Abstract
The objective of this systematic review was to identify and classify, from the available literature, non-conventional feed ingredients from terrestrial plants, animals, algae, and fungi which have been evaluated for their potential use for tilapia (Oreochromis spp.) production. For this purpose, 795 papers published in the Scopus and Web of Science databases between 2011 and 2021 were analyzed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology. Data on the growth rate (GR) and effects on weight gain (WG), specific growth rate (SGR) and feed conversion ratio (FCR); digestibility; fatty acid profile (FAP) of the fish carcass; and the survival rate (SR) were compiled in databases and summary tables. The results were refined according to different criteria, obtaining 144 documents that were pertinent for an in-depth analysis. From those, we found that 50.7% evaluated terrestrial plants, 22.2% animals, 13.9% algae, 9% fungi, and the remaining, combinations of some of the above categories. From the summarized results we concluded that most of the non-conventional sources analyzed have a positive potential impact as alternatives for producing tilapia. Survival was the most evaluated parameter, while digestibility was the least evaluated parameter.
Key Contribution:
This review provides a comprehensive overview of the results of more than a hundred evaluations that have been carried out on different non-conventional ingredients for feeding tilapia, offering a range of alternatives for fish farmers.
1. Introduction
Nowadays, animal production faces significant challenges arising from the need to reduce its negative environmental impact and effect on climate change. It also faces input scarcity and an increase in its prices, mainly due to the economic situation derived from the COVID-19 pandemic and the war in Ukraine. These conditions have increased the need to seek non-conventional, sustainable, and, hopefully, locally produced food sources that will reduce the costs and carbon footprint related to its production and transportation. In the case of aquaculture production, it is also necessary to minimize the use of raw materials from fishery to reduce their impact on marine ecosystems.
Global aquatic food consumption (excluding algae) grew at an annual average of 3.0% between 1961 and 2019, a rate that almost doubled the annual global population growth (1.6%) over the same period, with an annual per capita consumption of 20.5 kg in 2019. In the same year, aquatic food reached 17% of total animal protein and 7% of total protein consumed worldwide []. It is expected that, by 2030, 57% of fish will come from aquaculture, compared to 53% in 2018–2020. Likewise, it is estimated that by 2030, fish will remain critical to the global diet and play a key role in food security []. Nile tilapia (Oreochromis niloticus) is the third in the production of aquaculture species worldwide (9%), closely preceded by grass carp (Ctenopharyngodon idellus) with 11.8%, and silver carp (Hypophthalmichthys molitrix) with 10% [].
FAO [] has proposed a new approach to aquaculture called “Blue Transformation”, which aims, among other things, to develop and adopt sustainable aquaculture practices and improve capacities to achieve a more efficient and resilient aquaculture industry. The search for non-conventional and sustainable sources of fish feed allows progress toward achieving these objectives.
This paper presents the results of a systematic review aimed at identifying and classifying different raw materials from terrestrial plants, animals, algae, and fungi not commonly used in commercial fish feeds which have been evaluated to determine their potential use as ingredients for tilapia feed. To summarize this concept, we will call them non-conventional feeds, as opposed to traditional sources as fishmeal, soybean, fish, or corn oils, among others. These, in some cases, may be considered unsustainable or, in other cases, may be difficult to access for countries that do not produce, and therefore, must import them. In this case, situations such as those that occurred during the pandemic and post-pandemic, to cite two examples, can mean a shortage of supplies than can lead to a crisis in livestock production, and, in this specific case, in tilapia production.
To carry out an analysis in adequate depth, we have limited the scope of this manuscript to biological categories and therefore do not include chemical or mineral materials. Additionally, we exclude bacterial-based food sources since, if included, the analysis would exceed the length that can be adequately contained in a single article. Based on the results obtained, the digestibility results and the effects of diets and supplements on the fish in terms of growth rate, fatty acid profile, and survival rate were analyzed.
2. Materials and Methods
A systematic review was carried out based on an adaptation of the PRISMA methodology []. Bibliographic searches allow establishing data availability in systematic reviews, which aims to select and collect qualitative and quantitative data that will be available for analysis and will be used to determine eligibility depending on the topic of interest.
This process must be rigorous and replicable to ensure a minimal bias in searches []. Moreover, systematic reviews and meta-analyses synthesize data from existing primary research, and well-conducted reviews offer a practical solution to the problem of keeping up to date in any field of interest [].
The specific focus of this systematic review is on non-conventional feed sources for tilapia production. Thus, papers indexed in Scopus and Web of Science (WOS) databases were used as sources. In both databases, the search was carried out in the title, abstract, and keywords fields (TITLE-ABS-KEY), published from 1 January 2011 to 31 December 2021, in English, Portuguese, and Spanish.
The primary search term was “Tilapia”. It was applied in combination with any of the following: “nutrition”, “nutritional supplement”, “dietary supplement”, “dietary supplementation”, “nutritional supplementation”, “new ingredient”, and “insect”. In the Scopus database, particularly, the search was also limited by the type of paper: “ar” (reviewed articles), “re” (reviews), and “cp” (conference papers). However, it was impossible to limit the search by type of paper in the Web of Science database since the search engine does not have this option.
The search equations developed with the keywords of interest were as follows:
Scopus:
TITLE-ABS-KEY (tilapia AND (nutrition OR “nutritional supplement” OR “dietary supplementation” OR “dietary supplement” OR “nutritional supplementation” OR “novel ingredient” OR “insect”)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “cp”)) AND (LIMIT-TO (PUBYEAR, 2021) OR LIMIT-TO (PUBYEAR, 2020) OR LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT-TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016) OR LIMIT-TO (PUBYEAR, 2015) OR LIMIT-TO (PUBYEAR, 2014) OR LIMIT-TO (PUBYEAR, 2013) OR LIMIT-TO (PUBYEAR, 2012) OR LIMIT-TO (PUBYEAR, 2011)) AND (LIMIT-TO (LANGUAGE, “English”) OR LIMIT-TO (LANGUAGE, “Portuguese”) OR LIMIT-TO (LANGUAGE, “Spanish”)).
Web of Science:
(tilapia AND (nutrition OR “nutritional supplement” OR “dietary supplementation” OR “dietary supplement” OR “nutritional supplementation” OR “novel ingredient” OR “insect”)).
Using the results obtained from the search equation for Scopus and Web of Science, repeated papers were first discarded, and then a preliminary selection was made by reading the titles and abstracts. Thus, those unrelated to the topics of interest were discarded. The exclusion criteria used for this were:
- -
- The fish evaluated is not of the genus Oreochromis spp.
- -
- The article is not related to diet or supplement evaluation.
- -
- Only the effects of the ingredient on fish health were evaluated in the article.
- -
- Only effects on reproduction or sexual reversion were evaluated in the article.
After articles were eliminated based on the above criteria, it was also necessary to remove one article that had been retracted. A subsequent more in-depth reading allowed to identify other aspects that made some articles less interesting regarding the research objective:
- -
- The ingredients evaluated are not included in the scope of the study but are bacteria, minerals, or chemically synthesized compounds.
- -
- The ingredients evaluated are conventional. In the context of this paper, the term “conventional ingredient” is used to refer to an ingredient traditionally used in the production of commercial fish feed. For example, soybean, corn, fishmeal, and fish oil, among others. After these depuration stages, a preliminary selection of articles dedicated to feeding based on terrestrial plants (embryophytes), animals, algae, fungi, and some industrial by-products was obtained. The procedure followed is outlined in Figure 1.
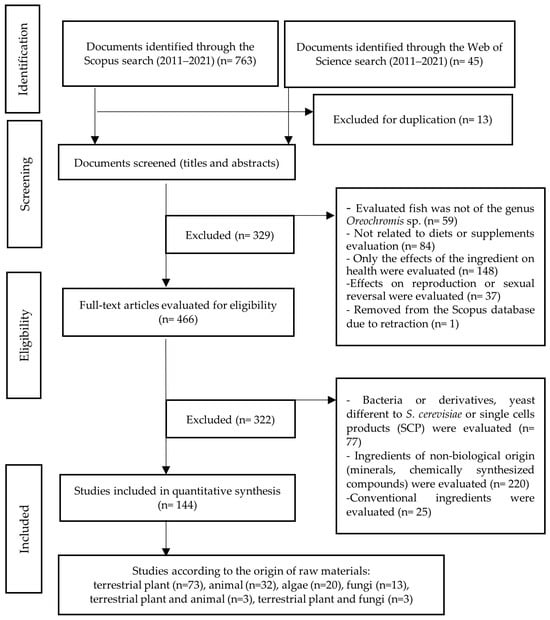 Figure 1. Document identification and selection flowchart. Source: Own elaboration.
Figure 1. Document identification and selection flowchart. Source: Own elaboration.
At the end, the papers that met the following criteria were selected:
- Raw material origin—the food source evaluated was algae, animal, fungus, plant, or insect.
- Raw material processing method—the type of raw material evaluated in the articles: by-product, excrement, seed, meal, cake, shell, bran, extract, oil, essential oil, leaves, powder, hydrolate, protein hydrolysate, the residue of slaughtering, syrup, and nanoparticles.
- Analyses carried out—the type of evaluation carried out in the study: growth, body composition, digestibility, feed conversion ratio, health, survival, food consumption, food cost, and sensory acceptance.
The selected articles were compiled in a database consisting of the following items: authors, title, year, country, affiliation, abstract, keywords, raw material origin, raw material processing method, analysis carried out, and food source. The purpose of the database was to identify and synthesize information of interest for the review. This information was also entered into VantagePoint software to obtain different qualitative and quantitative analyses.
Moreover, the resulting papers were used to create summary tables of findings related to the incidence of the supplement or diet evaluated on the fish growth, its survival, the ingredient digestibility, and the fatty acid profile of the fish at the end of the evaluation. To create the table regarding the evaluated ingredients’ incidence on tilapia’s growth rate, the results found in the reviewed papers related to weight gain (WG), specific growth rate (SGR), and feed conversion ratio (FCR) were recorded.
Following the PRISMA methodology, the presence of conflicts of interest and the sources of funding for the research were analyzed to establish whether they have any possible impact on the results obtained and to ensure the impartiality of the information. Furthermore, it is essential to highlight that analyses in which no quantitative data were found were not included to avoid errors in the evaluation of the certainty of the evidence.
Thus, tables and graphs were constructed to synthesize the information collected after examining the information grouped in these databases and the summary tables of findings. The most used way to analyze the different studies reviewed was to evaluate whether comparisons of the diets or supplements were made in relation to a commercial control and whether the highlight of this comparison showed a statistically significant difference (SSD) or not (No SSD). If there were differences, whether the diet or supplement evaluated had been superior (SSS) or inferior (SSI) compared to the control was recorded. The analysis of these sources in a disaggregated way, separating some of the feeding categories, allows us to go much deeper and bring up complementary data extracted from some of the other articles.
3. Results and Discussion
3.1. Analysis Criteria
As shown in Figure 1, with the equations for Scopus and WOS, 795 papers were obtained. A total of 651 were discarded for the various reasons mentioned above. Thus, 144 articles were finally obtained and classified according to the food source evaluated, resulting in 73 articles in the terrestrial plant category (50.7%); 32 in animal (22.2%); 20 in algae (13.9%); 13 in fungus (9.0%); 3 assessed terrestrial plant and animal (2.1%); and 3 evaluated terrestrial plant and fungus (2.1%).
The keywords most used by the authors of the reviewed papers can be seen graphically in the Word Cloud of Figure 2 as one of the results of VantagePoint software. These words largely coincide with the topics on which it was decided to delve in this review and confirm the correct choice of search terms.

Figure 2.
Keyword cloud of reviewed documents. Source: VantagePoint.
Figure 3 shows the number of publications per year according to the parameters previously defined for this review. A constant scientific production is noted from 2011 to 2017, with a small peak in 2014 and an increasing trend in publications starting in 2018. One possible explanation for this phenomenon is the increased interest in sustainability-related issues, which has been growing since the adoption of the Sustainable Development Goals in 2015 [] proposed by the United Nations General Assembly. The search for alternative ingredients to those originating from industrial agriculture and industrial fishing point, among others, to objectives such as responsible production and consumption, underwater life, and life of terrestrial ecosystems.
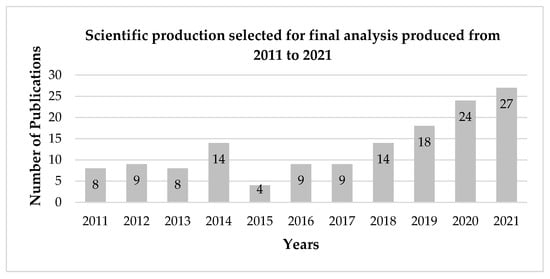
Figure 3.
Scientific production selected for final analysis produced from 2011 to 2021. Source: Own elaboration.
On the other hand, when analyzing the country of affiliation of the authors of the papers evaluated, it was found that the country with the highest scientific production was Brazil, with 20.67% (43 publications), followed by Egypt, with 18.27% (38 publications), Thailand with 7.69% (16 publications), and China and Saudi Arabia, with 5.29% each (11 publications). Figure 4 shows the countries of the authors of the publications and the frequency of publications expressed in colors.
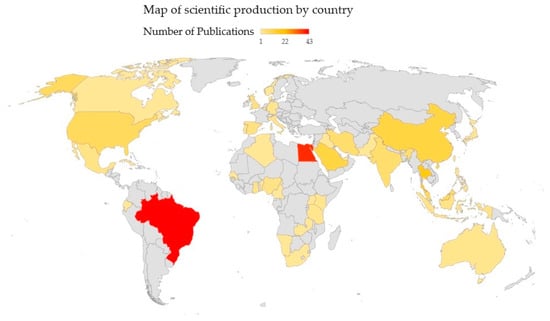
Figure 4.
Number of documents per country. Source: Own elaboration.
3.2. General Considerations
The most frequently used experimental conditions were identified, to establish reference frameworks for new studies based on the existing literature. 75% of the reviewed research was conducted with O. niloticus, 13.19% with red hybrid (O. niloticus × O. mossambicus), 6.25% with GIFT (O. niloticus genetically modified), 4.87% with O. mossambicus, and 0.69% with O. niloticus × O. aureus.
Moreover, 61.81% of the studies tested supplements, 37.5% produced concentrated feeds that included the raw material studied, and 0.69% did not report any information on this matter. The review showed that 64.58% of the studies started with fish weighing between 0 and 20 g, 15.28% between 21 and 40 g, and 16.67% with fish weighing more than 40 g. The great majority of the papers, except for five (3.47%), reported the initial weight of fish as an essential element to be evaluated. The most frequent duration interval for the experiments was from 31 to 60 days (46.53%), followed by 61–90 days with 22.92%, and 30 days or less corresponds to 16.67%. The most frequent range of the number of individuals per tank was between 11 and 20, with 35.42%, followed by 21–30, with 25%, and 0 to 10, with 18.1%.
Some of the non-conventional ingredients found were used to replace fishmeal, soybean, or similar protein sources. Conventional fat sources such fish oil, soybean oil or corn oil were the aim of other significant number of papers. The improvement of immune system, nutrient availability, antimicrobial and antioxidant activities were other goals that were aimed to using ingredients as essential oils or plant extracts.
According to the amount of data, raw materials show the highest number of papers in the analyses considered in this evaluation. Some of those that stand out for the terrestrial plants’ category are Linum usitatissimum L. and Helianthus annuus L., with the highest number of written articles, six and five publications, respectively. The next most frequent were Salvadora persica L. with 4, Moringa oleifera Lam. with three. In addition, there are Cocos nucifera L., Astragalus membranaceus Bunge, and Olea europaea L. with 2 evaluations each.
In the case of L. usitatissimum, H. annuus, C. nucifera, and O. europaea, extracted oil from these species was the most evaluated along with its impact on the growth and composition of the fish, especially on its fat content and fatty acid profile. For S. persica, its meal and its effect on the fish growth were evaluated. In A. membranaceus, the effects of polysaccharides extracted from the plant material on fish growth were evaluated. Also, its aqueous extract, in combination with that of other plants, was evaluated on growth and digestibility parameters.
The raw materials that stand out in the animal category are Hermetia illucens L. with 11 evaluations, followed by swine and poultry with 5 and 4 evaluations, respectively. Additionally, there is Penaeus vannamei Boone with 3 evaluations. In the fungi category, the highest number of mentions was obtained by Saccharomyces cerevisiae Meyen ex. Hansen, with 10 evaluations, and in the algae category, Arthrospira platensis Gomont stands out with 4 evaluations, most of them focused on growth and survival parameters.
Figure 5 shows the nine food sources with the highest number of mentions in the analyzed papers. 4 are terrestrial plants, 2 are of animal origin, 2 is algae, and 1 is fungi. It also shows that the Growth parameter reports the highest number of evaluations, followed by the Body Composition and Survival parameters.
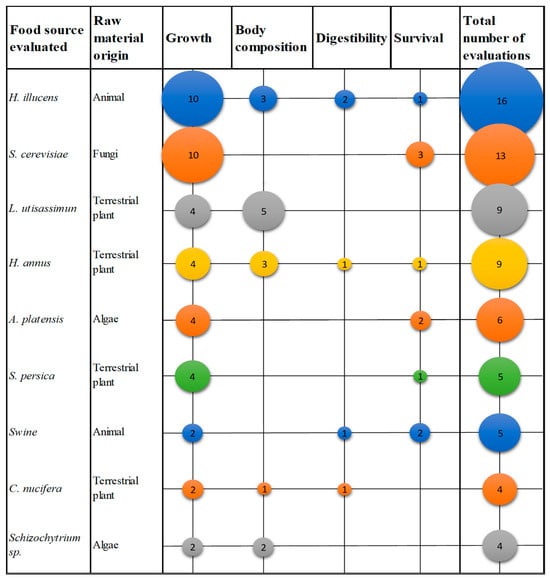
Figure 5.
Food source evaluated with the highest number of tests performed in the reviewed documents. Source: Own elaboration.
3.3. Parameters Studied in Fish
3.3.1. Growth
Fish growth was evaluated in 86.1% (124 articles) of the total papers. The indicators analyzed were Weight Gain percentage (WG) (Equation (1)), Specific Growth Rate (SGR) (Equation (2)), and Feed Conversion Ratio (FCR) (Equation (3)). These indicators were obtained from the papers reviewed or calculated by the authors of this review based on the available information. The formulas used were as follows:
Growth Rate
Weight Gain Percentage (%WG)
Specific Growth Rate (SGR)
Feed Conversion Ratio (FCR)
where
- Wi: initial weight
- Wf: final weight.
According to the processing method of the raw materials and how they were added to each diet, 31 different categories were obtained. There were 142 mentions related to them from the 124 articles where growth indicators were evaluated. The main processing methods were meal with 38.1% (54 mentions), extracts of different types with approximately 12% (17 mentions), and essential oils and oils in general with 4.9% (7 mentions) each.
Data were organized according to the origin of the food evaluated for each diet. 64 of the papers reported diets based on terrestrial plants only (51.6%), 25 of animal origin (20.2%), 16 of algae (12.9%), and 14 of fungi (11.3%). In one of the studies, a plant and a fungus were simultaneously evaluated (0.8%). Four of the analyzed feeds are by-products (3.2%), one of which evaluated a digestate and another a meal, both from terrestrial plant and animal waste. Furthermore, one study includes residues from pasta production and another from bee pollen.
The articles that evaluated growth using terrestrial plants, either individually or in combination with another type of food source for tilapia were 67, which can be seen in Table 1 (54.0% of the total number of papers reviewed and 84.8% of the total number of papers that evaluated terrestrial plants). Regarding food sources from animals, algae, fungi, and by-products, it was reported that 48.4% (60 papers) were about growth, equivalent to 84.5% of the total number of papers where these types of food sources were evaluated (71 articles).

Table 1.
Summary table of the different growth indicators evaluated in terrestrial plants as food sources with respect to commercial feeds. Source: Own elaboration.
Table 1 and Table 2 summarize the results of the different analyses performed by the authors, comparing them statistically with the commercial control used. Moreover, the tilapia species and the growth stage of the fish on which the evaluation was performed were recorded. In these tables these acronyms were used: WG Weight gain, SGR Specific growth rate and FCR Feed conversion ratio. In Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6, an arrow up (↑) is used to denote “statistically significant superior”, arrow down (↓) denotes “statistically significant inferior”, and (=), “not statistically significant different” from control. When no information was found in the corresponding parameter in the specific source, a hyphen (-) was used. It is important to consider that, in some works, different concentrations were studied. In the case where the parameters have different statistical differences with respect to the control for different concentrations, this is expressed using more than one symbol. For example, “=↑” means that for the first group of concentrations, there is not statistically significant difference, and in the second group of concentrations, the parameter is statistically significant superior with respect to control.

Table 2.
Summary table of the different growth indicators evaluated in animals, algae, fungi, and by-products as food sources with respect to commercial feeds. Source: Own elaboration.
Regarding growth, the most frequently evaluated plants were H. annuus, L. usitatissimum, and S. persica with four research each. M. oleifera with three, whereas A. membranaceus, C. sinensis, C. nucifera, C. longa, M. indica, O. europaea, and P. juliflora with two evaluations each.
It is noteworthy that studies with seed meal of H. annuus in 2013 and 2016 [,] showed no statistically significant differences (SSD) in Weight Gain and Specific Growth Rate concerning the control diet, while in a more recent study [] the results were statistically significant superior (SSS). Supplementation with the oil of this plant reported a statistically significant inferior Weight Gain (SSI) compared to the control [].
Trials with the seed extract of L. usitatissimum registered SSS weight gains compared to the control [], while its oil did not show SSD compared to the control in the other reviewed evaluations [,,]. In addition, S. persica meal recorded SSS results compared to the control in some of the evaluated diets [], while the other evaluated diets did not record SSD compared to their respective controls [,,].
In all cases where M. oleifera meal was evaluated, it recorded SSS results to the control []. When used as a seed, SSS weight gain and specific growth rate compared to the control were registered, while no SSD was found in these parameters when its ground and dried leaf was used []. In another experiment, leaves were supplemented in different percentages (4%, 8%, and 10%), registering results in weight gain SSS compared to the control, but without SSD in specific growth rate and feed conversion ratio [].
In the two studies reviewed which A. membranaceus was evaluated, SSS results were obtained compared to the control. One included polysaccharide obtained from such plants in the diet [], and the other used a mixture of aqueous extracts from four different plants, including the plant mentioned above []. The ethanolic extract of C. sinensis applied in diets registered SSS results compared to the control []. However, when it was added to the diet as green tea waste, no SSD occurred with the control [].
It is also noteworthy that in two reviewed studies where C. nucifera oil was added to the diets, contrasting results were registered in the control, SSS in one [] and SSI in the other []. Hydrolate of C. longa did not register SSD related to the control when added to the diets []. But when supplementation with curcumin was carried out, SSS were obtained [].
In the two studies that evaluated diets containing mango meal (M. indica), SSI growth results were found compared to the control in most treatments [,]. The O. europaea cake showed significantly superior results to the control, while its oil recorded SSI results []. P. juliflora meal did not show SSD results to the control in the two works where it was evaluated [,].
An outstanding element is that in most of the studies, the plants evaluated yielded weight gains that were not statistically significantly different from the control or higher. This suggests that there are a significant number of plant sources that deserve further exploration and may be viable alternatives to replace conventional ingredients in tilapia feed.
It is striking that a significant number of these positive results have been obtained with extracts and essential oils. This observation and the conclusions drawn by many of the researchers suggest that the improvement in weight gain rates is due to an improvement in the immune system of fish, and microbiological and antioxidant activities.
The raw material from animals with the highest number of evaluations and increasing trend was the black soldier fly, H. illucens, with nine mentions. It was used as meal and oil. The effect of its excrement was also evaluated in one paper. It is interesting that, in two evaluations, one made with oil and the other with meal, SSS was registered when the inclusion percentage was the lowest in the diets evaluated, but SSI when the highest inclusion percentage was evaluated [,]. Likewise, it showed SSI in WG and SGR in a single diet where such raw material was used as a meal []. Furthermore, in a single case study with different evaluated diets SSS was reported in FCR [].
The other mentions did not show SSD. P. vannamei, with three mentions, had SSI [] when used as a meal but SSS when the shell was used []. The other raw materials from animals most frequently evaluated in the reviewed papers are analyzed in the by-product category.
In the algae category, A. platensis stand out with four evaluations, showed SSS in all growth parameters for some of the diets when it was used as a meal [,]. In one of the works, A. platensis was evaluated in nanoparticle form and showed SSS in all diets []. As a meal, in some diets it reported SSS and SSI for the WG parameter, and SSS for SGR in others []. Evaluations of dried cells [] and meal [] of Schizochytrium sp. reported SSS for the WG in some of the diets evaluated.
In the fungi category, S. cerevisiae had nine evaluations, in which its use as an extract stands out [,] with SSS in all diets. Also, SSS was observed when evaluated as a meal [,] in all parameters for which data were reported. It should be noted that SSS was reported in all growth parameters when the supplementation with S. cerevisiae cell wall was used [].
The by-products from swine were mentioned four times. In this case, the only diet that showed differences from the control was the one that used pork fat [], having SSI in all of the evaluated parameters. Diets containing bovine meat and bone as a slaughter residue meal [] showed SSS in the WG parameter. Diets containing poultry feather meal [] showed SSI in the WG and SGR parameters where they were evaluated.
Within these categories, most of the algae evaluated showed promising results, while in animals, H. illucens and P vanamei showed the best results, although somewhat contradictory results among some studies. Within fungi, there are a significant number of promising results, with S. cerevisiae standing out. These results contrast with those obtained by the byproducts, since only those obtained with bone and meat slaughter residues obtaining outstanding results.
3.3.2. Fatty Acids Profile
This review also analyzed the impact of diets and supplements on the fatty acid profile of fish. It should be noted that, of the 144 papers initially reviewed, the fatty acid profile was evaluated in 13.2% (19 papers). Of these, 31.6% (6 papers) only reported data on the lipid profile of the diets fed to the fish, and 68.4% (13 papers) additionally reported the profile of the fish carcass at the end of the experiment. Two of them only reported the changes in the profiles at different moments of the experiment but not regarding a control diet, so they were not considered for this analysis. Therefore, for the fatty acid profile, 11 papers were analyzed. For terrestrial plants, five papers were found (45.5% of the 11 papers). Only oils of plant origin were evaluated in these papers, as reported in Table 3. For sources from animals, algae, fungi, and by-products, six articles were reviewed (54.5% of the 11 papers), which are summarized in Table 4.
For the preparation of Table 3 and Table 4, only the data of the most relevant indicators of the fatty acid profile concerning the food source were extracted. The data reported for the fish carcass were analyzed according to their impact on human food.

Table 3.
Summary table of the most relevant indicators of the fatty acid profile of the fish carcass after evaluation with supplements and vegetable oil diets. Source: Own elaboration.
Table 3.
Summary table of the most relevant indicators of the fatty acid profile of the fish carcass after evaluation with supplements and vegetable oil diets. Source: Own elaboration.
| Plant Species | H. annuus, L. usitatissimum, C. nucifera, O. europaea | B. napus, Salvia hispánica L. | H. annuus, Perilla frutescens (L.) Britton | H. annuus, L. usitatissimum, B. napus, C. sativum | Borago officinalis L., Oenothera biennis L. |
|---|---|---|---|---|---|
| Raw material processing method | Oil | Oil | Oil | Oil | Oil |
| Fish species | n | n | GIFT | GIFT | n |
| Stage | J | J | - | F | - |
| Omega 3 | - | ↑ | ↑ * | = | ↓ * |
| Omega 6 | - | ↑ | ↓ * | ↓ * | ↑ * |
| SFA | = | ↑ | = | = | = |
| MUFA | = | ↑ | ↓ * | = | = |
| PUFA | = | ↑ | ↑ * | = | = |
| LCPUFA | ↓ | - | - | = | - |
| Omega 3 | - | ↑ | - | = | - |
| Omega 6 | - | ↑ | - | ↑ * | - |
| n3/n6 | ↓ | ↑ | ↑ * | = | ↓ |
| Reference | [] | [] | [] | [] | [] |
Note: SFA: Saturated fatty acids, MUFA: Monounsaturated fatty acids, PUFA: Polyunsaturated fatty acid, LC: Long Chain. Fish species: n: O. niloticus, GIFT: niloticus GIFT (genetically modified). Stage: F: Fingerling, J: Juvenile. Statistically significant superior (↑), statistically significant inferior (↓), no statistically significant difference (=) compared to control, no information was found in the corresponding parameter (-). * The difference was only present in some of the diets evaluated.
The most relevant result on the fatty acid profile of the fish was obtained when the diet with B. napus and S. hispanica oil was evaluated []. This is the only case among those reviewed in which a statistically significant increase in all of the evaluated indicators was registered compared to the control diet. In addition, it was the only diet in which saturated fatty acids (SFA) increased significantly, contrary to the other articles in which no SSD were observed with the control [,,,]. This is important since there is a direct relationship between SFA intake and the risk of developing diseases such as diabetes in humans. Thus, FAO recommends replacing SFA with polyunsaturated fatty acids (PUFA) to reduce the risk of coronary heart disease. Moreover, replacing SFA of animal origin with monounsaturated fatty acids (MUFA) of plant origin improves insulin sensitivity and glycemic control in type 2 diabetes [].
In some of the diets that evaluated oils from H. annuus and P. frutescens [], a significant decrease in MUFA and a significant increase in PUFA were reported regarding to the control. Both fatty acid groups are good for human consumption, as they help reduce the risk of cardiovascular diseases and strokes. The increase in PUFA helps to reduce the concentration of LDL (low-density lipoproteins) cholesterol and the ratio of total cholesterol to HDL (high-density lipoproteins) cholesterol [].
On the other hand, Omega-6 was significantly inferior to the control when H. annuus oil was used as a supplement. Similar results were found for diets that included L. usitatissimum, B. napus, C. sativum [], and P. frutescens []. When diets that included H. annuus and P. frutescens [], and B. napus and S. hispanica [] were evaluated, statistically superior differences to the control in the ratio of Omegas n3/n6 were reported.
Regarding the LCPUFAs, diets that included H. annuus, L. usitatissimum, C. nucifera, and O. europaea [] showed SSI contents compared to the control, however, when H. annuus, L. usitatissimum, B. napus, and C. sativum [] were included in the diets, they did not report SSD. These fatty acids (mainly eicosapentaenoic acid 20:5n-3, EPA. and docosahexaenoic acid 22:6n-3, DHA) when come from oily fish diets, generally do not affect the concentrations of total cholesterol [].

Table 4.
Summary table of the most relevant indicators of the fatty acid profile of the fish carcass. Source: Own elaboration.
Table 4.
Summary table of the most relevant indicators of the fatty acid profile of the fish carcass. Source: Own elaboration.
| Raw Material Origin | Algae | Animal | Fungi | |||
|---|---|---|---|---|---|---|
| Food source | Schizochytrium sp. | N. salina | Schizochytrium sp. | H. illucens | T. molitor | Aurantiochytrium sp. |
| Raw material processing method | Meal | Meal | Dried whole cell | Meal | Meal | Meal |
| Fish species | n | n | n | n | n | GIFT |
| Stage | J | F | J | F | J | J |
| Omega 3 | ↑ | ↑ * | = | ↓ | ↓ * | ↑ |
| Omega 6 | = | ↓ * | = | = | ↑ | = |
| SFA | = | ↑ * | ↑ * | = | ↓ | = |
| MUFA | = | ↑ * | ↓ * | = | = | = |
| PUFA | = | - | = | = | = | ↑ * |
| LCPUFA | - | - | - | - | - | - |
| Omega 3 | - | - | = | - | - | ↑ |
| Omega 6 | - | - | ↑ | - | - | ↓ |
| n3/n6 | ↑ | ↑ * | ↓ | - | ↓ | ↑ |
| Reference | [] | [] | [] | [] | [] | [] |
Note: SFA: Saturated fatty acids, MUFA: Monounsaturated fatty acids, PUFA: Polyunsaturated fatty acid, LC: Long Chain. Fish species: n: O. niloticus, GIFT: niloticus—GIFT (genetically modified). Stage: F: Fingerling, J: Juvenile. Statistically significant superior (↑), statistically significant inferior (↓), no statistically significant difference (=) compared to control, no information was found about the corresponding parameter (-). * The difference was only present in some of the diets evaluated.
The most relevant result in the fatty acid profile of the fish when algae were evaluated was with the diet containing Schizochytrium sp. meal []. Through this diet, a statistically significant increase compared to the control diet was registered in the Omega 3 indicators and the n3/n6 ratio. The other parameters showed no difference compared to the control. The diet with N. salina [] also reported significantly higher values in the parameters mentioned above, which also happened with SFA and MUFA for some of the evaluated diets. The risk of developing diseases such as diabetes in humans directly relates to the intake of SFA-containing foods. Therefore, replacing SFA with PUFA [] is recommended to reduce the occurrence of arterial diseases.
In the evaluation of food source of animal origin, the diet with H. illucens meal [] stands out, which showed significantly lower values in its Omega 3 concentrations compared to the control, as the only parameter in which there were statistically significant differences. Some diets that used T. molitor meal also reported significantly lower values of this compound compared to the control []. In this study, all of the diets in which this meal was evaluated had significantly low SFA values and n3/n6. However, the use of the meal significantly increased the Omega 6 levels in all diets compared to the control. The lower SFA values obtained suggest that this dietary ingredient can potentially improve human health benefits, given the fatty acid profile of fish.
Only one case study of the fungi category showed SSD compared to the control; that is the case of Aurantiochytrium sp. meal []. In this study, some diets showed an increase in PUFA, which, as already mentioned, are beneficial to human health [].
3.3.3. Digestibility
Digestibility is usually calculated using the Apparent Digestibility Coefficient (ADC) of mainly dry matter (Equation (4)), protein (Equation (5)), and lipids (Equation (6)). Digestibility was evaluated in 11.1% (16 documents) of the total papers included in this review. Of these, 87.4% (14 documents) evaluated digestibility by ADC. In one article, the Digestive Enzyme Activity (DEA) method was used []. In another article, the Digestibility Coefficient method was used by mathematical calculation using the Acid Insoluble Ash method (AIA) [].
ADC DM: Apparent Dry Matter Digestibility (%)
ADC protein: Apparent Digestibility of protein (%)
ADC lipid: Apparent Digestibility of lipid (%)
For the case of terrestrial plants, digestibility was evaluated in seven papers (43.8% of the total reviewed which this parameter was evaluated). In five of the papers, it was evaluated by ADC and in four of them a comparison with a commercial diet was performed. Table 5 shows the results summarized for these four. In the fifth paper, comparisons were made between the two forms of processing (pelleting and extrusion) of supplements from M. esculenta scraps and hay of Manihot glaziovii Müll. Arg. The pelleting of M. esculenta from scraps of the same plant, in comparison with extrusion, was SSS for gross energy digestibility, SSI for dry matter digestibility, and no SSD for crude protein. The extrusion of M. glaziovii hay behaved in the same way, but in terms of gross energy and crude protein digestibility, it did not show SSD with respect to the pelleting [].
In addition to the data reported in Table 5, in the evaluation of okara, which is a pulp formed by the insoluble fraction of the seed remaining after the production of soy milk [], its digestibility was evaluated by ADC for lipids, and SSS values were obtained for all diets, except for the one in which 30% of dry okara was added, where no SSD were obtained. The ADC for phosphorus reported no SSD in most of the diets evaluated, SSI was recorded when the treatment with 30% dry okara was analyzed, and SSS when the diet contained 30% hydrolyzed okara.
In one of the papers reviewed, the digestibility of C. nucifera oil was evaluated with the DEA method, using lipase, amylase, and protease. When supplemented with 4% oil, no SSD were reported in terms of digestibility to the control, while with 2% and 3%, SSS digestibility indices compared to the control were recorded. When a concentration of 1% was used, lipase tests showed no SSD with the control, while SSS values of digestibility were found with the other enzymes []. The digestibility values of the diets with T. cacao, using the digestibility coefficient, were SSI to the control at all inclusion percentages [].

Table 5.
Summary table on digestibility assessment by Apparent Digestibility Coefficient method. Source: Own elaboration.
Table 5.
Summary table on digestibility assessment by Apparent Digestibility Coefficient method. Source: Own elaboration.
| Raw Material Origin | Food Source | Fish Species | Stage | Raw Material Processing Method | ADC | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Dry Matter | Protein | Energy | Lipid | ||||||
| Plant species | G. max | n | J | By-product: Okara | ↓= | ↓=↑ | ↓= | - | [] |
| E. ulmoides, A. membranaceus, L. japonica, C. pilosula | n × a | J | Extract | ↑ | ↑ | = | - | [] | |
| Unknown | n × m | F | Charcoal powder | ↑ | ↑ | - | - | [] | |
| M. sculenta | n | J | Root meal, Leaves meal | ↑ | ↓ | ↑ | - | [] | |
| Animal | H. illucens, C. putoria | n | F | Meal | = | ↓= | - | ↓= | [] |
| G. bimaculatus | n | F | Meal | - | ↑ | - | ↑ | [] | |
| H. illucens | n × m | J | Oil | - | = | - | ↓= | [] | |
| H. illucens | n | J | Meal | - | ↑ | - | - | [] | |
| Algae | Porphyra dioica C. Agardh, Ulva spp., Gracilaria vermiculophylla (Ohmi) Papenfuss, Sargassum muticum (Yendo) Fensholt1, | n | J | Meal | ↓ | ↓ | ↓ | ↓= | [] |
| Chlorella sorokiniana Shihira & R.W. Krauss | - | J | Meal | = | = | = | - | [] | |
| By-product | Poultry, Swine | n | J | Protein hydrolysates; Slaughter residues | =↑ | =↑ | =↑ | - | [] |
Note: Fish species: n: O. niloticus, n × m: red hybrid (O. niloticus × O. mosambicus), n × a: O. niloticus × O. aureus. Stage: F: Fingerling, J: Juvenile. Statistically significant superior (↑), statistically significant inferior (↓), no statistically significant difference (=) compared to control, no information was found about the corresponding parameter (-).
The digestibility of food sources from animals, algae, and by-products corresponds to 62.5% of the total that evaluate it (10 articles). In one of the papers, raw materials from plants and animals were evaluated simultaneously []. They all evaluated digestibility by the ADC method. The comparison and analysis concerning the control are shown in Table 5. Three evaluations were found for the insect H. illucens. Its meal reported an ADC protein SSS compared to the control [] while, in another work, the evaluation of the oil showed an ADC lipid SSI [] as its inclusion percentage increased. In the third work, H. illucens and C. putoria meals were evaluated, obtaining ADC protein and ADC lipid SSI to the control. Digestibility results of soldier fly meal inclusion in diets have shown conflicting results. Of course, it is understood that evaluations differ in the level of inclusion in the diet, the rearing and feeding conditions of the fly larvae, and the experimental conditions []. Diets using G. bimaculatus had SSS in ADC protein and lipid []. In addition, a study was found where the ADC of meals of N. cinerea, Z. morio, G. portentosa, G. assimilis, and T. molitor insects were evaluated, but only compared with each other and not with a commercial control [].
Two algae digestibility studies were reported, one using a mixture of P. dioica, Ulva spp., G. vermiculophylla, and S. muticum meals, reporting lower ADC than the control []. The other study used C. sorokiniana meal and found no SSD for any ADC []. In the by-product category, three studies were reported. Of these, only one made comparisons concerning commercial control. In this study, protein hydrolysates and slaughter residues from poultry and swine were used, and ADC values in dry matter, protein, and energy SSS were reported in some of the diets [], showing a beneficial effect of the hydrolysis process for the development of fish feed. Another study evaluated slaughter residue meal from goat and sheep and reported no SSD in ADC values in pelleted diets and SSI in ADC protein in diets processed by extrusion []. This study and the one conducted with dry protein hydrolysate from tilapia fillet waste [] did not perform a digestibility analysis with respect to a control diet. For this reason, they were not considered when constructing Table 5.
In general terms, no consistent relationships were found between digestibility and fish growth rates. However, in the study where H. illucens meal recorded low digestibility, it coincided with the lowest growth rates compared to the other studies reviewed where this insect was evaluated.
3.3.4. Survival Rate
Survival data were reported in 55.6% (80 articles) of the total number of papers initially selected. Of these, 67 articles (83.8%) did not show SSD with respect to the control diets, while 13 articles (16.2%) did. Eight articles had SSS and three had SSI. In two of the papers reviewed, treatments with SSS mortalities and others with SSI mortalities were reported simultaneously.
For terrestrial plants, survival data were reported in 44 articles. In 37 articles, the authors found no SSD, while in seven articles differences compared to the control were found. SSS survival rates were found in five of them and SSI in the other two. Survival was also reported in 16 papers that evaluated raw materials of animal origin, 11 from algae; 10 from fungi; and 1 where a combination of terrestrial plant and fungi was used. Statistical differences in some of the diets were reported in six of the eleven papers: five papers using algae and one using animal raw materials. Findings in this regard can be seen in Table 6. Survival rate (SR) is calculated using Equation (7).
It is noteworthy that some of the treatments where the alternative diets were evaluated, showed statistically significant increases in survival rates with respect to the control. The authors of these studies attribute this to different reasons, such as: stimulation of the production of total leukocytes, thus improving the immune system, which could have been induced by C. longa hydrolate []; increased nutrient availability and use of food and digestive enzyme activity due to the inclusion of polysaccharides derived from P. vera peels []; improved condition and integrity of livers and lower concentration of total heterotrophic bacteria and Pseudomonas sp. in the intestine, possibly caused by the essential oil of L. origanoides []; presence of phytochemicals in P. juliflora with antioxidant activity [] cited by [] and antimicrobial activity [,] cited by []; possible induction of the innate response against disease caused by Aeromonas veronii by the presence of antioxidants in M. nigra syrup []; increase in the quality of the medium by adding C. lentillifera at 2% []; stimulation of the fish immune system by adding tilapia fillet by-product in the diet at 8% [].

Table 6.
Summary table of survival rate assessed by diet. Source: Own elaboration.
Table 6.
Summary table of survival rate assessed by diet. Source: Own elaboration.
| Raw Material Origin | Fish Species | Stage | Food Source | Raw Material Processing Method | SSD | Reference |
|---|---|---|---|---|---|---|
| Plant species | n | F | C. longa | Hydrolate | ↑ | [] |
| n | F | L. origanoides | Essential oil | ↑ | [] | |
| n | F | S. persica | Derived | ↓= | [] | |
| n | J | M. nigra | Syrup | =↑ | [] | |
| n | J | P. juliflora | Meal | ↑ | [] | |
| n | J | P. vera | Extract, Seed | =↑ | [] | |
| n × m | F | Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg. | Seed | ↓= | [] | |
| Algae | n | F | C. lentillifera | Meal | =↑ | [] |
| n | J | C. vulgaris | Meal | ↓↑ | [] | |
| n × m | F | A. platensis | Meal | ↓↑ | [] | |
| n × m | F | A. platensis | Meal | ↓= | [] | |
| n × m | F | U. fasciata | Meal | =↑ | [] | |
| Animal | n | F | Oreochromis spp. | By-product; Hydrolysate | =↑ | [] |
Note: Fish species: n: O. niloticus, n × m: red hybrid (O. niloticus × O. mosambicus). Stage: F: Fingerling, J: Juvenile. Statistically significant superior (↑), statistically significant inferior (↓), No statistically significant difference (=).
In contrast, despite the reported antioxidant capacity of S. persica, significantly lower survival values were reported for fish fed a diet with this plant in the presence of high zinc concentrations []. Also, significantly lower survival rates were obtained in some of the diets evaluated in experiments carried out with H. brasiliensis seed derivatives. The authors attributed these results to the presence of toxic substances, such as hydrogen cyanide and cyanogenic glucoside []. Significantly lower survival rates are also reported when using the meals of the algae A. platensis [,], and C. vulgaris []. No explanation for these results was found in any of the cases by the authors.
In a significant number of experiments, the results were found to be dependent on the concentrations of the ingredients in the evaluated formulations. This was expressed throughout all of the tables above, since in some cases there were two or more symbols expressing that for a certain ingredient allowed obtaining significantly lower, equal, or higher results than the control.
Comparing the results recorded in Table 1 and Table 2 with those in Table 6, it was observed that the plants and algae that obtained outstanding results in terms of survival also presented weight gain rates equal to or higher than the control, which strengthens the conclusion that better fish health improves yields.
4. Conclusions
This systematic review shows a growing interest in research on different feed sources for tilapia and the evaluation of their effects on growth, digestibility, and product composition parameters. In recent years, the number of publications in this area has increased significantly.
O. niloticus is the most used tilapia species in the studies reported for this review. Weight gain was the most evaluated growth indicator. In more than 50% of the diets evaluated, results were found either without statistically significant differences or superior to the control. This leads to the conclusion that, most of the non-conventional sources analyzed have a positive potential impact as alternatives for producing tilapia. Among the non-conventional ingredients reviewed we found some protein sources that were used to replace fishmeal, soybean, and other protein sources. Other ingredients were used to replace conventional fat sources such as fish oil, soybean oil or corn oil, among others.
Ingredients such as essential oils or plant extracts were mainly used to improve the immune system, and to increase antimicrobial and antioxidant activities. This leads to an improvement in the weight gain and survival indexes of the fish.
As a source of non-conventional proteins, arthropods were predominant, especially black soldier fly (H. illucens) and white shrimp (P. vannamei) with 9 and 3 evaluations each, with predominant results without significant differences with respect to the control, replacing in the diets conventional ingredients such as fish meal, mainly.
The diets with canola oil (B. napus) and chia (S. hispanica), with which the fatty acid profile of the fish was evaluated, reported SSS data in all parameters of interest for this review. However, this was not the case for long-chain polyunsaturated fatty acids, where no data compared to the commercial diet were reported. Within these higher values, it was found that the proportion of SFA was also increased, which may represent a risk for the fish. In addition, given the fish’s fatty acid profile, including H. annuus and P. frutescens oils in fish diets may be beneficial for human health.
Digestibility was the least evaluated parameter by the authors reported in this review. For this one, three different ways of calculation were used, and it was possible to observe that the diets with the raw materials evaluated had statistically superior values with respect to the control. This makes the use of these food sources promising due to their higher digestibility rate compared to traditional foods.
Survival was the parameter most evaluated by the authors, reported in over 50% of the papers. No significant statistical difference was observed in more than 80% of these. Of these, more than 50% of the raw materials came from terrestrial plants. This, combined with the results of the other indices evaluated, leads to the conclusion that most of the terrestrial plants evaluated have potential benefits as alternative feed sources for tilapia, which should be further studied.
Some raw materials may pose a risk to tilapia survival. Therefore, caution in their use and further research is recommended. In this review, the plants S. persica and H. brasiliensis and the algae A. platensis, and C. vulgaris were identified in this category.
Author Contributions
C.D.Z.-H.: Investigation; Data curation; Formal analysis; Writing—original draft. C.A.H.: Conceptualization; Formal analysis; Funding acquisition; Project administration; Resources; Writing—review & editing. M.O.: Supervision; Methodology; Validation; Visualization; Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This review was conducted within the framework of the research program Technologies in Urban Agriculture, call for Minciencias 852, 2019; financed with resources from the PATRIMONIO AUTÓNOMO FONDO NACIONAL DE FINANCIAMIENTO PARA LA CIENCIA, LA TECNOLOGÍA Y LA INNOVACIÓN FRANCISCO JOSÉ DE CALDAS. Ministerio de Ciencia y Tecnología of Colombia and Universidad Pontificia Bolivariana. Contract number (Grant): 127-2021.
Data Availability Statement
The files where all the information related to the review was collected and organized can be accessed at this URL: https://www.dropbox.com/scl/fo/bxo35k1t8t1tnwy5nl7x9/h?rlkey=z5jv5i40o7zmuhpywy005yqij&dl=0 (accessed on 24 October 2023).
Acknowledgments
Thanks to the Universidad Pontificia Bolivarian for allowing the use of the databases for the search of information and to the analytical and context studies unit in charge of surveillance and strategic intelligence, with the implementation of the VantagePoint software, headed by Jaime Barajas and to the work team integrated by Diana Carolina Vásquez Osorio and Luis Miguel Rodríguez Ortiz for their commitment and support with the development of this research.
Conflicts of Interest
All authors have no conflict of interest to disclose.
References
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- OECD; FAO. OECD-FAO Agricultural Outlook 2021–2030. In OECD-FAO Agricultural Outlook; OECD: Paris, France, 2021. [Google Scholar] [CrossRef]
- FAO. Versión Resumida de El Estado Mundial de la Pesca y la Acuicultura 2022; Hacia la Transformación azul; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; McCulloch, M.; Gorman, J.D.; Pai, N.; Enanoria, W.; Kennedy, G.; Tharyan, P.; Colford, J.M. Systematic reviews and meta-analyses: An illustrated, step-by-step guide. Natl. Med. J. India 2004, 17, 86–95. [Google Scholar] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Mabrouk, H.; Labib, E.M.H.; Zaki, M.A. Response of Nile Tilapia mono-sex (Oreochromis niloticus) Fingerlings to Different Sources and Levels of Protein Using Garlic and Onion as Feed Phytophytoadditives. Arab. Gulf J. Sci. Res. 2011, 29, 146–159. [Google Scholar] [CrossRef]
- Agbo, N.W.; Adjei-Boateng, D.; Jauncey, K. The Potential of Groundnut (Arachis hypogaea L.) By-Products as Alternative Protein Sources in the Diet of Nile Tilapia (Oreochromis niloticus). J. Appl. Aquac. 2011, 23, 367–378. [Google Scholar] [CrossRef]
- Kareem, Z.H.; Abdelhadi, Y.M.; Christianus, A.; Karim, M.; Romano, N. Effects of some dietary crude plant extracts on the growth and gonadal maturity of Nile tilapia (Oreochromis niloticus) and their resistance to Streptococcus agalactiae infection. Fish Physiol. Biochem. 2015, 42, 757–769. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Sringarm, K.; Jaturasitha, S.; Yuangsoi, B.; Dawood, M.A.; Esteban, M.; Ringø, E.; Faggio, C. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish. Immunol. 2019, 93, 428–435. [Google Scholar] [CrossRef]
- Pessoa, M.; Avelar, J.; Nascimento, A.H.; Silva, K.; Soares, A.; Camargo, A.; Filho, D.F. Desempenho de tilápias-do-nilo alimentadas com farelo da casca de pequi. Arq. Bras. Med. Vet. Zootec. 2013, 65, 547–552. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Samir, F.; El-Naby, A.S.A.; Monier, M.N. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2018, 74, 19–25. [Google Scholar] [CrossRef]
- Mombach, P.I.; Adorian, T.J.; Goulart, F.R.; Martinelli, S.G.; Dalcin, M.O.; Veiverberg, C.A.; da Silva, L.P. The Effects of Fermentable Dietary Fiber on Performance and Metabolism of Nile Tilapia (Oreochromis niloticus). Braz. Arch. Biol. Technol. 2020, 63, e20190396. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Acar, Ü.; Yilmaz, S.; Aydin, Ö.D. Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2019, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Ali, M.F.; Amer, A.A.; Gewaily, M.S.; Mahmoud, M.M.; Alkafafy, M.; Assar, D.H.; Soliman, A.A.; Van Doan, H. The influence of coconut oil on the growth, immune, and antioxidative responses and the intestinal digestive enzymes and histomorphometry features of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2021, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Handajani, H.; Andriawan, S.; Gilang, R. Enrichment of commercial feed with plant proteins for Oreochromis niloticus diet: Digestibility and growth performance. AACL Bioflux 2021, 14, 2894–2904. Available online: https://www.bioflux.com.ro/docs/2021.2894-2904.pdf (accessed on 22 January 2022).
- Pereira, M.; Hess, J.D.; Rodhermel, J.C.B.; Farias, D.R.; Schleder, D.D.; Alves, L.; Bertoldi, F.C.; Chaban, A.; DE Andrade, J.I.A.; Jatobá, A. Curcuma longa hydrolate improves Nile tilapia survival in a recirculation rearing system, maintaining the animal homeostasis and modulating the gut microbial community. An. Acad. Bras. Ciências 2021, 93, e20210088. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Mahmoud, H.K.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 2018, 24, 1006–1014. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Sringarm, K.; Jaturasitha, S.; Khamlor, T.; Dawood, M.A.; Esteban, M.; Soltani, M.; Musthafa, M.S. Effects of elephant’s foot (Elephantopus scaber) extract on growth performance, immune response, and disease resistance of nile tilapia (Oreochromis niloticus) fingerlings. Fish Shellfish. Immunol. 2019, 93, 328–335. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.; Esteban, M.; Ringø, E.; Van Doan, H. The effect of fishwort (Houttuynia cordata) on skin mucosal, serum immunities, and growth performance of Nile tilapia. Fish Shellfish. Immunol. 2020, 98, 193–200. [Google Scholar] [CrossRef]
- Pallaya-Baleta, L.J.; Baleta, F.N.; Magistrado-Candelaria, P.; Plantado, L.C.; Baldo, D.E.B.; Navarro, M.C.; Encinas, J.L. Growth performance and economic viability of dietary inclusion of Ipomoea batatas L. shoot powder and extracts in the practical diets of Oreochromis niloticus L. Egypt. J. Aquat. Res. 2021, 48, 273–279. [Google Scholar] [CrossRef]
- Addam, K.G.S.; Pereira, S.A.; Jesus, G.F.A.; Cardoso, L.; Syracuse, N.; Lopes, G.R.; Lehmann, N.B.; Silva, B.C.; Sá, L.S.; Chaves, F.C.M.; et al. Dietary organic acids blend alone or in combination with an essential oil on the survival, growth, gut/liver structure and de hemato-immunological in Nile tilapia Oreochromis niloticus. Aquac. Res. 2019, 50, 2960–2971. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, K.; Zhong, H.; Ma, Y.; Guo, Z.; Tang, Z.; Liang, J.; Luo, Y.; Su, Z.; Wang, L. Effects of Lycium barbarum polysaccharides on immunological parameters, apoptosis, and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 97, 509–514. [Google Scholar] [CrossRef]
- Djeziri, M.; Nouri, L.; Kacher, M. An investigation on the effects of different diets on the growth performance of Nile tilapia, Oreochromis niloticus (Linné 1758). Iran J. Fish Sci. 2020, 19, 136–150. [Google Scholar] [CrossRef]
- Melo, J.F.B.; Seabra, A.G.L.; Souza, S.A.; Souza, R.C.; Figueiredo, R.A.C.R. Substituição do farelo de milho pela farinha de manga no desempenho da tilápia-do-nilo [Replacement of corn meal by mango in the dietary in performance of fingerlings of Nile-tilapia]. Arq. Bras. Med. Veterinária Zootec. 2012, 64, 177–182. [Google Scholar] [CrossRef]
- Elabd, H.; Soror, E.; El-Asely, A.; El-Gawad, E.A.; Abbass, A. Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: Effect on growth and stress indices. Egypt. J. Aquat. Res. 2019, 45, 265–271. [Google Scholar] [CrossRef]
- Parveen, S.; Rasool, F.; Akram, M.N.; Khan, N.; Ullah, M.; Mahmood, S.; Rabbani, G.; Manzoor, K. Effect of Moringa olifera leaves on growth and gut microbiota of Nile tilapia (Oreochromis niloticus). Braz. J. Biol. 2021, 84, e250916. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.E.J.; Tumbokon, B.L.M.; Cabanero, P.C. Nutritional Evaluation of Rhizoclonium riparium var implexum Meal to Replace Soybean in the Diet of Nile Tilapia Fry. Isr. J. Aquac.-Bamidgeh 2016, 68, 20818. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Amin, A.A.; Samir, F.; El-Ashram, A.; Dawood, M.A.O. Miswak (Salvadora persica) modulated the growth performance, antioxidative response, and histopathological damage induced by zinc toxicity in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 27, 31918–31932. [Google Scholar] [CrossRef]
- Toutou, M.M.; Soliman, A.A.; Elokaby, M.A.; Abdel-Rahim, M.M.; Abouelwafa, A.E.; Yones, A.E.M. The potential antimicrobial effects of dietary supplementation with Arak, Salvadora persica, on growth, health status, and pathogenic bacterial loads in Nile tilapia, Oreochromis niloticus fingerlings. Egypt. J. Aquat. Res. 2019, 45, 251–257. [Google Scholar] [CrossRef]
- Ashade, O.; Osineye, O. Effect of replacing maize with cocoa pod husk in the nutrition of Oreochromis niloticus. J. Fish. Aquat. Sci. 2010, 8, 73–79. [Google Scholar] [CrossRef][Green Version]
- Fakunmoju, F.; Babalola, O.; Ijimakinde, B.; Anjola, O.; Orowole, P. Effect of Substituting Maize with Bambara (Voandzeia subterrenea Thouars) Waste Meal in the Practical Diets of Tilapia niloticus Fingerlings. J. Fish. Aquat. Sci. 2016, 11, 185–189. [Google Scholar] [CrossRef][Green Version]
- Zahran, E.; Risha, E.; AbdelHamid, F.; Mahgoub, H.A.; Ibrahim, T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2014, 38, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Whangchai, N.; Ramaraj, R.; Whangchai, K.; Nomura, N.; Pimpimol, T. Innovative biorefinery concept for biogas-based digestate with rice bran protein-rich feed ingredient for tilapia production. Biomass-Convers. Biorefinery 2020, 12, 1639–1645. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Block, J.M.; Fracalossi, D.M. Mixes of plant oils as fish oil substitutes for Nile tilapia at optimal and cold suboptimal temperature. Aquaculture 2018, 497, 82–90. [Google Scholar] [CrossRef]
- De Souza, E.M.; De Souza, R.C.; Melo, J.F.; Da Costa, M.M.; De Souza, S.A.; De Souza, A.M.; Copatti, C.E. Cymbopogon flexuosus essential oil as an additive improves growth, biochemical and physiological responses and survival against Aeromonas hydrophila infection in nile tilapia. An. Acad. Bras. Cienc. 2020, 92, e20190140. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, R.; de Moraes, F.R.; Belo, M.A.d.A.; Pilarski, F.; de Moraes, J.R.E. Kinetics of chronic inflammation in Nile tilapia fed n-3 and n-6 essential fatty acids. Pesqui Agropecu Bras. 2013, 48, 313–319. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Lamtane, H.A.; Kishe-Machumu, M.A.; Joram, M.C.; Mbonde, A.S.; Mgana, H.F.; Mgaya, Y.D. Supplemental effects of mixed ingredients and rice bran on the growth performance, survival and yield of Nile tilapia, Oreochromis niloticus reared in fertilized earthen ponds. SpringerPlus 2016, 5, 5. [Google Scholar] [CrossRef]
- Palintorn, N.; Rujinanont, N.; Srisathaporn, A.; Gawborisut, S.; Wongkaew, P. Effects of dietary supplemented with flesh ripe fruit of local cultivated banana CV. Kluai Namwa on growth performance and meat quality of Nile tilapia. AACL Bioflux 2019, 12, 1578–1591. Available online: https://www.bioflux.com.ro/docs/2019.1578-1591.pdf (accessed on 20 February 2022).
- Kurian, A.; Van Doan, H.; Tapingkae, W.; Elumalai, P. Modulation of mucosal parameters, innate immunity, growth and resistance against Streptococcus agalactiae by enrichment of Nile tilapia (Oreochromis niloticus) diet with Leucas aspera. Fish Shellfish. Immunol. 2020, 97, 165–172. [Google Scholar] [CrossRef]
- de Rezende, R.A.E.; Soares, M.P.; Sampaio, F.G.; Cardoso, I.L.; Ishikawa, M.M.; Dallago, B.S.L.; Rantin, F.T.; Duarte, M.C.T. Phytobiotics blend as a dietary supplement for Nile tilapia health improvement. Fish Shellfish. Immunol. 2021, 114, 293–300. [Google Scholar] [CrossRef]
- Souza, R.C.; Melo, J.F.B.; Filho, R.M.N.; Campeche, D.F.B.; Figueiredo, R.A.C.R. Influencia da farinha de manga no crescimento e composição corporal da tilápia do nilo. Arch. Zootec. 2012, 62, 217–225. [Google Scholar] [CrossRef][Green Version]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; El-Houseiny, W.; Behairy, A.; Mansour, M.F.; Abd-Elhakim, Y.M. Ameliorative effects of Moringa oleifera seeds and leaves on chlorpyrifos-induced growth retardation, immune suppression, oxidative stress, and DNA damage in Oreochromis niloticus. Aquaculture 2019, 505, 225–234. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergün, S.; Yigit, M.; Yilmaz, E.; Ahmadifar, E. Dietary supplementation of black mulberry (Morus nigra) syrup improves the growth performance, innate immune response, antioxidant status, gene expression responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 107, 211–217. [Google Scholar] [CrossRef]
- Panprommin, D.; Kaewpunnin, W.; Insee, D. Effects of Holy Basil (Ocimum sanctum) Extract on the Growth, Immune Response and Disease Resistance against Streptococcus agalactiae of Nile Tilapia (Oreochromis niloticus). Int. J. Agric. Biol. 2016, 18, 677–682. [Google Scholar] [CrossRef]
- Voss, G.B.; Sousa, V.; Rema, P.; Pintado, M.E.; Valente, L.M.P. Processed By-Products from Soy Beverage (Okara) as Sustainable Ingredients for Nile Tilapia (O. niloticus) Juveniles: Effects on Nutrient Utilization and Muscle Quality. Animals 2021, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Hegazi, E.; Nassef, E.; El-Din, M.T.S.; Dawood, M.A.; Abdo, S.E.; Gewaily, M.S. Gut immune-related gene expression, histomorphometry and hematoimmunological assays in Nile tilapia (Oreochromis niloticus) fed Aspergillus oryzae fermented olive cake. Fish Shellfish. Immunol. 2021, 117, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Santo, A.H.E.; Brito, T.S.; Brandão, L.L.; Tavares, G.C.; Leibowitz, M.P.; Prado, S.A.; Ferraz, V.P.; Hoyos, D.C.M.; Turra, E.M.; Teixeira, E.A.; et al. Dietary supplementation of dry oregano leaves increases the innate immunity and resistance of Nile tilapia against Streptococcus agalactiae infection. J. World Aquac. Soc. 2020, 51, 418–436. [Google Scholar] [CrossRef]
- Freccia, A.; Sousa, S.M.d.N.; Meurer, F.; Butzge, A.J.; Mewes, J.K.; Bombardelli, R.A. Essential oils in the initial phase of broodstock diets of Nile tilapia. Rev. Bras. Zootec. 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Abdel-Latif, H.M.; Dawood, M.A. The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish Shellfish. Immunol. 2020, 106, 36–43. [Google Scholar] [CrossRef]
- Abdel-Razek, N.; Awad, S.M.; Abdel-Tawwab, M. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish Physiol. Biochem. 2019, 45, 1907–1917. [Google Scholar] [CrossRef]
- Nascimento, A.A.; Melo, J.F.B.; de Souza, A.M.; Melo, F.V.S.T. Inclusion of mesquite pod meal (Prosopis juliflora) in diets for nile tilapia (Oreochromis niloticus) juveniles. Bol. Inst. Pesca 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Silva, T.; Chung, S.; Araújo, T.; Azevedo, K.; Santos, M.; Bicudo, Á. Substituição do milho pelo farelo de algaroba (Prosopis juliflora) em dietas para juvenis de tilápia do Nilo cultivados em baixa temperatura. Rev. Bras. Cienc. Agrar. 2015, 10, 460–465. [Google Scholar] [CrossRef]
- El-Latif, A.M.A.; El-Gawad, E.A.A.; Soror, E.I.; Shourbela, R.M.; Zahran, E. Dietary supplementation with miswak (Salvadora persica) improves the health status of Nile tilapia and protects against Aeromonas hydrophila infection. Aquac. Rep. 2021, 19, 100594. [Google Scholar] [CrossRef]
- Lebda, M.A.; El-Hawarry, W.N.; Shourbela, R.M.; El-Far, A.H.; Shewita, R.S.; Mousa, S.A. Miswak (Salvadora persica) dietary supplementation improves antioxidant status and nonspecific immunity in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 88, 619–626. [Google Scholar] [CrossRef]
- Abbas, W.T.; Abumourad, I.M.; Mohamed, L.A.; Abbas, H.H.; Authman, M.; Soliman, W.S.; Elgendy, M.Y. The Role of the Dietary Supplementation of Fenugreek Seeds in Growth and Immunity in Nile Tilapia with or without Cadmium Contamination. Jordan J. Biol. Sci. 2019, 12, 649–656. [Google Scholar]
- Yunis-Aguinaga, J.; Claudiano, G.S.; Marcusso, P.F.; Manrique, W.G.; de Moraes, J.R.E.; de Moraes, F.R.; Fernandes, J.B. Uncaria tomentosa increases growth and immune activity in Oreochromis niloticus challenged with Streptococcus agalactiae. Fish Shellfish. Immunol. 2015, 47, 630–638. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; He, J.; Ma, X.Y.; Kpundeh, M.D.; Xu, P. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish. Immunol. 2015, 44, 504–514. [Google Scholar] [CrossRef]
- Teoh, C.-Y.; Ng, W.-K. Evaluation of the impact of dietary petroselinic acid on the growth performance, fatty acid composition, and efficacy of long chain-polyunsaturated fatty acid biosynthesis of farmed Nile tilapia. J. Agric. Food Chem. 2013, 61, 6056–6068. [Google Scholar] [CrossRef]
- Qiang, J.; Khamis, O.A.M.; Jiang, H.J.; Cao, Z.M.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W. Effects of dietary supplementation with apple peel powder on the growth, blood and liver parameters, and transcriptome of genetically improved farmed tilapia (GIFT, Oreochromis niloticus). PLoS ONE 2019, 14, e0224995. [Google Scholar] [CrossRef]
- Brum, A.; Pereira, S.A.; Owatari, M.S.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 2017, 468, 235–243. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergün, S. Dietary Supplementation with allspice Pimenta dioica reduces the occurrence of streptococcal disease during first feeding of mozambique tilapia fry. J. Aquat. Anim. Health 2014, 26, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Gobi, N.; Ramya, C.; Vaseeharan, B.; Malaikozhundan, B.; Vijayakumar, S.; Murugan, K.; Benelli, G. Oreochromis mossambicus diet supplementation with Psidium guajava leaf extracts enhance growth, immune, antioxidant response and resistance to Aeromonas hydrophila. Fish Shellfish. Immunol. 2016, 58, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, M.S.; Ali, A.R.J.; Kumar, M.S.A.; Paray, B.A.; Al-Sadoon, M.K.; Balasundaram, C.; Harikrishnan, R. Effect of Cucurbita mixta (L.) seed meal enrichment diet on growth, immune response and disease resistance in Oreochromis mossambicus. Fish Shellfish. Immunol. 2017, 68, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, M.S.; Asgari, S.M.; Kurian, A.; Elumalai, P.; Ali, A.R.J.; Paray, B.A.; Al-Sadoon, M.K. Protective efficacy of Mucuna pruriens (L.) seed meal enriched diet on growth performance, innate immunity, and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish. Immunol. 2018, 75, 374–380. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergün, S. Chickweed (Stellaria media) Leaf Meal as a Feed Ingredient for Tilapia (Oreochromis mossambicus). J. Appl. Aquac. 2013, 25, 329–336. [Google Scholar] [CrossRef]
- Michael, F.; Saleh, N.; Shalaby, S.; Sakr, E.; Abd-El-Khalek, D.; Elmonem, A.A. Effect of different dietary levels of commercial wood charcoal on growth, body composition and environmental loading of red tilapia hybrid. Aquac. Nutr. 2017, 23, 210–216. [Google Scholar] [CrossRef]
- Zheng, Q.; Han, C.; Zhong, Y.; Wen, R.; Zhong, M. Effects of dietary supplementation with green tea waste on growth, digestive enzyme and lipid metabolism of juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Fish Physiol. Biochem. 2017, 43, 361–371. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish. Immunol. 2019, 86, 260–268. [Google Scholar] [CrossRef]
- Botello-León, A.; Viana, M.T.; Téllez-Girón, E.; Pullés-Ariza, E.; Cisneros-Lopez, M.; Solano-Silveira, G.; Valdivie, M.; Miranda-Miranda, O.; Rodriguez-Valera, Y.; Cutiño-Espinoza, M.; et al. Fish meal substitution by protein sugar cane in diets for weight gain in red tilapia. Agrociencia 2011, 45, 23–31. Available online: https://www.researchgate.net/publication/289038710 (accessed on 12 February 2022).
- Poolsawat, L.; Yu, Y.; Li, X.; Zhen, X.; Yao, W.; Wang, P.; Luo, C.; Leng, X. Efficacy of phytogenic extracts on growth performance and health of tilapia (Oreochromis niloticus × O. aureus). Aquac. Fish. 2020, 7, 411–419. [Google Scholar] [CrossRef]
- Wachira, M.N.; Osuga, I.M.; Munguti, J.M.; Ambula, M.K.; Subramanian, S.; Tanga, C.M. Efficiency and improved profitability of insect-based aquafeeds for farming Nile tilapia fish (Oreochromis niloticus L.). Animals 2021, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Agbohessou, P.S.; Mandiki, S.N.; Gougbédji, A.; Megido, R.C.; Lima, L.-M.W.; Cornet, V.; Lambert, J.; Purcaro, G.; Francis, F.; Lalèyè, P.A.; et al. Efficiency of fatty acid-enriched dipteran-based meal on husbandry, digestive activity and immunological responses of Nile tilapia Oreochromis niloticus juveniles. Aquaculture 2021, 545, 737193. [Google Scholar] [CrossRef]
- Alves, A.P.D.C.; Paulino, R.R.; Pereira, R.T.; Costa, D.V.; Rosa, P.V. Nile tilapia fed insect meal: Growth and innate immune response in different times under lipopolysaccharide challenge. Aquac. Res. 2021, 52, 529–540. [Google Scholar] [CrossRef]
- Amer, A.A.; El-Nabawy, E.M.; Gouda, A.H.; Dawood, M.A.O. The addition of insect meal from Spodoptera littoralis in the diets of Nile tilapia and its effect on growth rates, digestive enzyme activity and health status. Aquac. Res. 2021, 52, 5585–5594. [Google Scholar] [CrossRef]
- Perera, A.D.; Bhujel, R.C. Field cricket (Gryllus bimaculatus) meal (FCM) to replace fishmeal in the diets for sex reversal and nursing of Nile tilapia (Oreochromis niloticus) fry. Aquac. Res. 2021, 52, 4946–4958. [Google Scholar] [CrossRef]
- Abu Bakar, N.-H.; Razak, S.A.; Taufek, N.M.; Alias, Z. Evaluation of black soldier fly (Hermetia illucens) prepupae oil as meal supplementation in diets for red hybrid tilapia (Oreochromis spp.). Int. J. Trop. Insect Sci. 2021, 41, 2093–2102. [Google Scholar] [CrossRef]
- Taufek, N.; Lim, J.; Abu Bakar, N. Comparative Evaluation of Hermetia illucens Larvae Reared on Different Substrates for Red tilapia Diet: Effect on Growth and Body Composition. J. Insects Food Feed. 2021, 7, 79–88. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Van Doan, H.; Paolucci, M. Replacement of fish meal by black soldier fly (Hermetia illucens) larvae meal: Effects on growth, haematology, and skin mucus immunity of Nile tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Schrimsher, C.; Beck, B.H. Use of dietary frass from black soldier fly larvae, Hermetia illucens, in hybrid tilapia (Nile x Mozambique, Oreocromis niloticus x O. mozambique) diets improves growth and resistance to bacterial diseases. Aquac. Rep. 2020, 17, 100373. [Google Scholar] [CrossRef]
- Wu, S. The growth performance, body composition and nonspecific immunity of Tilapia (Oreochromis niloticus) affected by chitosan. Int. J. Biol. Macromol. 2020, 145, 682–685. [Google Scholar] [CrossRef]
- Toriz-Roldan, A.; Ruiz-Vega, J.; García-Ulloa, M.; Hernández-Llamas, A.; Fonseca-Madrigal, J.; Rodríguez-González, H. Assessment of Dietary Supplementation Levels of Black Soldier Fly, Hermetia illucens, Pre-pupae Meal for Juvenile Nile Tilapia, Oreochromis niloticus. Southwest. Entomol. 2019, 44, 251–259. Available online: http://www.worldbank.org/ (accessed on 3 February 2022). [CrossRef]
- Dietz, C.; Liebert, F. Does graded substitution of soy protein concentrate by an insect meal respond on growth and N-utilization in Nile tilapia (Oreochromis niloticus)? Aquac. Rep. 2018, 12, 43–48. [Google Scholar] [CrossRef]
- Devic, E.; Leschen, W.; Murray, F.; Little, D. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing Black Soldier Fly (Hermetia illucens) larvae meal. Aquac. Nutr. 2018, 24, 416–423. [Google Scholar] [CrossRef]
- Orsi, R.O.; dos Santos, V.G.; Pezzato, L.E.; de Carvalho, P.L.; Teixeira, C.P.; Freitas, J.M.; Padovani, C.R.; Sartori, M.M.; Barros, M.M. Activity of Brazilian propolis against Aeromonas hydrophila and its effect on Nile tilapia growth, hematological and non-specific immune response under bacterial infection. An. Acad. Bras. Cienc. 2017, 89, 1785–1799. [Google Scholar] [CrossRef]
- Freccia, A.; Meurer, E.S.; Filho, J.C.; Jerônimo, G.T.; Emerenciano, M.G.C. Insect meal in tilapia fingerlings diets [Farinha de inseto em dietas de alevinos de tilápia]. Arch. Zootec. 2016, 62, 541–547. Available online: https://www.uco.es/ucopress/az/index.php/az/ (accessed on 5 February 2022).
- Sánchez-Muros, M.J.; De Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr. 2016, 22, 943–955. [Google Scholar] [CrossRef]
- Sing, K.W.; Kamarudin, M.S.; Wilson, J.J.; Sofian-Azirun, M. Evaluation of Blowfly (Chrysomya megacephala) Maggot Meal as an Effective, Sustainable Replacement for Fishmeal in the Diet of Farmed Juvenile Red Tilapia (Oreochromis spp.). Pak. Vet. J. 2014, 34, 288–292. Available online: https://www.researchgate.net/publication/285988706 (accessed on 6 February 2022).
- Qin, C.; Zhang, Y.; Liu, W.; Xu, L.; Yang, Y.; Zhou, Z. Effects of chito-oligosaccharides supplementation on growth performance, intestinal cytokine expression, autochthonous gut bacteria and disease resistance in hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Fish Shellfish. Immunol. 2014, 40, 267–274. [Google Scholar] [CrossRef]
- Fall, J.; Ndong, D.; Thengtseng, Y.; Sheen, S.-S. The effects of different protein sources on the growth of hybrid tilapia (Oreochromis niloticus × O. aureus) reared under fresh water and brackish water. Afr. J. Agric. Res. 2011, 6, 5024–5029. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Effects of chitosan nanoparticles on survival, growth and meat quality of tilapia, Oreochromis nilotica. Nanotoxicology 2011, 5, 425–431. [Google Scholar] [CrossRef]
- El-Asely, A.M.; Abbass, A.A.; Austin, B. Honey bee pollen improves growth, immunity and protection of Nile tilapia (Oreochromis niloticus) against infection with Aeromonas hydrophila. Fish Shellfish. Immunol. 2014, 40, 500–506. [Google Scholar] [CrossRef]
- Fadl, S.E.; El-Habashi, N.; Gad, D.M.; Elkassas, W.M.; Elbialy, Z.I.; Abdelhady, D.H.; Hegazi, S.M. Effect of adding Dunaliella algae to fish diet on lead acetate toxicity and gene expression in the liver of Nile tilapia. Toxin Rev. 2021, 40, 1155–1171. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alsaqufi, A.S.; Alkhamis, Y.A.; Al-Gazar, F.F.; Zaki, M.A.; Nour, A.A.M.; Ramadan, K.M.A. The evaluation of Arthrospira platensis bioactivity and their dietary supplementation to Nile tilapia vegetarian diet on growth performance, feed utilization, body composition and hemato-biochemical parameters. Ann. Anim. Sci. 2021, 21, 1061–1080. [Google Scholar] [CrossRef]
- Abdelghany, M.F.; El-Sawy, H.B.; El-Hameed, S.A.A.; Khames, M.K.; Abdel-Latif, H.M.; Naiel, M.A. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 107, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Matter, A. Nano spirulina dietary supplementation augments growth, antioxidative and immunological reactions, digestion, and protection of Nile tilapia, Oreochromis niloticus, against Aeromonas veronii and some physical stressors. Fish Physiol. Biochem. 2020, 46, 2143–2155. [Google Scholar] [CrossRef]
- Arisa, I.; Zulfikar, Z.; Muhammadar, M.; Nurfadillah, N.; Mellisa, S. Study on the addition of Caulerpa lentillifera on growth and survival rate of saline tilapia Oreochromis niloticus, L. IOP Conf. Ser. Earth Environ. Sci. 2020, 493, 012004. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; El-Sayed, B.M.; Mahsoub, Y.H.; El-Murr, A.E.I.; Neamat-Allah, A.N. Effect of Chlorella vulgaris enriched diet on growth performance, hemato-immunological responses, antioxidant and transcriptomics profile disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 102, 422–429. [Google Scholar] [CrossRef]
- dos Santos, S.K.A.; Schorer, M.; Moura, G.d.S.; Lanna, E.A.T.; Pedreira, M.M. Evaluation of growth and fatty acid profile of Nile tilapia (Oreochromis niloticus) fed with Schizochytrium sp. Aquac. Res. 2019, 50, 1068–1074. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Shangguan, J.; Chen, Q.; Li, W.; Lu, J. Growth performance, digestive enzyme activities and serum nonspecific immunity of the red tilapia (Oreochromis mossambicus × Oreochromis niloticus) fed diets supplemented with ultrafine powder of Enteromopha prolifera. J. Oceanol. Limnol. 2018, 36, 1843–1850. [Google Scholar] [CrossRef]
- Gbadamosi, O.K.; Lupatsch, I. Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia, Oreochromis niloticus. Algal Res. 2018, 33, 48–54. [Google Scholar] [CrossRef]
- Younis, E.-S.M.; Al-Quffail, A.S.; Al-Asgah, N.A.; Abdel-Warith, A.-W.A.; Al-Hafedh, Y.S. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J. Biol. Sci. 2018, 25, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Rodríguez, P.d.R.; Fajer-Ávila, E.J. The dietary effect of ulvan from Ulva clathrata on hematological-immunological parameters and growth of tilapia (Oreochromis niloticus). J. Appl. Phycol. 2017, 29, 423–431. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards Sustainable Aquafeeds: Complete Substitution of Fish Oil with Marine Microalga Schizochytrium sp. Improves Growth and Fatty Acid Deposition in Juvenile Nile Tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El-Shourbagy, I.; Shalaby, S.; Hosny, S. Effect of feeding Arthrospira platensis (Spirulina) on growth and carcass composition of hybrid red tilapia (Oreochromis niloticus x Oreochromis mossambicus). Turk. J. Fish Aquat. Sci. 2014, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.E.; Shalaby, S.M.; Sakr, E.M.; Elmonem, A.I.A.; Michael, F.R. Effect of Dietary Inclusion of Ulva fasciata on Red Hybrid Tilapia Growth and Carcass Composition. J. Appl. Aquac. 2014, 26, 197–207. [Google Scholar] [CrossRef]
- Costa, M.M.; Oliveira, S.T.L.; Balen, R.E.; Bueno Junior, G.; Baldan, L.T.; Silva, L.C.R.; Santos, L.D. Brown seaweed meal to nile tilapia fingerlings [alga marinha marrom para alevinos de tilápia do nilo]. Arch. Zootec. 2013, 62, 101–109. [Google Scholar] [CrossRef]
- Gomes, I.G.; Chaves, F.H.; Barros, R.N.; Moreira, R.L.; Teixeira, E.G.; Moreira, A.G.; Farias, W.R. Dietary supplementation with Spirulina platensis increases growth and color of red tilapia [Efecto de la suplementación dietaria con Spirulina platensis en el crecimiento y la coloración de la tilapia roja]. Rev. Colomb. Cienc. Pecu. 2012, 25, 462–471. [Google Scholar]
- Yossa, R.; Greiling, A.M.; Basiita, R.K.; Sakala, M.E.; Baumgartner, W.A.; Taylor, A.; Gatlin, D.M. Replacing fishmeal with a single cell protein feedstuff in Nile tilapia Oreochromis niloticus diets. Anim. Feed. Sci. Technol. 2021, 281, 115089. [Google Scholar] [CrossRef]
- Ching, J.J.; Shuib, A.S.; Abdullah, N.; Majid, N.A.; Taufek, N.M.; Sutra, J.; Azmai, M.N.A. Hot water extract of Pleurotus pulmonarius stalk waste enhances innate immune response and immune-related gene expression in red hybrid tilapia Oreochromis spp. following challenge with pathogen-associated molecular patterns. Fish Shellfish. Immunol. 2021, 116, 61–73. [Google Scholar] [CrossRef]
- Selim, K.M.; Reda, R.M.; Mahmoud, R.; El-Araby, I.E. Effects of nucleotides supplemented diets on growth performance and expressions of ghrelin and insulin-like growth factor genes in Nile tilapia, Oreochromis niloticus. J. Appl. Aquac. 2020, 32, 157–174. [Google Scholar] [CrossRef]
- Divya, M.; Gopi, N.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020, 139, 103917. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Zhao, W.; Xie, S.-W.; Xie, J.-J.; Zhang, Z.-H.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef]
- Abass, D.A.; Obirikorang, K.A.; Campion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus). Aquac. Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- Assem, H.; Khalifa, A.; Elsalhia, M. Physiological and microbiological indices as indicators of evaluating dietary fungi degraded date pits as a probiotic for cultured Nile tilapia Oreochromis niloticus fingerling and its effect on fish welfare. Egypt. J. Aquat. Res. 2014, 40, 435–441. [Google Scholar] [CrossRef]
- Salvador, R.; Claudiano, G.d.S.; Loureiro, B.A.; Marcusso, P.F.; Eto, S.F.; Pilarski, F.; Toazza, C.S.; de Moraes, J.R.E.; de Moraes, F.R. Desempenho e hematologia de tilápias-do-nilo alimentadas com Saccharomyces cerevisiae e vacinadas contra Streptococcus agalactiae. Pesquisa Agropecuária Brasileira 2013, 48, 892–898. [Google Scholar] [CrossRef]
- de Sousa Liranço, A.; Ciarlini, P.C.; Moraes, G.; Camargo, A.L.S.; Romagosa, E. Mannanoligosaccharide (mos) and ß-glucan (ß-glu) in Dietary Supplementation for Nile Tilapia Juveniles Dept in Cages. 2013. Available online: www.cobea.org.br (accessed on 2 February 2022).
- Abd Rahman, J.M.D.; Razak, S.A.; Sabaratnam, V. Effect of Mushroom Supplementation as a Prebiotic Compound in Super Worm Based Diet on Growth Performance of Red Tilapia Fingerlings. Sains Malays 2012, 41, 1197–1203. Available online: https://www.researchgate.net/publication/265042127 (accessed on 25 January 2022).
- Vechklang, K.; Lim, C.; Boonanuntanasarn, S.; Welker, T.; Ponchunchuwong, S.; Klesius, P.H.; Wanapu, C. Growth Performance and Resistance to Streptococcus iniae of Juvenile Nile Tilapia (Oreochromis niloticus) Fed Diets Supplemented with GroBiotic-A and Brewtech Dried Brewers Yeast. J. Appl. Aquac. 2012, 24, 183–198. [Google Scholar] [CrossRef]
- Koch, J.F.A.; Pezzato, L.E.; Barros, M.M.; Teixeira, C.P.; Junior, A.C.F.; Padovani, C.R. Levedura como pronutriente em dietas para matrizes e alevinos de tilápia-do-nilo. Rev. Bras. Zootec. 2011, 40, 2281–2289. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Batista, R.O.; Corrêa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Younis, N.A.; AbuBakr, H.O.; Ragaa, N.M.; Borges, L.L.; Bonato, M.A. Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egypt. J. Aquat. Res. 2018, 44, 333–341. [Google Scholar] [CrossRef]
- Tongmee, B.; Tongsiri, S.; Unpaprom, Y.; Ramaraj, R.; Whangchai, K.; Pugazhendhi, A.; Whangchai, N. Sustainable development of feed formulation for farmed tilapia enriched with fermented pig manure to reduce production costs. Sci. Total. Environ. 2021, 801, 149614. [Google Scholar] [CrossRef]
- Carvalho, K.V.; Luczinski, T.G.; Boscolo, W.R.; de Freitas, J.M.A.; Signor, A. Poultry byproducts and swine liver used in diets for Nile tilapia juveniles. Lat. Am. J. Aquat. Res. 2020, 48, 895–900. [Google Scholar] [CrossRef]
- Obirikorang, K.A.; Mensah, N.E.; Asiamah, E.A. Growth, feed utilization, and liver histology of juvenile Nile tilapia (Oreochromis niloticus) fed diets containing increasing levels of swine fat. J. Appl. Aquac. 2018, 30, 366–381. [Google Scholar] [CrossRef]
- Sary, C.; de Paris, L.D.; Bernardi, D.M.; Lewandowiski, V.; Signor, A.; Boscolo, W.R. Tilapia by-product hydrolysate powder in diets for Nile tilapia larvae. Acta Sci. Anim. Sci. 2017, 39, 1–6. [Google Scholar] [CrossRef][Green Version]
- Abimorad, E.G.; Castellani, D.; Gonçalves, G.S.; Romera, D.M.; Garcia, F.; Nascimento, T.M.T.D. Substituição parcial do farelo de soja pela farinha de carne e ossos em dietas para juvenis de tilápia-do-nilo. Pesqui Agropecu Bras. 2014, 49, 836–843. [Google Scholar] [CrossRef]
- Silva, T.R.M.; de Andrade, M.L.S.; Chung, S.; de Almeida Bicudo, Á.J. Substituição parcial do milho pelo resíduo de macarrão em dietas para tilápia-do-nilo [partial replacement of corn by pasta waste in diets for nile tilapia]. Bol. Inst. Pesca Sao Paulo 2014, 40, 669–676. [Google Scholar]
- Petenuci, M.E.; Schneider, V.V.; Lopes, A.P.; Gonçalves, R.M.; Dos Santos, V.J.; Matsushita, M.; Visentainer, J.V. Effect of Alpha-Linolenic Acid Sources in Diets for Nile Tilapia on Fatty Acid Composition of Fish Fillet Using Principal Component Analysis. J. Aquat. Food Prod. Technol. 2018, 27, 464–476. [Google Scholar] [CrossRef]
- Carbonera, F.; Bonafe, E.G.; Martin, C.A.; Montanher, P.F.; Ribeiro, R.P.; Figueiredo, L.C.; Almeida, V.C.; Visentainer, J.V. Effect of dietary replacement of sunflower oil with perilla oil on the absolute fatty acid composition in Nile tilapia (GIFT). Food Chem. 2014, 148, 230–234. [Google Scholar] [CrossRef]
- Visentainer, J.V.; França, P.B.; Aguiar, A.C.; Montanher, P.F.; Boroski, M.; de Souza, N.E. Incorporation and fatty acid composition in liver of Nile tilapia fed with flaxseed oil. Acta Sci. Technol. 2011, 33, 221–225. [Google Scholar] [CrossRef][Green Version]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation: 10–14 November 2008; FAO: Geneva, Switzerland, 2010. [Google Scholar]
- dos Santos, L.D.; Sousa, S.M.d.N.; da Silva, L.C.R.; Bombardelli, R.A.; Meurer, F. Efeitos da peletização e extrusão sobre a digestibilidade de ingredientes alternativos do Semi-árido Nordestino para a tilápia do Nilo. Semin. Cienc. Agrar. 2014, 35, 3367. [Google Scholar] [CrossRef][Green Version]
- Damasceno, F.M.; Pezzato, L.E. Nutritional value of root and leaves of cassava for Nile tilapia. Bol. Inst. Pesca Sao Paulo 2012, 38, 61–69. Available online: https://www.researchgate.net/publication/285958520 (accessed on 17 February 2022).
- Pereira, R.; Valente, L.M.P.; Sousa-Pinto, I.; Rema, P. Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal Res. 2012, 1, 77–82. [Google Scholar] [CrossRef]
- Barone, R.S.C.; Sonoda, D.Y.; Lorenz, E.K.; Cyrino, J.E.P. Digestibility and pricing of Chlorella sorokiniana meal for use in tilapia feeds. Sci. Agricola 2018, 75, 184–190. [Google Scholar] [CrossRef]
- dos Santos Cardoso, M.; Godoy, A.C.; Oxford, J.H.; Rodrigues, R.; Cardoso, M.d.S.; Bittencourt, F.; Signor, A.; Boscolo, W.R.; Feiden, A. Apparent digestibility of protein hydrolysates from chicken and swine slaughter residues for Nile tilapia. Aquaculture 2021, 530, 735720. [Google Scholar] [CrossRef]
- Fontes, T.V.; de Oliveira, K.R.B.; Gomes Almeida, I.L.; Maria Orlando, T.; Rodrigues, P.B.; Costa, D.V.D. Digestibility of Insect Meals for Nile Tilapia Fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Sirmah, P.; Mburu, F.; Iaych, K.; Dumarçay, S.; Gérardin, P. Potential antioxidant compounds from different parts of Prosopis juliflora. J. Trop. For. Sci. 2011, 23, 187–195. Available online: https://www.frim.gov.my/v1/JTFSOnline/jtfs/v23n2/187-195.pdf (accessed on 7 February 2022).
- Raja, K.; Saravanakumar, A.; Vijayakumar, R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Singh, R.; Saxena, P.; Mani, A. Evaluation of Antibacterial Activity of Prosopis juliflora (Sw.) Dc. Leaves. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.L.; Wee, W. Malaysian rubber (Hevea brasiliensis) seed as alternative protein source for red hybrid tilapia, Oreochromis spp., farming. AACL Bioflux 2017, 10, 32–37. Available online: https://www.bioflux.com.ro/docs/2017.32-37.pdf. (accessed on 24 February 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).