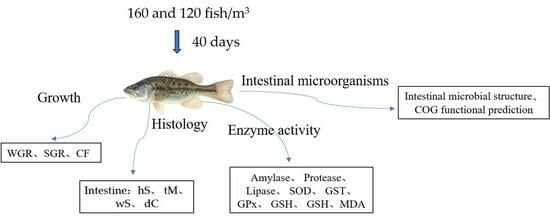
Effects of Stocking Density on Intestinal Health of Juvenile Micropterus salmoides in Industrial Aquaponics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Methods
2.3. Growth Performance Analysis
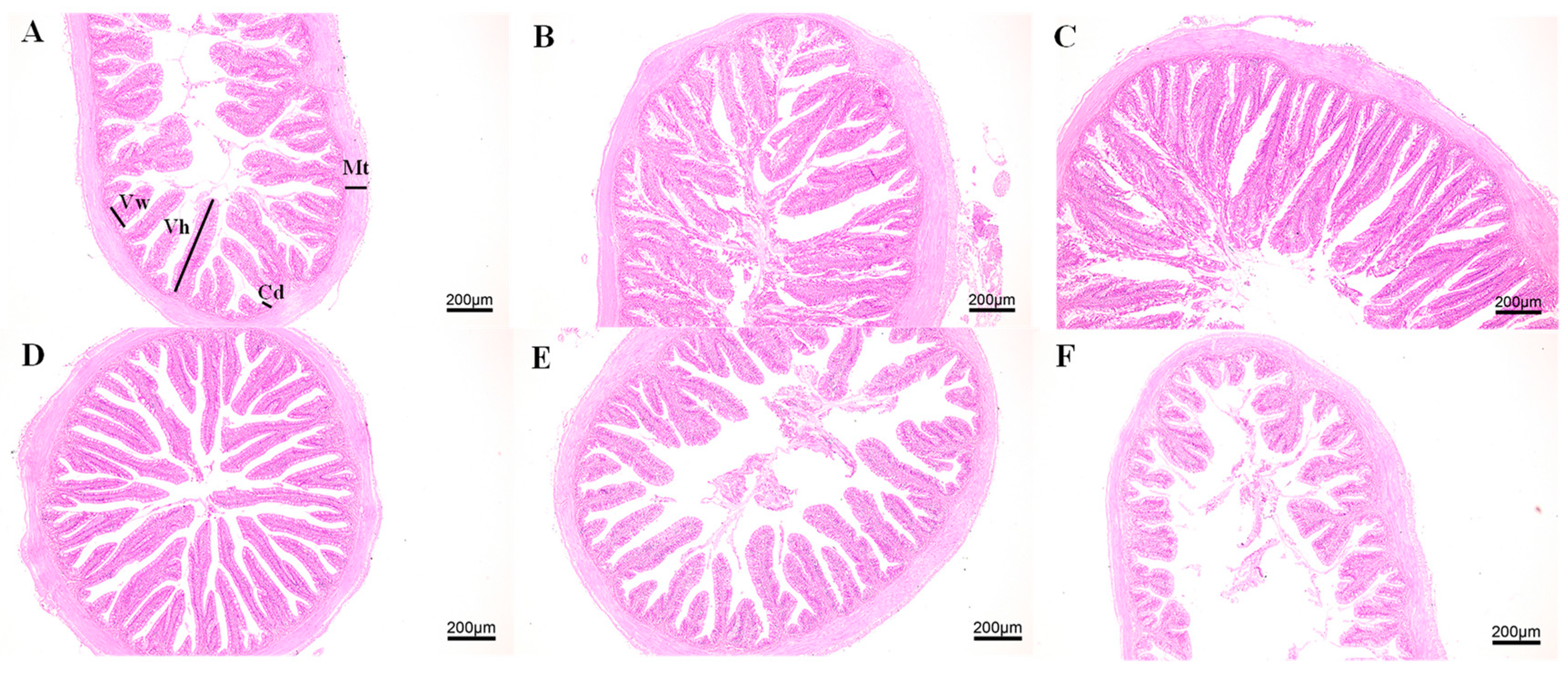
2.4. Histological Analysis
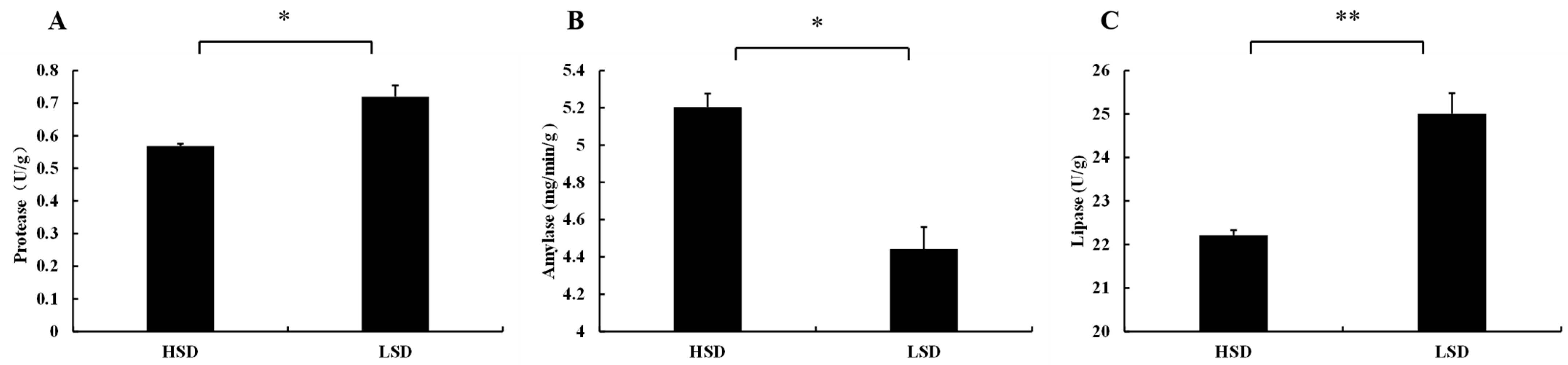
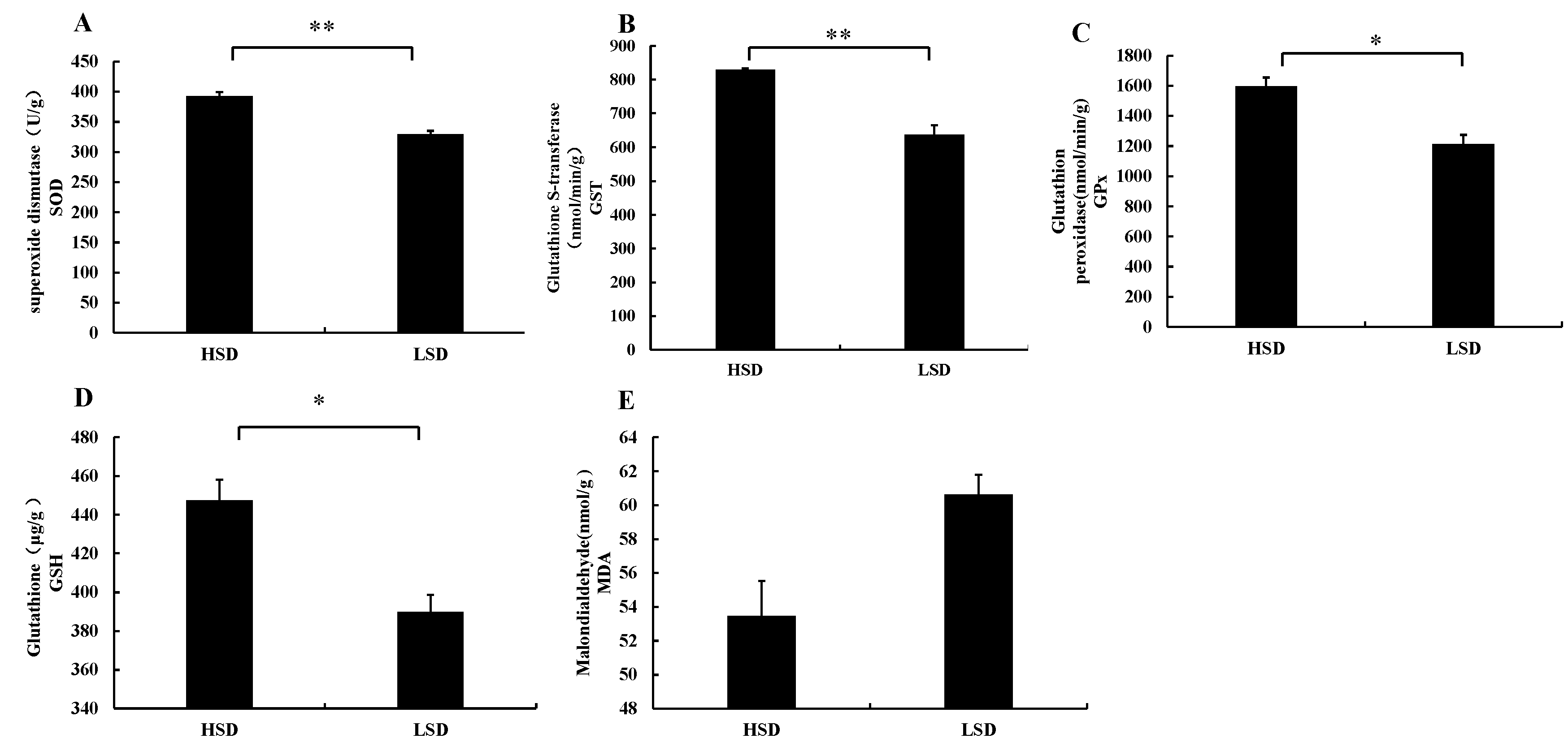
2.5. Determination of Enzyme Activity
2.6. Extraction of DNA from the Sample
2.7. High-Throughput Sequencing Analysis
2.8. Data Analysis
3. Results and Analysis
3.1. Growth and Intestinal Morphology of Juvenile M. salmoides Cultured at Different Densities
3.2. Changes in Enzyme Activities in Intestinal Tissues of Juvenile M. salmoides Cultured at Different Densities
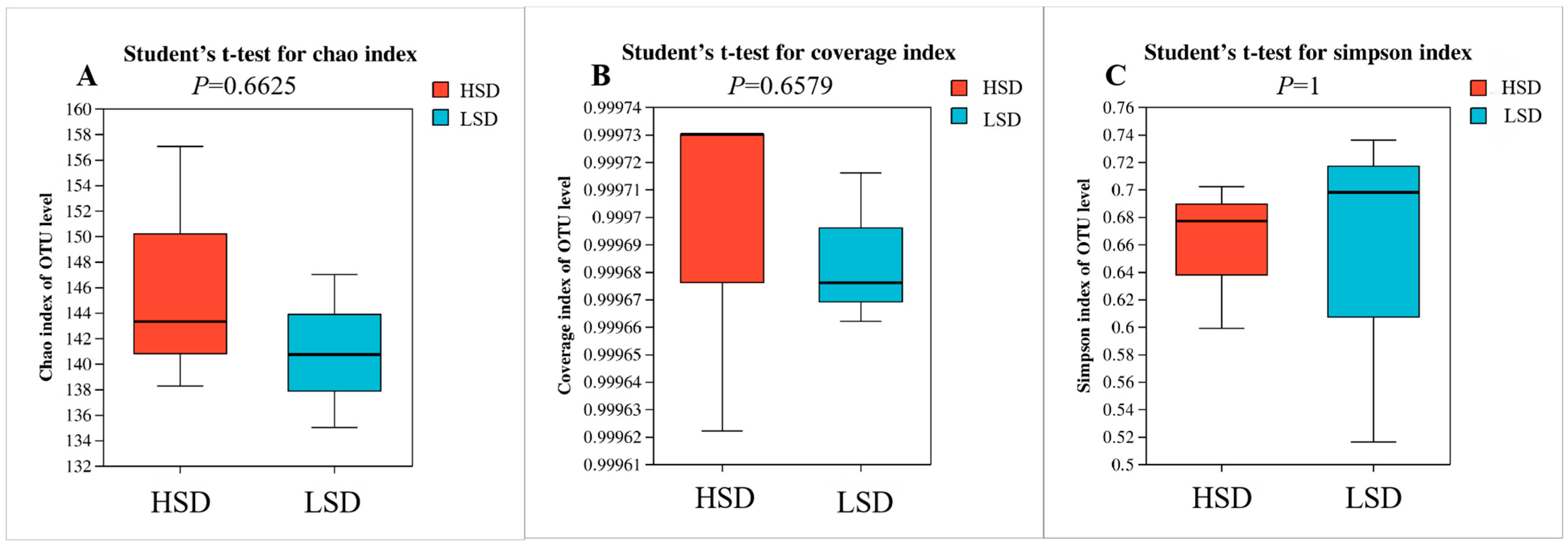
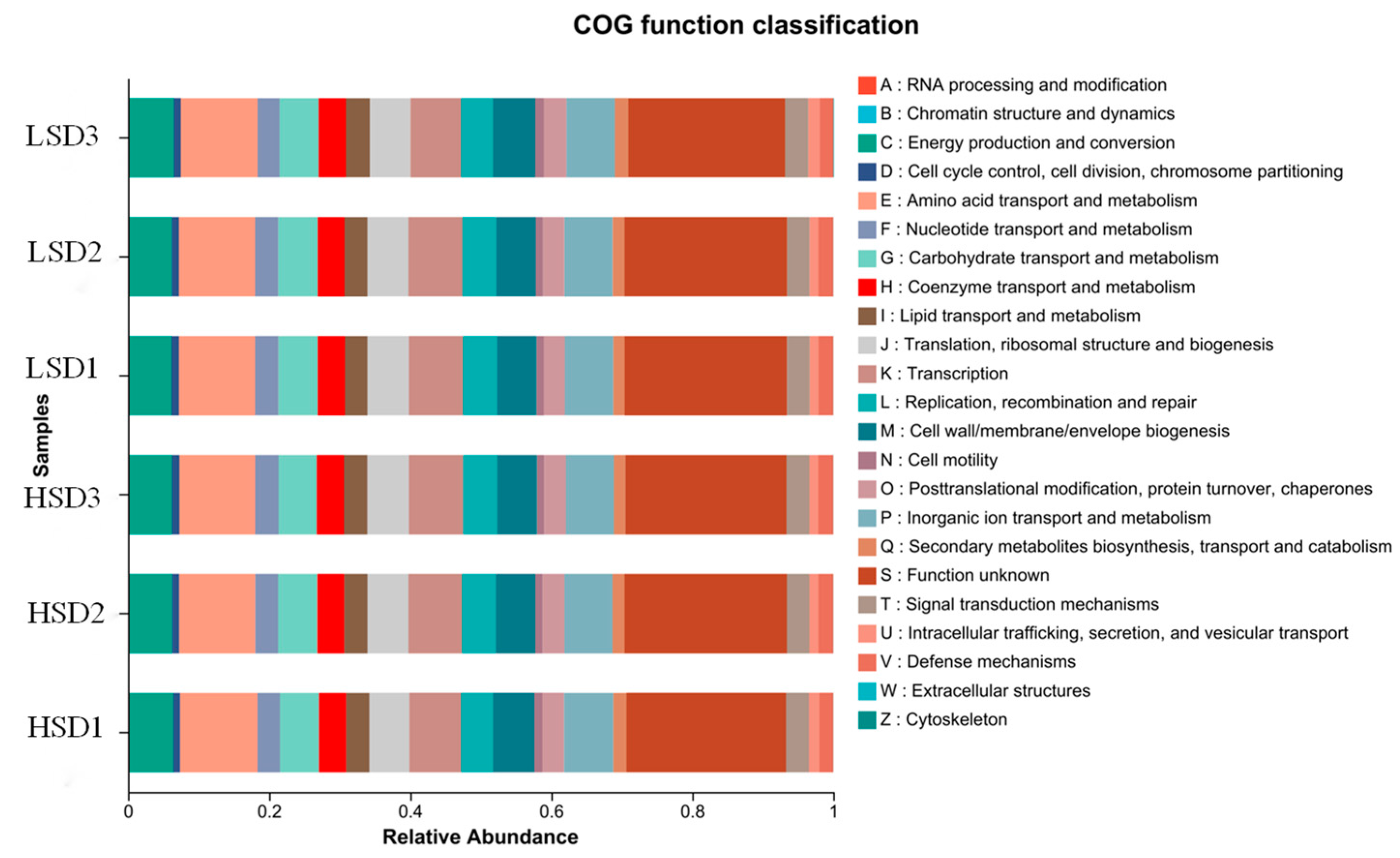
3.3. Intestinal Microbial Structure of Juvenile M. salmoides at Different Breeding Densities
4. Discussion
4.1. Effects of Culture Density on Growth and Intestinal Morphology of Juvenile M. salmoides
4.2. Effects of Different Culture Densities on Enzyme Activities in Intestinal Tissues of Juvenile M. salmoides
4.3. Effects of Different Culture Densities on Intestinal Microorganisms in Juvenile M. salmoides
4.4. Comparison of the Dominant Intestinal Microflora of M. salmoides under Different Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full name in English |
| HSD | 160 fish/m3, high stocking density |
| LSD | 120 fish/m3, low stocking density |
| IBW | initial average body weight |
| FBW | final average body weight |
| WGR | weight gain ratio |
| SGR | specific growth rate |
| CF | condition factor |
| hS | villus height |
| tM | muscular layer thickness |
| wS | villus width |
| dC | crypt depth |
| SOD | superoxide dismutase |
| GST | glutathione-S-transferase |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| MDA | malondialdehyde |
References
- Liu, C.; Zhao, L.P.; Shen, Y.Q. A systematic review of advances in intestinal microflora of fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Li, P.; Wang, C.; Liu, G.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet: Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota. Anim. Nutr. 2022, 8, 235–248. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.P.; Xie, S.W. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male). Fish Shellfish. Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef]
- Zhang, F.L.; Hao, Q.; Zhang, Q.S.; Lv, H.Y.; Yang, Y.L.; Chao, R.; Zhang, Z.; Zhou, Z. Influences of dietary Eucommia ulmoides leaf extract on the hepatic lipid metabolism, inflammation response, intestinal antioxidant capacity, intestinal microbiota, and disease resistance of the channel catfish (Ictalurus punctatus). Fish Shellfish. Immunol. 2022, 123, 75–84. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Xiao, S.; Bu, X.; Lin, Z.; Qi, C.; Qin, J.G.; Chen, L.Q. T-2 toxin in the diet suppresses growth and induces immunotoxicity in juvenile Chinese mitten crab (Eriocheir sinensis). Fish Shellfish. Immunol. 2020, 97, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.F.; Sun, R.J.; Zhang, L.; Li, B.S.; Wang, W.G.; Wu, Y.Y.; Wang, Y.L. Research progress of fish intestinal health. China Feed. 2015, 15, 19–22. [Google Scholar] [CrossRef]
- Øivind, B. Bacteria associated with early life stages of halibut, Hippoglossus hippoglossus L., inhibit growth of a pathogenic Vibrio sp. J. Fish Dis. 2010, 18, 31–40. [Google Scholar] [CrossRef]
- Chen, X.M.; Wang, G.Q.; Shan, X.F. Research Progress on the Relationship between Intestinal Barrier Damage and Intestinal Inflammation Development in Fish. J. Henan Agric. Sci. 2022, 51, 1–9. [Google Scholar] [CrossRef]
- Xu, G.F.; Chen, X.J.; Du, J.; Mou, Z.B. Fish Digestive System: It’s Structure, Function and The Distributions and Characteristics of Digestive Enzymes. Chin. J. Fish. 2009, 22, 49–55. [Google Scholar]
- Ji, Y.B.; Zhang, M.Y.; Wang, Y.; Wang, X.M.; Dai, W.; Wang, C.; Sun, X.L. Quantitative Histology Study on the Stomach and Intestines of Clarias gariepinus at Different Culture Densities. J. Anhui Agri Sci. 2013, 41, 5399–5401. [Google Scholar]
- Su, X. Study on Muscle Growth and Digestive Performance of Micropterus salmoides during Early Development. Master’s Thesis, Suzhou University, Suzhou, China, 2022. [Google Scholar]
- Comabella, Y.; Mendoza, R.; Aguilera, C.; Carrillo, O.; Hurtado, A.; García-Galano, T. Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiol. Biochem. 2006, 32, 147–157. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Lushchak, O.V.; Storey, J.M.; Storey, K.B. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int. J. Biochem. Cell Biol. 2005, 37, 1319–1330. [Google Scholar] [CrossRef]
- Feng, Z.D.; Zhong, Y.F.; He, G.L.; Sun, H.; Chen, Y.J.; Zhou, W.H.; Lin, S.M. Yeast culture improved the growth performance, liver function, intestinal barrier and microbiota of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2022, 120, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Miyuki, M.; Taiga, A.; Kenji, S.; Tomofumi, Y.; Kazuhisa, T.; Jun, K. Intestinal microbiota composition is altered according to nutritional biorhythms in the leopard coral grouper (Plectropomus leopardus). PLoS ONE 2018, 13, e0197256. [Google Scholar] [CrossRef]
- Li, J.Z.; Hou, J.; Zhang, P.F. Comparative study of intestinal microbial community structure in different species of carp in aquaponics system. South China Fish. Sci. 2016, 12, 42–50. [Google Scholar] [CrossRef]
- Parma, L.; Pelusio, N.F.; Gisbert, E.; Esteban, M.A.; D’Amico, F.; Soverini, M.; Candela, M.; Dondi, F.; Gatta, P.P.; Bonaldo, A. Effects of rearing density on growth, digestive conditions, welfare indicators and gut bacterial community of gilthead sea bream (Sparus aurata, L. 1758) fed different fishmeal and fish oil dietary levels. Aquaculture 2020, 518, 734854. [Google Scholar] [CrossRef]
- Wong, S.; Waldrop, T.; Summerfelt, S.; Davidson, J.; Barrows, F.; Kenney, P.B.; Welch, T.; Wiens, G.D.; Snekvik, K.; Rawls, J.F.; et al. Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl. Environ. Microbiol. 2013, 79, 4974–4984. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Spradlin, A.; Saha, S. Saline aquaponics: A review of challenges, opportunities, components, and system design. Aquaculture 2022, 555, 738173. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Debnath, S.; Ahmed, M.U.; Parvez, M.S.; Karmokar, A.K.; Nazmul, M. Effect of stocking density on growth performance and body composition of climbing perch (Anabas testudineus) in biofloc system. Aquacult Int. 2021, 30, 1089–1100. [Google Scholar] [CrossRef]
- Ezhilmathi, S.; Ahilan, B.; Uma, A.; Felix, N.; Cheryl, A.; Somu Sunder Lingam, R. Effect of stocking density on growth performance, digestive enzyme activity, body composition and gene expression of Asian seabass reared in recirculating aquaculture system. Aquac. Res. 2022, 53, 1963–1972. [Google Scholar] [CrossRef]
- Liu, B.L.; Lei, J.L.; Jia, R. A review: The influence of stocking density on fish welfare. Eng. Sci. China 2014, 16, 100–105. [Google Scholar]
- Zhu, C.K.; Jiang, F.F.; Zhai, W.J.; Chen, C.R.; Wei, M.Y.; Wu, M.R.; Wang, L.J.; Xu, J.R.; Xi, X.C.; Zhao, J.; et al. Effects of Stocking Density and Water Volume on Growth of Juvenile Ussuri Bullhead Pseudobagrus ussuriensis. Fish. Sci. 2019, 38, 682–687. [Google Scholar] [CrossRef]
- Liao, R.; Ou, Y.J.; Gou, X.W. A review: Influence of stocking density on fish welfare I mortality, growth, feeding and stress response. South China Fish. Sci. 2006, 6, 76–80. [Google Scholar]
- Liu, J.; Yan, Z.H.; Yao, B.L.; Li, Y.H. Research progress on the tissue structure, function, influencing factors and protective substances of fish intestine. Fish. Sci. Technol. Inf. 2023, 50, 121–127. [Google Scholar] [CrossRef]
- Noaillac-Depeyre, J.; Gas, N. Structure and function of the intestinal epithelial cells in the perch (Perca fluviatillis L.). Anat. Rec. 1979, 195, 621–627. [Google Scholar] [CrossRef]
- Sun, Y.X.; Dong, H.B.; Duan, Y.F.; Li, H.; Liu, Q.; Zhang, J.; Wang, W. Progresses in Stress Damage and Protection Studies on Fish Intestine. Trans. Oceanol. Limnol. 2019, 3, 174–183. [Google Scholar] [CrossRef]
- Farhangi, M.; Carter, C.G. Growth, physiological and immunological responses of rainbow trout (Oncorhynchus mykiss) to different dietary inclusion levels of dehulled lupin (Lupinus angustifolius). Aquac. Res. 2001, 32, 329–340. [Google Scholar] [CrossRef]
- Nie, G.X.; Wang, J.L.; Zhu, M.W.; Zhou, H.Q. The influences of xylanse added in wheat basal diet on intestine chymeviscosity and the development of villi and microvilli of Tilapia nilotica. J. Fish. China 2007, 1, 54–61. [Google Scholar]
- Zhong, Q.; Xu, D.D.; Wang, X.; Liu, C.d.; Zhou, H.H.; Mai, K.S.; He, K. Effects of Dietary Leucine and Glutamine on Growth and Intestinal Health of Juvenile Turbot (Scophthalmus maximus L.). Period. Ocean. Univ. China 2023, 53, 008–017. [Google Scholar] [CrossRef]
- Zhou, F.L. Analysis of Difference in Morphological Structure and Enzyme Activity on Different Parts of Black Carp Intestine. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Lundstedt, L.M.; José Fernando Bibiano, M.; Moraes, G. Digestive enzymes and metabolic profile of Pseudoplatystoma corruscans (Teleostei: Siluriformes) in response to diet composition. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, S.L.; Ju, C.X. Effects of temperature and pH on activity of digestive enzymes in Amphiprion ocellaris juvenile. Guangdong Agric. Sci. 2014, 41, 131–135. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Saghavi, H.; Monem, J. The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Booth, M.A.; Qin, J.G.; D'Antignana, T.; Thomson, M.J.S.; Stone, D.A.J. Temperature and dissolved oxygen influence growth and digestive enzyme activities of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac. Res. 2014, 45, 2010–2020. [Google Scholar] [CrossRef]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Yuan, J.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef]
- Peng, S.M.; Lin, S.Z.; Shi, Z.H.; Gao, Q.X.; Wang, J.G.; Sun, P.; Yin, F. Effects of rearing density on growth rate and digestive enzyme activity of juvenile Pampus argenteus. Mar. Fish. 2013, 35, 72–76. [Google Scholar] [CrossRef]
- Yu, Y.B.; Huang, W.Y.; Cui, M.C. Effects of stocking densities on growth performance, nutrient composition serum biochemical, digestive and metabolic enzymes activities of large yellow croaker (Larimichthys crocea). Fish. Mod. 2023, 50, 64–71. [Google Scholar]
- Wang, Y.N.; Zhang, Z.R.; Zheng, S.M. The carp compensate the influence of growth and hunger of amylase. Reserv. Fish. 2001, 5, 6–7. [Google Scholar] [CrossRef]
- Wu, W.Q.; Yao, J.M.; Zhao, J.; Chen, X.M.; Xia, C.G.; Niu, X.T.; Wang, G.Q. Effects of Astaxanthin on Growth, Intestinal Digestive Enzymes and Antioxidant Indexes of Juvenile Paramisgurnus dabryanus. J. Jilin Agric. Univ. 2021, 43, 482–487. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.G.; Zhang, J.R.; Gao, Y.J. Effects of stocking density on growth, feed utilization and intestinal oxidative stress resistance in juvenile Megalobrama pellegrini (Tchang, 1930). Prog. Fish. Sci. 2022, 43, 106–114. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Qu, J.H.; Chen, J.C.; Hu, G.D.; Wu, W. Dynamic changes of catalase and glutathione-s-transferase in the different tissues of tilapia exposed to phenol. Ecol. Environ. 2006, 15, 687–692. [Google Scholar] [CrossRef]
- Zang, L.X.; Ma, Y.; Huang, W.H.; Ling, Y.H.; Sun, L.M.; Wang, X.D.; Zeng, A.B.; Dahlgren, R.A.; Wang, C.H.; Wang, H.L. Dietary Lactobacillus plantarum ST-III alleviates the toxic effects of triclosan on zebrafish (Danio rerio) via gut microbiota modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef]
- Sahin, K.; Yazlak, H.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Sahin, N. The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture 2014, 418–419, 132–138. [Google Scholar] [CrossRef]
- Cantas, L.; Sørby, J.R.; Aleström, P.; Sørum, H. Culturable gut microbiota diversity in zebrafish. Zebrafish 2012, 9, 26–37. [Google Scholar] [CrossRef]
- Lyons, P.P.; Turnbull, J.F.; Dawson, K.A.; Crumlish, M. Phylogenetic and functional characterization of the distal intestinal microbiome of rainbow trout Oncorhynchus mykiss from both farm and aquarium settings. J. Appl. Microbiol. 2017, 122, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, L.H.; Jiang, J.H. Stocking densities on growth, antioxidant enzyme activities and intestinal microbiota of hybrid of Culter alburnus(♀) × Megalobrama terminalis(♂) in IPRA system. Acta Hydrobiol. Sin. 2023, 47, 479–487. [Google Scholar]
- Xu, S.H. The Growth and Physiological Response of Atlantic Salmon (Salmo salar) under the Conditions of Different Rearing Densities and Dissolved Oxygen. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Zhou, M.; Liang, R.; Mo, J.; Yang, S.; Gu, N.; Wu, Z.; Badu, V.S.; Li, J.; Huang, Y.; Lin, L. Effects of brewer’s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 2018, 484, 139–144. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhou, Y.F.; Fang, D.A.; Zhang, X.Z.; Zhang, L.; Liu, J.Y.; Zheng, Z.; You, Y. Effects of starvation and refeeding on intestinal microflora of chinese mitten crab (Eriocheir sinensis). Acta Hydrobiol. Sin. 2019, 43, 748–756. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef]
- Sellyei, B.; Varga, Z.; Cech, G.; Varga, Á.; Székely, C. Mycoplasma infections in freshwater carnivorous fishes in Hungary. J. Fish Dis. 2021, 44, 297–304. [Google Scholar] [CrossRef]
- Gnanadurai, R.; Fifer, H. Mycoplasma genitalium: A Review. Microbiology 2020, 166, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ye, W.; Tao, Y.; Zheng, T.; Qiang, J.; Li, Y.; Li, Y.; Liu, W.T.; Xu, P. Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora. Antioxidants 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.B.; Zhang, L.L.; Si, D.Y. Decomposition of amino acids by intestinal flora and the effect of the metabolites on the host. Siliao Gongye 2017, 38, 41–44. [Google Scholar] [CrossRef]
- Lin, K.T. Comparison of Intestinal Microbes between Feeding Broad Bead and Common Feed in Grass Carps. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Amato, K.R. Co-evolution in context: The importance of studying gut microbiomes in wild animals. Microbiome Sci. Med. 2013, 1, 10–29. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Wang, Q. Effect of Low Temperature on the Gut Microbiome Composition of Fish. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar]
- Zhai, W.Y.; Guo, A.N. Research progress on the fish intestinal microbiota. Henan Shuichan 2016, 4, 18–21+40. [Google Scholar]
- Fan, Z.; Wu, D.; Li, J.N.; Zhang, Y.Y.; Cui, Z.Y.; Li, T.B.; Zheng, X.H.; Liu, H.B.; Wang, L.S.; Li, H.Q. Assessment of fish protein hydrolysates in juvenile largemouth bass (Micropterus salmoides) diets: Effect on growth, intestinal antioxidant status, immunity, and microflora. Front. Nutr. 2022, 9, 816341. [Google Scholar] [CrossRef]
- Meng, X.L.; Cai, H.M.; Li, H.; You, F.; Jiang, A.X.; Hu, W.P.; Li, K.K.; Zhang, X.D.; Zhang, Y.M.; Chang, X.L.; et al. Clostridium butyricum-fermented Chinese herbal medicine enhances the immunity by modulating the intestinal microflora of largemouth bass (Micropterus salmoides). Aquaculture 2023, 562, 738768. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Zhu, X.X.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Huang, X.L.; Chen, D.F.; Yang, S.Y.; et al. Dietary methionine hydroxy analogue supplementation benefits on growth, intestinal antioxidant status and microbiota in juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 556, 738279. [Google Scholar] [CrossRef]
- Zhang, G.G. Effects of Different Lipid Sources and Protein Sources on Lipid Metabolism and Protein Metabolism of Largemouth Bass (Micropterus Salmoides). Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2019. [Google Scholar]
- Lu, Z.Y. Effect of Lysophospholipids or Alpha-Lipoic Acid Added to Diets with Different Lipid Levels on Lipid Metabolism in Largemouth Bass (Micropterus salmoides). Master’s Thesis, Guangdong Ocean University, Zhenjiang, China, 2022. [Google Scholar]
- Chang, K.; Gao, S.Y.; Tian, R.R. Effect of dietary sodium butyrate supplementation on intestinal microbiota composition of largemouth bass (Micropterus salmoides) fed high-soybean meal diets. China Feed. 2023, 1–7. [Google Scholar] [CrossRef]
- Bian, Y.R. Study on Partial Replacement of Fish Meal with Fermented Silkworm Pupae Meal in Feeding Largemouth Bass, Micropterus salmoides. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2021. [Google Scholar] [CrossRef]
- Huang, H. Fermented soybean meal instead of fish meal on growth, lipid Metabolism, serum non-specific immunity and intestinal flora of juvenile largemouth bass. Acta Hydrobiol. Sin. 2022, 46, 466–477. [Google Scholar] [CrossRef]
- Du, R.Y. Evaluation of Yeast Hydrolysate and Bacillus subtilis as Feed Additives in Largemouth Bass Micropterus salmoides. Master’s Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar] [CrossRef]
- Wang, S.G.; Yan, F.J.; Zhang, M.L.; Mao, S.Q.; Zhu, Y.A.; Fu, C.S.; Song, L.P.; Liu, F. Effects of two probiotics on growth, digestion, immunity, and intestinal microbial communities of largemouth bass (Micropterus salmoides). J. Fish. Sci. China 2023, 30, 479–491. [Google Scholar] [CrossRef]
- Zhu, S.J. Study on the Application of Dietary Enzymatically Treated Artemisia annua L. in Largemouth Bass, Micropterus salmoides. Master’s Thesis, Yangtze University, Jingzhou, China, 2022. [Google Scholar]
- Zhou, Y.L. Effects of Dietary Starch on Intestinal Health of Largemouth Bass, Micropterus salmoides. Master’s Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Feng, Z.D.; Liao, R.S.; Sun, H. Effects of enzymatic chicken pulp on liver antioxidant capacity, intestinal physical barrier and microflora of Micropterus salmoides. J. Fish. China 2022, 46, 1824–1835. [Google Scholar] [CrossRef]
- Guo, M.Y. Effects of Dietary Algae-Derived β–glucan on Growth, Non-Specific Immune and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2022. [Google Scholar] [CrossRef]
- Li, X. Effects of Small Peptides and Vitamin D3 on Growth, Liver Metabolism and Intestinal Microorganisms of Largemouth Bass Micropterus Salmoides. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Zhong, L.Q.; Wang, H.X.; Wang, H.M.; Zhang, S.Y.; Jiang, H.C.; Chen, X.H. Different diets on growth performance and intestinal bacterial community of largemouth bass (Micropterus salmoides). Acta Hydrobiol. Sin. 2022, 46, 1609–1617. [Google Scholar] [CrossRef]
- Dan, Y.; Yu, F.Q.; Li, S. Intestinal Microbial Diversity of Micropterus salmoides at Different Growth Stages. Southwest China J. Agric. Sci. 2021, 34, 2798–2803. [Google Scholar] [CrossRef]
- He, K. Study on Liver Transcriptome and Intestinal Microbes during the Weaning of Largemouth Bass in Pond. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2020. [Google Scholar] [CrossRef]
- Liu, M.; Lian, Q.P.; Ni, M.; Guo, A.H.; Yuan, J.L. Effects of inner-pond raceway aquaculture on the growth performance, antioxidant enzymes, digestive enzymes, digestive tract structure, and bacterial flora of largemouth bass (Micropterus salmoides). J. Fish. China 2021, 45, 2011–2028. [Google Scholar] [CrossRef]
- Larsen, A.M.; Mohammed, H.H.; Arias, C.R. Characterization of the gut microbiota of three commercially valuable warmwater fish species. J. Appl. Microbiol. 2014, 116, 1396–1404. [Google Scholar] [CrossRef]
- Chen, M.S.; Bao, X.X.; Yue, Y.H.; Yang, K.L.; Liu, H.; Yang, Y.; Yu, H.; Yu, Y.Y.; Duan, L. Combined effects of cadmium and nanoplastics on oxidative stress, histopathology, and intestinal microbiota in largemouth bass (Micropterus salmoides). Aquaculture 2023, 569, 739363. [Google Scholar] [CrossRef]

| Group | IBW (g) | FBW (g) | WGR (%) | SGR (%/Day) | CF (g/cm3) |
|---|---|---|---|---|---|
| HSD | 26.38 ± 3.48 | 93.50 ± 12.68 * | 274 ± 70 * | 3.15 ± 0.51 * | 2.77 ± 0.10 |
| LSD | 29.03 ± 3.21 | 83.89 ± 12.10 | 193 ± 39 | 2.62 ± 0.34 | 2.81 ± 0.17 |
| Group | Villus Height (μm) | Villus Width (μm) | Crypt Depth (μm) | Muscular Thickness (μm) |
|---|---|---|---|---|
| Foregut HSD | 415.98 ± 8.37 | 83.12 ± 4.54 * | 28.93 ± 1.49 | 97.92 ± 8.43 |
| Foregut LSD | 471.33 ± 36.7 | 65.26 ± 4.45 | 25.03 ± 1.45 | 80.85 ± 2.61 |
| Midgut HSD | 557.88 ± 19.44 ** | 97.07 ± 14.34 | 32.91 ± 2.35 | 168.28 ± 6.75 ** |
| Midgut LSD | 428.3 ± 16.47 | 88.86 ± 5.49 | 28.73 ± 1.34 | 102.3 ± 6.62 |
| Hindgut HSD | 668.52 ± 23.84 ** | 84.07 ± 10.17 | 28.13 ± 1.14 | 112.56 ± 12.57 |
| Hindgut LSD | 233.1 ± 31.64 | 74.22 ± 9.83 | 27.57 ± 1.46 | 102.06 ± 9.7 |
| No. | Experimental Site | Influence Factor | Dominant Flora (Phylum Level) | Dominant Flora (Genus Level) | References |
|---|---|---|---|---|---|
| 1 | Indoor recirculating aquaculture system/laboratory | Fish protein hydrolysates | Fusobateriota, Firmicutes, Cyanobacteria | Cetobaterium, Staphylococcus, Kocuria | [69] |
| 2 | Tanks/laboratory | Chinese herbal medicine | Proteobacteria, Firmicutes, Fusobacteria | [70] | |
| 3 | Concrete tanks | Methionine hydroxy analogue | Firmicutes, Bacteroidetes, Proteobacteria | Clostridiales, Faecalibacterium, Bifidobacterium | [71] |
| 4 | The blue circle cylinder/laboratory | Yeast culture | Proteobacteria, Firmicutes, Actinobacteria | Plesiomonas, Stenotrophomonas | [14] |
| 5 | Recirculating aquaculture systems/laboratory | Enzymatic hydrolysis Soybean meal and soybean meal | Firmicutes, Fusobacteria, Proteobacteria | Clostridium, Peptostreptococcaceae, Cetobacterium | [72] |
| 6 | Glass fiber tank/laboratory | Lysophospholipids | Fusobacteria, Proteobacteria, Tenericutes | Mycoplasma, Cetobacterium, Acinetobacter | [73] |
| 7 | Glass fiber tank/laboratory | Alpha-lipoic acid | Fusobacteria, Proteobacteria, Firmicutes | Cetobacterium, Lactobacillus, Lelliottia | [73] |
| 8 | Aquarium/laboratory | Sodium butyrate | Cyanobacteria, Firmicutes, Tenericutes | Mycoplasma, Fictibacillus, Lactobacillus | [74] |
| 9 | Temperature-controlled circulating aquaculture system/laboratory | Fermented silkworm pupae meal | Proteobacteria, Actinobacteria, Firmicutes | [75] | |
| 10 | Net cage | Fermented soybean meal | Tenericutes, Proteobacteria, Fusobacteria | Mycoplama, Plesiomonas, Cetobacterium | [76] |
| 11 | Aquarium/laboratory | Bacillus subtilis | Proteobacteria, Fusobacteria, Firmicutes | [77] | |
| 12 | Indoor recirculating aquaculture system | The probiotic Bacillus sp | Mycoplasma, Plesiomonas, Cetobacterium | [78] | |
| 13 | Net cage | Enzymatically treated Artemisia annua L. | Firmicutes, Fusobacteriota, Proteobacteria | [79] | |
| 14 | Aquarium/laboratory | Starch level | Proteobacteria, Actinobacteria, Firmicutes | Brevundimonas, Microbacteriaceae, Ralstonia | [80] |
| 15 | Indoor recirculating aquaculture system/laboratory | Enzymatic chicken pulp | Proteobacteria, Firmicutes, Bacteroidetes | [81] | |
| 16 | Glass fiber tank/laboratory | β–glucan | Fusobacteria, Firmicutes, Proteobacteria | [82] | |
| 17 | Net cage | Feed protein level and small peptide addition | Proteobacteria, Tenericutes, Firmicutes | Mollicutes, Mycoplasma, Achromobacter | [83] |
| 18 | Net cage | Vitamin D3 | Proteobacteria, Tenericutes, Firmicutes | Mycoplasma, Plesiomonas, Cetobacterium | [83] |
| 19 | Pond | Different diets | Firmicutes, Proteobacteria, Actinobacteriota | [84] | |
| 20 | Pond | Different Growth Stages | Proteobacteria, Fusobacteria, Firmicutes | Cetobacterium, Pseudomonas, Mycoplasma | [85] |
| 21 | Pond | Different weaning Stages | Firmicutes, Tenericutes, Fusobacteria | [86] | |
| 22 | Constant temperature recirculating aquaculture system | Different growth rates | Proteobacteria, Firmicutes | [11] | |
| 23 | In-pond Raceway Aquaculture | Still water culture | Proteobacteria, Firmicutes, Cyanobacteria | Acinetobacter, Delfti, Catenibacterium | [87] |
| 24 | In-pond Raceway Aquaculture | Flowing water culture | Firmicutes, Proteobacteria, Acfinobacteria | Mollicutes, Peptostreptococcaceae, Propionibacterium | [87] |
| 25 | Pond | Natural culture | Fusobacteria, Proteobacteria | Cetobacterium, Aeromonas, Fusobacterium | [88] |
| 26 | Indoor tank/laboratory | Dissolved oxygen | Proteobacteria, Spirochaetes, Fusobacteria | [62] | |
| 27 | Indoor recirculating aquaculture system/laboratory | Nanoplastics and Cd | Firmicutes, Tenericutes, Proteobacteria | Mycoplasma, Aurantimicrobium, Dubosiella | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Wang, Z.; Pu, D.; Li, P.; Wei, X.; Li, M.; Li, D.; Gao, L.; Zhai, X. Effects of Stocking Density on Intestinal Health of Juvenile Micropterus salmoides in Industrial Aquaponics. Fishes 2023, 8, 555. https://doi.org/10.3390/fishes8110555
Zheng J, Wang Z, Pu D, Li P, Wei X, Li M, Li D, Gao L, Zhai X. Effects of Stocking Density on Intestinal Health of Juvenile Micropterus salmoides in Industrial Aquaponics. Fishes. 2023; 8(11):555. https://doi.org/10.3390/fishes8110555
Chicago/Turabian StyleZheng, Jishu, Zhengxi Wang, Decheng Pu, Peiyuan Li, Xiuli Wei, Mai Li, Dongsheng Li, Lihong Gao, and Xuliang Zhai. 2023. "Effects of Stocking Density on Intestinal Health of Juvenile Micropterus salmoides in Industrial Aquaponics" Fishes 8, no. 11: 555. https://doi.org/10.3390/fishes8110555
APA StyleZheng, J., Wang, Z., Pu, D., Li, P., Wei, X., Li, M., Li, D., Gao, L., & Zhai, X. (2023). Effects of Stocking Density on Intestinal Health of Juvenile Micropterus salmoides in Industrial Aquaponics. Fishes, 8(11), 555. https://doi.org/10.3390/fishes8110555