Influence of Turbidity on Foraging Behaviour in Three-Spined Sticklebacks (Gasterosteus aculeatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sticklebacks for the Study
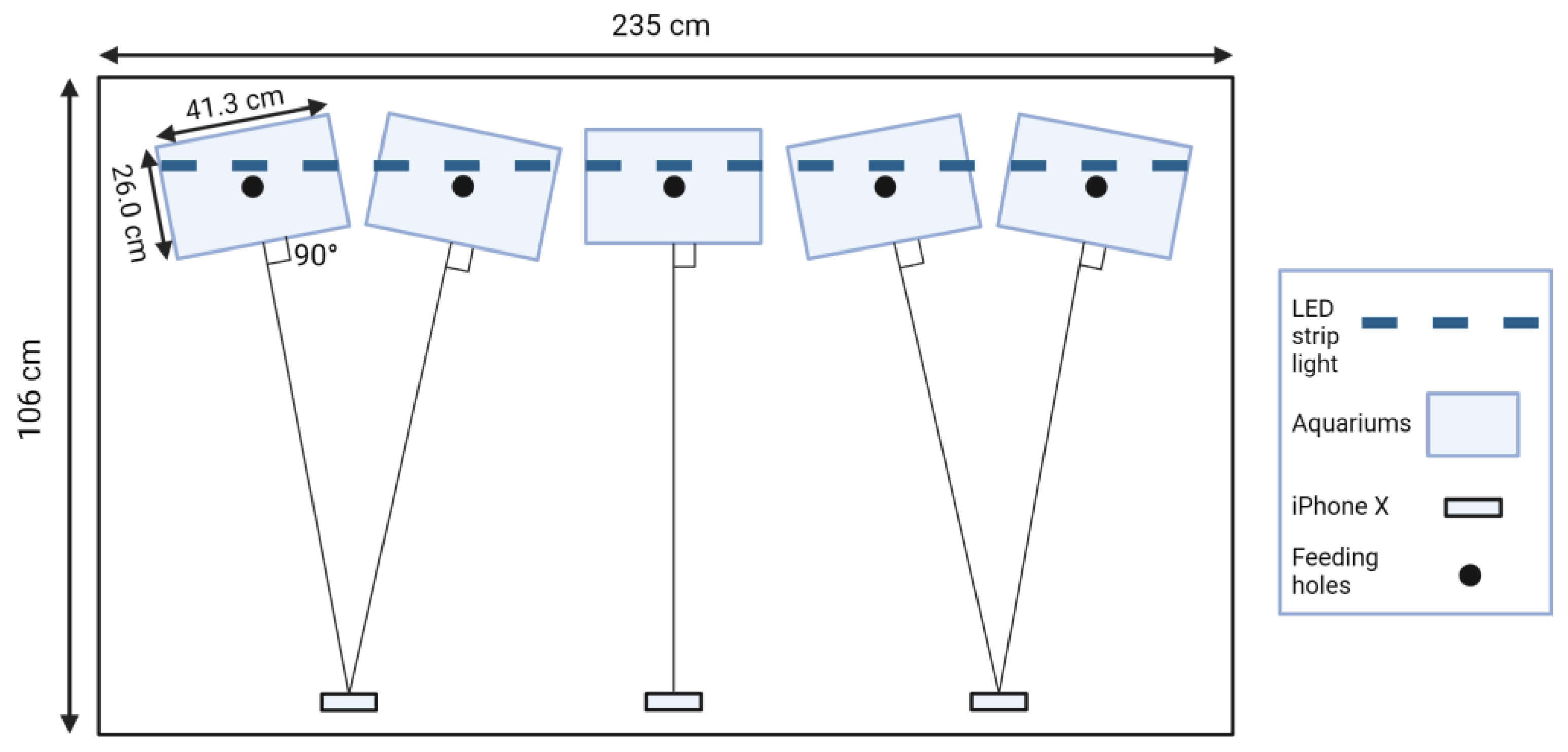
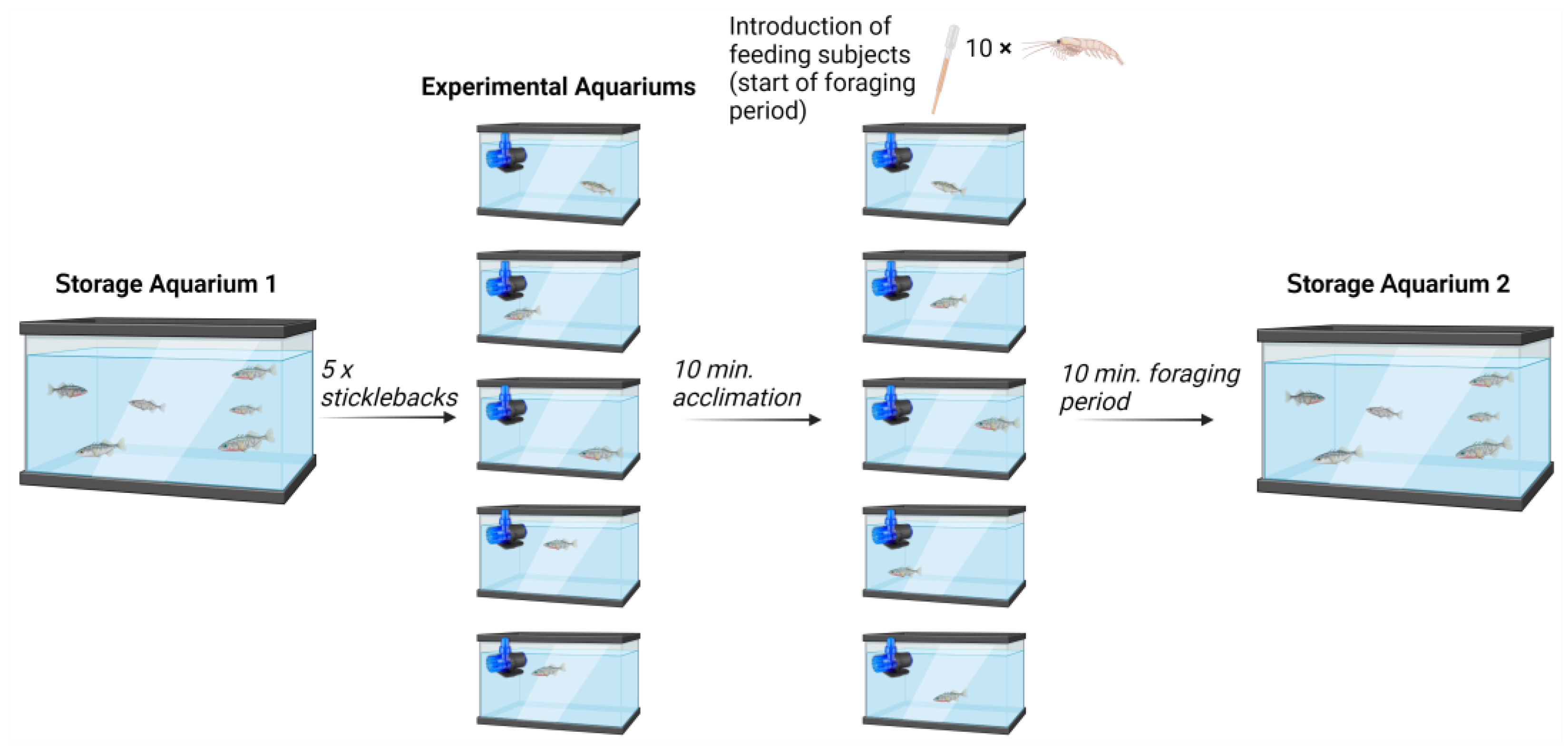
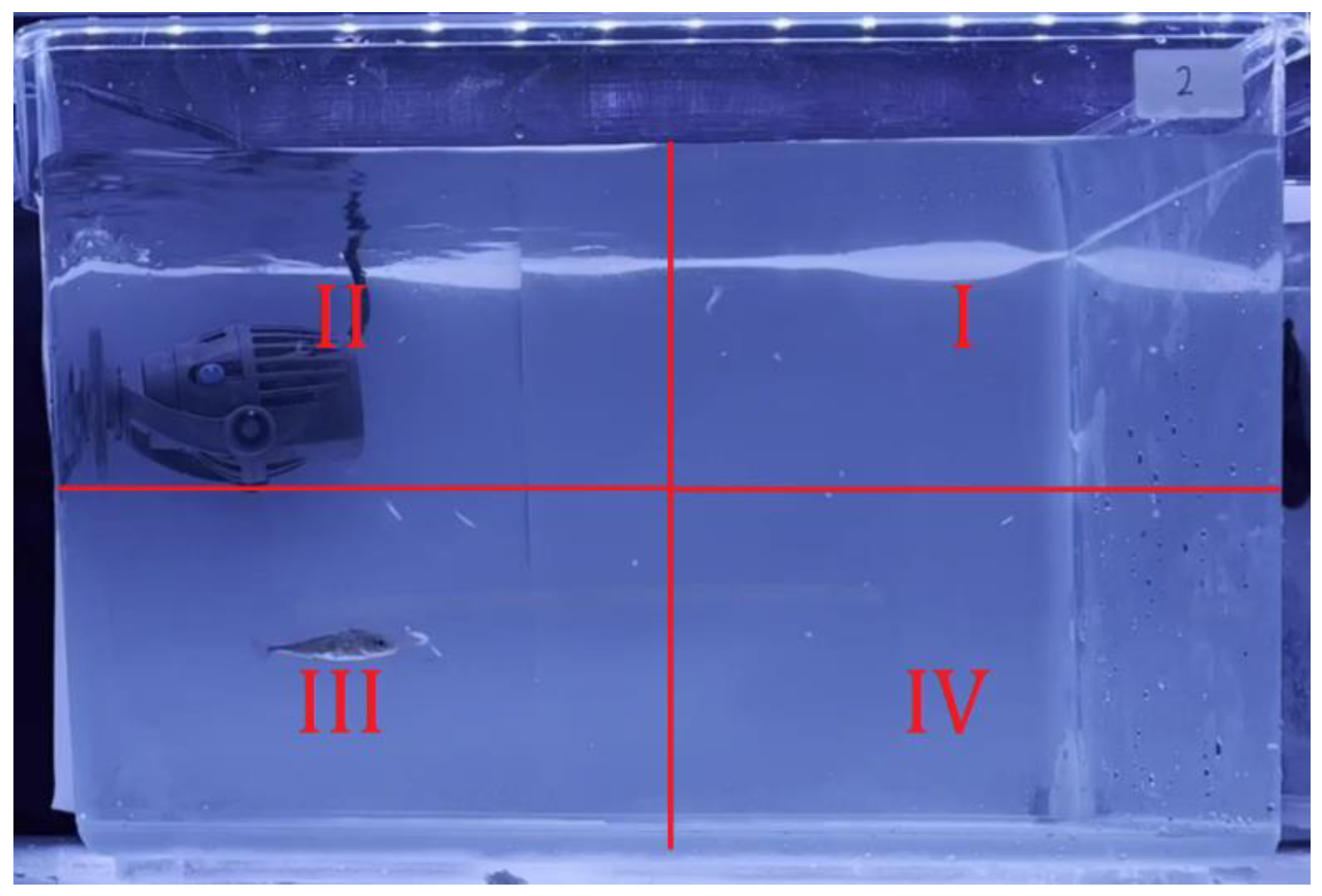
2.2. Design of Experimental Setup
2.3. Data and Statistical Analysis
3. Results
3.1. Sample Summary
3.2. The Influence of Change in Temperature on Foraging Behaviour and Activity
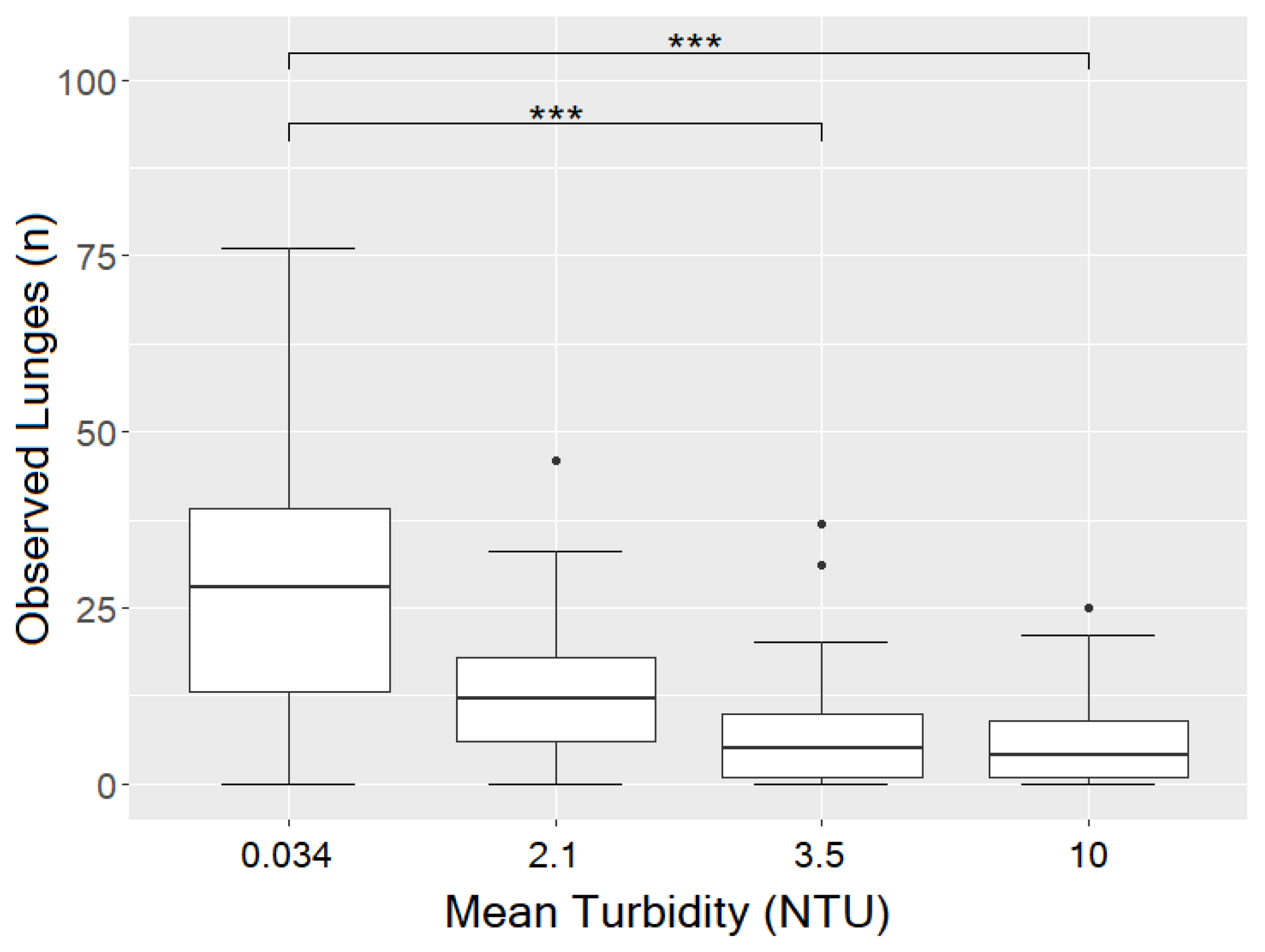
3.3. Influence of Turbidity on Observed Lunges
3.4. Behavioural Instability
3.5. The Influence of Turbidity on Activity and Time Spent in the Lower Half of Aquarium
4. Discussion
4.1. Methodology
4.2. The Influence of Temperature and Size
4.3. The Influence of Turbidity on Foraging Behaviour
4.4. The Influence of Turbidity on Activity and Vertical Placement
4.5. Behavioural Instability
- (a)
- The median absolute deviation increases with increasing levels of turbidity, with the exception of the highest turbidity (NTU 10), indicating a clear increase in the variability of feeding latency, which can be translated into a higher level of unpredictability in the time interval between a stimulus or opportunity for feeding and the initiation of feeding behaviour in an animal.
- (b)
- The reduction of kurtosis at the higher level of turbidity supports the increase in the median absolute deviation at the higher level of turbidity, as a reduction in kurtosis flattens the distribution curve and expands the tails of the distribution.
- (c)
- The reduction in the symmetry (skewness) observed at a higher level of turbidity indicates that at a low level of turbidity, the variation (of the time interval between a stimulus or opportunity for feeding and the initiation of feeding behaviour) is due to several long intervals of latency, which increase the tailness of the distribution on the right side. At higher levels of turbidity, the distributions tend to become more symmetric, which indicates that the increased variation observed at higher turbidity is not mainly due to higher skewness but is due to a flattening of the distribution and an increase of tailness on both sides of the distribution.
4.6. Eutrophication in Coastal Environments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Interval [s] | 0.03–2.15 NTU | 0.03–3.51 NTU | 0.03–10.12 NTU | 2.15–3.51 NTU | 2.15–10.12 NTU | 3.51–10.12 NTU |
|---|---|---|---|---|---|---|
| 0–59 | p > 0.05 | p < 0.001 | p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 |
| 60–119 | p < 0.05 | p < 0.001 | p < 0.001 | p > 0.05 | p > 0.05 | p > 0.05 |
| 120–179 | p > 0.05 | p < 0.001 | p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 |
| 180–239 | p > 0.05 | p < 0.01 | p < 0.001 | p > 0.05 | p > 0.05 | p > 0.05 |
| 240–299 | p > 0.05 | p < 0.05 | p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 |
| 300–359 | p > 0.05 | p < 0.01 | p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 |
| 360–419 | p > 0.05 | p < 0.05 | p < 0.01 | p > 0.05 | p > 0.05 | p > 0.05 |
| 420–479 | p > 0.05 | p > 0.05 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| 480–539 | p < 0.01 | p < 0.001 | p < 0.001 | p > 0.05 | p > 0.05 | p > 0.05 |
| 540–600 | p < 0.01 | p < 0.001 | p < 0.001 | p > 0.05 | p > 0.05 | p > 0.05 |
| Interval [s] | 0.03 NTU | 2.15 NTU | 3.51 NTU | 10.12 NTU |
|---|---|---|---|---|
| 0–59 and 240–299 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| 0–59 and 360–419 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| 0–59 and 420–479 | p < 0.001 | p > 0.05 | p > 0.05 | p > 0.05 |
| 0–59 and 480–539 | p < 0.05 | p > 0.05 | p > 0.05 | p < 0.01 |
| 0–59 and 540–600 | p < 0.05 | p < 0.01 | p > 0.05 | p < 0.05 |
| 60–119 and 420–479 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
References
- Wollschläger, J.; Neale, P.J.; North, R.L.; Striebel, M.; Zielinski, O. Editorial: Climate Change and Light in Aquatic Ecosystems: Variability and Ecological Consequences. Front. Mar. Sci. 2021, 8, 688712. [Google Scholar] [CrossRef]
- Gorokhovich, Y. Turbidity. In Encyclopedia of Estuaries; Kennish, M.J., Ed.; Springer: Dordrecht, Netherlands, 2016; pp. 720–721. [Google Scholar]
- Abrams, P.A. Optimal Traits When There are Several Costs: The Interaction of Mortality and Energy Costs in Determining Foraging Behavior. Behav. Ecol. 1993, 4, 246–259. [Google Scholar] [CrossRef]
- Vlieger, L.; Candolin, U. How Not to Be Seen: Does Eutrophication Influence Three-Spined Stickleback Gasterosteus aculeatus Sneaking Behaviour? J. Fish Biol. 2009, 75, 2163–2174. [Google Scholar] [CrossRef]
- Fischer, S.; Frommen, J.G. Eutrophication Alters Social Preferences in Three-Spined Sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 2013, 67, 293–299. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling Eutrophication Along the Freshwater-Marine Continuum: Dual Nutrient (N and P) Reductions are Essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef]
- McCrackin, M.L.; Jones, H.P.; Jones, P.C.; Moreno-Mateos, D. Recovery of Lakes and Coastal Marine Ecosystems from Eutrophication: A Global Meta-Analysis. Limnol. Oceanogr. 2017, 62, 507–518. [Google Scholar] [CrossRef]
- Riemann, B.; Carstensen, J.; Dahl, K.; Fossing, H.; Hansen, J.W.; Jakobsen, H.H.; Josefson, A.B.; Krause-Jensen, D.; Markager, S.; Stæhr, P.A.; et al. Recovery of Danish Coastal Ecosystems After Reductions in Nutrient Loading: A Holistic Ecosystem Approach. Estuaries Coasts 2016, 39, 82–97. [Google Scholar] [CrossRef]
- Marin-Diaz, B.; Bouma, T.J.; Infantes, E. Role of Eelgrass on Bed-Load Transport and Sediment Resuspension under Oscillatory Flow. Limnol. Oceanogr. 2020, 65, 426–436. [Google Scholar] [CrossRef]
- Ahnelt, H.; Ramler, D.; Madsen, M.Ø.; Jensen, L.F.; Windhager, S. Diversity and Sexual Dimorphism in the Head Lateral Line System in North Sea Populations of Threespine Sticklebacks, Gasterosteus aculeatus (Teleostei: Gasterosteidae). Zoomorphology 2021, 140, 103–117. [Google Scholar] [CrossRef]
- Barber, I. Sticklebacks as Model Hosts in Ecological and Evolutionary Parasitology. Trends Parasitol. 2013, 29, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Wootton, R.J.; Barber, I.; Przybylski, M.; Smith, C. Ecological Causes of Morphological Evolution in the Three-Spined Stickleback. Ecol. Evol. 2013, 3, 1717–1726. [Google Scholar] [CrossRef]
- Quesenberry, N.J.; Allen, P.J.; Cech, J.J., Jr. The Influence of Turbidity on Three-Spined Stickleback Foraging. J. Fish Biol. 2007, 70, 965–972. [Google Scholar] [CrossRef]
- Bretzel, J.B.; Geist, J.; Gugele, S.M.; Baer, J.; Brinker, A. Feeding Ecology of Invasive Three-Spined Stickleback (Gasterosteus aculeatus) in Relation to Native Juvenile Eurasian Perch (Perca fluviatilis) in the Pelagic Zone of Upper Lake Constance. Front. Environ. Sci. 2021, 9, 670125. [Google Scholar] [CrossRef]
- Olin, A.B.; Olsson, J.; Eklöf, J.S.; Eriksson, B.K.; Kaljuste, O.; Briekmane, L.; Bergström, U.; Ojaveer, H. Increases of Opportunistic Species in Response to Ecosystem Change: The Case of the Baltic Sea Three-Spined Stickleback. ICES J. Mar. Sci. 2022, 79, 1419–1434. [Google Scholar] [CrossRef]
- Tomczak, M.; Dinesen, G.E.; Hoffman, E.; Maar, M.; Støttrup, J.G. Integrated Trend Assessment of Ecosystem Changes in the Limfjord (Denmark): Evidence of a Recent Regime Shift? Estuar. Coast. Shelf Sci. 2013, 117, 178–187. [Google Scholar] [CrossRef]
- Sohel, S.; Lindström, K. Algal Turbidity Reduces Risk Assessment Ability of the Three-Spined Stickleback. Ethology 2015, 121, 548–555. [Google Scholar] [CrossRef]
- Pertoldi, C.; Bahrndorff, S.; Novicic, Z.K.; Rohde, P.D. The Novel Concept of “Behavioural Instability” and Its Potential Applications. Symmetry 2016, 8, 135. [Google Scholar] [CrossRef]
- Linder, A.C.; Lyhne, H.; Laubek, B.; Bruhn, D.; Pertoldi, C. Modeling Species-Specific Collision Risk of Birds with Wind Turbines: A Behavioral Approach. Symmetry 2022, 14, 2493. [Google Scholar] [CrossRef]
- Linder, A.C.; Lyhne, H.; Laubek, B.; Bruhn, D.; Pertoldi, C. Quantifying Raptors’ Flight Behavior to Assess Collision Risk and Avoidance Behavior to Wind Turbines. Symmetry 2022, 13, 2245. [Google Scholar] [CrossRef]
- Vollset, K.W.; Bailey, K.M. Interplay of Individual Interactions and Turbidity Affects the Functional Response of Three-Spined Sticklebacks Gasterosteus aculeatus. J. Fish Biol. 2011, 78, 1954–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Rice, W.R. Analyzing Tables of Statistical Tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Jolles, J.W.; Manica, A.; Boogert, N.J. Food Intake Rates of Inactive Fish are Positively Linked to Boldness in Three-Spined Sticklebacks Gasterosteus aculeatus. J. Fish Biol. 2016, 88, 1661–1668. [Google Scholar] [CrossRef]
- Sohel, S.; Mattila, J.; Lindström, K. Effects of Turbidity on Prey Choice of Three-Spined Stickleback Gasterosteus aculeatus. Mar. Ecol. 2017, 566, 159–167. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Barber, I.; Dochtermann, N.A. Non-Consumptive Effects of Predation: Does Perceived Risk Strengthen the Genetic Integration of Behaviour and Morphology in Stickleback? Ecol. Lett. 2020, 23, 107–118. [Google Scholar] [CrossRef]
- FitzGerald, G.J.; Wootton, R.J. Behavioural Ecology of Sticklebacks. In The Behaviour of Teleost Fishes; Pitcher, T.J., Ed.; Springer: Boston, MA, USA, 1986; pp. 409–432. [Google Scholar]
- Webster, M.M.; Atton, N.; Ward, A.J.W.; Hart, P.J.B. Turbidity and Foraging Rate in Threespine Sticklebacks: The Importance of Visual and Chemical Prey Cues. Behaviour 2007, 144, 1347–1360. [Google Scholar]
- Lefébure, R.; Larsson, S.; Byström, P. Temperature and Size-Dependent Attack Rates of the Three-Spined Stickleback (Gasterosteus aculeatus); Are Sticklebacks in the Baltic Sea Resource-Limited? J. Exp. Mar. Biol. Ecol. 2014, 451, 82–90. [Google Scholar] [CrossRef]
- Ajemian, M.J.; Sohel, S.; Mattila, J. Effects of Turbidity and Habitat Complexity on Antipredator Behavior of Three-Spined Sticklebacks (Gasterosteus aculeatus): Antipredator Behavior in Sticklebacks. Environ. Biol. Fishes 2015, 98, 45–55. [Google Scholar] [CrossRef]
- Engström-Öst, J.; Karjalainen, M.; Viitasalo, M. Feeding and Refuge Use by Small Fish in the Presence of Cyanobacteria Blooms. Environ. Biol. Fishes 2006, 76, 109–117. [Google Scholar] [CrossRef]
- Dinesen, G.E.; Timmermann, K.; Roth, E.; Markager, S.; Ravn-Jonsen, L.; Hjorth, M.; Holmer, M.; Støttrup, J.G. Mussel Production and Water Framework Directive Targets in the Limfjord, Denmark: An Integrated Assessment for Use in System-Based Management. Ecol. Soc. 2011, 16, 26. [Google Scholar] [CrossRef]
- Markager, S.; Storm, L.M.; Stedmon, C.A. Limfjordens Miljøtilstand 1985 til 2003. Sammenhæng Mellem Næringsstoftilførsler, Klima og Hydrografi Belyst ved Hjælp af Empiriske Modeller; Report 577; National Environmental Research Institute, Aarhus University: Aarhus, Denmark, 2006. [Google Scholar]
- Linder, A.C.; Gottschalk, A.; Lyhne, H.; Langbak, M.G.; Jensen, T.H.; Pertoldi, C. Using Behavioral Instability to Investigate Behavioral Reaction Norms in Captive Animals: Theoretical Implications and Future Perspectives. Symmetry 2020, 12, 603. [Google Scholar] [CrossRef]


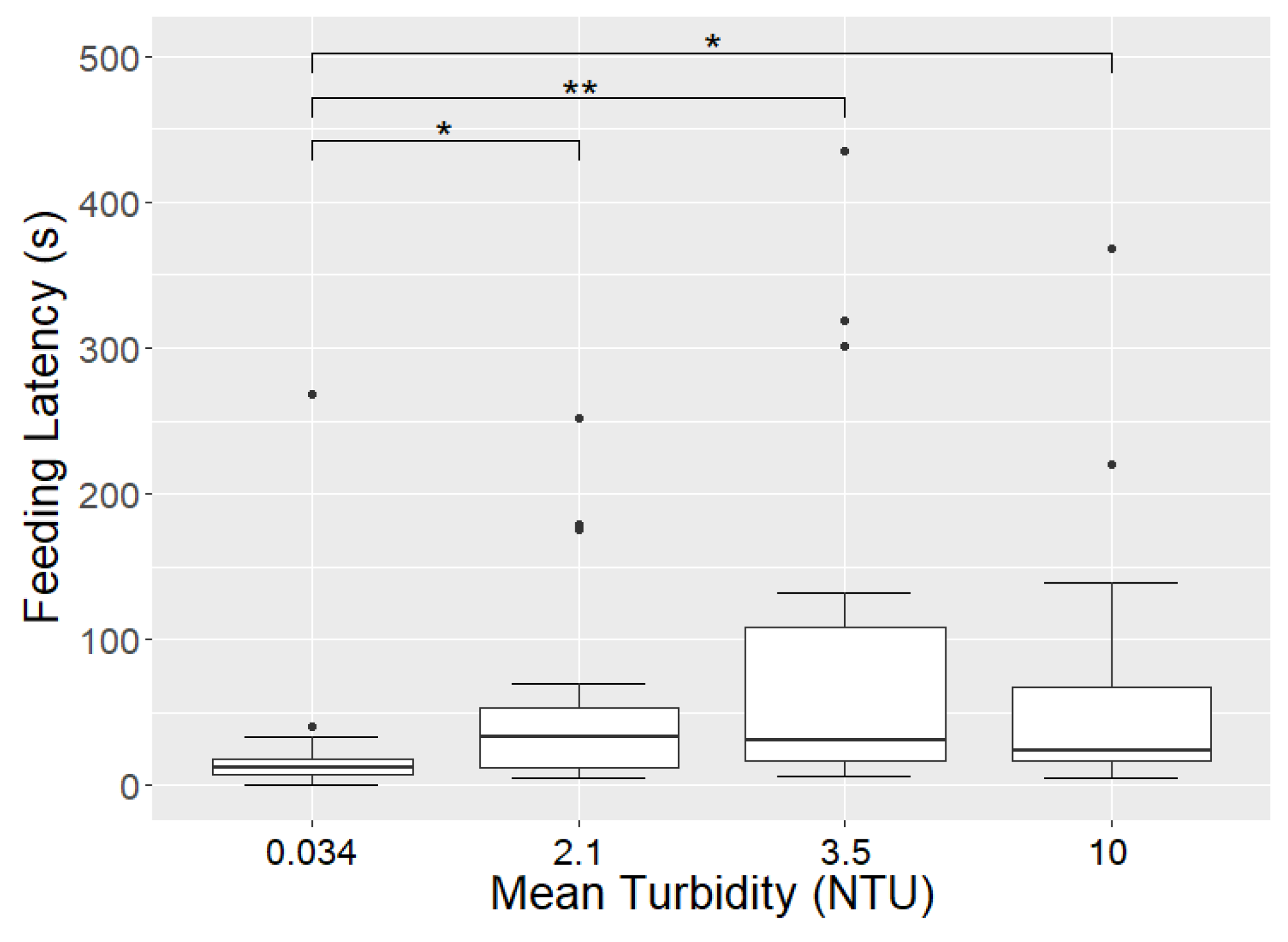


| 0.034 NTU | 2.1 NTU | 3.5 NTU | 10 NTU | |
|---|---|---|---|---|
| Turbidity (NTU) | 0.026 (n= 10) | 0.15 (n = 10) | 0.057 (n = 10) | 0.48 (n = 10) |
| C) | 0.037 (n = 5) | 0.025 (n = 5) | 0.037 (n = 5) | 0.020 (n = 5) |
| C) | 0.037 (n = 5) | 0.025 (n = 5) | 0.020 (n = 5) | 0.020 (n = 5) |
| 30 (n = 1) | 31 (n = 1) | 31 (n = 1) | 30 (n = 1) |
| 0.034 NTU | Observed Crossings (n) | Feeding Latency (s) | Observed Lunges (n) | tlow |
| Mean | 52 | 25 | 29 | 0.50 |
| SE | 7.0 | 11 | 3.9 | 0.064 |
| Min | 0 | 1 | 0 | 0 |
| Max | 138 | 268 | 76 | 0.96 |
| Sample size (n) | 24 | 23 | 24 | 24 |
| 2.1 NTU | Observed Crossings (n) | Feeding Latency (s) | Observed Lunges (n) | tlow |
| Mean | 40 | 52 | 13 | 0.62 |
| SE | 6.2 | 13 | 2.2 | 0.059 |
| Min | 0 | 5 | 0 | 0.078 |
| Max | 113 | 252 | 46 | 1 |
| Sample size (n) | 25 | 23 | 25 | 25 |
| 3.5 NTU | Observed Crossings (n) | Feeding Latency (s) | Observed Lunges (n) | tlow |
| Mean | 34 | 92 | 8.0 | 0.58 |
| SE | 5.6 | 27 | 1.9 | 0.070 |
| Min | 0 | 6 | 0 | 0.057 |
| Max | 93 | 435 | 37 | 1 |
| Sample size (n) | 25 | 20 | 25 | 25 |
| 10 NTU | Observed Crossings (n) | Feeding Latency (s) | Observed Lunges (n) | tlow |
| Mean | 17 | 66 | 6.8 | 0.65 |
| SE | 3.8 | 20 | 1.5 | 0.067 |
| Min | 0 | 5 | 0 | 0 |
| Max | 85 | 368 | 25 | 1 |
| Sample size (n) | 25 | 20 | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange Jensen, L.; Bjørn, T.; Hein Korsgaard, A.; Pertoldi, C.; Madsen, N. Influence of Turbidity on Foraging Behaviour in Three-Spined Sticklebacks (Gasterosteus aculeatus). Fishes 2023, 8, 609. https://doi.org/10.3390/fishes8120609
Lange Jensen L, Bjørn T, Hein Korsgaard A, Pertoldi C, Madsen N. Influence of Turbidity on Foraging Behaviour in Three-Spined Sticklebacks (Gasterosteus aculeatus). Fishes. 2023; 8(12):609. https://doi.org/10.3390/fishes8120609
Chicago/Turabian StyleLange Jensen, Lasse, Thomas Bjørn, Andreas Hein Korsgaard, Cino Pertoldi, and Niels Madsen. 2023. "Influence of Turbidity on Foraging Behaviour in Three-Spined Sticklebacks (Gasterosteus aculeatus)" Fishes 8, no. 12: 609. https://doi.org/10.3390/fishes8120609
APA StyleLange Jensen, L., Bjørn, T., Hein Korsgaard, A., Pertoldi, C., & Madsen, N. (2023). Influence of Turbidity on Foraging Behaviour in Three-Spined Sticklebacks (Gasterosteus aculeatus). Fishes, 8(12), 609. https://doi.org/10.3390/fishes8120609








