The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Rearing System and Water Preparation
2.3. BSFLM, Fish Meal, and Diet Formulation, Manufacturing, and Chemical Analysis
2.4. Shrimp Post-Larvae
2.5. Feeding and Water Quality
2.6. Shrimp Growth Performance
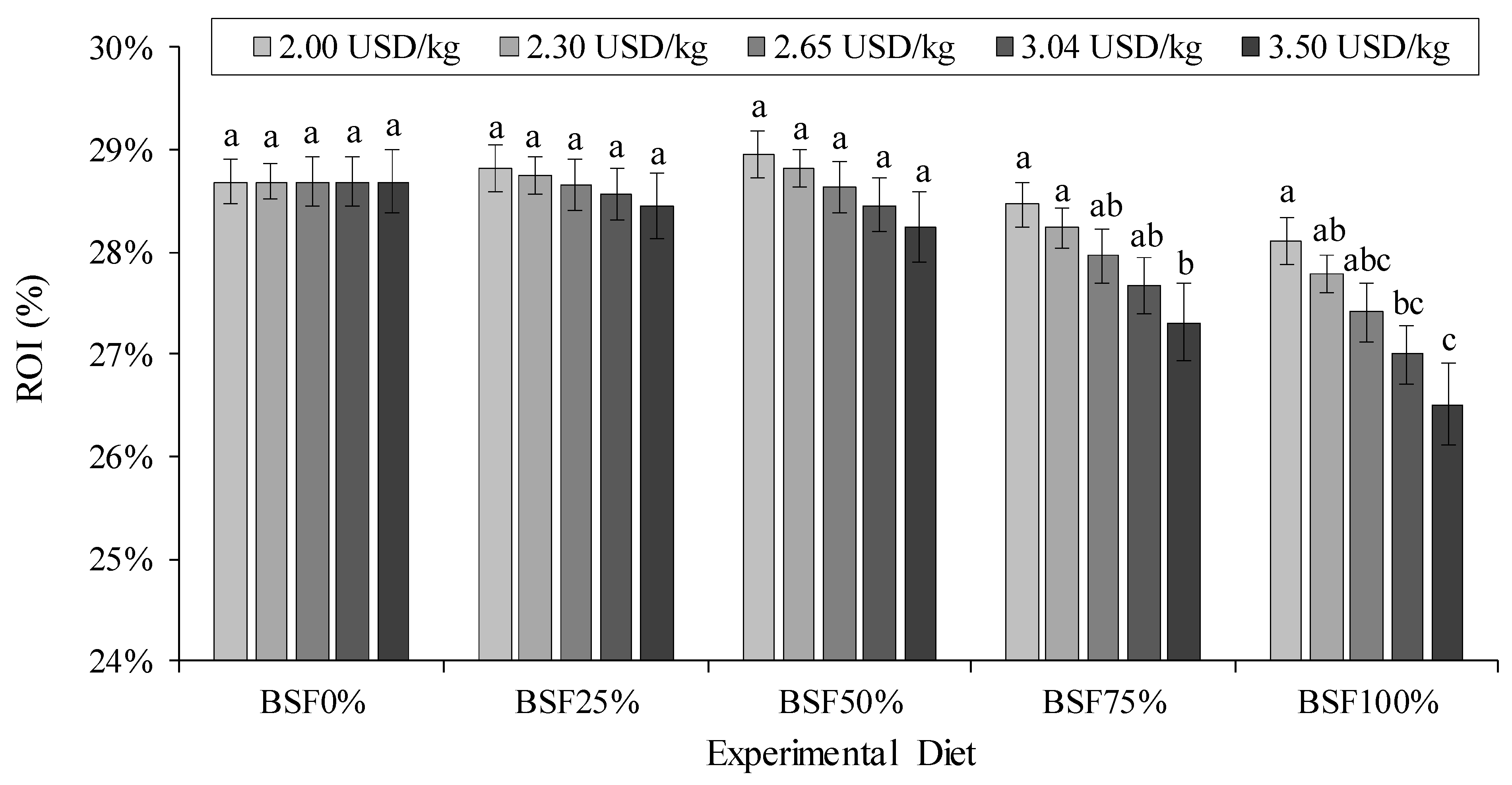
2.7. Price Sensitivity Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, A.J.P.; Dalen, L.L.; Leonardi, G.; Burri, L. Developing sustainable, cost-effective and high-performance shrimp feed formulations containing low fish meal levels. Aquac. Rep. 2022, 27, 101422. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Renteria, P.; Vizcaino, A.; Barroso, F.G. Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Rev. Aquac. 2020, 12, 186–203. [Google Scholar] [CrossRef]
- Siva Raman, S.; Stringer, L.C.; Bruce, N.C.; Chong, C.S. Opportunities, challenges and solutions for black soldier fly larvae-based animal feed production. J. Clean. Prod. 2022, 373, 133802. [Google Scholar] [CrossRef]
- Khalifah, A.; Abdalla, S.; Rageb, M.; Maruccio, L.; Ciani, F.; El-Sabrout, K. Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review. Animals 2023, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Shin, J.; Lee, K.J. Digestibility of insect meals for Pacific white shrimp (Litopenaeus vannamei) and their performance for growth, feed utilization and immune responses. PLoS ONE 2021, 16, e0260305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chi, S.; Zhang, S.; Dong, X.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Evaluation of the Dietary Black Soldier Fly Larvae Meal (Hermetia illucens) on Growth Performance, Intestinal Health, and Disease Resistance to Vibrio parahaemolyticus of the Pacific White Shrimp (Litopenaeus vannamei). Front. Mar. Sci. 2021, 8, 1072. [Google Scholar] [CrossRef]
- Cummins, V.C.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef]
- Richardson, A.; Dantas-Lima, J.; Lefranc, M.; Walraven, M. Effect of a black soldier fly ingredient on the growth performance and disease resistance of juvenile pacific white shrimp (Litopenaeus vannamei). Animals 2021, 11, 1450. [Google Scholar] [CrossRef]
- Keetanon, A.; Chuchird, N.; Phansawat, P.; Kitsanayanyong, L.; Chou, C.C.; Verstraete, P.; Ménard, R.; Richards, C.S.; Ducharne, F.; Rairat, T. Effects of black soldier fly larval meal on the growth performance, survival, immune responses, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). Aquac. Int. 2023. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Mo, W.; Wei, Z.; Huang, Y. Evaluation of defatted Hermetia illucens larvae meal for Litopenaeus vannamei: Effects on growth performance, nutrition retention, antioxidant and immune response, digestive enzyme activity and hepatic morphology. Aquac. Nutr. 2021, 27, 986–997. [Google Scholar] [CrossRef]
- He, Y.; Zhang, N.; Wang, A.; Wang, S.; Che, Y.; Huang, S.; Yi, Q.; Ma, Y.; Jiang, Y. Positive effects of replacing commercial feeds by fresh black soldier fly (Hermetia illucens) larvae in the diets of Pacific white shrimp (Litopenaeus vannamei): Immune enzyme, water quality, and intestinal microbiota. Front. Mar. Sci. 2022, 9, 987363. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Zhang, N.; Wang, S.; Wang, A.; Zuo, R.; Jiang, Y. Replacement of Commercial Feed with Fresh Black Soldier Fly (Hermetia illucens) Larvae in Pacific White Shrimp (Litopenaeus vannamei). Aquac. Nutr. 2022, 2022, 9130400. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Beck, B.H.; Peatman, E. Nutritional evaluation of frass from black soldier fly larvae as potential feed ingredient for Pacific white shrimp, Litopenaeus vannamei. Aquac. Rep. 2022, 27, 101353. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Soares, A.N.; Sabry-Neto, H.; Burri, L. Effect of dietary graded levels of astaxanthin krill oil and high protein krill meal on the growth performance and stress resistance of post larval Litopenaeus vannamei under hyper-intensive nursery culture. Aquac. Nutr. 2021, 27, 327–341. [Google Scholar] [CrossRef]
- Official Methods of Analysis of Aoac International; Oxford University Press: Oxford, UK, 2023. [CrossRef]
- Hagen, S.R.; Augustin, J.; Grings, E.; Tassinari, P. Precolumn phenylisothiocyanate derivatization and liquid chromatography of free amino acids in biological samples. Food Chem. 1993, 46, 319–323. [Google Scholar] [CrossRef]
- Road, R. An Pico-Tag. Water 1986, 8, 170–177. [Google Scholar]
- Engle, C.R.; McNevin, A.; Racine, P.; Boyd, C.E.; Paungkaew, D.; Viriyatum, R.; Tinh, H.Q.; Minh, H.N. Economics of Sustainable Intensification of Aquaculture: Evidence from Shrimp Farms in Vietnam and Thailand. J. World Aquac. Soc. 2017, 48, 227–239. [Google Scholar] [CrossRef]
- Xie, J.J.; Lemme, A.; He, J.Y.; Yin, P.; Figueiredo-Silva, C.; Liu, Y.J.; Xie, S.W.; Niu, J.; Tian, L.X. Fishmeal levels can be successfully reduced in white shrimp (Litopenaeus vannamei) if supplemented with DL-Methionine (DL-Met) or DL-Methionyl-DL-Methionine (Met-Met). Aquac. Nutr. 2018, 24, 1144–1152. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Sathishkumar, P.; Revathi, N. Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs. Aquaculture 2022, 553, 738095. [Google Scholar] [CrossRef]
- Vieira, C.C.F.; Pinto, R.C.C.; Diógenes, A.F.; Nunes, A.J.P. Apparent digestibility of protein and essential aminoacids from commonly used feed ingredients in Brazil for juvenile shrimp Litopenaeus vannamei. Revista Brasileira de Zootecnia 2022, 51, e20210177. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Guo, J.; Stein, H.H.; Allen Davis, D. Apparent energy, dry matter and amino acid digestibility of differently sourced soybean meal fed to Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 326–340. [Google Scholar] [CrossRef]
- Jory, D.E. Penaeid Shrimp Nursery Systems Penaeid Shrimp Nursery Systems. Aquac. Mag. 2020, 23, 1–5. [Google Scholar]

| Nutrients 1 | Composition (%, as-is) | |
|---|---|---|
| BSFLM | FML | |
| Dry matter | 96.70 | 92.45 |
| Crude protein | 57.77 | 67.08 |
| Ether extract | 7.58 | 10.88 |
| Total fiber | - | 0.13 |
| Crude ash | 9.87 | 14.07 |
| Nitrogen-free extract 2 | 9.87 | 0.29 |
| Gross energy (MJ/kg) 3 | 20.81 | 21.20 |
| Essential Amino Acids (EAA) | ||
| Arginine | 2.53 | 3.74 |
| Histidine | 1.51 | 1.66 |
| Isoleucine | 2.13 | 2.07 |
| Leucine | 3.60 | 3.62 |
| Lysine | 3.77 | 4.47 |
| Methionine | 0.92 | 1.69 |
| Methionine + Cysteine | 1.38 | 2.35 |
| Phenylalanine | 2.09 | 2.21 |
| Threonine | 2.13 | 2.45 |
| Tryptophan | 0.74 | 5.71 |
| Valine | 2.96 | 2.67 |
| Non-Essential Amino Acids (NEAA) | ||
| Alanine | 3.47 | 4.27 |
| Aspartic acid | 4.60 | 5.66 |
| Cystine | 0.46 | 0.66 |
| Glycine | 2.81 | 6.84 |
| Glutamic acid | 7.12 | 8.10 |
| Hydroxyproline | 0.28 | 1.55 |
| Proline | 3.10 | 3.64 |
| Serine | 2.26 | 2.74 |
| Taurine | 0.08 | 0.86 |
| Tyrosine | 3.11 | 1.59 |
| Sum EAA 2 | 22.38 | 30.29 |
| Sum NEAA | 27.29 | 31.64 |
| Sum EAA + NEAA | 49.67 | 61.93 |
| Ingredients | Diets/Ingredient Composition (%, as-is) | ||||
|---|---|---|---|---|---|
| BSF0% | BSF25% | BSF50% | BSF75% | BSF100% | |
| Soybean meal 1 | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Wheat flour 2 | 24.26 | 22.90 | 21.28 | 19.67 | 18.00 |
| Salmon meal 3 | 22.00 | 16.50 | 11.00 | 5.50 | - |
| BSF larvae meal 4 | - | 6.33 | 13.04 | 19.74 | 26.46 |
| Wheat gluten meal 5 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Soybean oil | 2.53 | 1.98 | 1.41 | 0.84 | 0.26 |
| Salmon oil | 2.39 | 2.65 | 2.90 | 3.15 | 3.40 |
| Krill meal 6 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Squid meal 7 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Potassium chloride | 1.51 | 1.51 | 1.52 | 1.53 | 1.54 |
| Magnesium sulphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sodium monophosphate 8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.28 |
| Vitamin–mineral premix 9 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.49 | 0.34 | 0.34 | 0.34 | 0.34 |
| Calcium carbonate | 0.36 | 0.60 | 0.82 | 1.04 | 1.03 |
| Synthetic binder 10 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Stay C, 35% 11 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| DL-Methionine 12 | 0.17 | 0.21 | 0.25 | 0.28 | 0.32 |
| Soy lecithin oil | 0.15 | 0.62 | 1.08 | 1.55 | 2.02 |
| L-Threonine 13 | 0.08 | 0.14 | 0.14 | 0.14 | 0.14 |
| L-Lysine 14 | 0.03 | 0.20 | 0.20 | 0.19 | 0.19 |
| Formulation cost (USD/kg) 15 | 1.09 | 1.19 | 1.29 | 1.39 | 1.50 |
| Proximate composition (%, as-is) | |||||
| Dry matter | 87.02 | 89.34 | 88.69 | 87.92 | 88.61 |
| Crude protein | 39.29 | 39.61 | 39.67 | 38.89 | 40.82 |
| Total lipids | 8.52 | 8.52 | 8.24 | 7.89 | 8.61 |
| Total fiber | 1.75 | 2.52 | 3.11 | 4.24 | 3.43 |
| Crude ash | 10.32 | 9.77 | 9.63 | 9.43 | 10.12 |
| Nitrogen-free extract 16 | 27.14 | 28.92 | 28.04 | 27.47 | 25.63 |
| Gross energy (MJ/kg) | 15.66 | 16.42 | 16.95 | 16.66 | 16.47 |
| Diets/Amino Acid Composition (%, as-is) | CV (%) | |||||
|---|---|---|---|---|---|---|
| Amino Acids | BSF0% | BSF25% | BSF50% | BSF75% | BSF100% | |
| Essential Amino Acids (EAA) | ||||||
| Arginine | 2.32 | 2.29 | 2.28 | 2.09 | 2.34 | 4.4 |
| Histidine | 0.84 | 0.85 | 0.9 | 0.96 | 1.04 | 9.1 |
| Isoleucine | 1.50 | 1.49 | 1.55 | 1.5 | 1.61 | 3.3 |
| Leucine | 2.58 | 2.63 | 2.71 | 2.55 | 2.81 | 4.0 |
| Lysine | 2.26 | 2.34 | 2.37 | 2.21 | 2.45 | 4.0 |
| Methionine | 0.85 | 0.91 | 0.94 | 0.87 | 1.03 | 7.7 |
| Met + Cys 1 | 1.39 | 1.46 | 1.48 | 1.37 | 1.56 | 5.2 |
| Phenylalanine | 1.69 | 1.69 | 1.72 | 1.65 | 1.77 | 2.6 |
| Threonine | 1.55 | 1.63 | 1.69 | 1.55 | 1.79 | 6.2 |
| Valine | 1.74 | 1.76 | 1.86 | 1.82 | 1.95 | 4.6 |
| Non-Essential Amino Acids (NEAA) | ||||||
| Alanine | 1.91 | 1.91 | 1.94 | 1.84 | 2.04 | 3.8 |
| Aspartic acid | 3.37 | 3.44 | 3.50 | 3.2 | 3.62 | 4.6 |
| Cystine | 0.54 | 0.55 | 0.54 | 0.5 | 0.53 | 3.6 |
| Glycine | 2.67 | 2.50 | 2.35 | 2.05 | 2.35 | 9.6 |
| Glutamic acid | 7.47 | 7.54 | 7.54 | 6.98 | 7.66 | 3.6 |
| Hydroxyproline | 0.34 | 0.24 | 0.15 | 0.09 | 0.11 | 55.7 |
| Proline | 2.30 | 2.30 | 2.33 | 2.2 | 2.37 | 2.7 |
| Serine | 1.83 | 1.88 | 1.90 | 1.76 | 1.97 | 4.2 |
| Taurine | 0.20 | 0.15 | 0.11 | 0.07 | 0.09 | 41.7 |
| Tyrosine | 1.15 | 1.28 | 1.42 | 1.46 | 1.54 | 11.3 |
| Sum EAA 2 | 15.33 | 15.59 | 16.02 | 15.20 | 16.79 | 4.1 |
| Sum NEAA | 21.78 | 21.79 | 21.78 | 20.15 | 22.28 | 3.8 |
| Sum EAA + NEAA | 37.11 | 37.38 | 37.80 | 35.35 | 39.07 | 3.6 |
| Diet/Composition (%, as-is) | |||||
|---|---|---|---|---|---|
| Fatty Acids | BSF0% | BSF25% | BSF50% | BSF75% | BSF100% |
| 4:0 | <0.001 | <0.001 | <0.001 | - | - |
| 6:0 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 8:0 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 10:0 | <0.001 | - | 0.01 | 0.01 | 0.01 |
| 11:0 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 12:0 | - | 0.13 | 0.28 | 0.41 | 0.41 |
| 13:0 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 14:0 | 0.12 | 0.15 | 0.18 | 0.20 | 0.22 |
| 15:0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 16:0 | 1.32 | 1.33 | 1.32 | 1.31 | 1.44 |
| 17:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 18:0 | 0.43 | 0.41 | 0.38 | 0.36 | 0.41 |
| 20:0 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 21:0 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 |
| 22:0 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 23:0 | 0.01 | 0.01 | 0.01 | - | 0.01 |
| 24:0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 15:1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 17:1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 16:1n-7 | 0.14 | 0.14 | 0.14 | 0.14 | 0.16 |
| 18:1n-9 | 2.55 | 2.46 | 2.30 | 2.15 | 2.41 |
| 20:1n-9 | 0.11 | 0.10 | 0.09 | 0.09 | 0.10 |
| 22:1n-9 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 |
| 24:1n-9 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 |
| 18:2n-6 | 2.63 | 2.63 | 2.45 | - | - |
| 20:2n-6 | 0.05 | 0.05 | 0.04 | 0.05 | 0.06 |
| 18:3n-6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 20:3n-6 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 |
| 20:4n-6 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 |
| 18:3n-3 | 0.34 | 0.33 | 0.32 | 0.31 | 0.33 |
| 20:3n-3 | 0.01 | 0.01 | - | 0.01 | 0.01 |
| 20:5n-3 | 0.21 | 0.19 | 0.18 | 0.16 | 0.19 |
| 22:6n-3 | 0.31 | 0.30 | 0.25 | 0.19 | 0.25 |
| ∑ n-3 1 | 0.87 | 0.83 | 0.75 | 0.67 | 0.78 |
| ∑ n-6 2 | 2.74 | 2.74 | 2.58 | 0.08 | 0.11 |
| ∑ SFA 3 | 2.00 | 2.16 | 2.30 | 2.41 | 2.62 |
| ∑ MUFA 4 | 2.87 | 2.77 | 2.59 | 2.43 | 2.73 |
| ∑ PUFA 5 | 3.06 | 3.05 | 2.88 | 0.38 | 0.42 |
| ∑ HUFA 6 | 0.55 | 0.52 | 0.45 | 0.37 | 0.47 |
| Shrimp Stage | Shrimp Body Weight (BW, mg) | Feeding Rate (% of BW per Day) | Estimated Survival (%) | DWG (mg) | |
|---|---|---|---|---|---|
| From | To | ||||
| PL10 | 1 | 2 | 35.0 | 100 | 0.1 |
| PL11 | 2 | 3 | 35.0 | 100 | 0.2 |
| PL12 | 3 | 6 | 35.0 | 100 | 0.3 |
| PL13 | 6 | 9 | 34.5 | 100 | 0.4 |
| PL14 | 9 | 13 | 33.0 | 100 | 0.5 |
| PL15 | 13 | 17 | 32.0 | 100 | 0.6 |
| PL16 | 17 | 22 | 31.0 | 99 | 0.7 |
| PL17 | 22 | 28 | 26.0 | 99 | 0.8 |
| PL18 | 28 | 35 | 25.0 | 99 | 1.0 |
| PL19 | 35 | 43 | 24.0 | 99 | 1.0 |
| PL20 | 43 | 52 | 23.0 | 99 | 2.0 |
| PL21 | 52 | 62 | 22.0 | 99 | 2.0 |
| PL22 | 62 | 73 | 20.0 | 98 | 3.0 |
| PL23 | 73 | 86 | 18.0 | 98 | 3.0 |
| PL24 | 86 | 101 | 17.0 | 98 | 4.0 |
| PL25 | 101 | 118 | 16.0 | 98 | 4.0 |
| PL26 | 118 | 137 | 13.0 | 98 | 6.0 |
| PL27 | 137 | 158 | 12.0 | 98 | 6.0 |
| PL28 | 158 | 182 | 11.5 | 97 | 7.0 |
| PL29 | 182 | 207 | 11.0 | 97 | 7.0 |
| PL30 | 207 | 232 | 10.5 | 97 | 8.0 |
| Juvenile | 232 | 259 | 10.0 | 97 | 8.0 |
| Juvenile | 259 | 286 | 10.0 | 97 | 9.0 |
| Juvenile | 286 | 314 | 9.5 | 97 | 9.0 |
| Juvenile | 314 | 342 | 9.0 | 96 | 10.0 |
| Juvenile | 342 | 372 | 8.5 | 96 | 10.0 |
| Juvenile | 372 | 402 | 8.0 | 96 | 11.0 |
| Juvenile | 402 | 434 | 7.5 | 96 | 11.0 |
| Juvenile | 434 | 466 | 7.0 | 96 | 12.0 |
| Juvenile | 466 | 500 | 6.8 | 96 | 12.0 |
| Juvenile | 500 | 534 | 6.5 | 95 | 13.0 |
| Juvenile | 534 | 570 | 6.3 | 95 | 13.0 |
| Juvenile | 570 | 608 | 6.1 | 95 | 14.0 |
| Juvenile | 608 | 648 | 5.9 | 95 | 14.0 |
| Juvenile | 648 | 688 | 5.7 | 95 | 15.0 |
| Juvenile | 688 | 730 | 5.5 | 95 | 15.0 |
| Juvenile | 730 | 772 | 5.3 | 94 | 16.0 |
| Juvenile | 772 | 817 | 5.1 | 94 | 16.0 |
| Juvenile | 817 | 862 | 4.9 | 94 | 17.0 |
| Juvenile | 862 | 909 | 4.7 | 94 | 17.0 |
| Growth Parameters | Diets | Mean ± SD | p ANOVA | ||||
|---|---|---|---|---|---|---|---|
| BSF0% | BSF25% | BSF50% | BSF75% | BSF100% | |||
| Initial body weight (mg) | 2.7 ± 0.2 | 2.6 ± 0.2 | 2.7 ± 0.3 | 2.7 ± 0.2 | 2.6 ± 0.3 | 2.7 ± 0.2 | 0.608 |
| Final survival (%) | 89.5 ± 7.5 | 89.1 ± 4.6 | 94.0 ± 9.7 | 90.8 ± 7.7 | 88.9 ± 7.9 | 90.5 ± 7.6 | 0.562 |
| Gained yield (mg/m3) | 726 ± 34 b | 762 ± 47 a,b | 791 ± 52 a | 776 ± 38 a | 749 ± 58 a,b | - | 0.047 |
| Growth (mg/day) | 14.2 ± 0.8 | 14.8 ± 1.3 | 14.7 ± 1.3 | 15.0 ± 1.5 | 14.8 ± 0.8 | 14.7 ± 1.1 | 0.653 |
| AFI a (g/stocked shrimp) | 0.66 ± 0.03 | 0.67 ± 0.04 | 0.67 ± 0.03 | 0.68 ± 0.02 | 0.68 ± 0.02 | 0.67 ± 0.03 | 0.561 |
| FCR b | 1.25 ± 0.04 b | 1.20 ± 0.06 a,b | 1.16 ± 0.06 a | 1.20 ± 0.05 a,b | 1.24 ± 0.08 b | - | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.J.P.; Yamamoto, H.; Simões, J.P.; Pisa, J.L.; Miyamoto, N.; Leite, J.S. The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture. Fishes 2023, 8, 605. https://doi.org/10.3390/fishes8120605
Nunes AJP, Yamamoto H, Simões JP, Pisa JL, Miyamoto N, Leite JS. The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture. Fishes. 2023; 8(12):605. https://doi.org/10.3390/fishes8120605
Chicago/Turabian StyleNunes, Alberto J. P., Hiroshi Yamamoto, João Paulo Simões, João Luiz Pisa, Nelson Miyamoto, and Jordana Sampaio Leite. 2023. "The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture" Fishes 8, no. 12: 605. https://doi.org/10.3390/fishes8120605
APA StyleNunes, A. J. P., Yamamoto, H., Simões, J. P., Pisa, J. L., Miyamoto, N., & Leite, J. S. (2023). The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture. Fishes, 8(12), 605. https://doi.org/10.3390/fishes8120605







