Reproductive Characteristics of Pseudecheneis sulcatus (Siluriforms: Sisoridae) in the Lower Yarlung Zangbo River, Tibet
Abstract
:1. Introduction
2. Materials and Methods
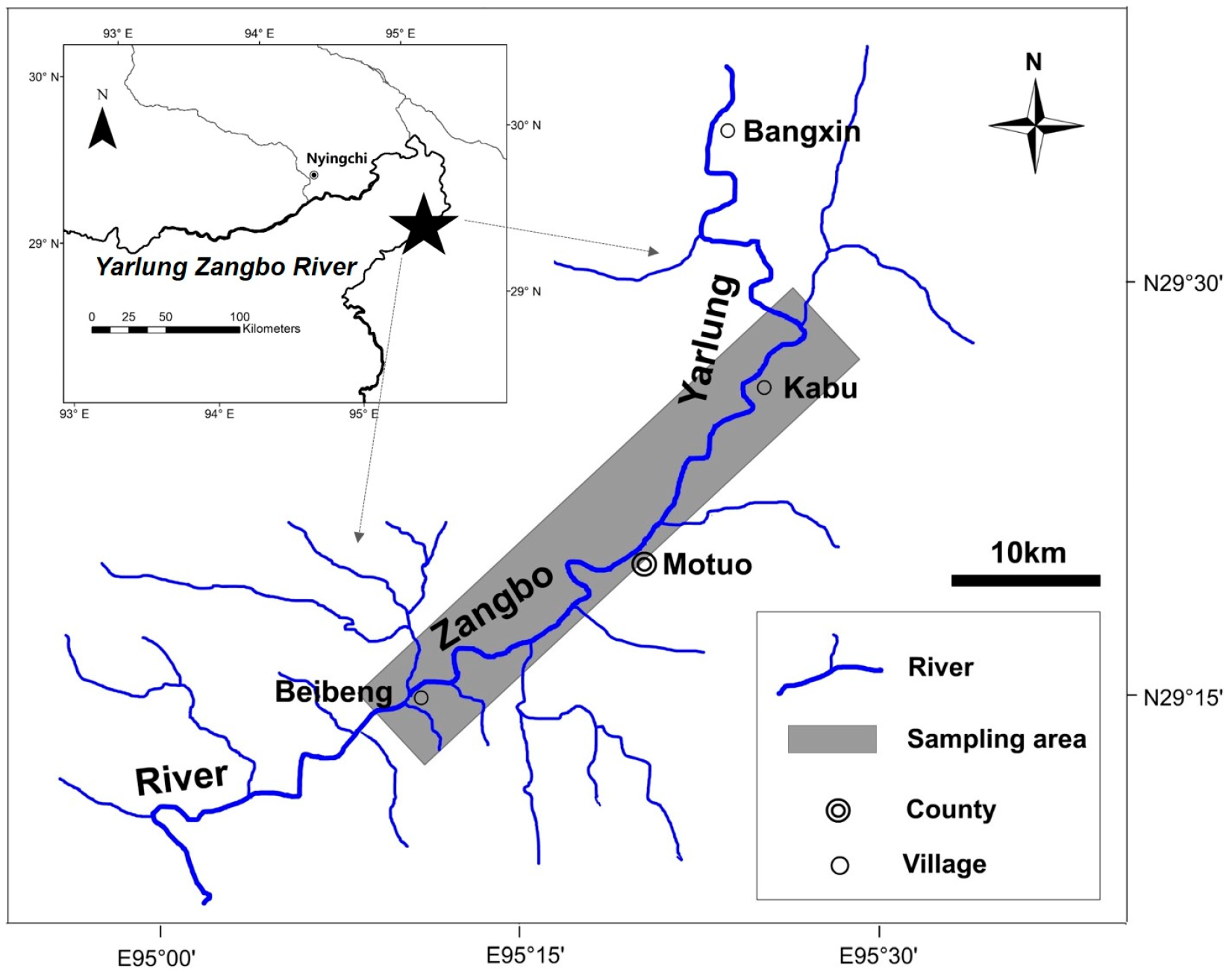
2.1. Study Area and Fish Samplings
2.2. Size at Sexual Maturity
2.3. Spawning Season
2.4. Fecundity
2.5. Statistical Analysis
3. Results
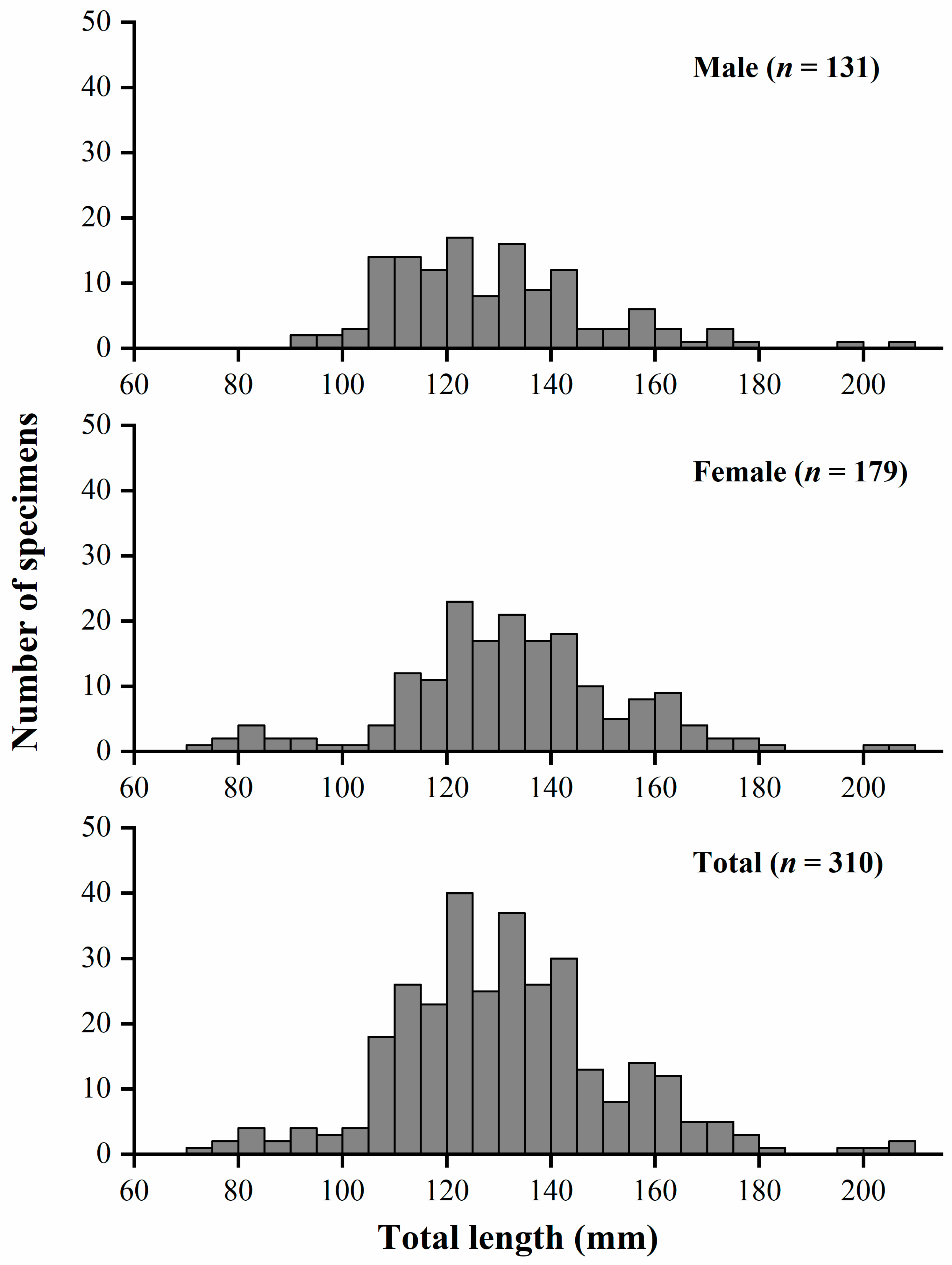
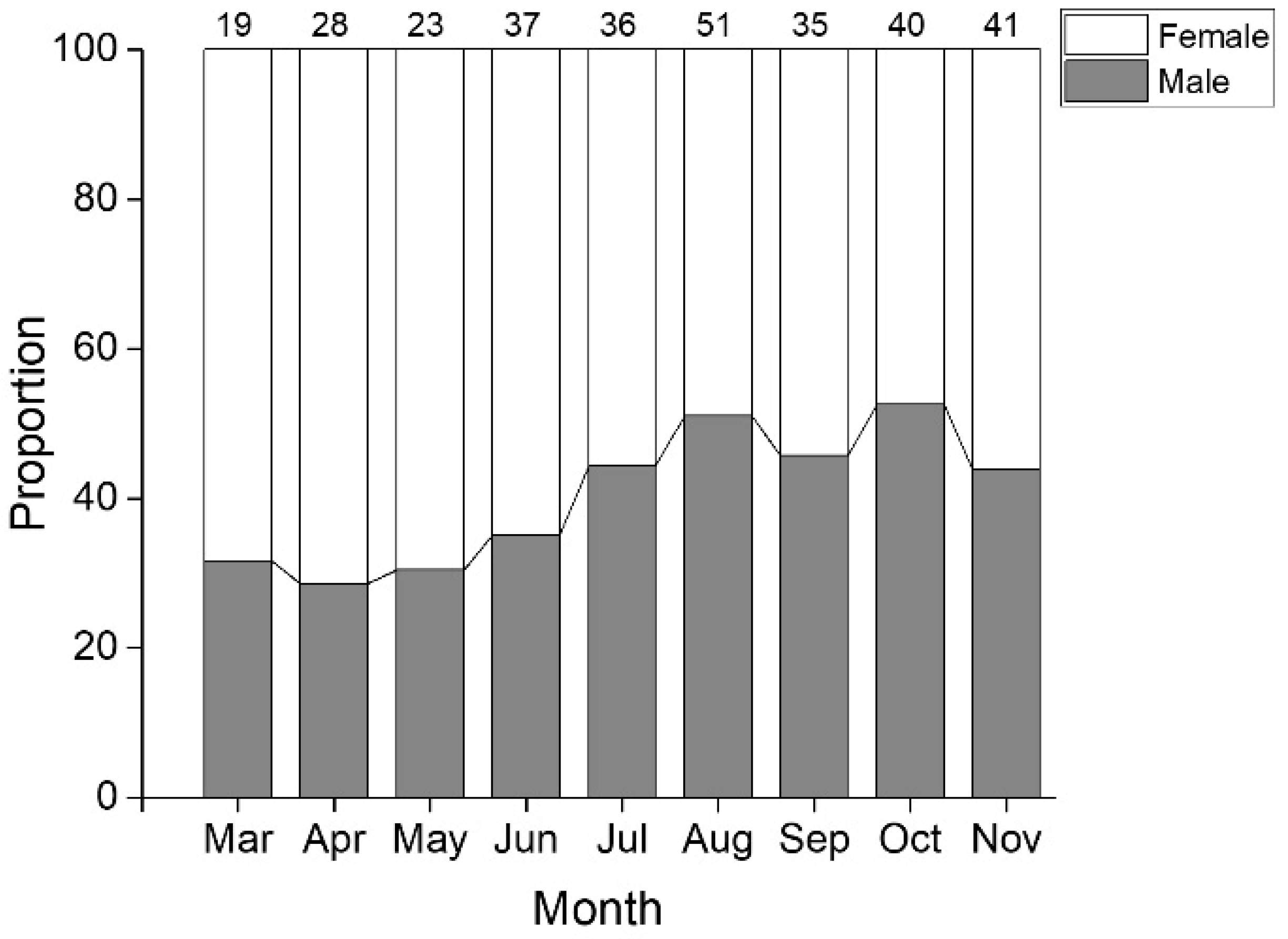
3.1. Distribution of Length Frequency and Sex Ratio
3.2. Length at Sexual Maturity
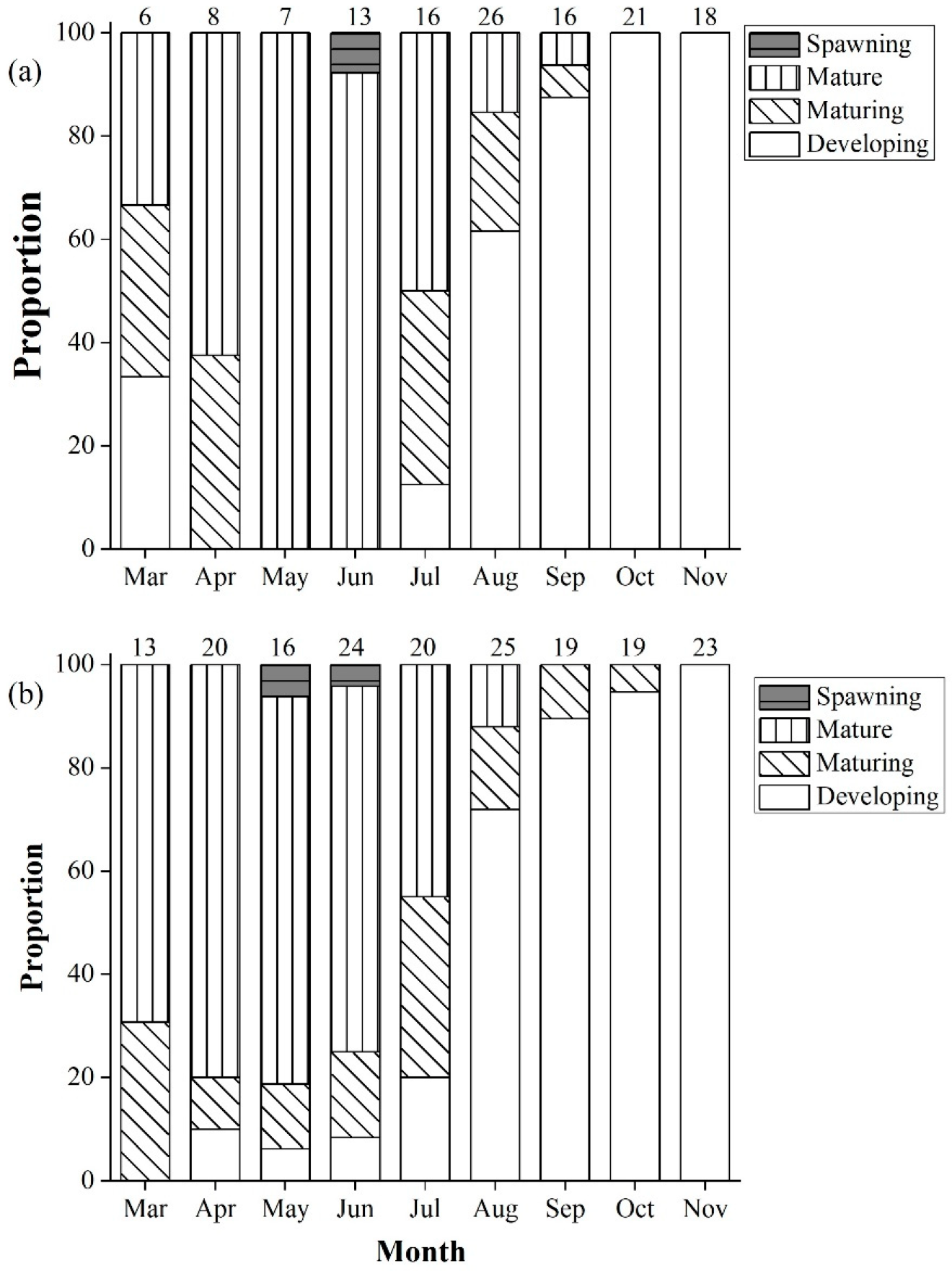
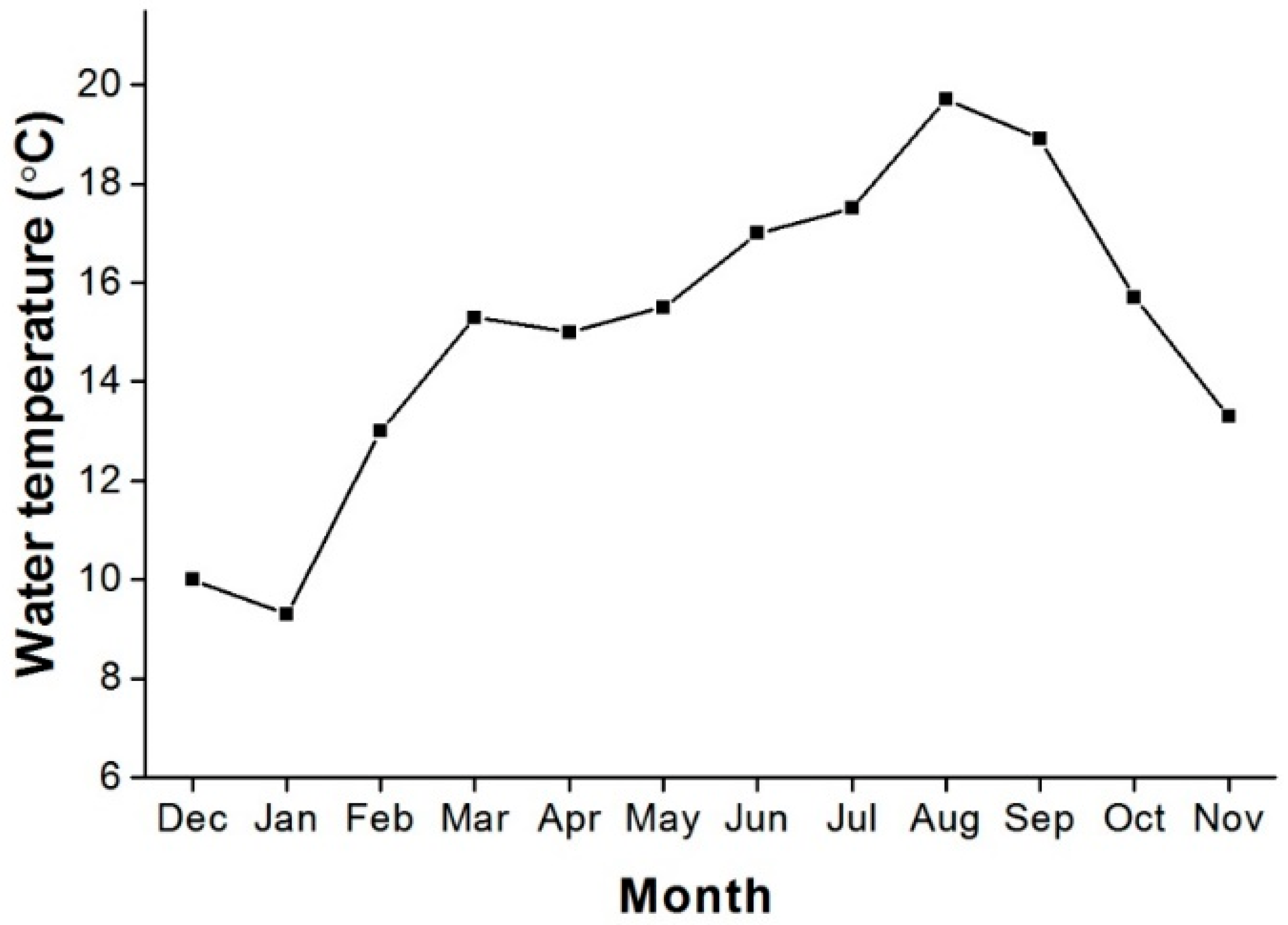
3.3. Spawning Season
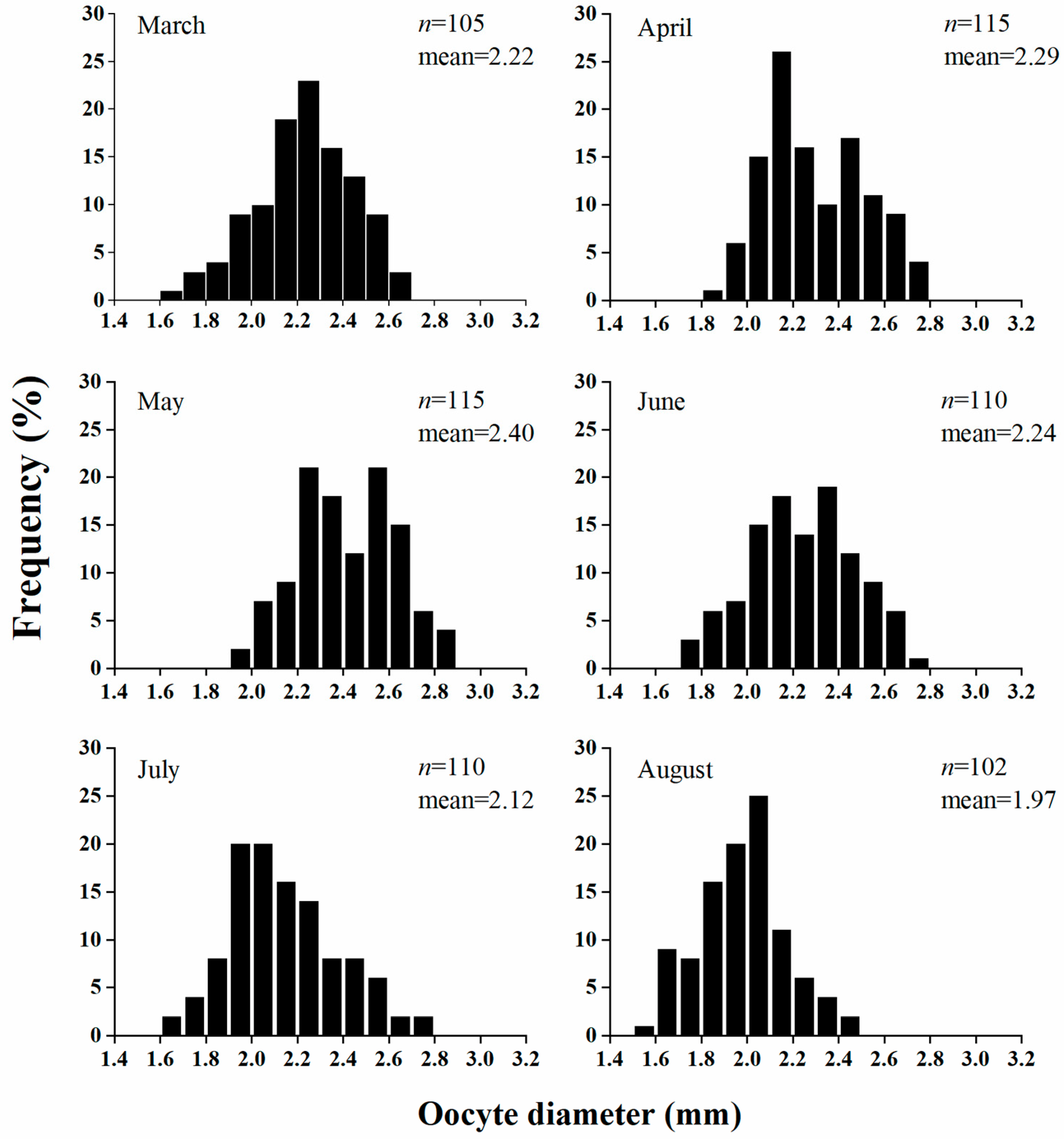
3.4. Fecundity
4. Discussion
4.1. Sex Ratio and Size at First Maturity
4.2. Gonadosomatic Index (GSI) and Spawning Season
4.3. Reproductive Effort and Fecundity
4.4. Reproductive Characteristics and Protection Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K. The World’s Forgotten Fishes; WWF International: Gland, Switzerland, 2021. [Google Scholar]
- Comizzoli, P.; Brown, J.L.; Holt, W. Reproductive Sciences in Animal Conservation, 2nd ed.; Springer: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar]
- Wootton, R.J.; Smith, C. Reproductive Biology of Teleost Fishes; Wiley: Oxford, UK, 2015. [Google Scholar]
- Angermeier, P.L. Ecological attributes of extinction-prone species: Loss of freshwater fishes of Virginia. Conserv. Biol. 1995, 9, 143–158. [Google Scholar] [CrossRef]
- Gao, D. The Greatest Canyon in the World: History, Resources and Their Associations with Natural Environment and Mankind; Zhejiang Education Publishing House: Hangzhou, China, 2001. [Google Scholar]
- Wu, P.P.; Wang, Z.; Jia, N.X.; Dong, S.Q.; Qu, X.Y.; Qiao, X.G.; Liu, C.C.; Guo, K. Vegetation classification and distribution patterns in the south slope of Yarlung Zangbo Grand Canyon National Nature Reserve, Eastern Himalayas. Plants 2022, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, M.Z.; Wang, J.; Gong, Z.; Liu, M.; Liu, H.Z.; Lin, P.C. Species composition and longitudinal patterns of fish assemblages in the middle and lower Yarlung Zangbo River, Tibetan Plateau, China. Ecol. Indic. 2021, 125, 107542. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.; Zhou, W.; Zhang, M.H. Distribution, habits and resources conservation of catfish Pseudecheneis (Siluriforms: Sisoridae) in China. J. Guangxi Norm. Univ. 2018, 36, 111–117. [Google Scholar]
- Jin, H.Y.; Li, L.; Jin, X.; Wang, N.M.; Ma, Q.Z.; Yin, J.S.; Ma, B. Age and growth of Pseudecheneis sulcatus in lower reach of Yarlung Zangbo River of Medog county reach. Freshw. Fish. 2020, 50, 10–17. [Google Scholar]
- Das, D.; Nag, T.C. Adhesion by paired pectoral and pelvic fins in a mountain-stream catfish, Pseudocheneis sulcatus (Sisoridae). Environ. Biol. Fishes 2004, 71, 1–5. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Pan, J.F.; Zhou, W.; Zhang, Y.P.; Zhang, Q. Taxonomy and molecular evolution of genus Pseudecheneis in family Sisoridae inferred from partial mitochondrial DNA sequences. J. Southwest For. Coll. 2007, 27, 45–51. [Google Scholar]
- Li, L.; Ma, B.; Gong, J.; Chen, Z.; Cheng, L.; Wu, S.; Ji, F. Length-weight relationships of 6 fish species from the lower reaches of the YarlungZangbo River, Southwest China. J. Appl. Ichthyol. 2018, 34, 1062–1064. [Google Scholar] [CrossRef]
- Dey, A.; Choudhury, H.; Basumatary, S.; Bharali, R.C.; Sarma, D. Length–weight relationships of three freshwater fish species from the Kameng River (Brahmaputra basin) in Arunachal Pradesh, Northeast India. J. Appl. Ichthyol. 2018, 34, 1002–1003. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, T.B.; Zhou, C.W.; Pan, Y.Z.; Liu, F.; Mou, Z.B. Length-weight relationships of three small fish species caught in the Yarlung Zangbo River basin, China. J. Appl. Ichthyol. 2018, 34, 1009–1010. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Z.H.; Zhao, X.T.; Xiao, K.Y. Glacial dammed lakes in the Tsangpo River during late Pleistocene, southeastern Tibet. Quat. Int. 2013, 298, 114–122. [Google Scholar] [CrossRef]
- Somerton, D.A. A computer technique for estimating the size of sexual maturity in crabs. Can. J. Fish. Aquat. Sci. 1980, 37, 1488–1494. [Google Scholar] [CrossRef]
- Ding, C.Z.; Chen, Y.F.; He, D.K.; Yao, J.L.; Chen, F. Reproductive biology of Glyptosternon maculatum in Yarlung Tsangpo River in Tibet, China. Acta Hydrobiol. Sin. 2010, 34, 762–768. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Chen, Y.; Paloheimo, J.E. Estimating fish length and age at 50% maturity using a logistic type model. Aquat. Sci. 1994, 56, 206–219. [Google Scholar] [CrossRef]
- Brewer, S.K.; Rabeni, C.F.; Papoulias, D.M. Comparing histology and gonadosomatic index for determining spawning condition of small-bodied riverine fishes. Ecol. Freshw. Fish 2008, 17, 54–58. [Google Scholar] [CrossRef]
- Kirczuk, L.; Dziewulska, K.; Czernieewski, P.; Brysiewicz, A.; Government, I. Reproductive potential of stone moroko (Pseudorasbora parva, Temminck et Schlegel, 1846) (Teleostei: Cypriniformes: Gobionidae) inhabiting Central Europe. Animals 2021, 11, 2627. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1999. [Google Scholar]
- Gong, Z.; Chen, L.; Wang, J.; Liu, H.Z. The reproductive characteristics of Garra tibetana, an endemic labeonine fish in the lower Yarlung Tsangpo River, Tibet, China. Fishes 2022, 7, 104. [Google Scholar] [CrossRef]
- Ma, B.S.; Xie, C.X.; Huo, B.; Yang, X.F.; Chen, S.S. Reproductive biology of Schizothorax o’connori (Cyprinidae: Schizothoracinae) in the Yarlung Zangbo River, Tibet. Zool. Stud. 2012, 51, 1066–1076. [Google Scholar]
- Bagenal, T.B. Methods for Assessment of Fish Production in Fresh Waters, 3rd ed.; Blackwell Scientific Publishing: Oxford, UK, 1978; pp. 165–201. [Google Scholar]
- Adebisi, A.A. The relationships between fecundities, gonadosomatic indices and egg sizes of some fishes of Ogun River, Nigeria. Arch. Für Hydrobiol. 1987, 111, 151–156. [Google Scholar] [CrossRef]
- Fryxell, D.C.; Arnett, H.A.; Apgar, T.M.; Kinnison, M.T.; Palkovacs, E.P. Sex ratio variation shapes the ecological effects of a globally introduced freshwater fish. Proc. R. Soc. B-Biol. Sci. 2015, 282, 20151970. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Hu, H.M.; Liu, F.; Li, M.Z.; Liu, H.Z. Reproductive characteristics of Psilorhynchus homaloptera Hora and Mukerji, 1935 (Cyprinidae: Psilorhynchidae) in the lower YarlungZangbo River, Tibet. J. Oceanol. Limnol. 2021, 39, 1513–1522. [Google Scholar] [CrossRef]
- Huo, B.; Xie, C.X.; Ma, B.S.; Yang, X.F.; Huang, H.P. Reproductive biology of Oxygymnocypris stewartii in the Yarlung Zangbo River in Tibet, China. Environ. Biol. Fishes. 2013, 96, 481–493. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.M.; Liang, L.Y.; Wang, Y.M.; Peng, P.L.; Xie, B.W. Individual Fecundity of Euchiloglanis kishinouyei in the Zumuzu River. Sichuan J. Zool. 2019, 38, 305–310. [Google Scholar]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Selection for increased male size predicts variation in sexual size dimorphism among fish species. Proc. R. Soc. B-Biol. Sci. 2020, 287, 20192640. [Google Scholar] [CrossRef]
- Wang, J. Fish community structure in the lower reach Yarlung Tsangpo River and the biological traits of Schizothorax curvilabiatusa. Doctoral Dissertation, University of Chinese Academy of Sciences, Wuhan, China, 2018. [Google Scholar]
- Morbey, Y.E. Female-biased dimorphism in size and age at maturity is reduced at higher latitudes in lake whitefish Coregonus clupeaformis. J. Fish Biol. 2018, 93, 40–46. [Google Scholar] [CrossRef]
- Duarte, C.M.; Alcaraz, M. To produce many small or few large eggs: A size-independent reproductive tactic of fish. Oecologia 1989, 80, 401–404. [Google Scholar] [CrossRef]
- Flores, A.; Wiff, R.; Ganias, K.; Marshall, C.T. Accuracy of gonadosomatic index in maturity classification and estimation of maturity ogive. Fish. Res. 2019, 210, 50–62. [Google Scholar] [CrossRef]
- Teletchea, F.; Fostier, A.; Kamler, E.; Gardeur, J.N.; Bail, P.; Jalabert, B.; Fontaine, P. Comparative analysis of reproductive traits in 65 freshwater fish species: Application to the domestication of new fish species. Rev. Fish. Biol. Fish. 2009, 19, 403–430. [Google Scholar] [CrossRef]
- Chowdhary, S.T.; Shahid, I. Maturity and breeding of Labeogonius (Ham.) from Brahmaputra. J. Biosph. 2018, 7, 44–54. [Google Scholar]
- Saxena, N.; Patiyal, R.S.; Rawat, K.D.; Tiwari, V.K. Population dynamics and reproductive biology of Barilius bendelisis (Cyprinidae: Cypriniformes) from river Gaula of Central Indian Himalaya. Rev. Biol. Trop. 2016, 64, 1287–1295. [Google Scholar] [CrossRef]
- Patimar, R.; Abdoli, A.; Kiabi, B.H. Biological characteristics of the introduced sawbelly, Hemiculter leucisculus (Basilewski, 1855), in three wetlands of northern Iran: Alma-Gol, Adji-Gol and Ala-Gol. J. Appl. Ichthyol. 2008, 24, 617–620. [Google Scholar] [CrossRef]
- Dobriyal, A.K.; Singh, H.R. Reproductive biology of a hillstream catfish, Glyptothorax madraspatanum (Day), from the Garhwal, Central Himalaya, India. Aquac. Res. 1993, 24, 699–706. [Google Scholar] [CrossRef]
- Amini, M.; Bahrami, S.; Bakhshi, F.; Tahmasebi, A.H.; Shahrani, F. Growth, reproduction and feeding biology of an endemic sucker catfish, Glyptothorax silviae Coad, 1981 (Actinopterygii: Sisoridae), in the Maroon River, Iran. Int. J. Aquat. Biol. 2014, 2, 1–8. [Google Scholar]
- Liu, Y.T.; Tian, S.K.; Leng, Y.; Xue, C.J.; Li, X.S.; Fu, G.H. Study on the biological characterstics of wild Bagarius yarrelli. Mod. Agric. Sci. Technol. 2010, 18, 302–307. [Google Scholar]
- Coburn, M.M. Egg diameter variation in Eastern North American minnows (Pisces: Cyprinidae): Correlation with vertebral number, habitat and spawning behavior. Ohio J. Sci. 1986, 86, 110–120. [Google Scholar]
- Beck, S.V.; Räsänen, K.; Kristjánsson, B.K.; Skúlason, S.; Jónsson, Z.O.; Tsinganis, M.; Leblanc, C.A. Variation in egg size and offspring phenotype among and within seven Arctic charr morphs. Ecol. Evol. 2022, 12, e9427. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Werner, R.G. Fishery Science: The Unique Contributions of Early Life Stages; Blackwell Science: Hoboken, NJ, USA, 2002. [Google Scholar]
- Nierenberg, W.A. Encyclopedia of Environmental Biology; Academic Press: San Diego, CA, USA, 1995; Volume 2, pp. 49–65. [Google Scholar]




| Species | River Drainage | Max Standard Length (mm) | Oocyte Diameter (mm) | Absolute Fecundity (Eggs) | Relative Fecundity (Eggs/g) |
|---|---|---|---|---|---|
| Pseudecheneis sulcatus | Yarlung Zangbo River | 177 | 2.39 | 911 | 40.4 |
| Glyptothorax madraspatanum | Western Nayar River | 152 | 1.62 | 3213 | 128 |
| Glyptosternon maculaturn | Lhasa River | 243 | 2.83 | 1244 | 14.7 |
| Glyptothorax silviae | Maroon River | 96 | 1.29 | 1129 | 105 |
| Euchiloglanis kishinouyei | Dadu River | 168 | 4.38 | 248 | 6.6 |
| Bagarius yarrelli | Lancang River | 800 | 1.20 | 640,200 | 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Hu, H.; Gong, Z.; Wang, J.; Gao, X. Reproductive Characteristics of Pseudecheneis sulcatus (Siluriforms: Sisoridae) in the Lower Yarlung Zangbo River, Tibet. Fishes 2023, 8, 106. https://doi.org/10.3390/fishes8020106
Lin P, Hu H, Gong Z, Wang J, Gao X. Reproductive Characteristics of Pseudecheneis sulcatus (Siluriforms: Sisoridae) in the Lower Yarlung Zangbo River, Tibet. Fishes. 2023; 8(2):106. https://doi.org/10.3390/fishes8020106
Chicago/Turabian StyleLin, Pengcheng, Huaming Hu, Zheng Gong, Jian Wang, and Xin Gao. 2023. "Reproductive Characteristics of Pseudecheneis sulcatus (Siluriforms: Sisoridae) in the Lower Yarlung Zangbo River, Tibet" Fishes 8, no. 2: 106. https://doi.org/10.3390/fishes8020106
APA StyleLin, P., Hu, H., Gong, Z., Wang, J., & Gao, X. (2023). Reproductive Characteristics of Pseudecheneis sulcatus (Siluriforms: Sisoridae) in the Lower Yarlung Zangbo River, Tibet. Fishes, 8(2), 106. https://doi.org/10.3390/fishes8020106





