 141,120 | 161,458 | 123,194 | 149,368 | 196,570 | 139,359 | 136,833 | 113,875 | 110,373 | 104,090 |
| Africa | Crustaceans | 6977 | 6382 | 11527 | 12339 | 3732 | 4643 | 6171 | 5581 | 4888 | 7618 |
| Africa | Demersal Marine Fish | 166,047 | 175,332 | 159,849 | 170,154 | 211,781 | 239,006 | 325,176 | 347,135 | 362,527 | 453,845 |
| Africa | Freshwater and Diadromous Fish | 1,220,024 | 1,305,182 | 1,444,882 | 1,530,740 | 1,557,634 | 1,728,811 | 1,691,354 | 1,879,181 | 1,903,076 | 1,781,492 |
| Africa | Marine Fish NEI | 19 | 15 | 17 | 1 | - | - | - | - | - | - |
| Africa | Molluscs excl. Cephalopods | 2741 | 3269 | 3903 | 4475 | 4919 | 5959 | 5527 | 5836 | 7351 | 5994 |
| Africa | Pelagic Marine Fish | 350 | 841 | 380 | 549 | 199 | 218 | - | - | - | 1194 |
| Americas | Aquatic Animals NEI | 900 | 891 | 743 | 857 | 821 | 664 | 706 | 591 | 478 | 370 |
| Americas | Aquatic Plants | 15,443 | 5029 | 13,332 | 13,641 | 12,818 | 15,727 | 17,694 | 22,244 | 28,067 | 25,315 |
| Americas | Crustaceans | 618,364 | 637,099 | 614,514 | 703,004 | 792,808 | 792,922 | 861,838 | 1,000,688 | 1,164,234 | 1,266,090 |
| Americas | Demersal Marine Fish | 1859 | 2076 | 2425 | 2130 | 2070 | 1931 | 2026 | 4286 | 4468 | 5300 |
| Americas | Freshwater and Diadromous Fish | 844,409 | 914,932 | 937,817 | 1,082,143 | 1,017,448 | 1,042,779 | 1,092,918 | 1,130,032 | 1,149,848 | 1,179,727 |
| Americas | Freshwater and Diadromous Fish | 751,734 | 910,725 | 875,063 | 1,010,734 | 990,251 | 892,436 | 1,008,046 | 1,040,804 | 1,137,781 | 1,225,123 |
| Americas | Marine Fish NEI | - | - | 601 | 714 | 619 | 611 | 628 | 1276 | 1518 | 1340 |
| Americas | Molluscs excl. Cephalopods | 552,648 | 521,578 | 546,649 | 538,136 | 464,780 | 573,990 | 609,277 | 659,382 | 727,752 | 688,077 |
| Americas | Pelagic Marine Fish | 4929 | 3247 | 8579 | 11,056 | 10,386 | 11,681 | 8571 | 11,936 | 9022 | 9206 |
| Asia | Aquatic Animals NEI | 713,393 | 777,976 | 829,036 | 828,958 | 843,550 | 906,681 | 923,813 | 914,231 | 972,685 | 1,052,345 |
| Asia | Aquatic Plants | 21,597,225 | 24,480,914 | 27,837,570 | 28,878,515 | 30,841,267 | 31,473,785 | 32,442,126 | 33,281,704 | 34,427,039 | 34,916,316 |
| Asia | Crustaceans | 5,176,715 | 5,369,903 | 5,595,283 | 6,030,841 | 6,312,175 | 6,871,655 | 7,759,255 | 8,454,936 | 9,370,474 | 9,951,148 |
| Asia | Demersal Marine Fish | 783,216 | 846,950 | 896,385 | 970,249 | 1,051,938 | 1,094,310 | 1,240,763 | 1,301,177 | 1,422,748 | 1,519,924 |
| Asia | Freshwater and Diadromous Fish | 32,715,476 | 34,944,744 | 37,387,252 | 39,060,979 | 40,836,410 | 42,593,525 | 43,736,146 | 45,211,394 | 46,419,231 | 47,260,766 |
| Asia | Marine Fish NEI | 552,319 | 560,543 | 596,918 | 653,765 | 639,088 | 676,692 | 718,286 | 724,768 | 761,000 | 858,180 |
| Asia | Molluscs excl. Cephalopods | 266,545 | 264,060 | 259,436 | 521,244 | 259,516 | 257,793 | 226,562 | 209,427 | 201,725 | 192,671 |
| Asia | Molluscs excl. Cephalopods | 12,257,179 | 12,873,857 | 13,453,885 | 13,928,542 | 14,404,018 | 15,278,828 | 15,706,153 | 15,859,689 | 15,721,931 | 16,158,709 |
| Asia | Pelagic Marine Fish | 313,580 | 350,480 | 355,587 | 326,088 | 333,196 | 342,557 | 379,443 | 386,760 | 402,389 | 390,617 |
| Asia | Others | 50,658 | 19,601 | 30,923 | 46,218 | 39,158 | 53,018 | 64,143 | 25,589 | 631 | 473 |
| Europe | Aquatic Animals NEI | 250 | 323 | 349 | 928 | 917 | 2200 | 2196 | 2827 | 5052 | 6671 |
| Europe | Aquatic Plants | 2071 | 2869 | 2678 | 2819 | 2531 | 1640 | 2032 | 5373 | 11388 | 21792 |
| Europe | Cephalopods | 3 | 5 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | - |
| Europe | Crustaceans | 3462 | 3293 | 3213 | 3275 | 3373 | 3373 | 3368 | 3444 | 3585 | 3563 |
| Europe | Demersal Marine Fish | 181,102 | 178,726 | 174,583 | 169,504 | 168,358 | 183,819 | 194,580 | 197,370 | 205,799 | 198,140 |
| Europe | Freshwater and Diadromous Fish | 1,840,173 | 2,065,497 | 2,009,110 | 2,127,983 | 2,157,742 | 2,149,894 | 2,177,825 | 2,173,721 | 2,391,459 | 2,440,403 |
| Europe | Marine Fish NEI | 2422 | 2219 | 1519 | 1229 | 1474 | 1263 | 1396 | 3124 | 1633 | 5823 |
| Europe | Molluscs excl. Cephalopods | 618,528 | 579,284 | 563,639 | 620,721 | 620,430 | 628,579 | 646,253 | 687,470 | 621,891 | 578,712 |
| Europe | Pelagic Marine Fish | 8536 | 8840 | 11,710 | 10,849 | 15,376 | 18,840 | 20,479 | 28,289 | 23,265 | 29,303 |
| Oceania | Aquatic Animals NEI | 3536 | 2841 | 3645 | 1354 | 5593 | 4772 | 3993 | 973 | 2899 | 2844 |
| Oceania | Aquatic Plants | 12,508 | 17,400 | 16,864 | 23,229 | 20,336 | 15,153 | 9,750 | 10,200 | 10,190 | 10,065 |
| Oceania | Crustaceans | 5824 | 5956 | 5613 | 5700 | 6862 | 6543 | 6434 | 6108 | 6619 | 8597 |
| Oceania | Demersal Marine Fish | 7 | 10 | 9 | 13 | 24 | 29 | 37 | 35 | 29 | 31 |
| Oceania | Freshwater and Diadromous Fish | 58,030 | 63,777 | 61,396 | 58,749 | 67,695 | 75,911 | 74,909 | 86,605 | 77,750 | 89,267 |
| Oceania | Marine Fish NEI | 3625 | 1538 | 890 | 579 | 1098 | 2018 | 2294 | 2487 | 2951 | 3068 |
| Oceania | Molluscs excl. Cephalopods | 120,789 | 104,624 | 101,947 | 114,684 | 94,316 | 111,818 | 118,272 | 101,904 | 113,966 | 116,363 |
| Oceania | Pelagic Marine Fish | 5800 | 7087 | 7486 | 7544 | 8418 | 8895 | 8100 | 8000 | 8252 | 8345 |
| Oceania | Others | 2868 | 2574 | 2610 | 1985 | 1799 | 1218 | 1210 | 1210 | 1210 | 1210 |
Appendix C. The Groups of Species of Global Fishery Outputs by World Bank Income Classification
| Country | ASFIS Species | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIC | Aquatic Animals NEI | 48,811 | 36,548 | 27,504 | 30,081 | 30,625 | 26,925 | 25,660 | 24,092 | 32,551 | 30,805 |
| HIC | Aquatic Plants | 966,192 | 1,143,999 | 982,130 | 1,060,976 | 1,146,877 | 1,299,863 | 1,342,762 | 1,413,017 | 1,408,800 | 1,352,987 |
| HIC | Cephalopods | 16 | 14 | 8 | 12 | 16 | 11 | 27 | 30 | 15 | 10 |
| HIC | Crustaceans | 52,647 | 68,312 | 87,599 | 85,636 | 91,186 | 90,787 | 102,791 | 108,862 | 100,242 | 105,987 |
| HIC | Demersal Marine Fish | 251,175 | 269,771 | 331,714 | 309,347 | 338,080 | 351,024 | 379,259 | 395,435 | 409,371 | 376,880 |
| HIC | Freshwater and Diadromous Fish | 2,369,963 | 2,399,951 | 2,466,081 | 2,525,324 | 2,494,064 | 2,556,448 | 2,651,428 | 2,670,068 | 2,755,598 | 2,681,088 |
| HIC | Marine Fish NEI | 14,426 | 14,149 | 16,816 | 14,915 | 16,366 | 17,363 | 20,717 | 20,268 | 23,766 | 26,330 |
| HIC | Molluscs excl. Cephalopods | 1,791,519 | 1,756,050 | 1,913,145 | 1,990,836 | 1,897,647 | 2,074,066 | 2,200,864 | 2,000,511 | 2,033,211 | 2,053,695 |
| HIC | Pelagic Marine Fish | 168,737 | 178,161 | 172,808 | 176,810 | 182,098 | 186,136 | 191,863 | 180,244 | 182,320 | 163,949 |
| HIC | Others | 902 | 1311 | 1985 | 1865 | 2916 | 2447 | 2237 | 1926 | 1889 | 2167 |
| LIC | Aquatic Animals NEI | - | - | - | 10 | 50 | 51 | 101 | 101 | 101 | 126 |
| LIC | Aquatic Plants | 444,995 | 446,565 | 450,325 | 446,015 | 445,765 | 449,800 | 448,690 | 448,720 | 448,200 | 449,400 |
| LIC | Crustaceans | 5411 | 7928 | 7359 | 6679 | 8214 | 9810 | 9456 | 8757 | 3789 | 4822 |
| LIC | Freshwater and Diadromous Fish | 19,374 | 23,191 | 25,504 | 26,503 | 31,808 | 54,645 | 73,458 | 75,553 | 101,041 | 121,874 |
| LIC | Molluscs excl. Cephalopods | 60,000 | 60,000 | 60,000 | 60,000 | 60,000 | 60,020 | 60,020 | 60,120 | 60,220 | 60,220 |
| LMIC | Aquatic Animals NEI | - | 25 | 40 | 42 | 96 | 116 | 114 | 490 | 28,184 | 31,240 |
| LMIC | Aquatic Plants | 1,109,060 | 1,255,327 | 1,345,709 | 1,733,369 | 2,372,211 | 2,752,820 | 3,342,265 | 3,947,178 | 4,841,142 | 5,886,379 |
| LMIC | Crustaceans | 586,820 | 648,564 | 760,193 | 893,952 | 1,036,632 | 1,118,234 | 1,096,986 | 1,165,178 | 1,145,230 | 1,126,726 |
| LMIC | Demersal Marine Fish | 115,193 | 138,286 | 162,099 | 158,087 | 187,350 | 249,381 | 269,106 | 239,061 | 245,392 | 185,150 |
| LMIC | Freshwater and Diadromous Fish | 4,675,907 | 5,030,920 | 5,552,321 | 6,478,141 | 7,083,636 | 7,713,803 | 8,447,715 | 9,925,845 | 10,281,891 | 11,744,244 |
| LMIC | Marine Fish NEI | 28,990 | 35,083 | 40,292 | 49,281 | 55,576 | 118,670 | 86,617 | 254,231 | 181,647 | 214,524 |
| LMIC | Molluscs excl. Cephalopods | 85,243 | 101,822 | 131,814 | 191,384 | 187,263 | 196,324 | 223,507 | 234,065 | 235,880 | 171,143 |
| LMIC | Pelagic Marine Fish | 16 | 17 | 170 | 98 | 262 | 528 | 950 | 2649 | 2298 | 956 |
| LMIC | Others | - | 1 | 2867 | 12,992 | 16,349 | 16,349 | 16,001 | 19,663 | 15,857 | 58,079 |
| UPMIC | Aquatic Animals NEI | 135,033 | 151,258 | 305,783 | 347,348 | 397,554 | 440,677 | 482,865 | 593,162 | 662,794 | 731,465 |
| UPMIC | Aquatic Plants | 8,423,150 | 9,039,743 | 9,836,715 | 10,639,745 | 10,866,403 | 11,150,404 | 11,204,110 | 11,452,738 | 11,957,703 | 12,485,559 |
| UPMIC | Crustaceans | 1,334,997 | 1,493,820 | 2,150,790 | 2,406,102 | 2,644,992 | 3,134,759 | 3,591,913 | 3,736,422 | 4,045,746 | 4,244,243 |
| UPMIC | Demersal Marine Fish | 35,160 | 32,451 | 311,401 | 367,557 | 421,232 | 458,067 | 488,113 | 500,107 | 507,807 | 532,554 |
| UPMIC | Freshwater and Diadromous Fish | 14,044,467 | 14,894,818 | 14,952,631 | 15,993,666 | 16,923,806 | 17,836,569 | 18,706,196 | 19,666,546 | 20,707,444 | 21,337,143 |
| UPMIC | Marine Fish NEI | 436,590 | 493,848 | 167,941 | 178,714 | 214,533 | 201,665 | 225,212 | 275,700 | 241,725 | 230,807 |
| UPMIC | Molluscs excl. Cephalopods | 8,350,940 | 8,948,175 | 9,242,944 | 9,593,783 | 9,968,309 | 10,309,682 | 10,546,385 | 10,706,741 | 11,059,622 | 11,443,192 |
| UPMIC | Pelagic Marine Fish | 532 | 521 | 25,079 | 30,114 | 32,061 | 66,588 | 76,295 | 82,848 | 118,350 | 134,924 |
| UPMIC | Others | 10 | 5 | 26 | 39 | 43 | 4891 | 5175 | 4589 | 3344 | 3015 |
| HIC | Aquatic Animals NEI | 28,640 | 24,111 | 26,583 | 27,926 | 48,658 | 65,945 | 55,691 | 60,249 | 50,739 | 51,548 |
| HIC | Aquatic Plants | 1,362,855 | 1,471,995 | 1,567,462 | 1,475,191 | 1,610,419 | 1,758,272 | 2,187,546 | 2,123,924 | 2,183,809 | 2,180,819 |
| HIC | Cephalopods | 3 | 5 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | - |
| HIC | Crustaceans | 95,620 | 86,244 | 87,359 | 112,789 | 118,834 | 131,493 | 138,867 | 162,821 | 172,917 | 160,956 |
| HIC | Demersal Marine Fish | 353,688 | 360,612 | 364,777 | 378,679 | 381,007 | 385,915 | 408,172 | 405,625 | 417,456 | 415,385 |
| HIC | Freshwater and Diadromous Fish | 2,923,440 | 3,337,750 | 3,226,558 | 3,468,081 | 3,428,471 | 3,293,062 | 3,445,749 | 3,470,853 | 3,753,319 | 3,880,933 |
| HIC | Marine Fish NEI | 22,421 | 9782 | 11,260 | 13,486 | 9249 | 10,252 | 11,436 | 13,014 | 11,976 | 16,566 |
| HIC | Molluscs excl. Cephalopods | 2,002,476 | 1,972,154 | 1,839,422 | 2,001,602 | 1,966,128 | 2,055,385 | 2,133,868 | 2,226,744 | 2,187,898 | 2,088,811 |
| HIC | Pelagic Marine Fish | 167,390 | 193,669 | 188,172 | 175,143 | 186,149 | 190,080 | 192,760 | 201,496 | 197,455 | 202,262 |
| HIC | Others | 2877 | 2593 | 2631 | 2004 | 1818 | 1238 | 1230 | 1231 | 1229 | 1226 |
| LIC | Aquatic Animals NEI | 136 | 141 | 146 | 146 | 175 | 186 | 264 | 215 | 215 | 215 |
| LIC | Aquatic Plants | 447,129 | 446,842 | 450,035 | 509,110 | 517,497 | 570,613 | 570,617 | 608,547 | 612,075 | 611,164 |
| LIC | Crustaceans | 6066 | 5096 | 5377 | 4696 | 3452 | 4139 | 5439 | 4947 | 4308 | 5273 |
| LIC | Demersal Marine Fish | 6 | 75 | 145 | 165 | 15 | 15 | 15 | 15 | 15 | 15 |
| LIC | Freshwater and Diadromous Fish | 113,084 | 131,021 | 133,449 | 148,061 | 157,777 | 164,447 | 173,820 | 167,677 | 168,143 | 195,506 |
| LIC | Marine Fish NEI | - | - | - | 65 | 65 | 65 | 80 | 120 | 120 | 120 |
| LIC | Molluscs excl. Cephalopods | 60,220 | 60,220 | 60,270 | 60,270 | 60,270 | 60,270 | 62,320 | 62,420 | 62,420 | 62,420 |
| LMIC | Aquatic Animals NEI | 5074 | 5609 | 7218 | 20735 | 7093 | 6850 | 8410 | 7269 | 14,090 | 13,224 |
| LMIC | Aquatic Plants | 7,182,520 | 8,468,204 | 11,012,765 | 11,809,920 | 13,060,792 | 12,604,842 | 12,109,910 | 11,943,968 | 11,407,845 | 11,210,375 |
| LMIC | Crustaceans | 1,377,612 | 1,386,460 | 1,751,470 | 2,016,426 | 2,142,801 | 2,338,030 | 2,775,903 | 2,844,304 | 3,187,139 | 3,436,716 |
| LMIC | Demersal Marine Fish | 183,898 | 195,679 | 188,546 | 191,449 | 236,946 | 258,227 | 396,200 | 378,561 | 389,048 | 477,291 |
| LMIC | Freshwater and Diadromous Fish | 12,326,745 | 13,860,385 | 15,189,862 | 15,926,170 | 16,705,250 | 17,899,023 | 18,964,905 | 20,590,216 | 21,756,243 | 22,060,390 |
| LMIC | Marine Fish NEI | 262,837 | 250,932 | 243,287 | 253,792 | 193,009 | 232,871 | 255,316 | 259,814 | 279,827 | 288,311 |
| LMIC | Molluscs excl. Cephalopods | 185,297 | 206,242 | 253,919 | 258,627 | 294,898 | 333,637 | 349,787 | 429,493 | 352,229 | 357,712 |
| LMIC | Pelagic Marine Fish | 1007 | 12,610 | 2571 | 3242 | 1999 | 1857 | 1704 | 1507 | 3639 | 5647 |
| LMIC | Others | 48,449 | 17,251 | 29,091 | 44,399 | 37,505 | 51,406 | 63,181 | 24,863 | - | - |
| UPMIC | Aquatic Animals NEI | 684,230 | 752,170 | 799,827 | 783,292 | 794,979 | 841,374 | 866,445 | 850,952 | 916,132 | 997,303 |
| UPMIC | Aquatic Plants | 12,775,863 | 14,280,629 | 14,963,377 | 15,273,351 | 15,884,812 | 16,711,935 | 17,740,362 | 18,756,957 | 20,383,328 | 21,075,219 |
| UPMIC | Crustaceans | 4,332,032 | 4,544,821 | 4,385,933 | 4,621,235 | 4,853,851 | 5,205,465 | 5,716,847 | 6,458,675 | 7,185,428 | 7,634,064 |
| UPMIC | Demersal Marine Fish | 594,538 | 646,550 | 679,622 | 741,559 | 816,077 | 874,821 | 958,119 | 1,065,722 | 1,188,976 | 1,284,455 |
| UPMIC | Freshwater and Diadromous Fish | 22,064,478 | 22,875,602 | 24,165,553 | 25,328,941 | 26,335,607 | 27,126,752 | 27,196,708 | 27,292,974 | 27,401,423 | 27,839,930 |
| UPMIC | Marine Fish NEI | 273,117 | 303,601 | 345,398 | 388,945 | 439,956 | 437,397 | 455,771 | 458,706 | 475,179 | 563,415 |
| UPMIC | Molluscs excl. Cephalopods | 11,570,434 | 12,108,048 | 12,775,836 | 13,407,238 | 13,526,672 | 14,407,664 | 14,765,973 | 14,804,994 | 14,792,062 | 15,231,576 |
| UPMIC | Pelagic Marine Fish | 164,792 | 164,216 | 192,999 | 177,701 | 179,428 | 190,254 | 222,128 | 231,982 | 241,834 | 230,755 |
| UPMIC | Others | 2199 | 2331 | 1812 | 1800 | 1634 | 1592 | 941 | 705 | 612 | 456 |
References
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.-P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E. Rebuilding marine life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Agricultural. Available online: http://www.fao.org/faostat/en/#data (accessed on 17 August 2022).
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8. 5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef] [PubMed]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.; Heinze, C.; Ilyina, T.; Seferian, R. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Lotze, H.K.; Tittensor, D.P.; Bryndum-Buchholz, A.; Eddy, T.D.; Cheung, W.W.; Galbraith, E.D.; Barange, M.; Barrier, N.; Bianchi, D.; Blanchard, J.L. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 12907–12912. [Google Scholar] [CrossRef]
- Cheung, W.W.L. The future of fishes and fisheries in the changing oceans. J. Fish Biol. 2018, 92, 790–803. [Google Scholar] [CrossRef]
- Holsman, K.; Haynie, A.; Hollowed, A.; Reum, J.; Aydin, K.; Hermann, A.; Cheng, W.; Faig, A.; Ianelli, J.; Kearney, K. Ecosystem-based fisheries management forestalls climate-driven collapse. Nat. Commun. 2020, 11, 4579. [Google Scholar] [CrossRef]
- McCauley, D.J.; Pinsky, M.L.; Palumbi, S.R.; Estes, J.A.; Joyce, F.H.; Warner, R.R. Marine defaunation: Animal loss in the global ocean. Science 2015, 347, 1255641. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Bianchi, G.; Gislason, H.; Graham, K.; Hill, L.; Jin, X.; Koranteng, K.; Manickchand-Heileman, S.; Paya, I.; Sainsbury, K.; Sanchez, F. Impact of fishing on size composition and diversity of demersal fish communities. J. Mar. Sci. 2000, 57, 558–571. [Google Scholar] [CrossRef]
- Jennings, S.; Blanchard, J.L. Fish abundance with no fishing: Predictions based on macroecological theory. J. Anim. Ecol. 2004, 73, 632–642. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Rome FAO: Rome, Italy, 2012. [Google Scholar]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. Res. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Tai, T.C. End overfishing and increase the resilience of the ocean to climate change. Front. Mar. Sci. 2020, 7, 523. [Google Scholar] [CrossRef]
- Sissenwine, M.; Symes, D. Reflections on the common fisheries policy. In Report to the General Directorate for Fisheries Maritime Affairs of the European Commission; European Commission: Brussels, Belgium, 2007; p. 75. [Google Scholar]
- Pauly, D.; Watson, R.; Alder, J. Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 5–12. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Skerritt, D.J.; Schuhbauer, A.; Villasante, S.; Cisneros-Montemayor, A.M.; Sinan, H.; Burnside, D.; Abdallah, P.R.; Abe, K.; Addo, K.A. WTO must ban harmful fisheries subsidies. Science 2021, 374, 544. [Google Scholar] [CrossRef]
- Agnew, D.J.; Pearce, J.; Pramod, G.; Peatman, T.; Watson, R.; Beddington, J.R.; Pitcher, T.J. Estimating the worldwide extent of illegal fishing. PloS ONE 2009, 4, e4570. [Google Scholar] [CrossRef]
- Wang, Z.; Leung, K.M.Y.; Sung, Y.-H.; Dudgeon, D.; Qiu, J.-W. Recovery of tropical marine benthos after a trawl ban demonstrates linkage between abiotic and biotic changes. Commun. Biol. 2021, 4, 212. [Google Scholar] [CrossRef]
- Jennings, S.; Dinmore, T.A.; Duplisea, D.E.; Warr, K.J.; Lancaster, J.E. Trawling disturbance can modify benthic production processes. J. Anim. Ecol. 2001, 70, 459–475. [Google Scholar] [CrossRef]
- Schuhbauer, A.; Chuenpagdee, R.; Cheung, W.W.; Greer, K.; Sumaila, U.R. How subsidies affect the economic viability of small-scale fisheries. Mar. Policy 2017, 82, 114–121. [Google Scholar] [CrossRef]
- Conrad, J.M.; Rondeau, D. Bioeconomics of a marine disease. Mar. Resour. Econ. 2015, 30, 393–416. [Google Scholar] [CrossRef]
- Welcomme, R. Principles and approaches for river fisheries management. In Management and Ecology of River Fisheries; John Wiley & Sons: New York, NY, USA, 2000; pp. 331–345. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Jensen, O.P.; Ricard, D.; Palumbi, S.R. Unexpected patterns of fisheries collapse in the world’s oceans. Proc. Natl. Acad. Sci. USA 2011, 108, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B. What was natural in the coastal oceans? Proc. Natl. Acad. Sci. USA 2001, 98, 5411–5418. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W. Role of marine mammals in aquatic ecosystems. Mar. Ecol. Prog. Ser. 1997, 158, 267–274. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Magnan, A.K.; Bopp, L.; Cheung, W.W.; Duarte, C.M.; Hinkel, J.; Mcleod, E.; Micheli, F.; Oschlies, A.; Williamson, P. Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 2018, 5, 337. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Lowndes, J.S.; Rockwood, R.C.; Selig, E.R.; Selkoe, K.A. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef]
- Le Quesne, W.J.F.; Jennings, S. Predicting species vulnerability with minimal data to support rapid risk assessment of fishing impacts on biodiversity. J. Appl. Ecol. 2012, 49, 20–28. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Tai, T.C.; Lam, V.W.; Cheung, W.W.; Bailey, M.; Cisneros-Montemayor, A.M.; Chen, O.L.; Gulati, S.S. Benefits of the Paris Agreement to ocean life, economies, and people. Sci. Adv. 2019, 5, eaau3855. [Google Scholar] [CrossRef]
- Coll, M.; Libralato, S.; Tudela, S.; Palomera, I.; Pranovi, F. Ecosystem overfishing in the ocean. PLoS ONE 2008, 3, e3881. [Google Scholar] [CrossRef]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef]
- Bailey, M.; Sumaila, U. Destructive fishing and fisheries enforcement in eastern Indonesia. Mar. Ecol. Prog. Ser. 2015, 530, 195–211. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and rebuilding of European fisheries. Mar. Policy 2018, 93, 159–170. [Google Scholar] [CrossRef]
- McGarvey, R. Demand-side fishery management: Integrating two forms of input control. Mar. Policy 2003, 27, 207–218. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; Food Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Kumar, A.B.; Deepthi, G. Trawling and by-catch: Implications on marine ecosystem. Curr. Sci. 2006, 90, 922–931. [Google Scholar]
- De Groot, S. The impact of bottom trawling on benthic fauna of the North Sea. Ocean. Manag. 1984, 9, 177–190. [Google Scholar] [CrossRef]
- Foden, J.; Rogers, S.I.; Jones, A.P. Human pressures on UK seabed habitats: A cumulative impact assessment. Mar. Ecol. Prog. Ser. 2011, 428, 33–47. [Google Scholar] [CrossRef]
- Jones, J. Environmental impact of trawling on the seabed: A review. New Zealand J. Mar. Freshw. Res. 1992, 26, 59–67. [Google Scholar] [CrossRef]
- Thrush, S.F.; Dayton, P.K. Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu. Rev. Ecol. Syst. 2002, 33, 449–473. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Jennings, S.; Sciberras, M.; Szostek, C.L.; Hughes, K.M.; Ellis, N.; Rijnsdorp, A.D.; McConnaughey, R.A.; Mazor, T.; Hilborn, R. Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proc. Natl. Acad. Sci. USA 2017, 114, 8301–8306. [Google Scholar] [CrossRef]
- Pusceddu, A.; Grémare, A.; Escoubeyrou, K.; Amouroux, J.; Fiordelmondo, C.; Danovaro, R. Impact of natural (storm) and anthropogenic (trawling) sediment resuspension on particulate organic matter in coastal environments. Cont. Shelf Res. 2005, 25, 2506–2520. [Google Scholar] [CrossRef]
- Dureuil, M.; Boerder, K.; Burnett, K.A.; Froese, R.; Worm, B.J.S. Elevated trawling inside protected areas undermines conservation outcomes in a global fishing hot spot. Science 2018, 362, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Goode, S.L.; Rowden, A.A.; Bowden, D.A.; Clark, M.R. Resilience of seamount benthic communities to trawling disturbance. Mar. Environ. Res. 2020, 161, 105086. [Google Scholar] [CrossRef] [PubMed]
- Abdulqader, E.A.; Abdurahiman, P.; Mansour, L.; Harrath, A.H.; Qurban, M.A.; Rabaoui, L. Bycatch and discards of shrimp trawling in the Saudi waters of the Arabian Gulf: Ecosystem impact assessment and implications for a sustainable fishery management. Fish. Res. 2020, 229, 105596. [Google Scholar] [CrossRef]
- Gamaza-Márquez, M.A.; Pennino, M.G.; Torres, M.A.; Acosta, J.J.; Erzini, K.; Sobrino, I. Discard practices in the gulf of Cadiz multispecies trawl fishery. Implications for the EU ‘landing obligation’. Mar. Policy 2020, 118, 104008. [Google Scholar] [CrossRef]
- Cashion, T.; Al-Abdulrazzak, D.; Belhabib, D.; Derrick, B.; Divovich, E.; Moutopoulos, D.K.; Noël, S.-L.; Palomares, M.L.D.; Teh, L.C.L.; Zeller, D.; et al. Reconstructing global marine fishing gear use: Catches and landed values by gear type and sector. Fish. Res. 2018, 206, 57–64. [Google Scholar] [CrossRef]
- Roda, P.; Gilman, E.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P. Third Assessment of Global Marine Fisheries Discards; FAO: Rome, Italy, 2019. [Google Scholar]
- Eigaard, O.R.; Bastardie, F.; Hintzen, N.T.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; Catarino, R.; Dinesen, G.E.; Egekvist, J.; Fock, H.O.; Geitner, K. The footprint of bottom trawling in European waters: Distribution, intensity, and seabed integrity. J. Mar. Sci. 2017, 74, 847–865. [Google Scholar] [CrossRef]
- Belhabib, D.; Cheung, W.W.; Kroodsma, D.; Lam, V.W.; Underwood, P.J.; Virdin, J. Catching industrial fishing incursions into inshore waters of Africa from space. Fish Fish. 2020, 21, 379–392. [Google Scholar] [CrossRef]
- de Juan, S.; Demestre, M.; Thrush, S. Defining ecological indicators of trawling disturbance when everywhere that can be fished is fished: A Mediterranean case study. Mar. Policy 2009, 33, 472–478. [Google Scholar] [CrossRef]
- Zhang, X.; Vincent, A.C.J. China’s policies on bottom trawl fisheries over seven decades (1949–2018). Mar. Policy 2020, 122, 104256. [Google Scholar] [CrossRef]
- Blanchard, C. Fragmentation in high seas fisheries: Preliminary reflections on a global oceans governance approach. Mar. Policy 2017, 84, 327–332. [Google Scholar] [CrossRef]
- Gjerde, K.M.; Dotinga, H.; Hart, S.; Molenaar, E.J.; Rayfuse, R.; Warner, R. Regulatory and Governance Gaps in the International Regime for the Conservation and Sustainable Use of Marine Biodiversity in Areas beyond National Jurisdiction; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Haward, M.G.; Vince, J. Oceans Governance in the Twenty-First Century: Managing the Blue Planet; Edward Elgar Publishing: Cheltenham, UK, 2008. [Google Scholar]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B. Ecosystem-based fishery management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Cormier, R.; Elliott, M. SMART marine goals, targets and management—Is SDG 14 operational or aspirational, is ‘Life Below Water’ sinking or swimming? Mar. Pollut. Bull. 2017, 123, 28–33. [Google Scholar] [CrossRef]
- Link, J.S.; Browman, H.I. Operationalizing and implementing ecosystem-based management. ICES J. Mar. Sci. 2017, 74, 379–381. [Google Scholar] [CrossRef]
- Sardà, R.; O’Higgins, T.; Cormier, R.; Diedrich, A.; Tintoré, J. A proposed ecosystem-based management system for marine waters: Linking the theory of environmental policy to the practice of environmental management. Ecol. Soc. 2014, 19, 51. [Google Scholar] [CrossRef]
- Long, R.D.; Charles, A.; Stephenson, R.L. Key principles of ecosystem-based management: The fishermen’s perspective. Fish Fish. 2017, 18, 244–253. [Google Scholar] [CrossRef]
- Hughes, T.P.; Bellwood, D.R.; Folke, C.; Steneck, R.S.; Wilson, J. New paradigms for supporting the resilience of marine ecosystems. Trends Ecol. Evol. 2005, 20, 380–386. [Google Scholar] [CrossRef]
- Clark, C.W.; Munro, G.R.; Sumaila, U.R. Subsidies, buybacks, and sustainable fisheries. J. Environ. Econ. Manag. 2005, 50, 47–58. [Google Scholar] [CrossRef]
- Bank, W. The Sunken Billions: The Economic Justification for Fisheries Reform; The World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Sumaila, U.R.; Lam, V.; Le Manach, F.; Swartz, W.; Pauly, D. Global fisheries subsidies: An updated estimate. Mar. Policy 2016, 69, 189–193. [Google Scholar] [CrossRef]
- Da-Rocha, J.-M.; García-Cutrín, J.; Prellezo, R.; Sempere, J. The social cost of fishery subsidy reforms. Mar. Policy 2017, 83, 236–242. [Google Scholar] [CrossRef]
- Cisneros-Montemayor, A.M.; Sanjurjo, E.; Munro, G.R.; Hernández-Trejo, V.; Rashid Sumaila, U. Strategies and rationale for fishery subsidy reform. Mar. Policy 2016, 69, 229–236. [Google Scholar] [CrossRef]
- Morgan, R. An investigation of constraints upon fisheries diversification using the Analytic Hierarchy Process (AHP). Mar. Policy 2017, 86, 24–30. [Google Scholar] [CrossRef]
- Law, S.H.; Azman-Saini, W.N.W.; Ibrahim, M.H. Institutional quality thresholds and the finance—Growth nexus. J. Bank. Financ. 2013, 37, 5373–5381. [Google Scholar] [CrossRef]
- Hansen, B.E. Inference When a Nuisance Parameter Is Not Identified Under the Null Hypothesis. Econometrica 1996, 64, 413–430. [Google Scholar] [CrossRef]
- Hansen, B.E. Sample Splitting and Threshold Estimation. Econometrica 2000, 68, 575–603. [Google Scholar] [CrossRef]
- Pradhan, T.; Chaudhuri, K.S. A dynamic reaction model of a two-species fishery with taxation as a control instrument: A capital theoretic analysis. Ecol. Model. 1999, 121, 1–16. [Google Scholar] [CrossRef]
- Pradhan, T.; Chaudhuri, K.S. Bioeconomic harvesting of a schooling fish species: A dynamic reaction model. Korean J. Comput. Appl. Math. 1999, 6, 127–141. [Google Scholar] [CrossRef]
- Clark, C.W. Bioeconomic Modelling of Fisheries Management; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Milazzo, M. Subsidies in World Fisheries: A Reexamination; World Bank Publications: Washington, DC, USA, 1998; Volume 23. [Google Scholar]
- Tao, L.S.R.; Lui, K.K.Y.; Lau, E.T.C.; Ho, K.K.Y.; Mak, Y.K.Y.; Sadovy de Mitcheson, Y.; Leung, K.M.Y. Trawl ban in a heavily exploited marine environment: Responses in population dynamics of four stomatopod species. Sci. Rep. 2018, 8, 17876. [Google Scholar] [CrossRef]
- Pipitone, C.; Badalamenti, F.; D’Anna, G.; Patti, B. Fish biomass increase after a four-year trawl ban in the Gulf of Castellammare (NW Sicily, Mediterranean Sea). Fish. Res. 2000, 48, 23–30. [Google Scholar] [CrossRef]
- Fiorentino, F.; Badalamenti, F.; D’anna, G.; Garofalo, G.; Gianguzza, P.; Gristina, M.; Pipitone, C.; Rizzo, P.; Fortibuoni, T. Changes in spawning-stock structure and recruitment pattern of red mullet, Mullus barbatus, after a trawl ban in the Gulf of Castellammare (central Mediterranean Sea). J. Mar. Sci. 2008, 65, 1175–1183. [Google Scholar] [CrossRef]
- Anticamara, J.A.; Go, K.T. Spatio-temporal declines in Philippine fisheries and its implications to coastal municipal fishers’ catch and income. Front. Mar. Sci. 2016, 3, 21. [Google Scholar] [CrossRef]
- Srinivasan, U.T.; Cheung, W.W.L.; Watson, R.; Sumaila, U.R. Food security implications of global marine catch losses due to overfishing. J. Bioeconomics 2010, 12, 183–200. [Google Scholar] [CrossRef]
- Beddington, J.R.; Agnew, D.J.; Clark, C.W. Current problems in the management of marine fisheries. Science 2007, 316, 1713–1716. [Google Scholar] [CrossRef]
- Purcell, S.W.; Mercier, A.; Conand, C.; Hamel, J.-F.; Toral-Granda, M.V.; Lovatelli, A.; Uthicke, S. Sea cucumber fisheries: Global analysis of stocks, management measures and drivers of overfishing. Fish Fish. 2013, 14, 34–59. [Google Scholar] [CrossRef]
- Brashares, J.S.; Abrahms, B.; Fiorella, K.J.; Golden, C.D.; Hojnowski, C.E.; Marsh, R.A.; McCauley, D.J.; Nuñez, T.A.; Seto, K.; Withey, L. Wildlife decline and social conflict. Science 2014, 345, 376–378. [Google Scholar] [CrossRef]
- Greene, C.H.; Meyer-Gutbrod, E.L.; McGarry, L.P.; Hufnagle Jr, L.C.; Chu, D.; McClatchie, S.; Packer, A.; Jung, J.-B.; Acker, T.; Dorn, H. A wave glider approach to fisheries acoustics: Transforming how we monitor the nation’s commercial fisheries in the 21st century. Oceanography 2014, 27, 168–174. [Google Scholar] [CrossRef]
- Fujita, R.; Cusack, C.; Karasik, R.; Takade-Heumacher, H.; Baker, C. Technologies for Improving Fisheries Monitoring; Environmental Defense Fund: San Francisco, CA, USA, 2018; pp. 1–71. [Google Scholar]
- Punt, A.E.; Ralston, S. A management strategy evaluation of rebuilding revision rules for overfished rockfish stocks. In Proceedings of the 2005 Lowell Wakefield Symposium-Biology, Assessment, and Management of North Pacific Rockfishes, University of Alaska Fairbanks, Anchorage, AK, USA, 13–15 September 2005; pp. 329–351. [Google Scholar]
- Mohn, R.; Chouinard, G. Harvest control rules for stocks displaying dynamic production regimes. ICES J. Mar. Sci. 2007, 64, 693–697. [Google Scholar] [CrossRef]
- Johnson, J.; Allain, V.; Bell, J.; Lehodey, P.; Nicol, S.; Senina, I. Impacts of climate change on oceanic fisheries relevant to the Pacific Islands. Pac. Mar. Clim. Change Rep. Card: Sci. Rev. 2018, 2018, 177–188. [Google Scholar]
- Pinsky, M.L.; Reygondeau, G.; Caddell, R.; Palacios-Abrantes, J.; Spijkers, J.; Cheung, W.W. Preparing ocean governance for species on the move. Science 2018, 360, 1189–1191. [Google Scholar] [CrossRef]
- Richards, M. From Disaster to Sustainability: The Story of the Pacific Groundfish; All Graduate Plan B and other Reports; Utah State University: Logan, UT, USA, 2017. [Google Scholar]
- Shen, C.; Chen, T. Impact of fuel subsidies on bottom trawl fishery operation in China. Mar. Policy 2022, 138, 104977. [Google Scholar] [CrossRef]
- Chai, P.; Hu, Q.; Wei, X. Influence of Fishery Subsidies on Fishing: Empirical Test Based on China’s Provincial Panel Data. Fishes 2021, 6, 40. [Google Scholar] [CrossRef]
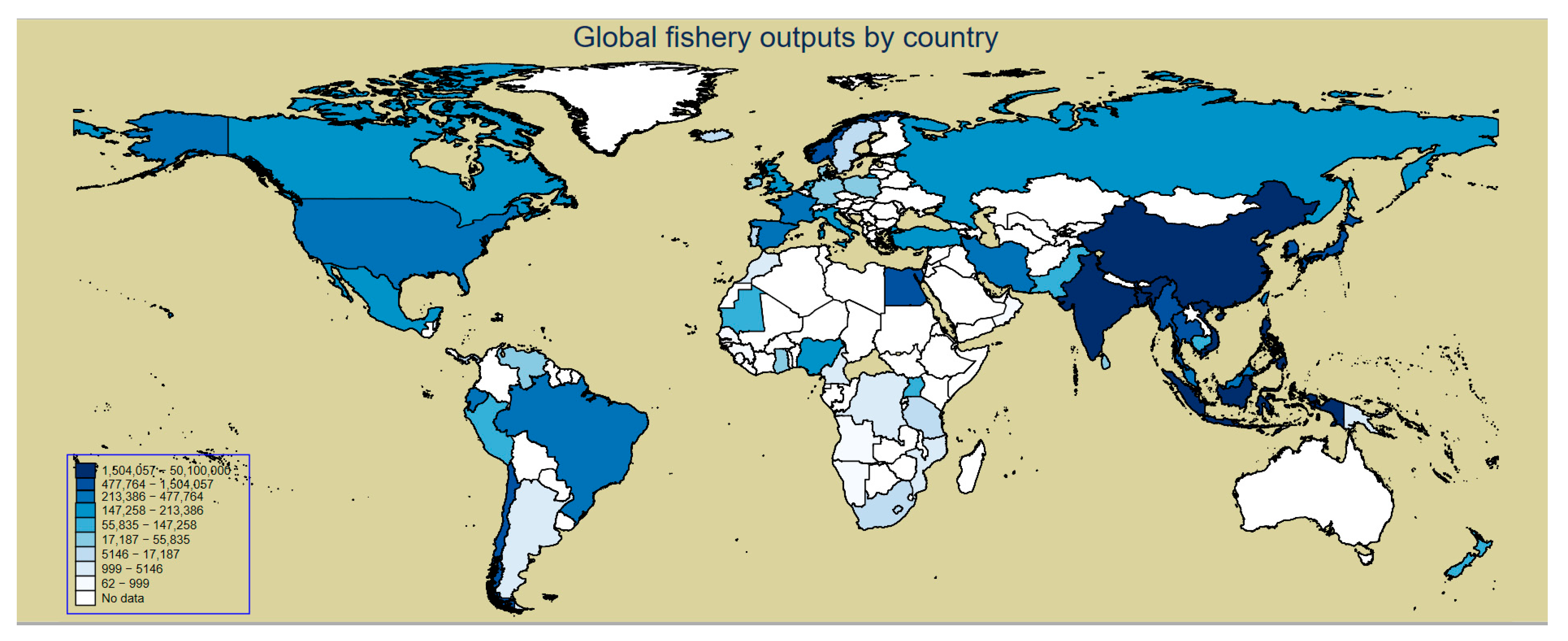
| Panel A: The mean value of variables by year. | |||||||||||||||||
| Year | Observations | Global Fishery Outputs | Overfishing | Trawling | NFV (Number of Fishing Vessels) | NF (Number of Fishers) | GDP Growth Rate | Poverty Rate | |||||||||
| 2001 | 62 | 716,897 | 74.33 | 80.18 | 39,653 | 527,542 | 47.67 | 18.07 | |||||||||
| 2002 | 62 | 766,203 | 74.29 | 79.81 | 40,292 | 528,153 | 48.79 | 18.02 | |||||||||
| 2003 | 62 | 812,125 | 73.33 | 80.74 | 40,124 | 537,879 | 49.20 | 17.68 | |||||||||
| 2004 | 62 | 881,533 | 71.94 | 80.77 | 38,535 | 557,982 | 50.77 | 17.23 | |||||||||
| 2005 | 62 | 935,467 | 70.31 | 81.88 | 40,880 | 541,681 | 50.18 | 16.30 | |||||||||
| 2006 | 62 | 995,938 | 67.93 | 80.98 | 40,030 | 564,652 | 50.87 | 16.02 | |||||||||
| 2007 | 62 | 1,048,080 | 66.17 | 81.49 | 40,641 | 530,685 | 50.71 | 15.61 | |||||||||
| 2008 | 62 | 1,109,958 | 65.92 | 81.28 | 41,920 | 543,191 | 48.49 | 14.92 | |||||||||
| 2009 | 62 | 1,168,047 | 65.57 | 81.42 | 42,913 | 558,465 | 45.21 | 14.16 | |||||||||
| 2010 | 62 | 1,234,563 | 65.22 | 81.57 | 41,097 | 571,106 | 49.76 | 13.17 | |||||||||
| 2011 | 62 | 1,292,839 | 65.76 | 82.09 | 41,275 | 577,512 | 49.25 | 12.87 | |||||||||
| 2012 | 62 | 1,397,460 | 65.65 | 82.94 | 40,776 | 563,890 | 48.73 | 11.30 | |||||||||
| 2013 | 62 | 1,505,387 | 64.97 | 81.64 | 38,495 | 561,428 | 48.67 | 11.13 | |||||||||
| 2014 | 62 | 1,578,629 | 65.77 | 82.22 | 42,363 | 579,659 | 48.72 | 11.31 | |||||||||
| 2015 | 62 | 1,647,904 | 68.16 | 83.04 | 41,624 | 579,574 | 48.25 | 10.64 | |||||||||
| 2016 | 62 | 1,715,094 | 69.02 | 82.19 | 41,379 | 576,410 | 48.06 | 10.96 | |||||||||
| 2017 | 62 | 1,778,825 | 69.82 | 81.87 | 41,824 | 577,776 | 48.60 | 10.49 | |||||||||
| 2018 | 62 | 1,836,535 | 70.07 | 81.75 | 40,657 | 576,055 | 48.20 | 9.75 | |||||||||
| 2019 | 62 | 1,897,315 | 69.24 | 81.69 | 39,214 | 572,649 | 47.70 | 10.15 | |||||||||
| 2020 | 62 | 1,941,143 | 68.92 | 81.77 | 39,721 | 559,559 | 41.49 | 9.92 | |||||||||
| Panel B: The mean value of variables by world region. | |||||||||||||||||
| Region | Observations | Global Fishery Outputs | Overfishing | Trawling | NFV (Number of Fishing Vessels) | NF (Number of Fishers) | GDP Growth Rate | Poverty Rate | |||||||||
| Africa | 280 | 29,353 | 73.61 | 87.90 | 9150 | 191,327 | 50.92 | 39.15 | |||||||||
| Europe and Central Asia | 40 | 74,129 | 60.04 | 99.91 | 1004 | 85,834 | 50.13 | 5.42 | |||||||||
| South East Asia | 260 | 5,535,225 | 81.11 | 72.97 | 120,028 | 2,199,454 | 51.71 | 12.15 | |||||||||
| Latin America and the Caribbean (LAC) | 140 | 120,545 | 64.97 | 95.24 | 13,525 | 157,712 | 48.77 | 6.83 | |||||||||
| The Middle East and North Africa (MENA) | 80 | 297,380 | 81.30 | 72.63 | 29,505 | 211,453 | 49.49 | 4.08 | |||||||||
| OECD | 420 | 326,311 | 56.35 | 76.95 | 29,423 | 49,295 | 44.28 | 0.68 | |||||||||
| Oceania | 20 | 2829 | 86.03 | 105.00 | 514 | 248,000 | 50.09 | 40.90 | |||||||||
| Note: GFO is the global fishing outputs (tons live weight); Overfishing is the fish caught from overexploited or collapsed stocks (% of the total catch); Trawling is the fish caught by trawling or dredging (% of fish caught by trawling); NFV is the global number of fishing vessels (number of motorized vessels propelled by engines); NF is the global number of fishers (number); GDP growth rate is the gross domestic product annual growth rate (annual %); Poverty rate is the proportion of poverty headcount at national poverty lines (% of population). All variables are reported clearly in Appendix A. | |||||||||||||||||
| Panel C: The mean values of variables by country. | |||||||||||||||||
| Country | Observations | Global Fishery Outputs | Over-Fishing | Trawling | NFV (Number of Fishing Vessels) | NF (Number of Fishers) | GDP Growth Rate | Poverty Rate | Country | Observations. | Global Fishery Outputs | Over-Fishing | Trawling | NFV (Number of Fishing Vessels) | NF (Number of Fishers) | GDP Growth Rate | Poverty Rate |
| Angola | 20 | 553 | 88.52 | 68.65 | 2467 | 84,676 | 51.17 | 17.08 | Mozambique | 20 | 1735 | 83.66 | 105.00 | 1096 | 224,555 | 52.49 | 70.95 |
| Argentina | 20 | 2727 | 58.79 | 53.11 | 1490 | 19,887 | 47.61 | 2.34 | Myanmar | 20 | 748,168 | 85.57 | 54.63 | 15,090 | 2,909,199 | 55.47 | 3.47 |
| Bangladesh | 20 | 1,525,550 | 98.27 | 90.67 | 27,206 | 1,500,570 | 52.07 | 19.35 | Namibia | 20 | 426 | 75.59 | 68.42 | 218 | 19,906 | 49.27 | 22.60 |
| Belize | 20 | 5147 | 44.69 | 104.95 | 586 | 3074 | 48.08 | 5.51 | Netherlands | 20 | 55,836 | 48.22 | 75.79 | 840 | 2526 | 47.24 | 0.10 |
| Brazil | 20 | 419,035 | 81.80 | 91.02 | 31,591 | 848,764 | 48.09 | 6.83 | New Zealand | 20 | 103,553 | 51.07 | 58.47 | 1420 | 1847 | 48.71 | 0.15 |
| Cambodia | 20 | 110,637 | 69.95 | 33.11 | 56,417 | 501,575 | 53.17 | 33.95 | Nigeria | 20 | 184,074 | 85.72 | 96.20 | 36,779 | 1,122,825 | 51.40 | 44.92 |
| Cameroon | 20 | 1235 | 48.25 | 96.41 | 8669 | 173,570 | 50.10 | 25.99 | Norway | 20 | 1,034,438 | 76.66 | 82.06 | 6619 | 12,746 | 47.54 | 0.19 |
| Canada | 20 | 169,108 | 55.17 | 80.02 | 19,795 | 41,199 | 48.28 | 0.22 | Oman | 20 | 333 | 97.55 | 98.19 | 15,089 | 42,455 | 48.88 | 10.58 |
| Chile | 20 | 962,062 | 60.00 | 104.94 | 13,218 | 53,883 | 49.33 | 2.07 | Pakistan | 20 | 127,439 | 62.93 | 105.00 | 13,502 | 371,384 | 50.09 | 13.94 |
| China | 20 | 50,100,000 | 81.34 | 55.21 | 592,406 | 8,709,106 | 54.78 | 12.67 | Panama | 20 | 7495 | 52.61 | 104.84 | 8463 | 36,459 | 50.95 | 4.84 |
| Congo, DP | 20 | 3072 | 43.64 | 105.00 | 19,424 | 275,254 | 51.28 | 81.69 | Papua New Guinea | 20 | 2829 | 86.03 | 105.00 | 514 | 248,000 | 50.09 | 40.90 |
| Denmark | 20 | 37,026 | 59.95 | 66.71 | 2686 | 2300 | 47.21 | 0.21 | Peru | 20 | 73,430 | 94.71 | 104.63 | 4181 | 85,841 | 50.17 | 9.56 |
| Ecuador | 20 | 311,597 | 64.85 | 105.00 | 16,444 | 63,653 | 49.00 | 7.37 | Philippines | 20 | 2,149,586 | 83.65 | 97.81 | 183,824 | 1,927,922 | 50.86 | 10.39 |
| Egypt | 20 | 937,234 | 66.93 | 52.32 | 4583 | 139,358 | 50.33 | 2.30 | Poland | 20 | 36,934 | 38.38 | 66.82 | 839 | 4534 | 49.56 | 0.60 |
| France | 20 | 213,387 | 74.21 | 76.70 | 5845 | 15,120 | 46.93 | 0.29 | Portugal | 20 | 9526 | 22.01 | 68.65 | 7178 | 17,423 | 46.38 | 0.43 |
| Georgia | 20 | 999 | 49.90 | 98.97 | 26 | 2200 | 51.05 | 10.51 | Russian Federati | 20 | 147,259 | 70.18 | 100.86 | 1982 | 169,469 | 49.21 | 0.33 |
| Germany | 20 | 40,757 | 45.51 | 88.83 | 1660 | 2954 | 47.08 | 0.01 | Senegal | 20 | 534 | 63.50 | 87.15 | 7199 | 65,597 | 50.09 | 36.97 |
| Ghana | 20 | 25,459 | 63.04 | 97.16 | 12,689 | 234,408 | 51.99 | 34.84 | Sierra Leone | 20 | 62 | 98.25 | 88.09 | 7442 | 63,570 | 51.58 | 46.98 |
| Guinea | 20 | 315 | 90.71 | 82.08 | 1398 | 33,406 | 50.68 | 34.37 | South Africa | 20 | 6294 | 61.19 | 80.89 | 1784 | 17,748 | 48.21 | 22.13 |
| Iceland | 20 | 11,300 | 50.24 | 85.72 | 1731 | 4633 | 48.68 | 0.05 | Spain | 20 | 282,526 | 54.54 | 59.32 | 10,357 | 32,710 | 47.13 | 0.81 |
| India | 20 | 4,424,787 | 94.49 | 53.51 | 118,084 | 7,521,681 | 52.01 | 27.82 | Sri Lanka | 20 | 17,187 | 84.94 | 105.00 | 25,568 | 223,890 | 50.73 | 5.05 |
| Indonesia | 20 | 8,326,168 | 81.64 | 63.16 | 386,143 | 3,609,297 | 50.99 | 17.52 | Sweden | 20 | 10,154 | 65.13 | 77.89 | 1382 | 1646 | 48.03 | 0.48 |
| Iran | 20 | 250,713 | 70.38 | 103.38 | 79,420 | 552,670 | 48.96 | 0.94 | Taiwan | 20 | 315,074 | 81.97 | 87.39 | 21,872 | 243,565 | 49.68 | 0.46 |
| Ireland | 20 | 46,859 | 58.59 | 91.89 | 1764 | 5547 | 11.22 | 0.26 | Tanzania | 20 | 8592 | 80.33 | 105.00 | 14,149 | 174,718 | 52.29 | 48.82 |
| Italy | 20 | 158,380 | 37.03 | 50.60 | 12,029 | 30,454 | 45.85 | 1.11 | Thailand | 20 | 1,107,688 | 52.24 | 88.24 | 18,243 | 182,686 | 49.53 | 2.64 |
| Japan | 20 | 1,140,500 | 51.09 | 85.39 | 266,099 | 196,766 | 46.56 | 0.27 | Turkey | 20 | 196,146 | 56.96 | 73.39 | 17,813 | 62,021 | 11.84 | 0.98 |
| Korea, Rep. | 20 | 1,504,057 | 71.60 | 67.56 | 75,352 | 145,059 | 49.80 | 0.22 | Uganda | 20 | 70,290 | 60.96 | 76.21 | 6464 | 80,487 | 52.31 | 50.07 |
| Malaysia | 20 | 381,815 | 79.55 | 72.61 | 44,522 | 120,701 | 50.43 | 0.61 | United Kingdom | 20 | 199,784 | 60.65 | 78.95 | 6504 | 12,389 | 47.26 | 0.26 |
| Mauritania | 20 | 108,299 | 87.13 | 74.29 | 8328 | 107,849 | 49.99 | 10.68 | United States | 20 | 477,764 | 68.92 | 82.05 | 76,140 | 149,443 | 47.79 | 1.00 |
| Mexico | 20 | 162,436 | 77.41 | 94.20 | 88,624 | 239,990 | 47.50 | 4.52 | Venezuela | 20 | 24,384 | 57.30 | 103.13 | 31,922 | 46,306 | 47.53 | 11.39 |
| Morocco | 20 | 1240 | 90.34 | 36.62 | 18,929 | 111,330 | 49.80 | 2.52 | Vietnam | 20 | 2,658,570 | 97.92 | 42.25 | 57,484 | 771,327 | 52.48 | 10.06 |
| Variables | Measurement Unit | Obs. | Mean | Std Dev. | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Log of global fishing outputs (GFO) | Tons live weight | 1240 | 10.754 | 3.029 | 0.693 | 18.071 |
| Log of global number of fishing vessels (NFV) | Number of motorized vessels propelled by engines | 1240 | 9.047 | 1.867 | 3.258 | 13.453 |
| Log of global number of fishers (NF) | Number of fishers | 1240 | 11.184 | 2.082 | 5.298 | 16.182 |
| Log of SDG14_ overfishing | % of total catch | 1240 | 4.171 | 0.366 | 2.194 | 4.604 |
| Log of SDG14_trawling | % of fish caught by trawling | 1240 | 4.361 | 0.312 | 1.609 | 4.654 |
| High income (HIC) | Dummy variable | 1240 | 0.323 | 0.468 | 0.000 | 1.000 |
| Lower-middle income (LMIC) | Dummy variable | 1240 | 0.339 | 0.473 | 0.000 | 1.000 |
| Upper-middle income (UMIC) | Dummy variable | 1240 | 0.242 | 0.428 | 0.000 | 1.000 |
| Technological progress (Time trend) | Year from 2001–2020 | 1240 | 10.5 | 5.768 | 1.000 | 20.000 |
| Log of GDP growth rate | % of annual | 1240 | 3.851 | 0.319 | 0.270 | 4.284 |
| Log of Poverty rate | % of population | 1240 | 2.024 | 1.192 | 0.693 | 4.538 |
| Variables | GFO | NFV | NF | SDG14 Overfishing | SDG14 Trawling | HIC | LMIC | UMIC | GDP Growth Rate | Poverty Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| GFO | 1 | |||||||||
| NFV | 0.601 | 1 | ||||||||
| NF | 0.419 | 0.720 | 1 | |||||||
| SDG14 Overfishing | −0.563 | 0.216 | 0.437 | 1 | ||||||
| SDG14 Trawling | −0.205 | −0.151 | −0.822 | −0.138 | 1 | |||||
| HIC | 0.139 | −0.981 | −0.549 | −0.363 | −0.706 | 1 | ||||
| LMIC | 0.204 | 0.193 | 0.484 | 0.304 | −0.137 | −0.494 | 1 | |||
| UMIC | 0.336 | −0.538 | 0.201 | −0.0949 | 0.129 | −0.390 | −0.404 | 1 | ||
| GDP growth rate | −0.305 | 0.678 | 0.190 | 0.124 | −0.331 | −0.144 | 0.179 | −0.100 | 1.000 | |
| Poverty rate | −0.409 | 0.170 | 0.381 | 0.296 | 0.149 | −0.620 | 0.406 | −0.476 | 0.228 | 1 |
| Sample Split | SDG14 Overfishing | SDG14 Trawling |
|---|---|---|
| Model 1 | Model 2 | |
| The Lagrange multiplier test for no threshold | 139.600 | 118.718 |
| Bootstrap p-value | 0.0004 | 0.0001 |
| Threshold estimate | 4.4456 | 4.5516 |
| 95% Confidence Interval | (4.396, 4.455) | (4.5077, 4.5523) |
| Variables | Panel GLS without threshold | Threshold Model 1 = SDG14 Overfishing | Threshold Model 2 = SDG14 Trawling | ||
|---|---|---|---|---|---|
| Regime 1 | Regime 2 | Regime 1 | Regime 2 | ||
| Overfishing ≤ 4.4456 | Overfishing > 4.4456 | Trawling ≤ 4.5516 | Trawling > 4.5516 | ||
| Constant | −4.402 ** | −2.548 ** | −10.46 ** | −5.106 ** | −2.981 ** |
| [−9.150] | [−5.332] | [−10.524] | [−14.489] | [−4.869] | |
| Logarithm (NFV) | 0.552 ** | 0.412 ** | 0.776 ** | 0.728 ** | 0.283 ** |
| [10.023] | [7.501] | [7.399] | [18.473] | [7.170] | |
| Logarithm (NF) | 0.605 ** | 0.623 ** | 0.927 ** | 0.576 ** | 0.599 ** |
| [9.967] | [10.237] | [7.786] | [16.919] | [9.160] | |
| High income (HIC) | 4.557 ** | 4.017 ** | −0.358 | 4.400 ** | 4.515 ** |
| [17.689] | [14.543] | [−0.596] | [13.925] | [12.504] | |
| Lower-middle income (LMIC) | 1.755 ** | 0.951 ** | 1.634 ** | 1.932 ** | 1.985 ** |
| [7.867] | [3.814] | [4.385] | [5.944] | [12.962] | |
| Upper-middle income (UMIC) | 3.031 ** | 2.221 ** | 3.400 ** | 2.515 ** | 3.513 ** |
| [13.079] | [8.872] | [7.913] | [8.040] | [22.514] | |
| Technological progress (Time trend) | 0.0574 ** | 0.0659 ** | 0.0255 | 0.0435 ** | 0.0809 ** |
| [5.482] | [6.554] | [1.184] | [10.515] | [8.786] | |
| Observationss | 1240 | 905 | 335 | 829 | 411 |
| Wald χ2 | 7848 ** | 4489 ** | 3731 ** | 6797 ** | 1186 ** |
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Sample Split | SDG14 Overfishing | SDG14 Trawling |
|---|---|---|
| Model 1 | Model 2 | |
| The Lagrange multiplier test for no threshold | 139.600 | 96.704 |
| Bootstrap p-value | 0.0004 | 0.0001 |
| Threshold estimate | 4.4456 | 4.4038 |
| 95% Confidence Interval | (4.396, 4.455) | (4.1596, 4.4073) |
| Variables | Panel GLS without Threshold | Threshold Model 1 = SDG14 Overfishing | Threshold Model 2 = SDG14 Trawling | ||
|---|---|---|---|---|---|
| Regime 1 | Regime 2 | Regime 1 | Regime 2 | ||
| Overfishing ≤ 4.4456 | Overfishing > 4.4456 | Trawling ≤ 4.4038 | Trawling > 4.4038 | ||
| Constant | 2.326 ** | 3.901 ** | −5.234 ** | −0.0769 ** | 4.826 ** |
| [3.234] | [16.980] | [−7.584] | [−0.295] | [18.108] | |
| Logarithm (NFV) | 0.493 ** | 0.423 ** | 0.488 ** | 0.966 ** | 0.243 ** |
| [10.422] | [20.701] | [7.710] | [28.849] | [9.677] | |
| Logarithm (NF) | 0.602 ** | 0.457 ** | 1.203 ** | 0.215 ** | 0.699 ** |
| [13.014] | [25.262] | [17.205] | [7.593] | [21.714] | |
| Logarithm of GDP growth rate | 0.00163 | 0.164 ** | −0.274 * | 0.392 ** | −0.331 ** |
| [0.009] | [3.327] | [−1.911] | [7.926] | [−4.678] | |
| Logarithm of poverty rate | −1.433 ** | −1.322 ** | −1.096 ** | −0.861 ** | −1.438 ** |
| [−25.124] | [−58.985] | [−18.631] | [−19.457] | [−38.016] | |
| Technological progress (Time trend) | 0.0117 | 0.0202 ** | −0.00578 | 0.0379 ** | −0.00385 |
| [1.195] | [5.644] | [−0.515] | [8.648] | [−0.789] | |
| Observations | 1240 | 905 | 335 | 558 | 682 |
| Wald χ2 | 6932 ** | 6216 ** | 3137 ** | 5044 ** | 5705 ** |
| Prob > χ2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, C.-V.; Wang, H.-C.; Chen, S.-H.; Lee, J.-M. The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes 2023, 8, 71. https://doi.org/10.3390/fishes8020071
Pham C-V, Wang H-C, Chen S-H, Lee J-M. The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes. 2023; 8(2):71. https://doi.org/10.3390/fishes8020071
Chicago/Turabian StylePham, Ca-Van, Hui-Cheng Wang, Sheng-Hung Chen, and Jie-Min Lee. 2023. "The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective" Fishes 8, no. 2: 71. https://doi.org/10.3390/fishes8020071
APA StylePham, C.-V., Wang, H.-C., Chen, S.-H., & Lee, J.-M. (2023). The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes, 8(2), 71. https://doi.org/10.3390/fishes8020071






