Genome-Wide Identification, Phylogenetic Analysis and Expression Pattern Profiling of the Aquaporin Family Genes in Leuciscus waleckii
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Sequence and Aquaporin Sequence Retrieval
2.2. Aqp Gene Nomenclature
2.3. Construction of Phylogenetic Tree Aqp Genes in Six Species
2.4. Analysis of Phylogenetic Relationships, Gene Structure, and Motifs of LwAqps
2.5. Differential Expression Calculation
2.5.1. Differential Expression Analysis between DL and WSL L. waleckii
2.5.2. Indoor Experiment of Alkaline Stress and Post Alkaline-Stress Recovery in DL L. waleckii
2.6. Statistics of SNPs and Fixation Index (Fst) of Aqp Gene
2.7. Comparison of Bases and Amino Acids at the Top 5% Site of Fst Value of Aqp Gene in DL and WSL L. waleckii
2.8. Amino Acid Sequence Alignment of aqp3 in Multiple Species
2.9. Aqp3a Three-Dimensional Structure Prediction
2.10. Quantitative Reverse-Transcription PCR (qRT-PCR)
3. Results
3.1. Identification of Aqp Genes
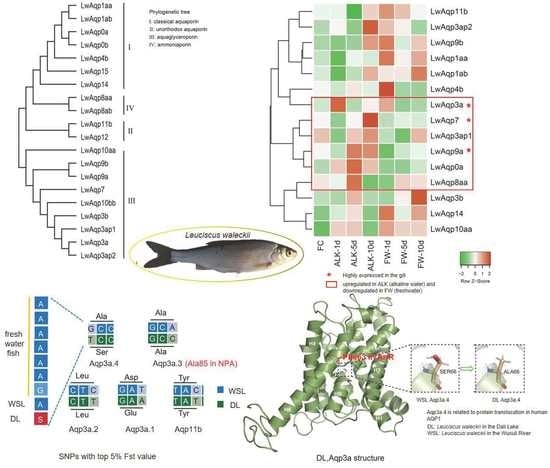
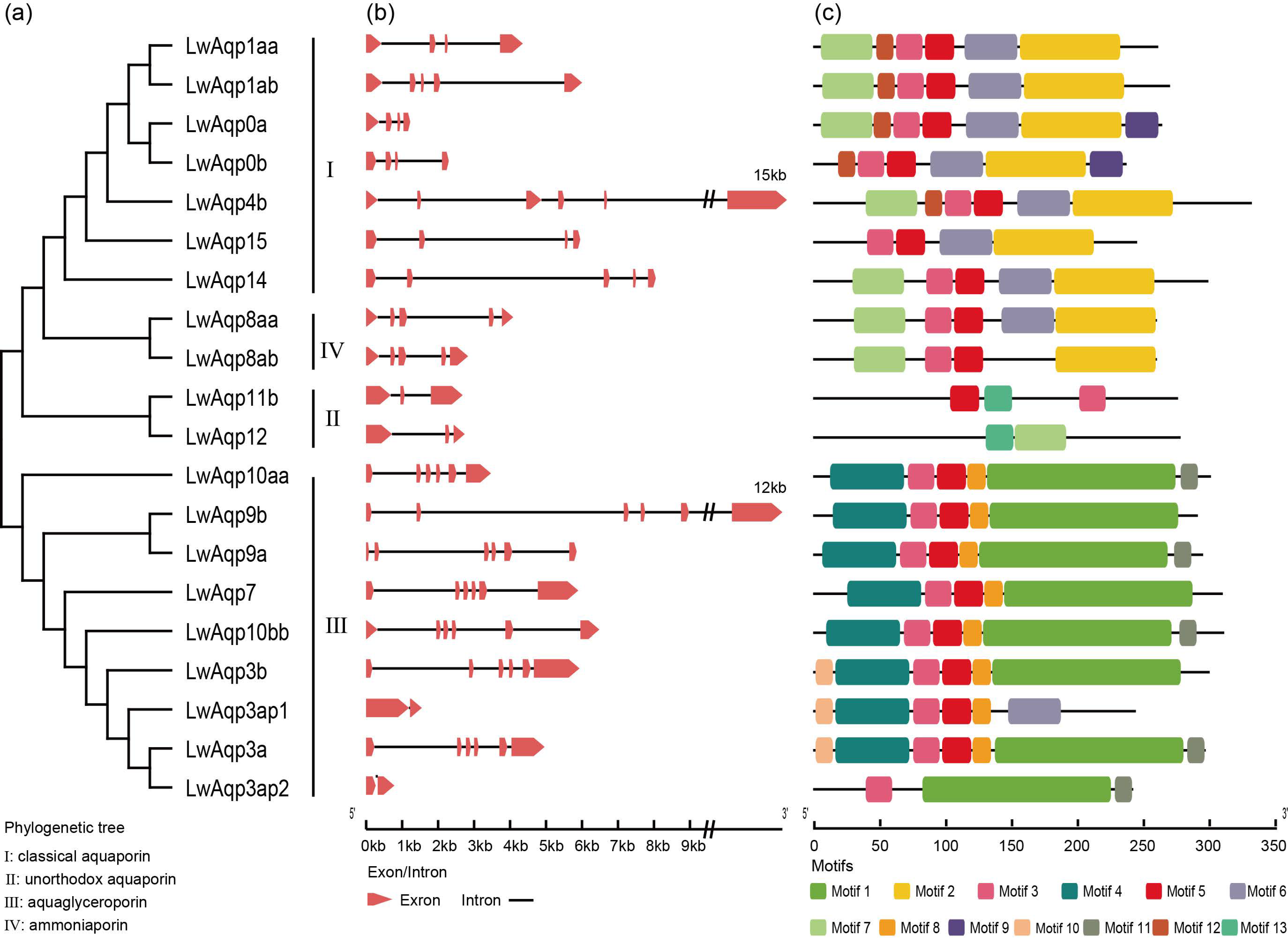
3.2. Evolution and Classification of the Aqp Gene Family
3.3. Analysis of Phylogenetic Relationships, Gene Structure, and Motifs
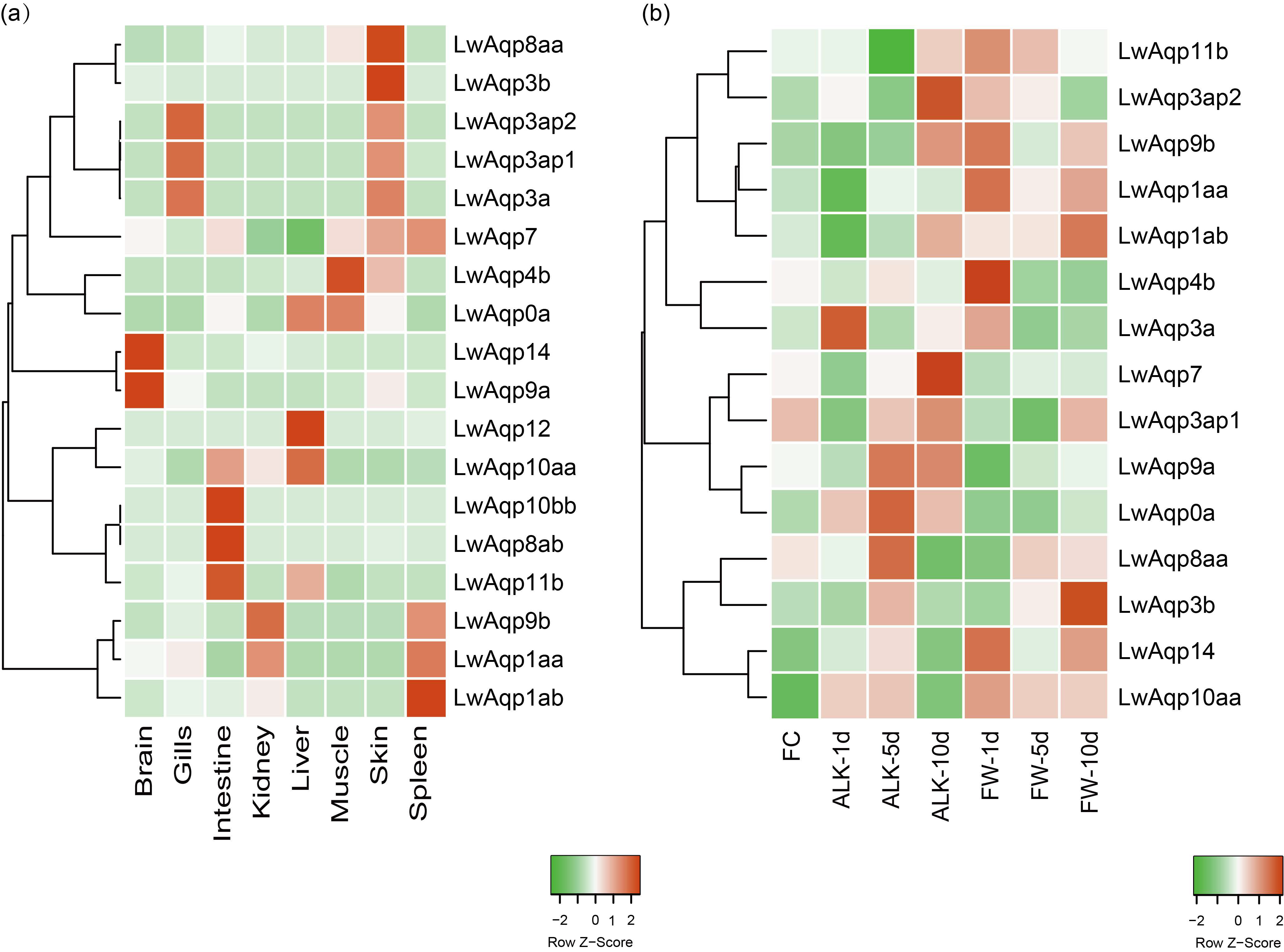
3.4. Analysis of the Aqp Gene Tissue Expression Profile
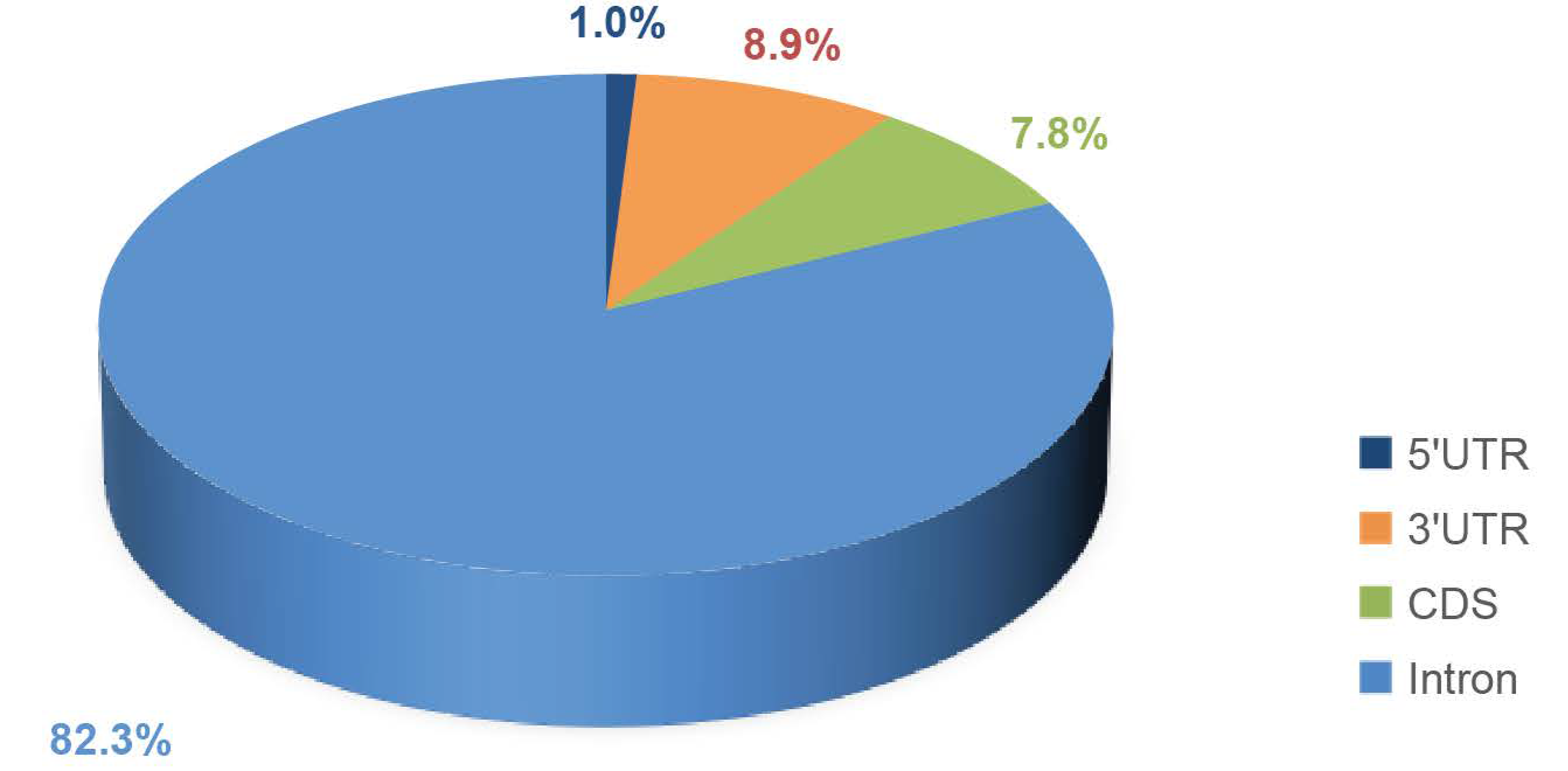
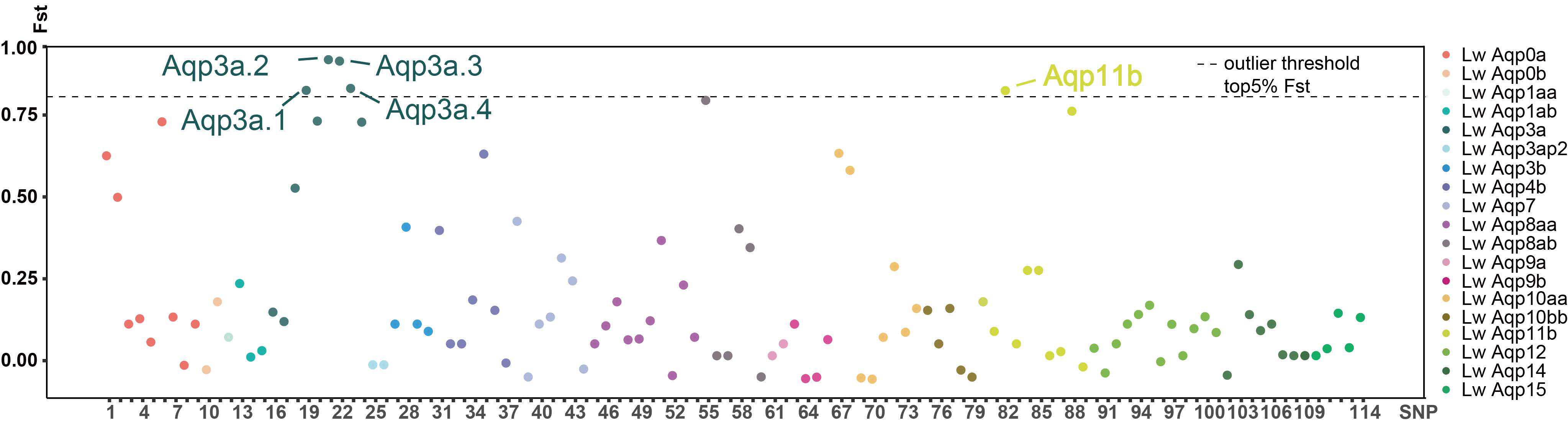
3.5. Classification of the SNP Markers of Aqp Gene and Statistics of the Fst Value in CDS Region
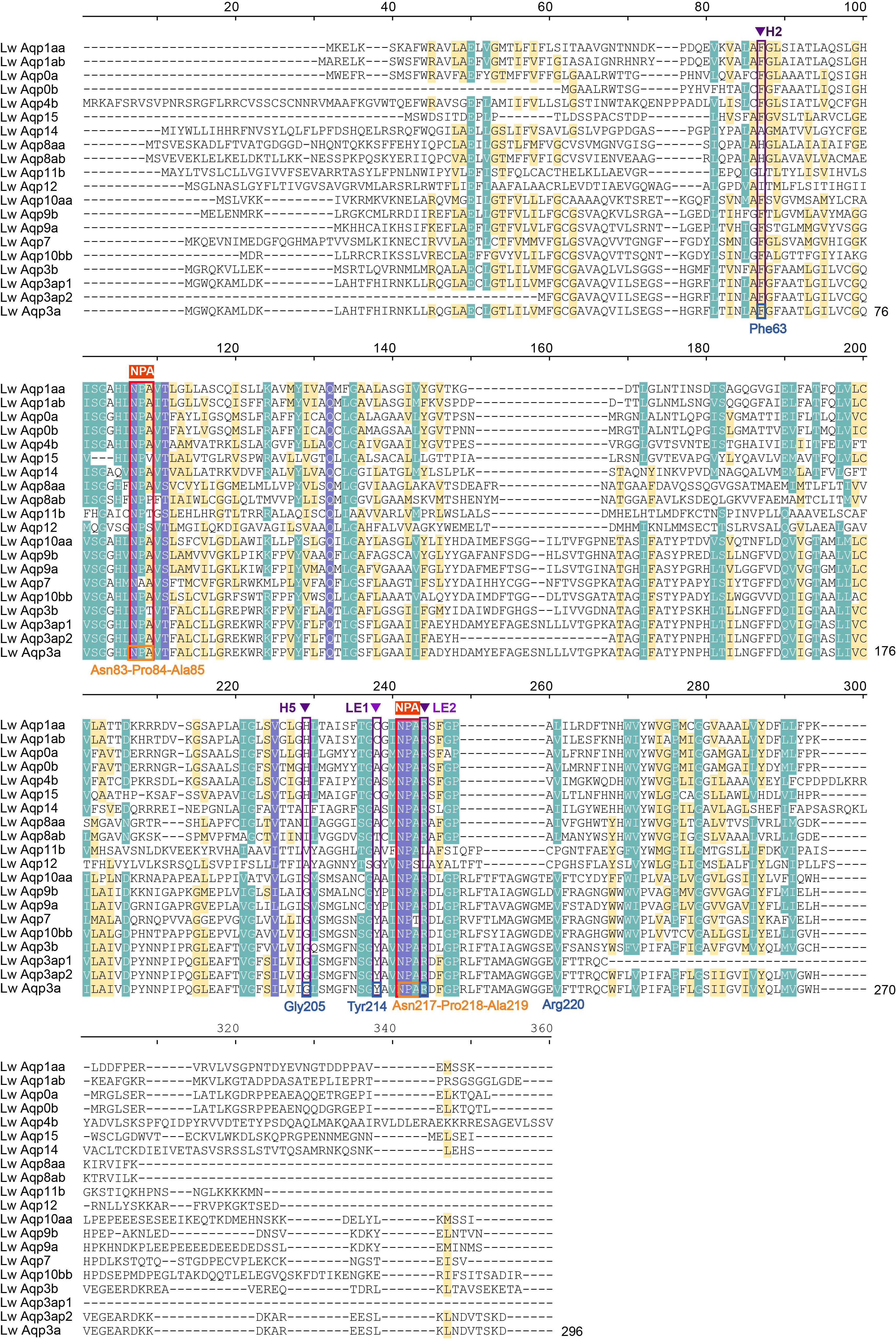
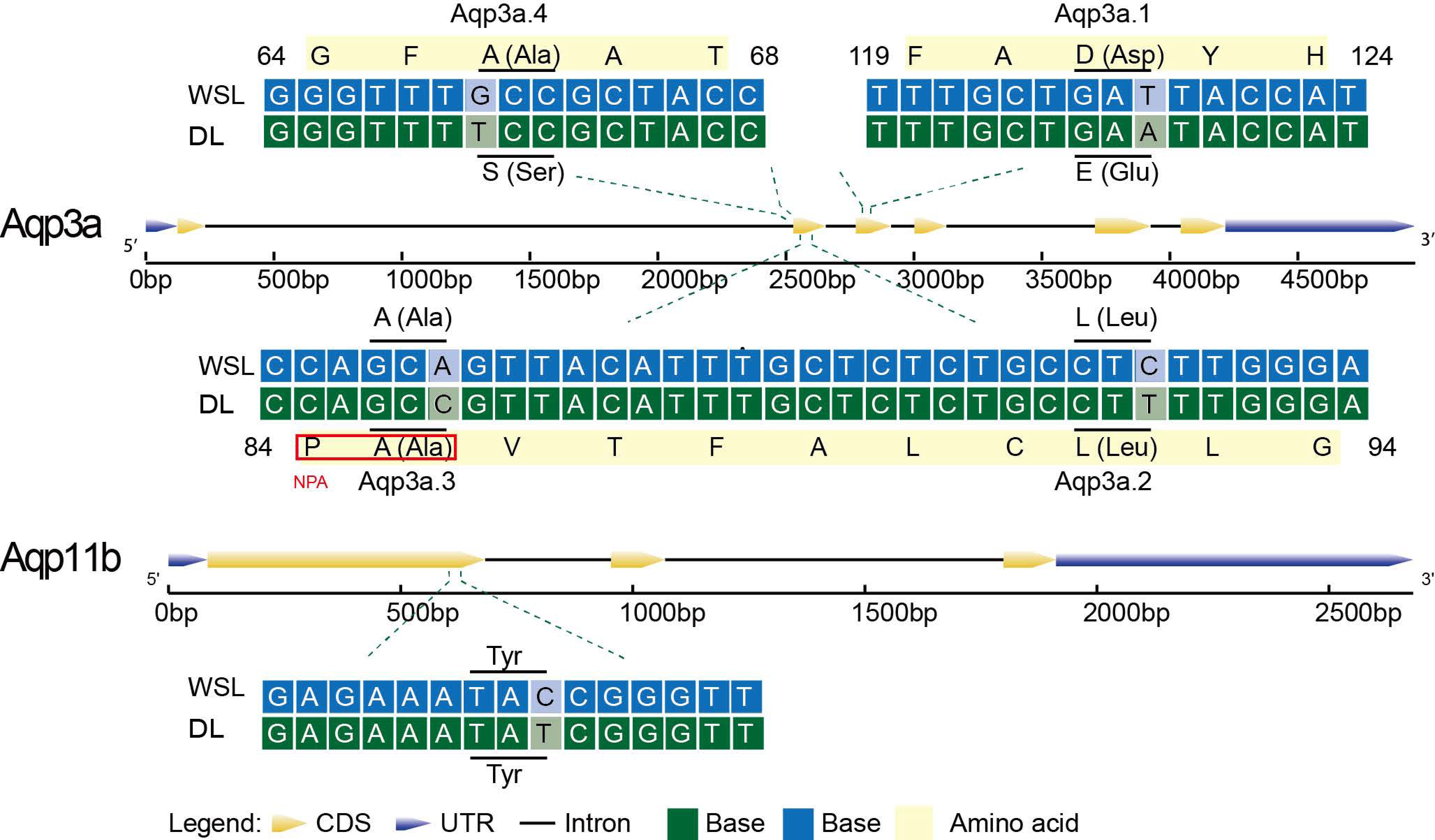
3.6. Base and Amino Acid Mutation Analysis of the Aqp Gene
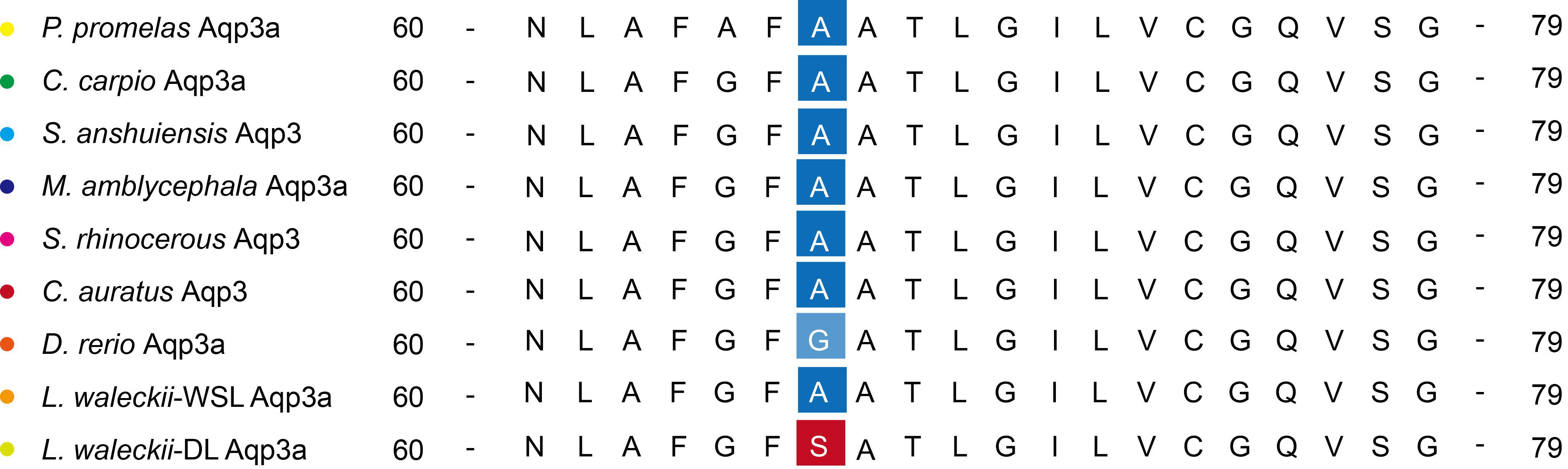
3.7. Amino Acid Sequence Alignment of Aqp3 in Multiple Species
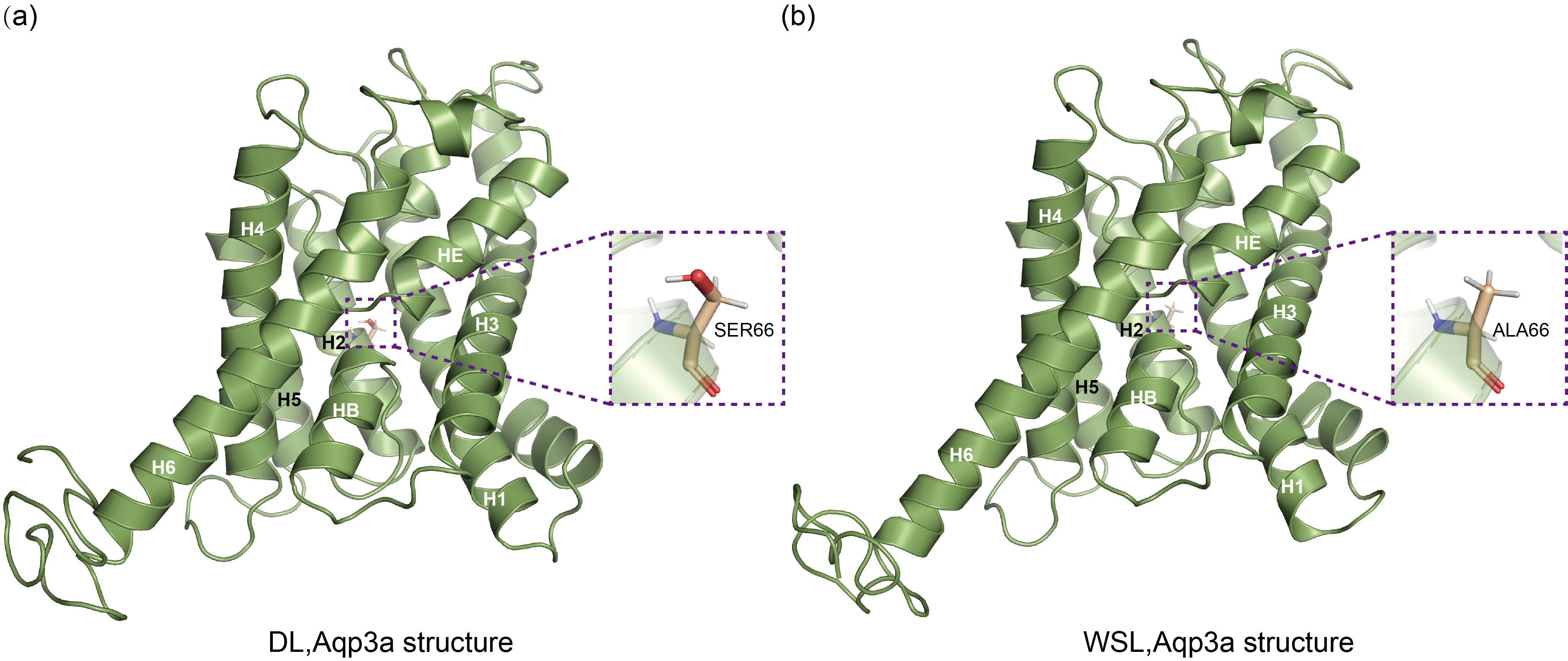
3.8. Prediction of the Three-Dimensional Structure of Aqp3a
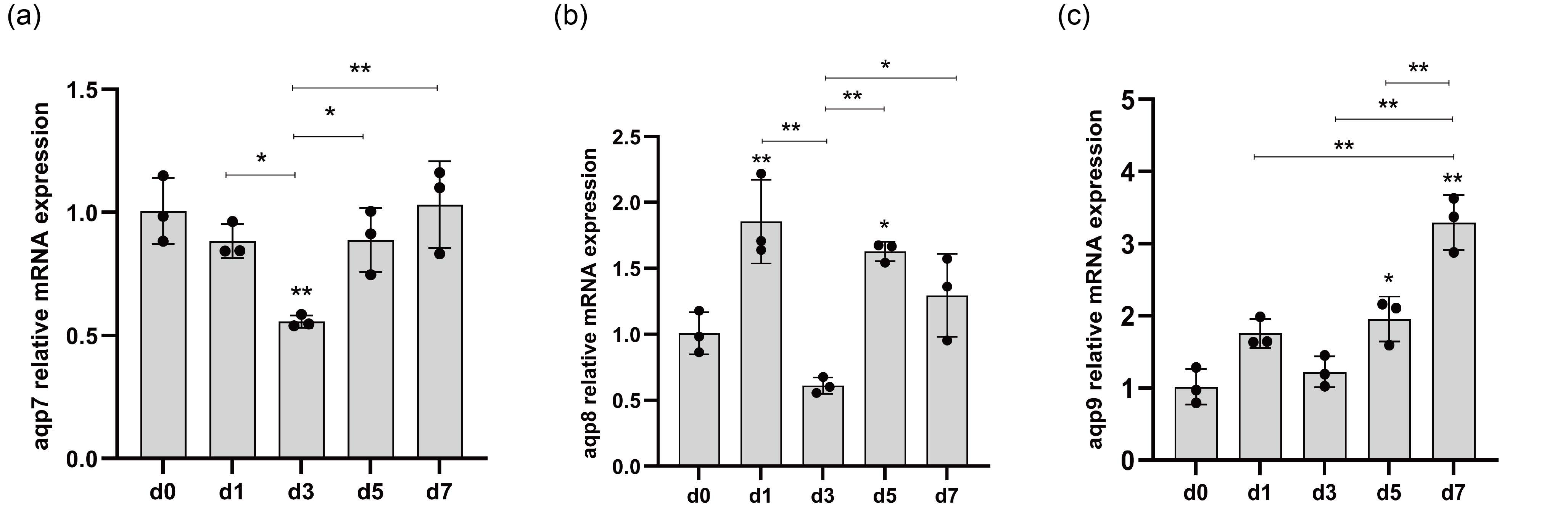
3.9. qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Finn, R.N.; Chauvigné, F.; Hlidberg, J.B.; Cutler, C.P.; Cerdà, J. The lineage-specific evolution of aquaporin gene clusters facilitated tetrapod terrestrial adaptation. PLoS ONE 2014, 9, e113686. [Google Scholar] [CrossRef]
- Chauvigné, F.; Yilmaz, O.; Ferré, A.; Fjelldal, P.G.; Finn, R.N.; Cerdà, J. The vertebrate Aqp14 water channel is a neuropeptide-regulated polytransporter. Commun. Biol. 2019, 2, 462. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerda, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef]
- MacIver, B.; Cutler, C.P.; Yin, J.; Hill, M.G.; Zeidel, M.L.; Hill, W.G. Expression and functional characterization of four aquaporin water channels from the European eel (Anguilla anguilla). J. Exp. Biol. 2009, 212, 2856–2863. [Google Scholar] [CrossRef]
- Engelund, M.B.; Chauvigné, F.; Christensen, B.M.; Finn, R.N.; Cerdà, J.; Madsen, S.S. Differential expression and novel permeability properties of three aquaporin 8 paralogs from seawater-challenged Atlantic salmon smolts. J. Exp. Biol. 2013, 216, 3873–3885. [Google Scholar] [CrossRef]
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and selective ammonia transport by aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301. [Google Scholar] [CrossRef]
- Cerdà, J.; Chauvigné, F.; Finn, R.N. The physiological role and regulation of aquaporins in teleost germ cells. In Aquaporins; Springer: Berlin/Heidelberg, Germany, 2017; pp. 149–171. [Google Scholar] [CrossRef]
- Fujiyoshi, Y.; Mitsuoka, K.; De Groot, B.L.; Philippsen, A.; Grubmüller, H.; Agre, P.; Engel, A. Structure and function of water channels. Curr. Opin. Struct. Biol. 2002, 12, 509–515. [Google Scholar] [CrossRef]
- Sui, H.; Han, B.-G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Pickel, S.U.; Jennings, J.; Törnroth-Horsefield, S.; Conner, M.T.; Bill, R.M.; Conner, A.C. Water channel pore size determines exclusion properties but not solute selectivity. Sci. Rep. 2019, 9, 20369. [Google Scholar] [CrossRef]
- Hamdi, M.; Sanchez, M.A.; Beene, L.C.; Liu, Q.; Landfear, S.M.; Rosen, B.P.; Liu, Z. Arsenic transport by zebrafish aquaglyceroporins. BMC Mol. Biol. 2009, 10, 104. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on Aqp1 and Aqp3 expression. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef]
- Fabra, M.; Raldúa, D.; Power, D.M.; Deen, P.M.; Cerda, J. Marine fish egg hydration is aquaporin-mediated. Science 2005, 307, 545. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhao, J.; Tang, S.; Zhao, Y.; Gu, Y.; Chen, X. Genomic and expression characterization of aquaporin genes from Siniperca chuatsi. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 38, 100819. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, Y.K.; Kim, Y.K. Characterization of aquaporin-1ab (Aqp1ab) mRNA in mud loach (Misgurnus mizolepis) exposed to heavy metal and immunostimulant stimuli. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 270, 111226. [Google Scholar] [CrossRef]
- Ip, Y.K.; Soh, M.M.; Chen, X.L.; Ong, J.L.; Chng, Y.R.; Ching, B.; Wong, W.P.; Lam, S.H.; Chew, S.F. Molecular characterization of branchial aquaporin 1aa and effects of seawater acclimation, emersion or ammonia exposure on its mRNA expression in the gill, gut, kidney and skin of the freshwater climbing perch, Anabas testudineus. PLoS ONE 2013, 8, e61163. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, L.-J.; Wu, M.-X.; Peng, G.-F.; Zhang, Y.-L. Aquaporins1 and 3 in the tissues of Paramisgurnus dabryanus and their expression profiles in response to ammonia and drought. Front. Mar. Sci. 2022, 9, 1776. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Li, J.; Shen, Y. Transcriptomic Analysis of the Liver and Brain in Grass Carp (Ctenopharyngodon idella) Under Heat Stress. Mar. Biotechnol. 2022, 34, 856–870. [Google Scholar] [CrossRef]
- Parisi, M.G.; Maisano, M.; Cappello, T.; Oliva, S.; Mauceri, A.; Toubiana, M.; Cammarata, M. Responses of marine mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) after infection with the pathogen Vibrio splendidus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Geng, K.; Zhang, Z. The geomorphic characteristics and evolution of the lakes in Dalairuoer area of Neimenggu Plateau during the Holocene. J. Beijing Norm. Univ. Nat. Sci. 1988, 4, 94–101. (In Chinese) [Google Scholar]
- Wang, S.; Kuang, Y.; Liang, L.; Sun, B.; Zhao, X.; Zhang, L.; Chang, Y. Resequencing and SNP discovery of Amur ide (Leuciscus waleckii) provides insights into local adaptations to extreme environments. Sci. Rep. 2021, 11, 5064. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, R.; Sun, X.; Liang, L.; Chen, J.; Huang, J.; Dou, X.; Tao, R. Genetic analysis of population differentiation and adaptation in Leuciscus waleckii. Genetica 2013, 141, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.P.; Wright, P.A.; Iwama, G.K.; Wood, C.M. The physiological adaptations of the Lahontan cutthroat trout (Oncorhynchus clarki henshawi) following transfer from well water to the highly alkaline waters of Pyramid Lake, Nevada (pH 9.4). Physiol. Zool. 1994, 67, 355–380. [Google Scholar] [CrossRef]
- He, Q.; Chang, Y.; Su, B.; Sun, B.; Sun, X.; Liang, L. Effects of carbonate alkalinities on oxygen consumption, ammonia excretion and ammonia excretion gene expression in Leuciscus waleckii Dybowski. J. Shanghai Ocean Univ. 2016, 25, 551–558. (In Chinese) [Google Scholar] [CrossRef]
- Xu, J.; Li, J.T.; Jiang, Y.; Peng, W.; Yao, Z.; Chen, B.; Jiang, L.; Feng, J.; Ji, P.; Liu, G.; et al. Genomic basis of adaptive evolution: The survival of Amur Ide (Leuciscus waleckii) in an extremely alkaline environment. Mol. Biol. Evol. 2017, 34, 145–159. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Liang, L.; Su, B.; Zhang, Y.; LIEW, H.J.; Sun, B.; Zhang, L.; Chang, Y. Distinctive metabolite profiles in migrating Amur ide (Leuciscus waleckii) reveal changes in osmotic pressure, gonadal development, and energy allocation strategies. Front. Environ. Sci. 2022, 10, 997827. [Google Scholar] [CrossRef]
- Holm, L.M.; Jahn, T.P.; Møller, A.L.; Schjoerring, J.K.; Ferri, D.; Klaerke, D.A.; Zeuthen, T. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflügers. Archiv. 2005, 450, 415–428. [Google Scholar] [CrossRef]
- Fan, Z.; Peng, F.; Li, J.; Wu, D.; Zhang, Y.; Wang, C.a.; Wang, L.; Xu, Q. Effects of α-ketoglutarate on growth performance, antioxidant capacity and ammonia metabolization against chronic carbonate alkalinity stress in Songpu mirror carp (Cyprinus carpio Songpu). Aquacult. Res. 2020, 51, 2029–2040. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, P.; Sun, X. Ammonia Eexcretion Related Genes Expression of Common Carp Under the Stress of Carbonate Alkalinity. Biotechnol. Bull. 2013, 4, 185. (In Chinese) [Google Scholar]
- Chang, Y.-M.; Tang, R.; Dou, X.-J.; Tao, R.; Sun, X.-W.; Liang, L.-Q. Transcriptome and expression profiling analysis of Leuciscus waleckii: An exploration of the alkali-adapted mechanisms of a freshwater teleost. Mol. Biosyst. 2014, 10, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ma, D.; Zhu, H.; Liu, Z.; Gao, F. Transcriptomic response to three osmotic stresses in gill of hybrid tilapia (Oreochromis mossambicus female× O. urolepis hornorum male). BMC Genom. 2020, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, H.; Chen, L.; Zhou, K.; Ying, C.; Lai, Q. Transcriptomic profiles of Japanese medaka (Oryzias latipes) in response to alkalinity stress. Genet. Mol. Res. 2012, 11, 2200–2246. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ge, Q.; Wang, J.; Li, M.; Liu, P.; Li, J.; Li, J. Comparative Transcriptomic and Proteomic Analysis of Exopalaemon carinicauda in Response to Alkalinity Stress. Front. Mar. Sci. 2021, 8, 1523. [Google Scholar] [CrossRef]
- Dong, C.; Chen, L.; Feng, J.; Xu, J.; Mahboob, S.; Al-Ghanim, K.; Li, X.; Xu, P. Genome wide identification, phylogeny, and expression of aquaporin genes in common carp (Cyprinus carpio). PLoS ONE 2016, 11, e0166160. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Xu, L.; Wang, S.; Jiang, Y.; Zhao, Z.; Zhang, Y.; Li, J.; Dong, C.; Xu, P.; et al. Gene expression changes leading extreme alkaline tolerance in Amur ide (Leuciscus waleckii) inhabiting soda lake. BMC Genom. 2013, 14, 682. [Google Scholar] [CrossRef]
- Yilmaz, O.; Chauvigné, F.; Ferré, A.; Nilsen, F.; Fjelldal, P.G.; Cerdà, J.; Finn, R.N. Unravelling the complex duplication history of deuterostome glycerol transporters. Cells 2020, 9, 1663. [Google Scholar] [CrossRef]
- Zeuthen, T.; MacAulay, N. Transport of water against its concentration gradient: Fact or fiction? Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 373–381. [Google Scholar] [CrossRef]
- Shen, Y.; He, Y.; Bi, Y.; Chen, J.; Zhao, Z.; Li, J.; Chen, X. Transcriptome analysis of gill from Lateolabrax maculatus and aqp3 gene expression. Aquac. Fish. 2019, 4, 247–254. [Google Scholar] [CrossRef]
- Boj, M.; Chauvigné, F.; Ribes, E.; Cerdà, J. AQUAPORIN-1aa and-8b are differentially localized in gilthead sea bream (Sparus aurata) spermatozoa and play distinct roles during the activation of sperm motility. In Proceedings of the 10th International Symposium on Reproductive Physiology of Fish, Expanding the Khowledge Base of Reproductive Success: From Genes to the Environment, Olhao, Portugal, 25–30 May 2014. [Google Scholar]
- Madsen, S.S.; Olesen, J.H.; Bedal, K.; Engelund, M.B.; Velasco-Santamaría, Y.M.; Tipsmark, C.K. Functional characterization of water transport and cellular localization of three aquaporin paralogs in the salmonid intestine. Front. Physiol. 2011, 2, 56. [Google Scholar] [CrossRef]
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K.; Fujiyoshi, Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta 2007, 1768, 688–693. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.; Martins, A.; Mósca, A.; Wijma, H.; Prista, C.; Soveral, G.; Casini, A. Exploring the gating mechanisms of aquaporin-3: New clues for the design of inhibitors? Mol. BioSyst. 2016, 12, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Smolin, N.; Li, B.; Beck, D.A.; Daggett, V. Side-chain dynamics are critical for water permeation through aquaporin-1. Biophys. J. 2008, 95, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.; Helm, A.; Turnbull, I.; Gulati, H.; Yang, B.; Verkman, A.S.; Skach, W.R. Identification of sequence determinants that direct different intracellular folding pathways for aquaporin-1 and aquaporin-4. J. Biol. Chem. 2000, 275, 34157–34165. [Google Scholar] [CrossRef]
- Lu, Y.; Turnbull, I.R.; Bragin, A.; Carveth, K.; Verkman, A.; Skach, W.R. Reorientation of aquaporin-1 topology during maturation in the endoplasmic reticulum. Mol. Biol. Cell 2000, 11, 2973–2985. [Google Scholar] [CrossRef]
- Buck, T.M.; Skach, W.R. Differential stability of biogenesis intermediates reveals a common pathway for aquaporin-1 topological maturation. J. Biol. Chem. 2005, 280, 261–269. [Google Scholar] [CrossRef]
- Daniel, C.J.; Conti, B.; Johnson, A.E.; Skach, W.R. Control of Translocation through the Sec61 Translocon by Nascent Polypeptide Structure within the Ribosome*♦. J. Biol. Chem. 2008, 283, 20864–20873. [Google Scholar] [CrossRef]
- Conner, A.C.; Bill, R.M.; Conner, M.T. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol. 2013, 30, 101–112. [Google Scholar] [CrossRef]
- Garcia, N.; Gondran, C.; Menon, G.; Mur, L.; Oberto, G.; Guerif, Y.; Dal Farra, C.; Domloge, N. Impact of AQP3 inducer treatment on cultured human keratinocytes, ex vivo human skin and volunteers. Int. J. Cosmet. Sci. 2011, 33, 432–442. [Google Scholar] [CrossRef]


| Primer Name | Sequence (5′–3′) |
|---|---|
| aqp7-F | TGTTGCTGTGTCTGATGGCT |
| aqp7-R | CATTGCCTGCCCTGAAAACC |
| aqp8aa-F | GACTCACCGTGACTGCAAATA |
| aqp8aa-R | AAGGCTGACAGTGACCAAAG |
| aqp9a-F | GGAATGCTGGTTCTCTGTATTCTG |
| aqp9a-R | AGGGATCCACCACCAGTAATC |
| Gene Name | Chromosome Localization | Gene Length/bp | CDS Sequence Length/bp | Amino Acid Number/aa |
|---|---|---|---|---|
| aqp0a | Chr19 | 1225 | 792 | 264 |
| aqp0b | Chr19 | 2297 | 711 | 237 |
| aqp1aa | Chr7 | 4353 | 783 | 261 |
| aqp1ab | Chr7 | 6001 | 810 | 270 |
| aqp3a | Chr4 | 4958 | 891 | 296 |
| aqp3ap1 | Chr7 | 1546 | 732 | 244 |
| aqp3ap2 | Chr1 | 783 | 727 | 242 |
| aqp3b | Chr14 | 5929 | 900 | 299 |
| aqp4b | Chr16 | 14,964 | 996 | 331 |
| aqp7 | Chr14 | 5896 | 930 | 309 |
| aqp8aa | Chr18 | 4088 | 781 | 260 |
| aqp8ab | Chr18 | 2832 | 781 | 259 |
| aqp9a | Chr24 | 5851 | 886 | 294 |
| aqp9b | Chr3 | 11,935 | 873 | 290 |
| aqp10aa | Chr15 | 3468 | 903 | 301 |
| aqp10bb | Chr11 | 6481 | 933 | 310 |
| aqp11b | Chr8 | 2681 | 828 | 276 |
| aqp12 | Chr5 | 2739 | 834 | 277 |
| aqp14 | Chr19 | 8051 | 897 | 299 |
| aqp15 | Chr1 | 5951 | 735 | 245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, F.; Liang, L.; Wang, S.; Liew, H.; Chang, Y.; Zhang, L. Genome-Wide Identification, Phylogenetic Analysis and Expression Pattern Profiling of the Aquaporin Family Genes in Leuciscus waleckii. Fishes 2023, 8, 107. https://doi.org/10.3390/fishes8020107
Zhan F, Liang L, Wang S, Liew H, Chang Y, Zhang L. Genome-Wide Identification, Phylogenetic Analysis and Expression Pattern Profiling of the Aquaporin Family Genes in Leuciscus waleckii. Fishes. 2023; 8(2):107. https://doi.org/10.3390/fishes8020107
Chicago/Turabian StyleZhan, Feifei, Liqun Liang, Shuangyi Wang, Honjung Liew, Yumei Chang, and Limin Zhang. 2023. "Genome-Wide Identification, Phylogenetic Analysis and Expression Pattern Profiling of the Aquaporin Family Genes in Leuciscus waleckii" Fishes 8, no. 2: 107. https://doi.org/10.3390/fishes8020107
APA StyleZhan, F., Liang, L., Wang, S., Liew, H., Chang, Y., & Zhang, L. (2023). Genome-Wide Identification, Phylogenetic Analysis and Expression Pattern Profiling of the Aquaporin Family Genes in Leuciscus waleckii. Fishes, 8(2), 107. https://doi.org/10.3390/fishes8020107