The Early Allometric Growth and Osteological Ontogeny of Pot-Bellied Seahorse (Hippocampus abdominalis, L. 1827) under Mass-Scale Captive Breeding Conditions in North China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing Conditions for Experimental Animal
2.2. Sampling and Skeletal Staining
2.3. Observations and Measurements
2.4. Statistical Analysis
3. Results
3.1. Allometric Growth
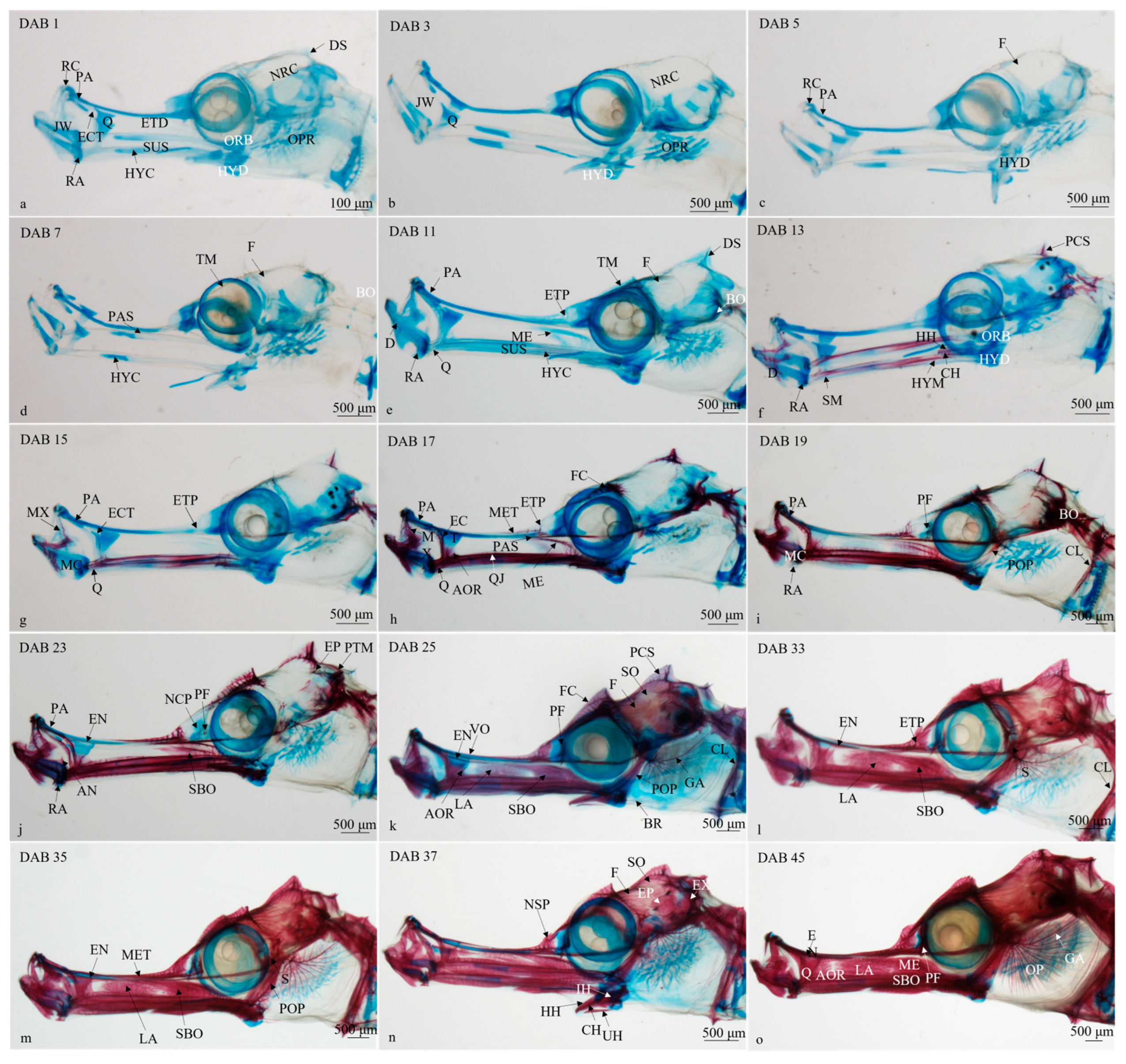
3.2. Timing and Progression of Skeletal Development
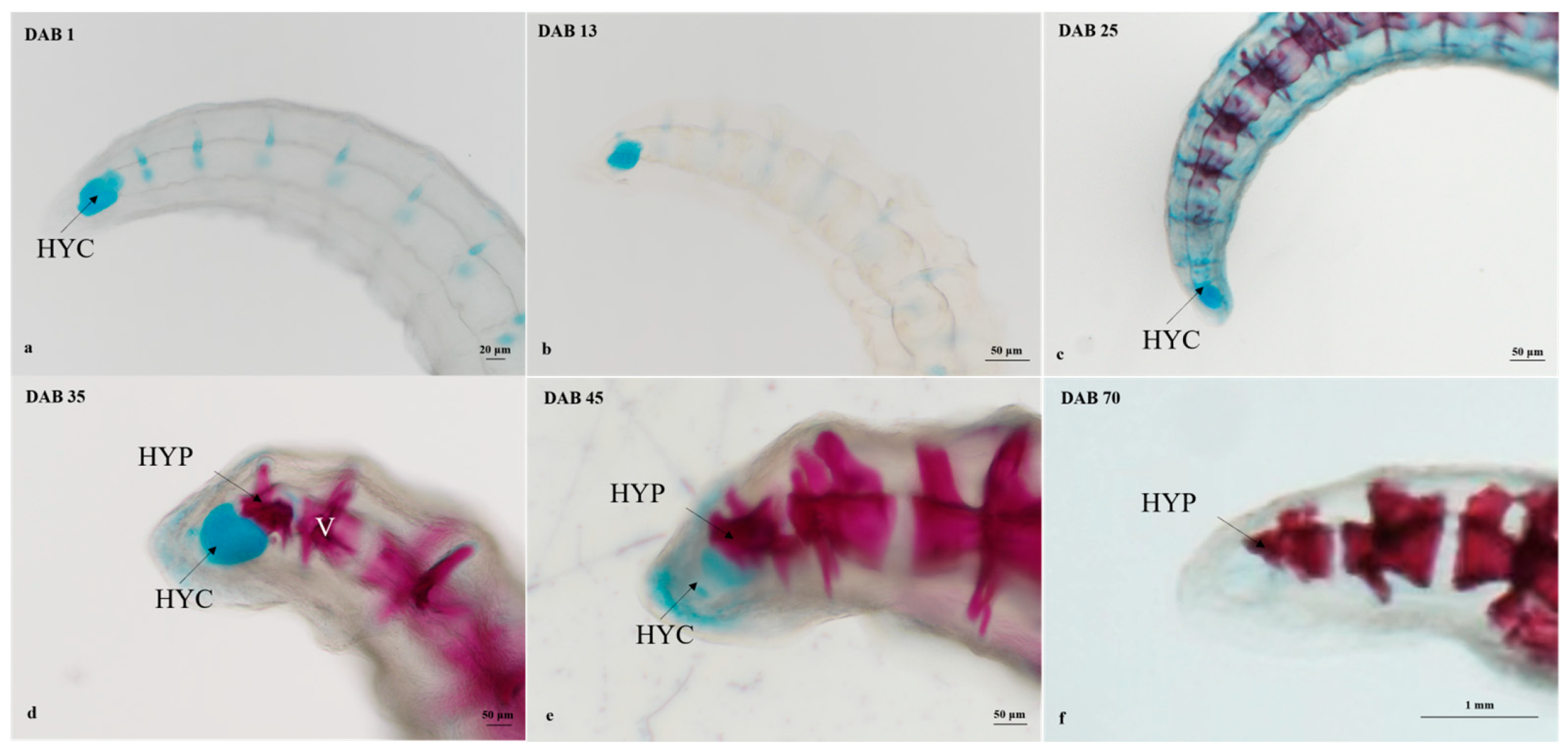
3.2.1. Timing and Progression of Cranium Development
- (1)
- DAB 1–7 (SL 19.21 ± 1.43 mm to SL 24.68 ± 2.86 mm; Figure 2a–c)
- (2)
- DAB 8–11 (SL 24.16 ± 2.07 to SL 28.14 ± 2.94 mm; Figure 2d)
- (3)
- DAB 12–13 (SL 26.48 ± 0.63 mm; Figure 2e)
- (4)
- DAB 14–17 (SL 28.34 ± 3.87 mm; Figure 2f,g)
- (5)
- DAB 18–23 (SL 27.76 ± 0.80 to SL 31.27 ± 4.05 mm; Figure 2h,i)
- (6)
- DAB 24–25 (SL 31.27 ± 1.93 mm; Figure 2j)
- (7)
- DAB 26–35 (SL 38.89 ± 1.98 to SL 40.55 ± 3.33 mm; Figure 2k,l)
- (8)
- DAB 36–45 (SL 49.14 ± 3.53 to SL 54.87 ± 4.70 mm; Figure 2m,n)
3.2.2. Timing and Progression of Coronet Development
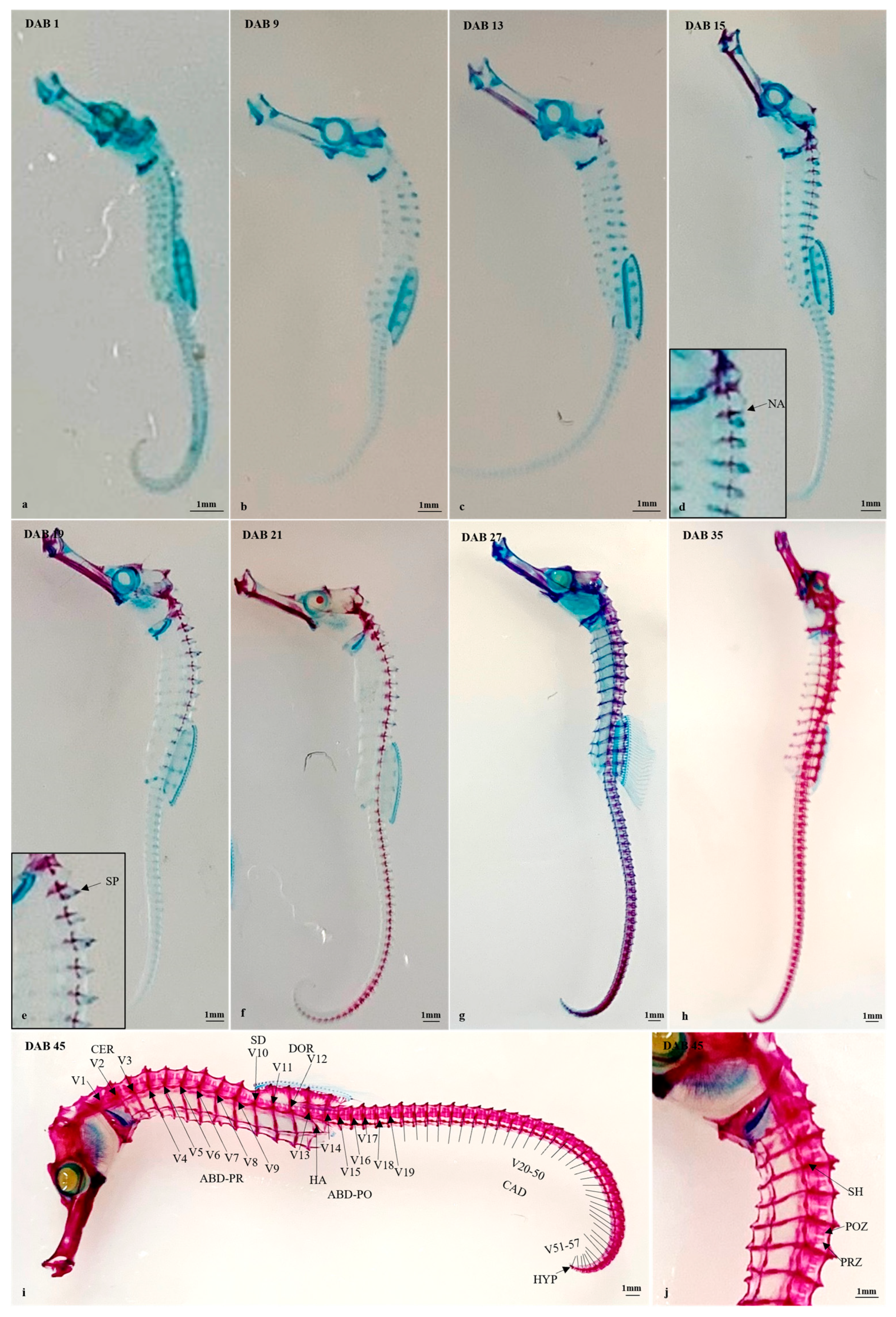
3.2.3. Timing and Progression of Vertebral Column Development
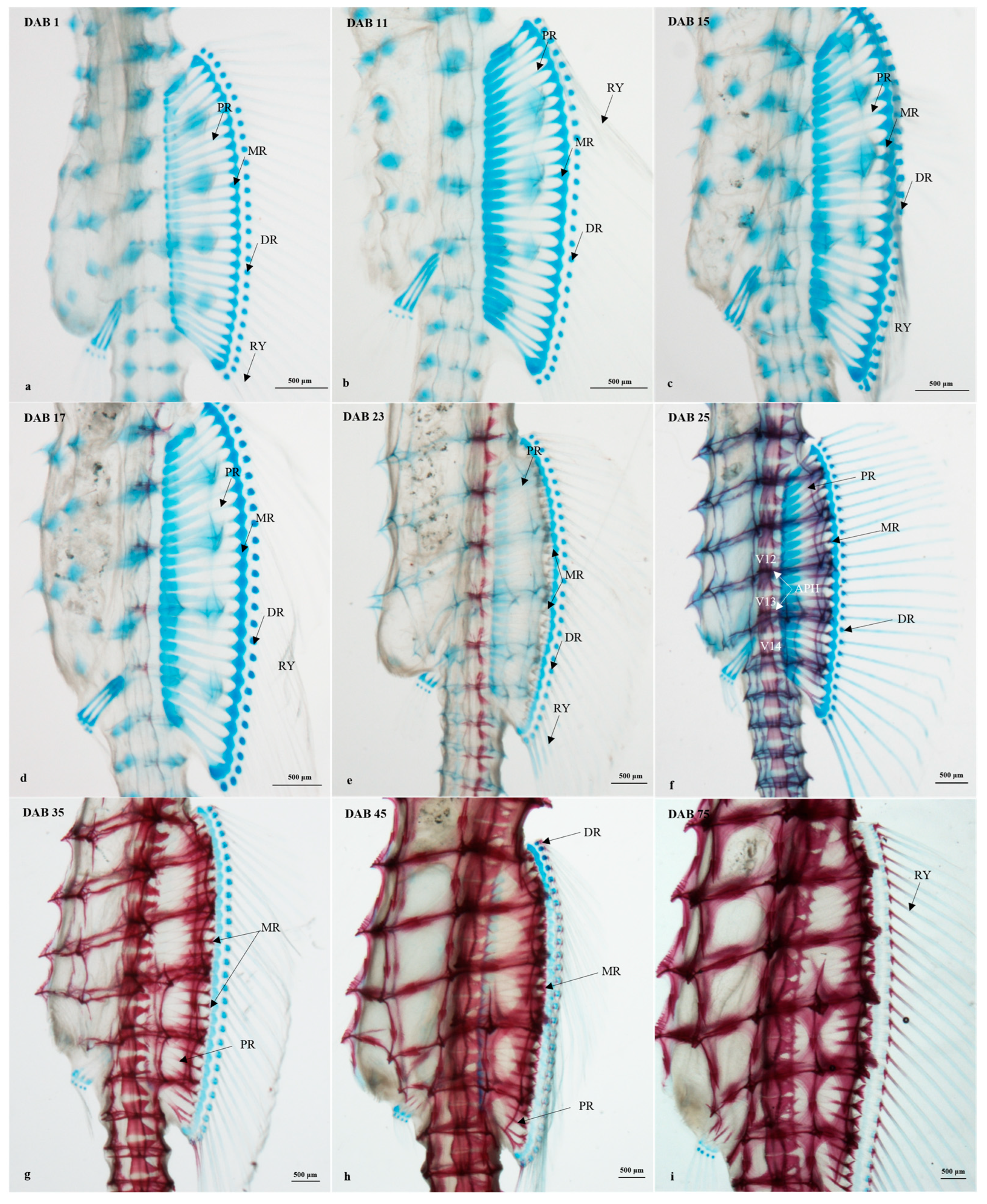
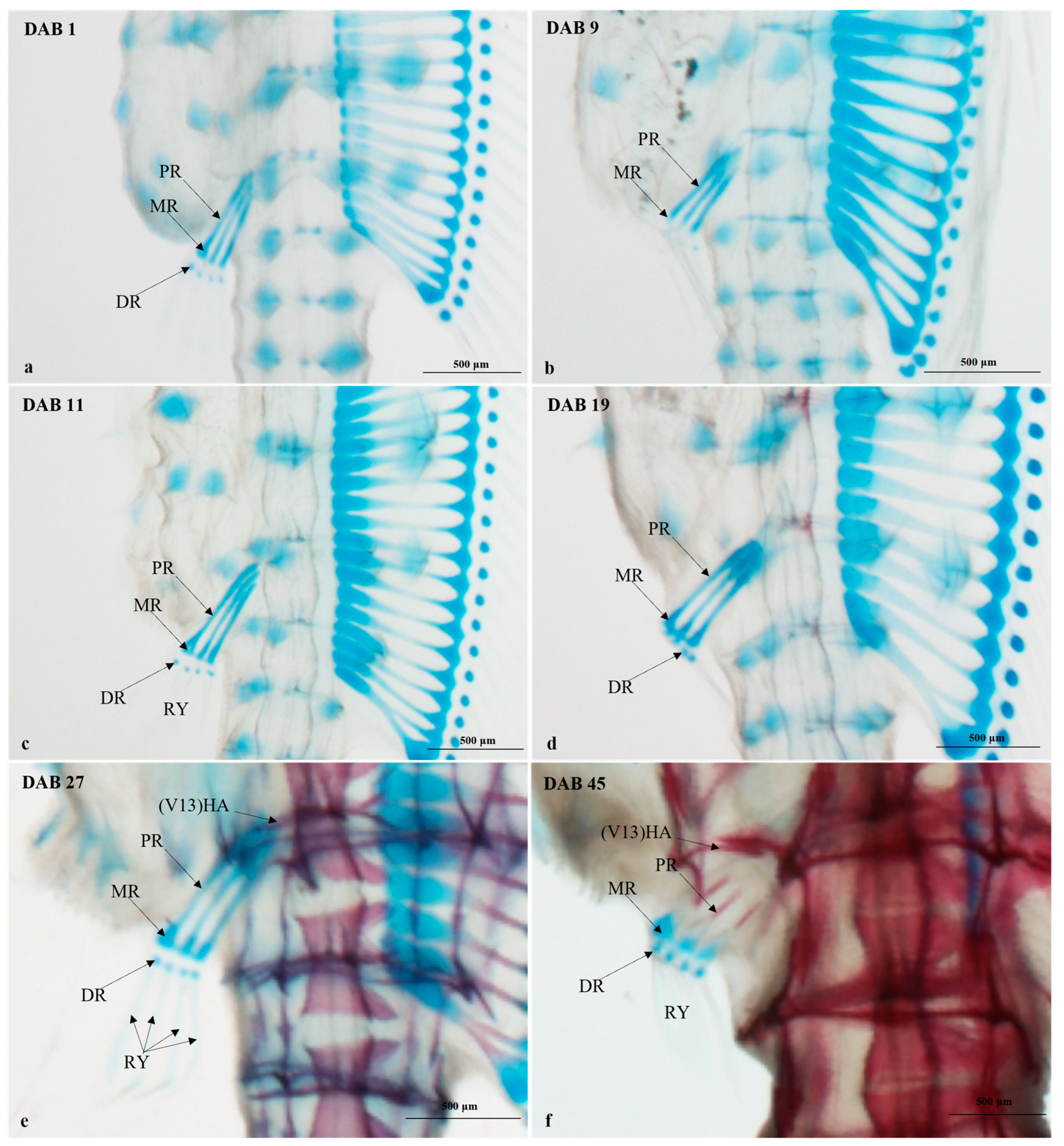
3.2.4. Timing and Progression of Fin Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, J.; Marcoval, M.A.; Bazzini, S.M.; Vallina, M.V.; Marco, S.G.D. Coastal Marine Biodiversity: Challenges and Threats. In Marine Ecology in a Changing World; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Woods, C.M.C. Improving initial survival in cultured seahorses, Hippocampus abdominalis Leeson, 1827 (Teleostei: Syngnathidae). Aquaculture 2000, 190, 377–388. [Google Scholar] [CrossRef]
- Lourie, S.A.; Stanley, H.F.; Vincent, A.C.J.; Hall, H.J.; Pritchard, J.C.; Casey, S.P. Seahorses: An Identification Guide to the World’s Species and Their Conservation; Project Seahorse: Vancouver, BC, Canada, 1999. [Google Scholar]
- Job, S.D.; Do, H.H.; Meeuwig, J.J.; Hall, H.J. Culturing the oceanic seahorse, Hippocampus kuda. Aquaculture 2002, 214, 333–341. [Google Scholar] [CrossRef]
- Johannesbury, A.C.o. Convention on international trade in endangered species of wild fauna and flora. J. Int. Wildl. Law Policy 2005, 8, 115–127. [Google Scholar]
- Koldewey, H.J.; Martin-Smith, K.M. A global review of seahorse aquaculture. Aquaculture 2010, 302, 131–152. [Google Scholar] [CrossRef]
- Cohen, F.P.A.; Valenti, W.C.; Planas, M.; Calado, R.J. Seahorse Aquaculture, Biology and Conservation: Knowledge Gaps and Research Opportunities. Rev. Fish. Sci. Aquac. 2016, 25, 100–111. [Google Scholar] [CrossRef]
- He, L.; Jianfei, Q.; Lin, J.; Chen, X.; Luo, H.; Wang, Q.; Leyun, Z. Artificial propagation and seedling technique of Hippocampus abdominalis. J. Appl. Oceanogr. 2022, 41, 701–707. [Google Scholar]
- Nur, F.A.H.; Christianus, A.; Abdullah, A.R.; Zakaria, M.H.; Saad, C.R. Effects of thyroxine, cod liver oil and potassium iodide on growth and survival of juvenile seahorse, Hippocampus barbouri. Aquaculture 2018, 49, 867–873. [Google Scholar] [CrossRef]
- Murugan, A.; Dhanya, S.; Sreepada, R.A.; Rajagopal, S.; Balasubramanian, T. Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 2009, 290, 87–96. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Lin, T.; Li, S. Culturing low quality juveniles of the lined seahorse, Hippocampus erectus. Aquac. Rep. 2023, 30, 101561. [Google Scholar] [CrossRef]
- Huang, J.; Qin, G.; Zhang, B.; Tan, S.; Sun, J.; Lin, Q. Effects of food, salinity, and ammonia-nitrogen on the physiology of juvenile seahorse (Hippocampus erectus) in two typical culture models in China. Aquaculture 2020, 520, 734965. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Lin, T.; Li, S.; Shen, F.; Wang, Y. Culturing the lined seahorse (Hippocampus erectus) in extensive shrimp ponds and cages. J. World Aquac. Soc. 2023. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, H.; Yang, N.; Feng, H.; Li, Y.; Han, B. Isolation, identification, and biological control in vitro of tail rot pathogen strain from Hippocampus kuda. PLoS ONE 2020, 15, e0232162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Zheng, F.; Wang, B.; Liu, H.; Zhang, P.; Xin, M. Identification and characterization of the pathogen associated with skin ulcer syndrome in lined seahorse Hippocampus erectus. Aquac. Res. 2019, 51, 989–999. [Google Scholar] [CrossRef]
- Willadino, L.; Souza-Santos, L.P.; Mélo, R.C.S.; Brito, A.P.; Barros, N.C.S.; Araújo-Castro, C.M.V.; Galvão, D.B.; Gouveia, A.; Regis, C.G.; Cavalli, R.O. Ingestion rate, survival and growth of newly released seahorse Hippocampus reidi fed exclusively on cultured live food items. Aquaculture 2012, 360–361, 10–16. [Google Scholar] [CrossRef]
- Celino, F.T.; Hilomen-Garcia, G.V.; del Norte-Campos, A.G.C. Feeding selectivity of the seahorse, Hippocampus kuda (Bleeker), juveniles under laboratory conditions. Aquac. Res. 2012, 43, 1804–1815. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Molina, L.; Socorro, J.; Herrera, R.; Fernández-Palacios, H.; Soledad Izquierdo, M. Live prey first feeding regimes for short-snouted seahorse Hippocampus hippocampus (Linnaeus, 1758) juveniles. Aquac. Res. 2010, 41, e8–e19. [Google Scholar] [CrossRef]
- Vargas-Abúndez, J.A.; Simões, N.; Mascaró, M. Feeding the lined seahorse Hippocampus erectus with frozen amphipods. Aquaculture 2018, 491, 82–85. [Google Scholar] [CrossRef]
- Schubert, P.; Vogt, L.; Eder, K.; Hauffe, T.; Wilke, T. Effects of Feed Species and HUFA Composition on Survival and Growth of the Longsnout Seahorse (Hippocampus reidi). Front. Mar. Sci. 2016, 3, 53. [Google Scholar] [CrossRef]
- Planas, M.; Olivotto, I.; González, M.J.; Laurà, R.; Angeletti, C.; Amici, A.; Zarantoniello, M. Pre-breeding Diets in the Seahorse Hippocampus reidi: How Do They Affect Fatty Acid Profiles, Energetic Status and Histological Features in Newborn? Front. Mar. Sci. 2021, 8, 58. [Google Scholar] [CrossRef]
- Del Vecchio, G.; Otero-Ferrer, F.; Pascual, C.; Rosas, C.; Simoes, N.; Mascaró, M. Effect of starvation on survival and biochemical profile of newborn juvenile lined seahorses, Hippocampus erectus (Perry, 1810). Aquac. Res. 2019, 50, 3729–3740. [Google Scholar] [CrossRef]
- Hora, M.d.S.C.d.; Joyeux, J.-C. Closing the reproductive cycle: Growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 2009, 292, 37–41. [Google Scholar] [CrossRef]
- Avidan, C.; Day, S.W.; Holzman, R. A power amplification dyad in seahorses. Proc. Biol. Sci. 2023, 290, 20230520. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.G.; Foster, S.J.; Vincent, A.C.J. A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (Family Syngnathidae). Rev. Fish Biol. Fish. 2019, 29, 197–221. [Google Scholar] [CrossRef]
- Taylor, W.R.; Vandyke, G.C. Revised Procedures for Staining and Clearing Small Fishes and Other Vertebrates For Bone And Cartilage Study. Cybium 1985, 9, 107–119. [Google Scholar]
- Dingerkus, G.; Uhler, L.D. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977, 52, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Azzarello, M.Y. A Comparative Study of the Developmental Osteology of Syngnathus scovelli and Hippocampus zosterae (Pisces, Syngnathidae) and Its Phylogenetic Implications. Ph.D. Thesis, University of South Florida, St. Petersburg, FL, USA, 1990. [Google Scholar]
- Silveira, R.B.J.Z. Desenvolvimento osteológico de Hippocampus reidi Ginsburg (Pisces, Syngnathiformes, Syngnathidae), em laboratório: II. Período juvenil. Rev. Bras. De Zool. 2000, 17, 515–531. [Google Scholar] [CrossRef]
- Arratia, G.; Schultze, H.P.; Casciotta, J.J. Vertebral column and associated elements in dipnoans and comparison with other fishes: Development and homology. J. Morphol. 2001, 250, 101–172. [Google Scholar] [CrossRef]
- Bruner, E.; Bartolino, V. Morphological Variation in the Seahorse Vertebral System. Int. J. Morphol. 2008, 26, 247–262. [Google Scholar] [CrossRef]
- Leysen, H.; Jouk, P.; Brunain, M.; Christiaens, J.; Adriaens, D.J. Cranial architecture of tube-snouted Gasterosteiformes (Syngnathus rostellatus and Hippocampus capensis). J. Morphol. 2010, 271, 255–270. [Google Scholar] [CrossRef]
- Leysen, H.; Christiaens, J.; Kegel, B.D.; Boone, M.N.; Hoorebeke, L.V.; Adriaens, D.J. Musculoskeletal structure of the feeding system and implications of snout elongation in Hippocampus reidi and Dunckerocampus dactyliophorus. J. Fish Biol. 2011, 78, 1799–1823. [Google Scholar] [CrossRef]
- Novelli, B.; Otero-Ferrer, F.; Socorro, J.A.; Caballero, M.J.; Segade-Botella, A.; Molina Dominguez, L. Development of short-snouted seahorse (Hippocampus hippocampus, L. 1758): Osteological and morphological aspects. Fish Physiol. Biochem. 2017, 43, 833–848. [Google Scholar] [CrossRef]
- Fuiman, L.A. Growth gradients in fish larvae. J. Fish Biol. 1983, 23, 117–123. [Google Scholar] [CrossRef]
- Mathias, J.A.; Li, S. Feeding Habits of Walleye Larvae and Juveniles: Comparative Laboratory and Field Studies. Trans. Am. Fish. Soc. 1982, 111, 722–735. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Adriaens, D. Comparative developmental osteology of the seahorse skeleton reveals heterochrony amongst Hippocampus sp. and progressive caudal fin loss. Evodevo 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Bergert, B.A.; Wainwright, P.C.J. Morphology and kinematics of prey capture in the syngnathid fishes Hippocampus erectus and Syngnathus floridae. Mar. Biol. 1997, 127, 563–570. [Google Scholar] [CrossRef]
- Choo, C.K.; Liew, H.C. Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. J. Fish Biol. 2006, 69, 426–445. [Google Scholar] [CrossRef]
- Lv, X.; Xu, S.; Liu, Q.; Wang, X.; Yang, J.; Song, Z.; Li, J. Osteological ontogeny and allometric growth in larval and juvenile turbot (Scophthalmus maximus). Aquaculture 2019, 498, 351–363. [Google Scholar] [CrossRef]
- He, T.; Xiao, Z.Z.; Liu, Q.H.; Li, J. Allometric growth in rock bream larvae (Temminck et Schlegel 1844). J. Fish. China 2012, 36, 1242. [Google Scholar] [CrossRef]
- Osse, J.W.M.; van den Boogaart, J.G.M. Dynamic morphology of fish larvae, structural implications of friction forces in swimming, feeding and ventilation. J. Fish Biol. 1999, 55, 156–174. [Google Scholar] [CrossRef]
- Gagna, M.R.; Wold, P.A.; Bardal, T.; Øie, G.; Open, E. Allometric growth and development of organs in ballan wrasse (Labrus bergylta Ascanius, 1767) larvae in relation to different live prey diets and growth rates. Biol. Open 2016, 5, 1241–1251. [Google Scholar] [CrossRef]
- Shuiqing, W.U.; Jiaer, L.I.; Youjun, O.U.; Guomin, L.V.; Jianghua, L. Allometric growth of hybrid grouper (Epinephelus coioides ♀ × E. lanceolatus ♂) larvae and juveniles. J. Fish. Sci. China 2014, 21, 503–510. [Google Scholar]
- Yu-Fu, W.; Zhi-Zhong, X.; Qing-Hua, L.; Jie-Ming, Z.; Zun-Fang, P.; Wen-Hui, M.A.; Dao-Yuan, M.A.; Shi-Hong, X.U.; Yong-Shuang, X.; Jun, L.I.J. Allometric growth pattern during early ontogeny of spotted knifejaw (Oplegnathus punctatus). Mar. Sci. 2016, 40, 43–48. [Google Scholar]
- De Lussanet, M.H.; Muller, M. The smaller your mouth, the longer your snout: Predicting the snout length of Syngnathus acus, Centriscus scutatus and other pipette feeders. J. R. Soc. Interface 2007, 4, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Optimization principles applied to the mechanism of neurocranium levation and mouth bottom depression in bony fishes (Halecostomi). J. Theor. Biol. 1987, 126, 343–368. [Google Scholar] [CrossRef]
- Detwyler, R.; Houde, E.D. Food selection by laboratory-reared larvae of the scaled sardine Harengula pensacolae (Pisces, Clupeidae) and the bay anchovy Anchoa mitchilli (Pisces, Engraulidae). Mar. Biol. 1970, 7, 214–222. [Google Scholar] [CrossRef]
- Teixeira, R.L.; Musick, J.A. Trophic ecology of two congeneric pipefishes (Syngnathidae) of the lower York River, Virginia. Environ. Biol. Fishes 1995, 43, 295–309. [Google Scholar] [CrossRef]
- Porter, M.M.; Adriaens, D.; Hatton, R.L.; Meyers, M.A.; McKittrick, J. BIOMECHANICS. Why the seahorse tail is square. Science 2015, 349, aaa6683. [Google Scholar] [CrossRef]
- Praet, T.; Adriaens, D.; Cauter, S.V.; BertMasschaele; Beule, M.D.; Verhegghe, B. Inspiration from nature: Dynamic modelling of the musculoskeletal structure of the seahorse tail. Int. J. Numer. Methods Biomed. Eng. 2012, 28, 1028. [Google Scholar] [CrossRef]
- Porter, M.M.; Novitskaya, E.; Castro-Cesena, A.B.; Meyers, M.A.; McKittrick, J. Highly deformable bones: Unusual deformation mechanisms of seahorse armor. Acta Biomater 2013, 9, 6763–6770. [Google Scholar] [CrossRef]


| Growth | AGR (mm/day) |
|---|---|
| DAB | SL |
| 1–30 | 0.58 ± 0.02 b |
| 30–54 | 1.4 ± 0.01 a |
| 54–100 | 0.17 ± 0.07 c |
| Skeletal Elements | Time (DAB) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 11 | 15 | 19 | 23 | 27 | 31 | 35 | 39 | 41 | 45 | 55 | 65 | 75 | ||||
| Branchiocranium | ||||||||||||||||||
| Angular | ||||||||||||||||||
| Antorbital | ||||||||||||||||||
| Cleithrum | ||||||||||||||||||
| Dentary | ||||||||||||||||||
| Ectopterygoid | ||||||||||||||||||
| Endopterygoid | ||||||||||||||||||
| Ethmoid plate | ||||||||||||||||||
| Hyomandibular | ||||||||||||||||||
| Lachrymal | ||||||||||||||||||
| Maxillary | ||||||||||||||||||
| Meckel’s cartilage | ||||||||||||||||||
| Mesethmoid | ||||||||||||||||||
| Metapterygoid | ||||||||||||||||||
| Nasal spine | ||||||||||||||||||
| Operculum | ||||||||||||||||||
| Palatine | ||||||||||||||||||
| Parasphenoid | ||||||||||||||||||
| Quadrate | ||||||||||||||||||
| Retroarticular | ||||||||||||||||||
| Rostral cartilage | ||||||||||||||||||
| Sub-orbital | ||||||||||||||||||
| Symplectic | ||||||||||||||||||
| Vomeral | ||||||||||||||||||
| Neurocranium | ||||||||||||||||||
| Basioccipital | ||||||||||||||||||
| Coronet | ||||||||||||||||||
| Epiotic | ||||||||||||||||||
| Exoccipital | ||||||||||||||||||
| Frontal | ||||||||||||||||||
| Frontal crests | ||||||||||||||||||
| Post-temporal | ||||||||||||||||||
| Precoronet spine | ||||||||||||||||||
| Sphenotic | ||||||||||||||||||
| Supra-occipital | ||||||||||||||||||
| Legend | Absence | Cartilage | Cartilage-bone | bone | ||||||||||||||
| Skeletal Elements | Time (DAB) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 11 | 15 | 19 | 23 | 27 | 31 | 35 | 39 | 41 | 45 | 55 | 65 | 75 | |||||||||
| Axial skeleton/shields | |||||||||||||||||||||||
| Abdominal pre-dorsal vertebrae | |||||||||||||||||||||||
| Abdominal post-dorsal vertebrae | |||||||||||||||||||||||
| Caudal vertebrae | |||||||||||||||||||||||
| cervical vertebrae | |||||||||||||||||||||||
| Dermal body plates | |||||||||||||||||||||||
| Dorsal vertebrae | |||||||||||||||||||||||
| Haemal arch | |||||||||||||||||||||||
| Hypural plate | |||||||||||||||||||||||
| Neural arch | |||||||||||||||||||||||
| Post-zygapophysis | |||||||||||||||||||||||
| Pre-zygapophysis | |||||||||||||||||||||||
| Shields | |||||||||||||||||||||||
| Legend | Absence | Cartilage | Cartilage-bone | bone | |||||||||||||||||||
| Skeletal Elements | Time (DAB) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 11 | 15 | 19 | 23 | 27 | 31 | 35 | 39 | 41 | 45 | 55 | 65 | 75 | ||||||||||
| Appendicular skeleton | ||||||||||||||||||||||||
| Distal radial | anal fin | |||||||||||||||||||||||
| dorsal fin | ||||||||||||||||||||||||
| Caudal fin | ||||||||||||||||||||||||
| Medial radial | anal fin | |||||||||||||||||||||||
| dorsal fin | ||||||||||||||||||||||||
| Caudal fin | ||||||||||||||||||||||||
| Proximal radial | anal fin | |||||||||||||||||||||||
| dorsal fin | ||||||||||||||||||||||||
| Caudal fin | ||||||||||||||||||||||||
| ray | anal fin | |||||||||||||||||||||||
| dorsal fin | ||||||||||||||||||||||||
| Caudal fin | ||||||||||||||||||||||||
| Legend | Absence | Cartilage | Cartilage-bone | bone | ||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Tang, X.; Zhang, Y.; Wang, W.; Qin, S.; Liu, Q.; Mei, J. The Early Allometric Growth and Osteological Ontogeny of Pot-Bellied Seahorse (Hippocampus abdominalis, L. 1827) under Mass-Scale Captive Breeding Conditions in North China. Fishes 2023, 8, 604. https://doi.org/10.3390/fishes8120604
Shi X, Tang X, Zhang Y, Wang W, Qin S, Liu Q, Mei J. The Early Allometric Growth and Osteological Ontogeny of Pot-Bellied Seahorse (Hippocampus abdominalis, L. 1827) under Mass-Scale Captive Breeding Conditions in North China. Fishes. 2023; 8(12):604. https://doi.org/10.3390/fishes8120604
Chicago/Turabian StyleShi, Xuehui, Xinyi Tang, Yichao Zhang, Wenqi Wang, Siyong Qin, Qinghua Liu, and Jie Mei. 2023. "The Early Allometric Growth and Osteological Ontogeny of Pot-Bellied Seahorse (Hippocampus abdominalis, L. 1827) under Mass-Scale Captive Breeding Conditions in North China" Fishes 8, no. 12: 604. https://doi.org/10.3390/fishes8120604
APA StyleShi, X., Tang, X., Zhang, Y., Wang, W., Qin, S., Liu, Q., & Mei, J. (2023). The Early Allometric Growth and Osteological Ontogeny of Pot-Bellied Seahorse (Hippocampus abdominalis, L. 1827) under Mass-Scale Captive Breeding Conditions in North China. Fishes, 8(12), 604. https://doi.org/10.3390/fishes8120604






