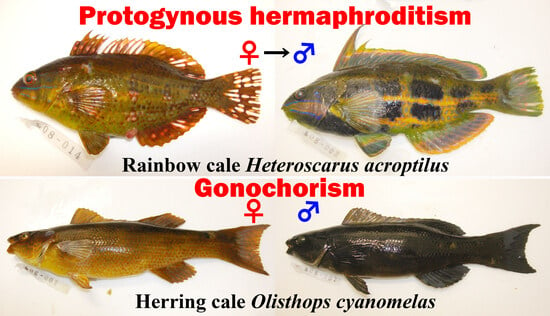
Reproductive Behavior and Sexual Patterns in Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae) at Rocky Reefs in Temperate Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Site
2.2. Underwater Observations
2.3. Specimen Collection
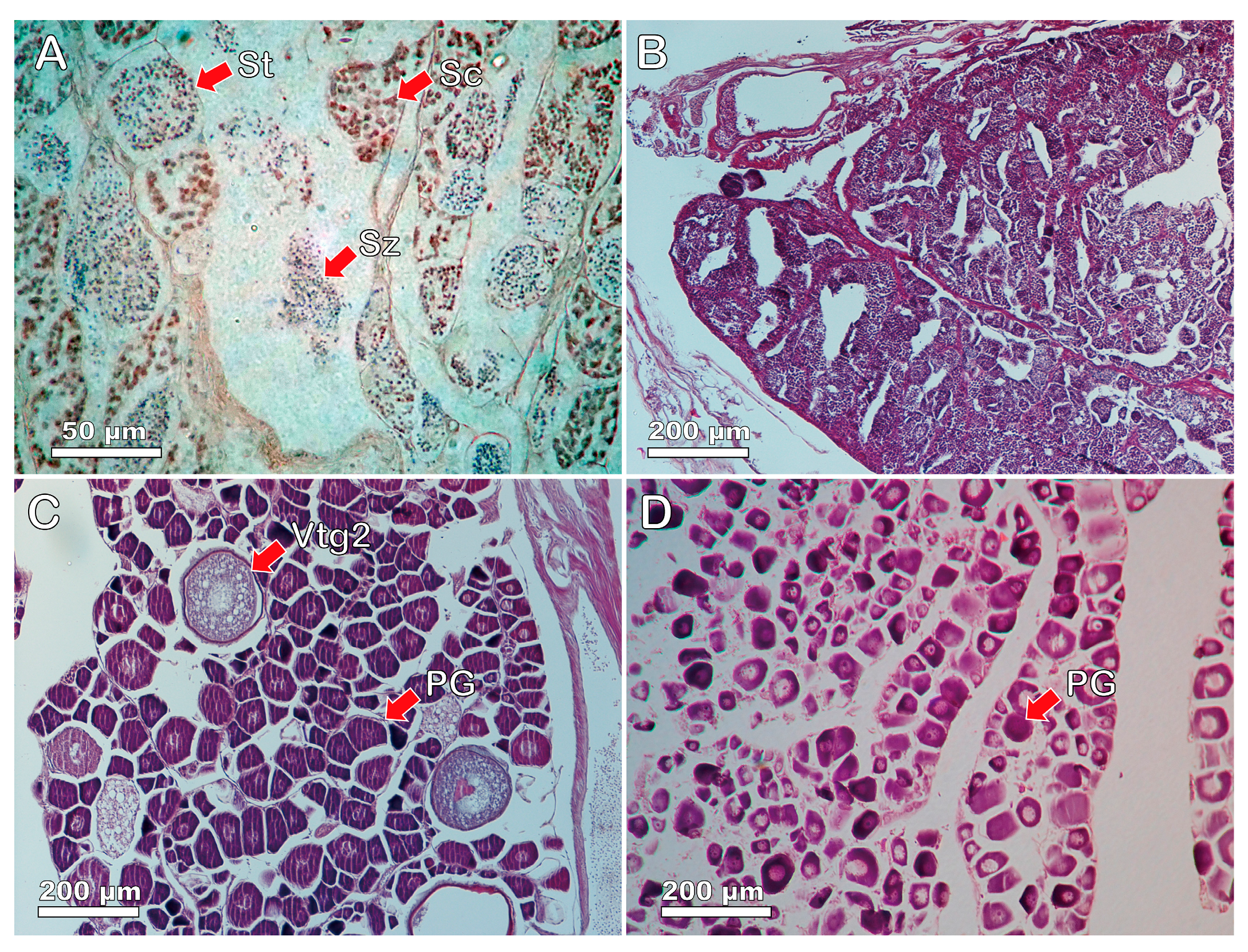
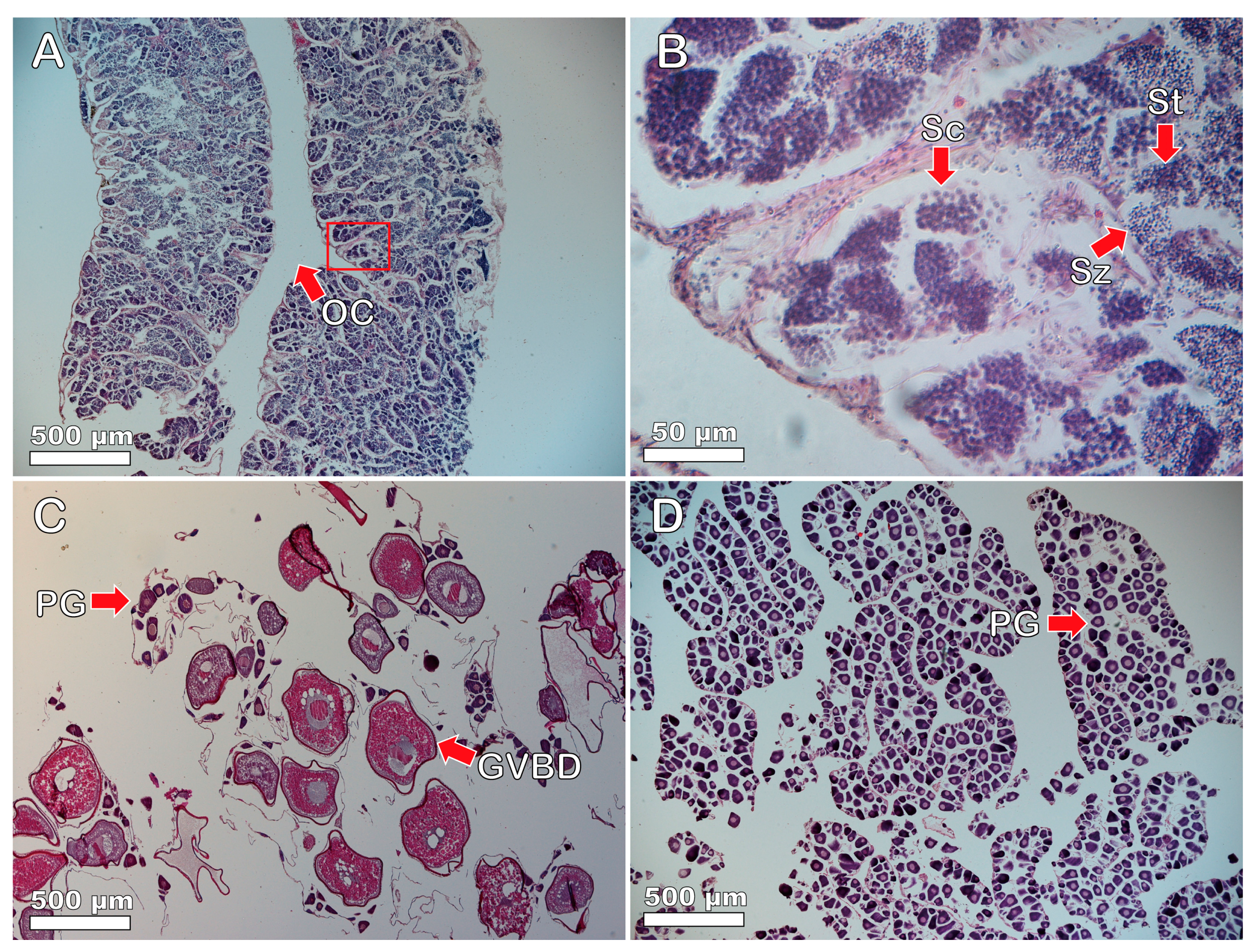
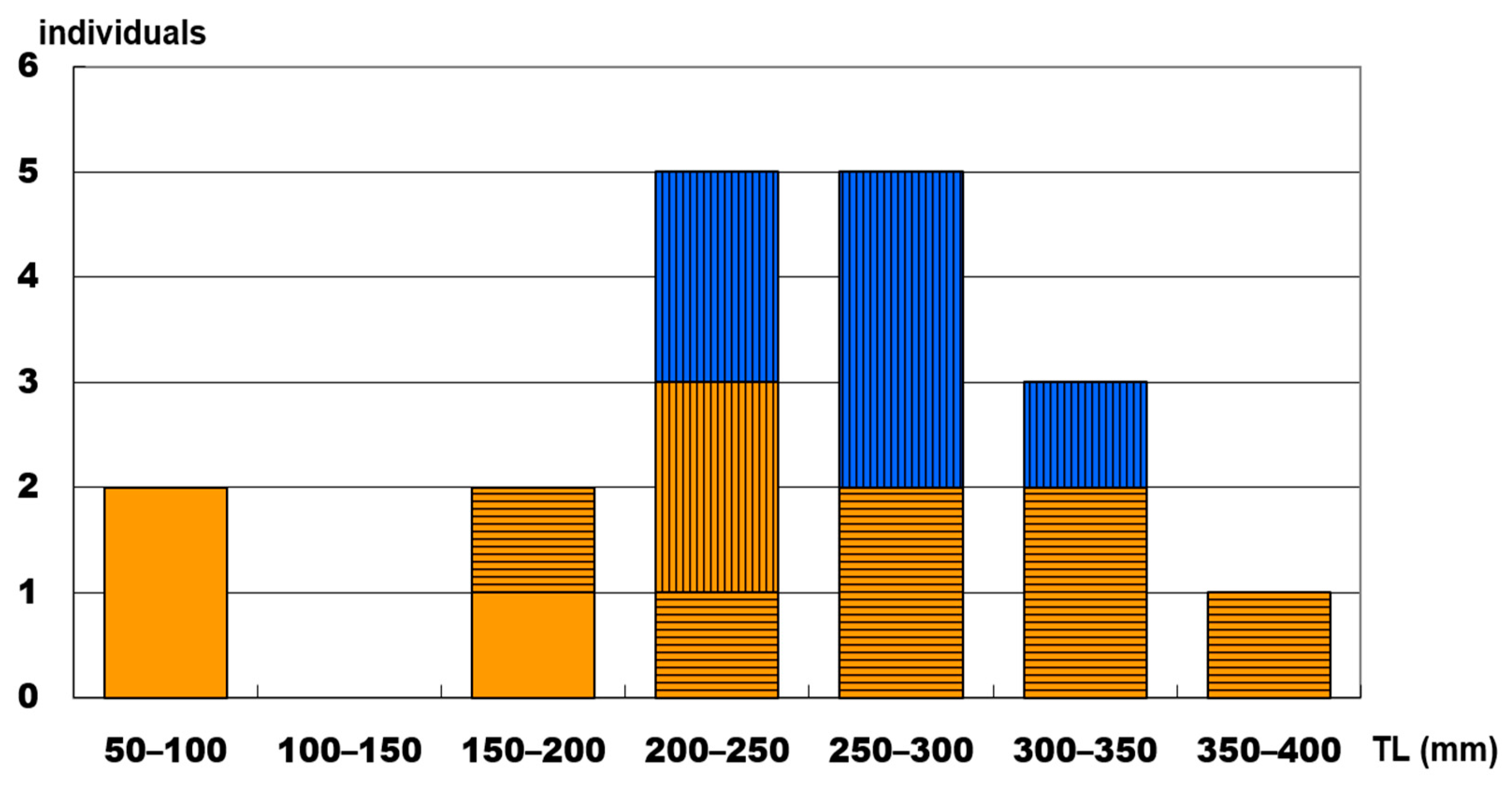
2.4. Gonad Histology
3. Results
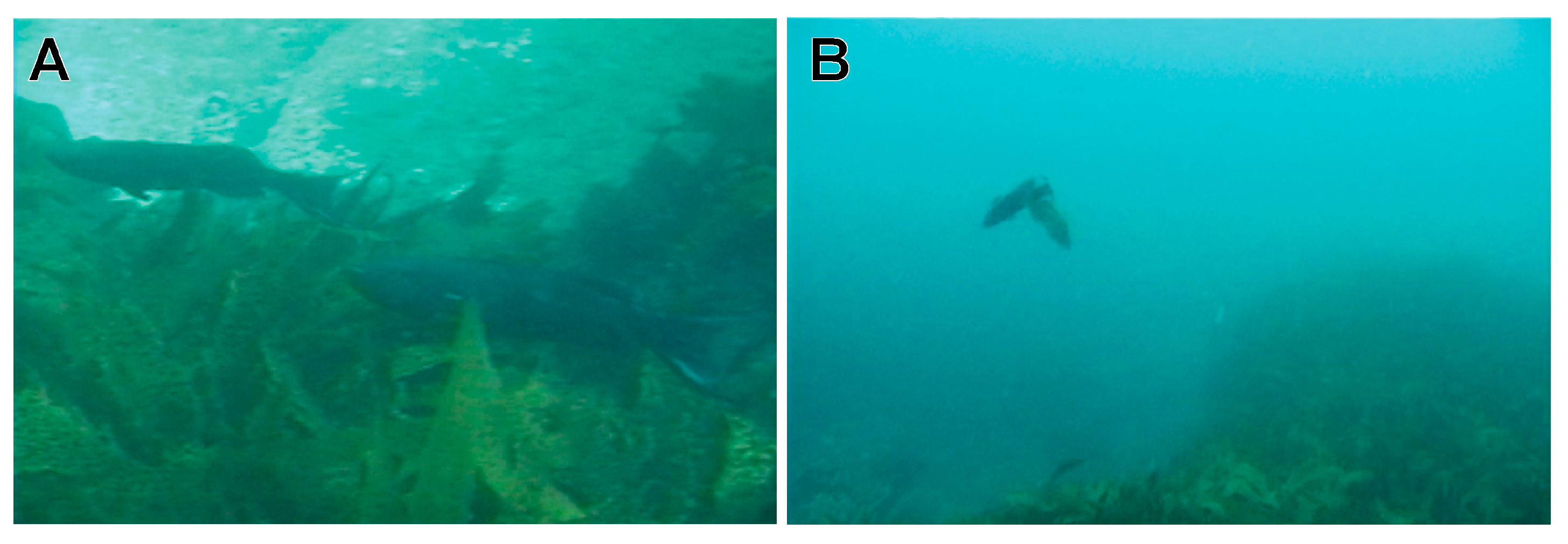
3.1. Reproductive Behavior of Heteroscarus acroptilus
3.2. Reproductive Behavior of Olisthops cyanomelas
3.3. Gonadal Tissue and Sexual Systems of Heteroscarus acroptilus
3.4. Gonadal Tissue and Sexual Patterns of Olisthops cyanomelas
4. Discussion
4.1. Reproductive Behavior
4.2. Sexual Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Gomon, M.; Paxton, J.R. A Revision of the Odacidae, a Temperate Australian-New Zealand Labroid Fish Family; Indo-Pacific Fishes; Bishop Museum: Honolulu, HI, USA, 1985; Volume 8, pp. 1–57. [Google Scholar]
- Clements, K.D.; Alfaro, M.E.; Fessler, J.L.; Westneat, M.W. Relationships of the temperate Australasian labrid fish tribe Odacini (Perciformes; Teleostei). Mol. Phylogenet. Evol. 2004, 32, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Westneat, M.W.; Alfaro, M.E. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 2005, 36, 370–390. [Google Scholar] [CrossRef] [PubMed]
- Clements, K.D.; Choat, J.H. Influence of season, ontogeny and tide on the diet of the temperate marine herbivorous fish Odax pullus (Odacidae). Mar. Biol. 1993, 117, 213–220. [Google Scholar] [CrossRef]
- Trip, E.D.L.; Raubenheimer, D.; Clements, K.D.; Choat, J.H. Reproductive demography of a temperate protogynous and herbivorous fish, Odax pullus (Labridae, Odacini). Mar. Freshw. Res. 2011, 62, 176–186. [Google Scholar] [CrossRef]
- Trip, E.D.; Clements, K.D.; Raubenheimer, D.; Choat, J.H. Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. J. Anim. Ecol. 2014, 83, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Trip, E.D.; Clements, K.D.; Raubenheimer, D.; Choat, J.H. Reproductive biology of an odacine labrid, Odax pullus. J. Fish Biol. 2011, 78, 741–761. [Google Scholar] [CrossRef] [PubMed]
- Paulin, C.; Roberts, C. The Rockpool Fishes of New Zealand Te Ika Aaria o Aotearoa; Museum of New Zealand Te Papa Tongarewa: Wellington, New Zealand, 1992. [Google Scholar]
- Andrew, N.L.; Jones, G.P. Patch formation by herbivorous fish in a temperate Australian kelp forest. Oecologia 1990, 85, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.R. The Adaptive Significance of Sequential Hermaphroditism in Animals. Am. Nat. 1975, 109, 61–82. [Google Scholar] [CrossRef]
- Warner, R.R. Mating behavior and hermaphroditism in coral reef fishes. Am. Sci. 1984, 72, 128–136. [Google Scholar]
- Gomon, M.F.; Glover, J.C.M.; Kuiter, R.H. Fishes of Australia’s Southern Coast; State Print: Adelaide, Australia, 1994. [Google Scholar]
- Gomon, M.F.; Bray, D.J.; Kuiter, R.H. Fishes of Australia’s Southern Coast; New Holland Publishers: Wahroonga, Australia, 2008. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Kawase, H.; Sunobe, T. Photos of Collected Specimens and Their Gonadal Tissues of Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae, Pisces), Inhabiting Rocky Reefs in Temperate Australia. figshare. 2023. [Google Scholar] [CrossRef]
- Okiyama, M. (Ed.) An Atlas of Early Stage Fishes in Japan, 2nd ed.; Tokai University Press: Hadano, Japan, 2014. [Google Scholar]
- Morton, J.K.; Gladstone, W.; Hughes, J.M.; Stewart, J. Comparison of the life histories of three co-occurring wrasses (Teleostei: Labridae) in coastal waters of south-eastern Australia. Mar. Freshw. Res. 2008, 59, 560–574. [Google Scholar] [CrossRef]
- Nakazono, A. Studies on the Sex Reversal and Spawning Behavior of Five Species of Japanese Labrid Fishes; Report of Fishery Research Laboratory; Kyushu University: Fukuoka, Japan, 1979; Volume 4, pp. 1–64. [Google Scholar]
- Kinoshita, Y. Studies on hibernation and sleep of wrasses. Zool. Mag. 1935, 47, 795–799. [Google Scholar]
- Kuwamura, T.; Sunobe, T.; Sakai, Y.; Kadota, T.; Sawada, K. Hermaphroditism in fishes: An annotated list of species, phylogeny, and mating system. Ichthyol. Res. 2020, 67, 341–360. [Google Scholar] [CrossRef]
- Sunobe, T. Protandry in fishes. In Hermaphroditism and Mating Systems in Fish; Kuwamura, T., Sawada, K., Sunobe, T., Sakai, Y., Kadota, T., Eds.; Springer: Singapore, 2022; pp. 63–85. [Google Scholar]
- Taborsky, M.; Hudde, B.; Wirtz, P. Reproductive behaviour and ecology of Symphodus (Crenilabrus) Ocellatus, a European wrasse with four types of male behaviour. Behaviour 1987, 102, 82–117. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawase, H.; Sunobe, T. Reproductive Behavior and Sexual Patterns in Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae) at Rocky Reefs in Temperate Australia. Fishes 2023, 8, 491. https://doi.org/10.3390/fishes8100491
Kawase H, Sunobe T. Reproductive Behavior and Sexual Patterns in Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae) at Rocky Reefs in Temperate Australia. Fishes. 2023; 8(10):491. https://doi.org/10.3390/fishes8100491
Chicago/Turabian StyleKawase, Hiroshi, and Tomoki Sunobe. 2023. "Reproductive Behavior and Sexual Patterns in Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae) at Rocky Reefs in Temperate Australia" Fishes 8, no. 10: 491. https://doi.org/10.3390/fishes8100491
APA StyleKawase, H., & Sunobe, T. (2023). Reproductive Behavior and Sexual Patterns in Two Cales, Heteroscarus acroptilus and Olisthops cyanomelas (Odacidae) at Rocky Reefs in Temperate Australia. Fishes, 8(10), 491. https://doi.org/10.3390/fishes8100491