Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock and Egg Collection
2.2. Embryos and Larval Rearing
2.3. Broodstock Feed and Proximal Analysis
2.4. Sample Collection
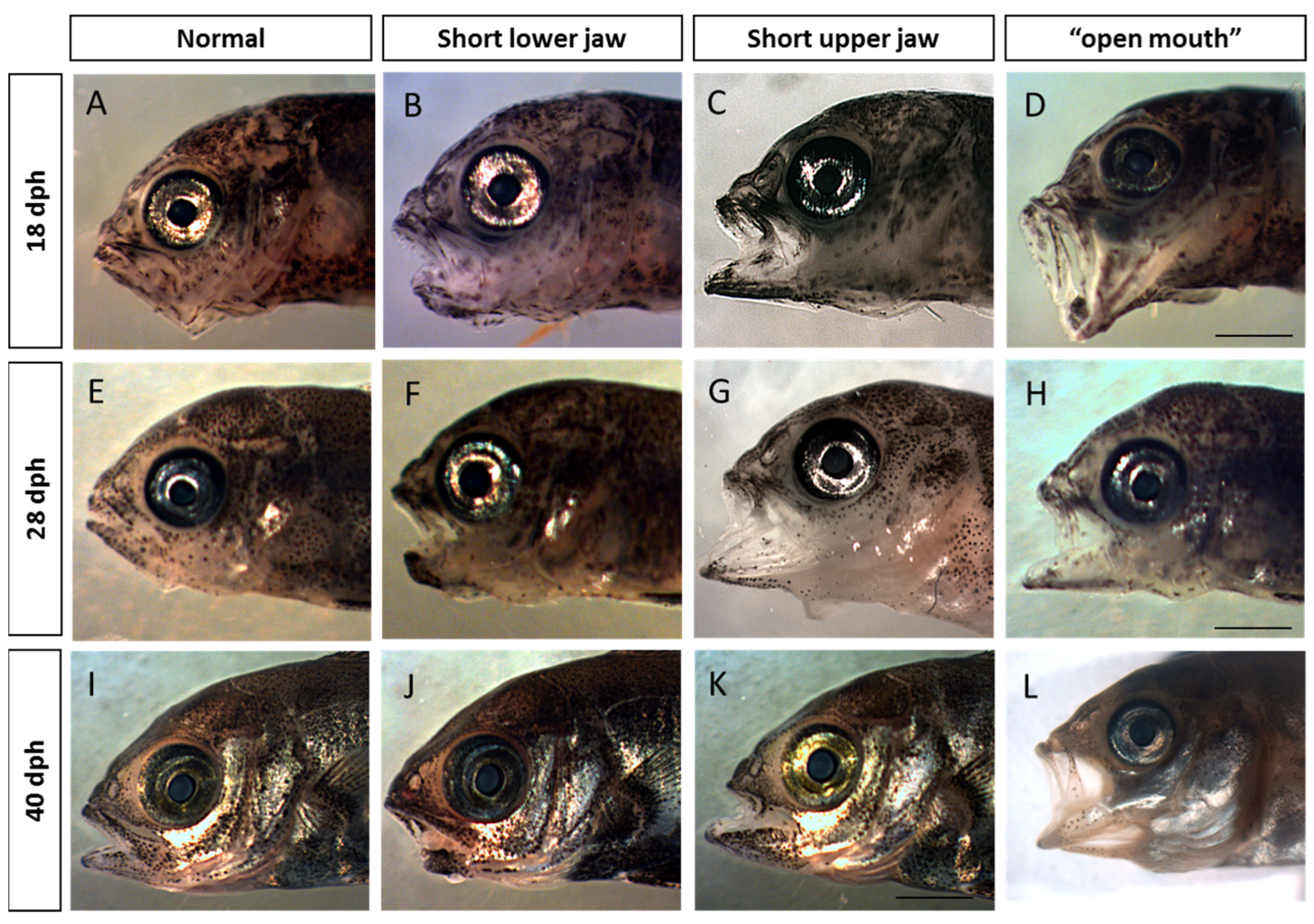
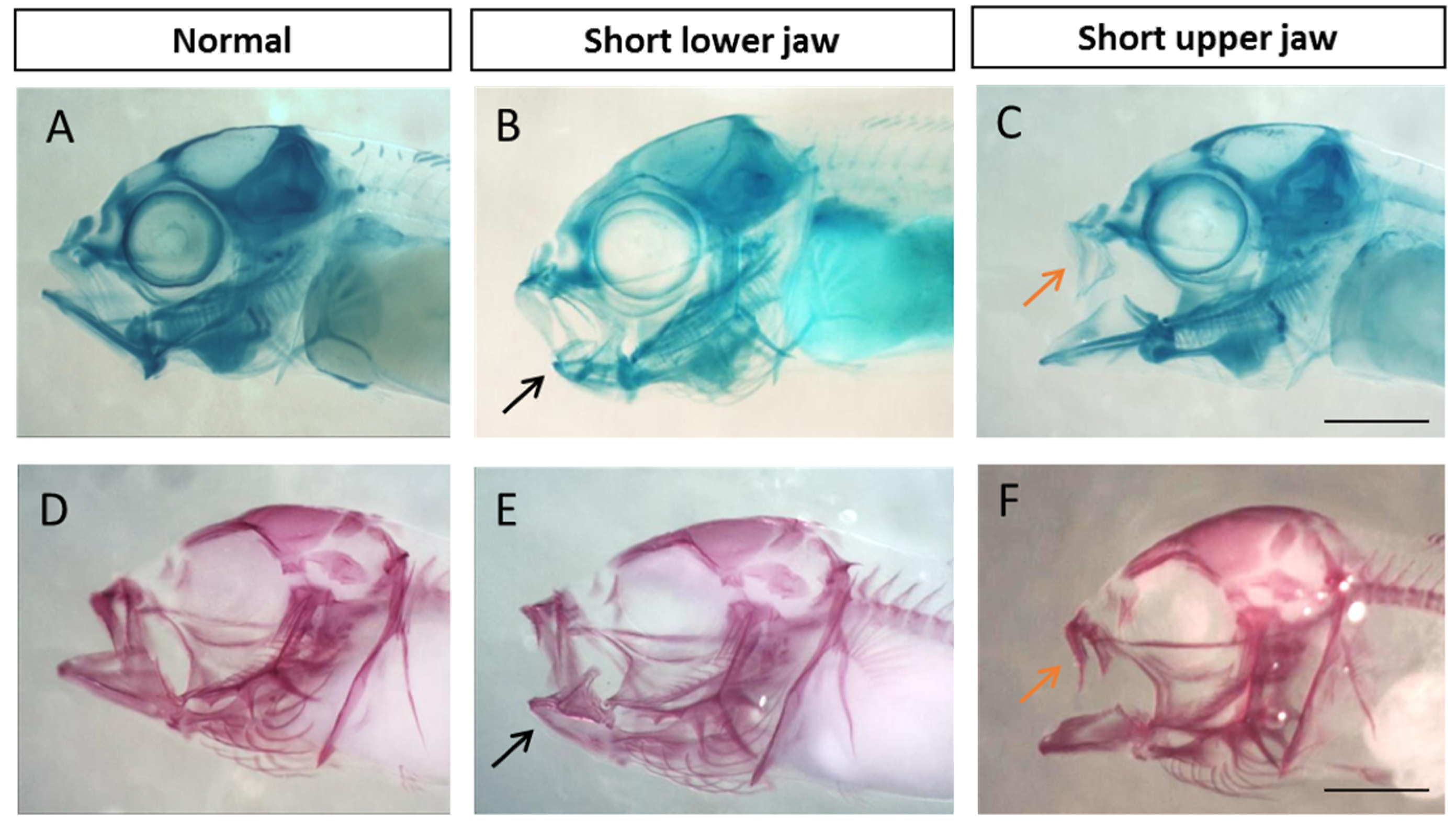
2.5. Visualization and Malformation Analysis
2.6. Lipid and Fatty Acid Analysis of Whole Body
2.7. Gene Expression Analysis Based on Transcriptomic Data
2.7.1. RNA Isolation
2.7.2. cDNA Library Preparation, Sequencing, and Mapping for Transcriptome Analysis
2.7.3. Differential Expression Analysis of Lipid Metabolism-Related Genes
2.8. Statistical Analysis
3. Results
3.1. Malformation Types and Frequency during Early, Middle, and Late Spawning Events
3.2. Proximate Composition of Diets Used to Feed S. lalandi Broodstock
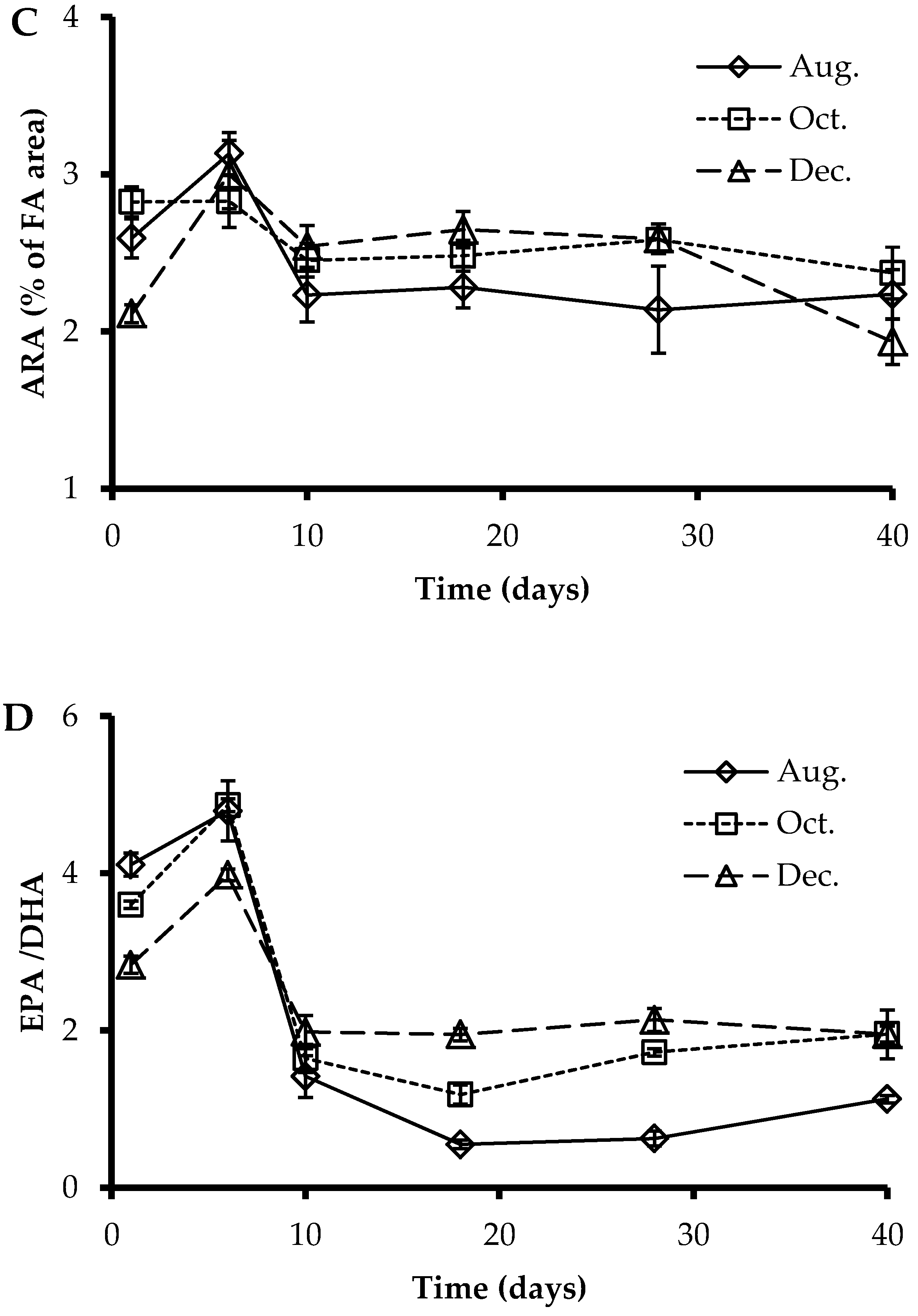
3.3. Fatty Acids Profile of Larvae at Sampling Points
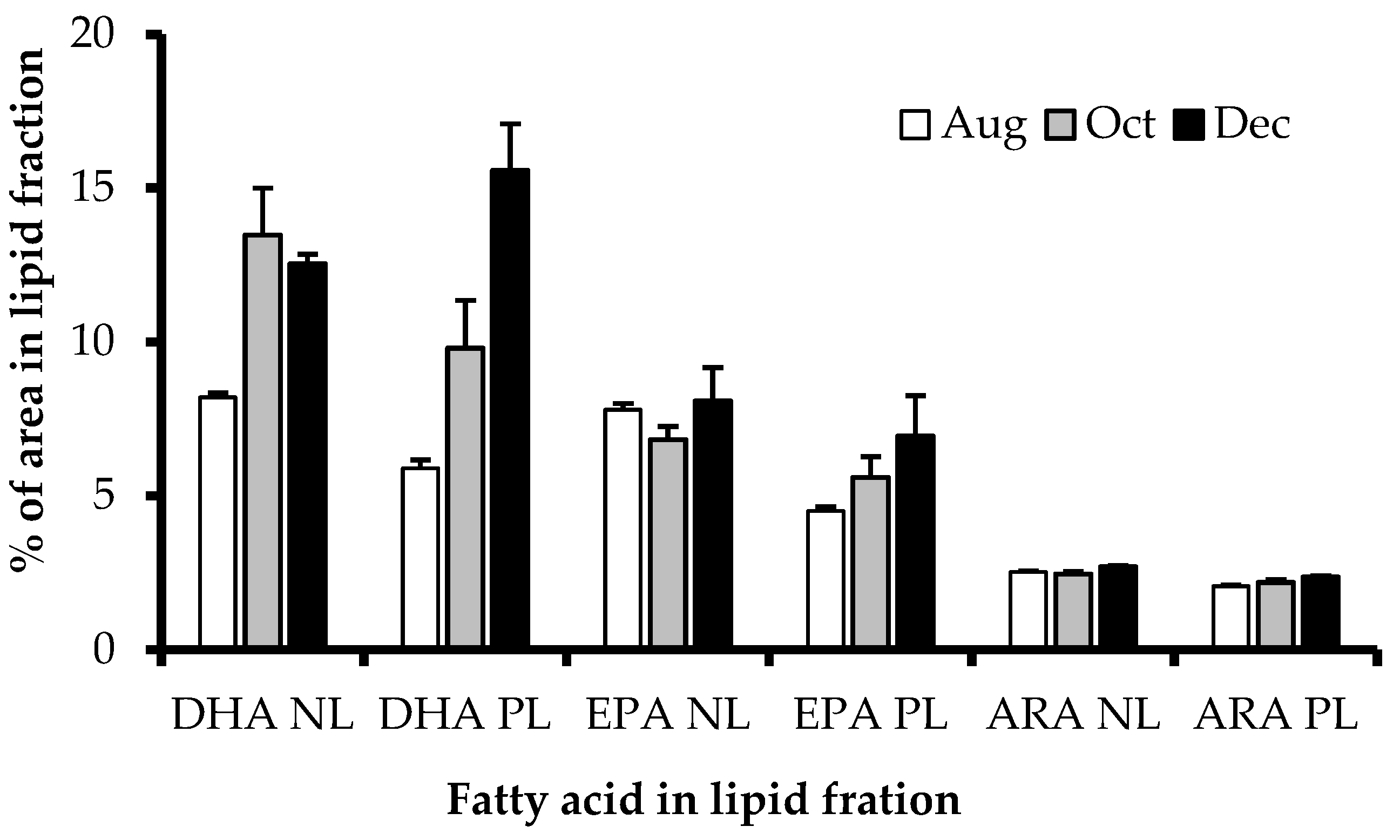
3.4. EPA, DHA, and ARA Content in Polar and Neutral Lipid Fraction
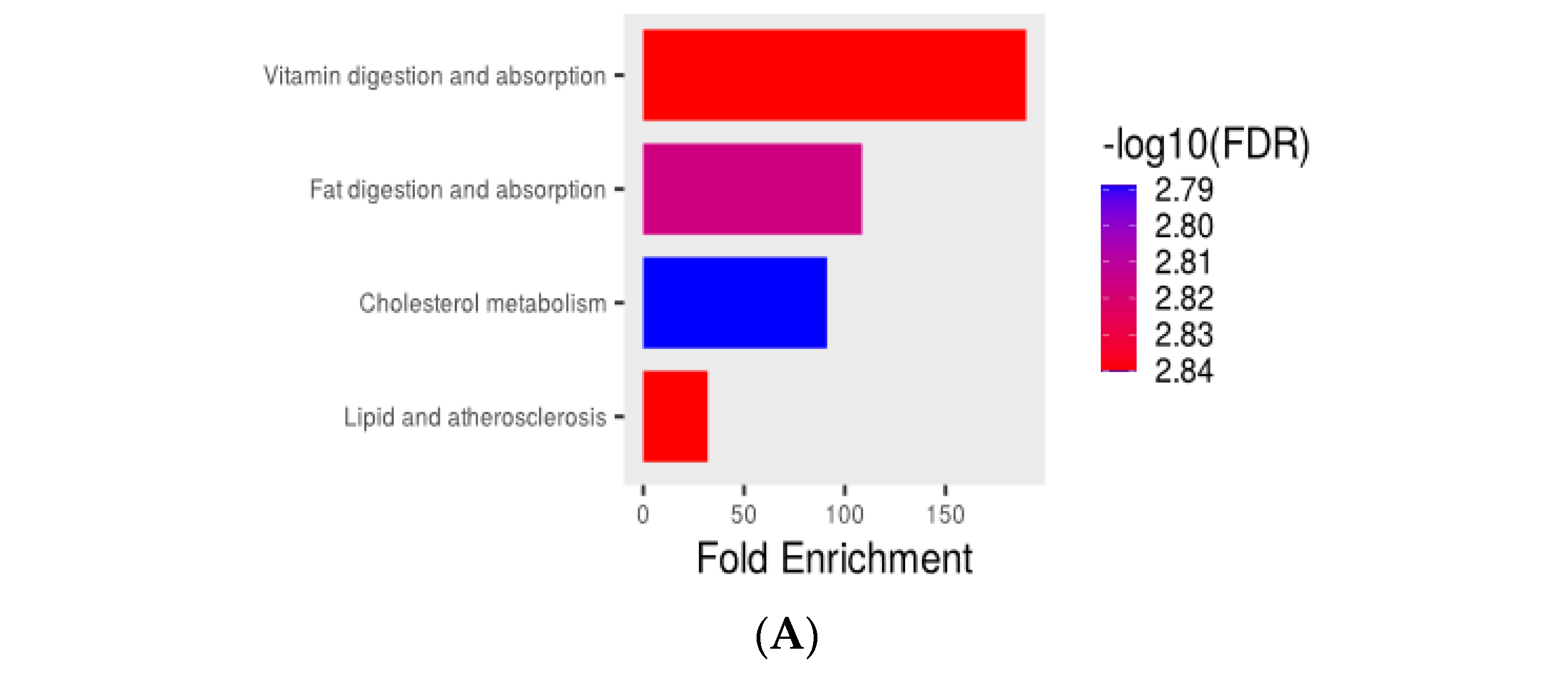
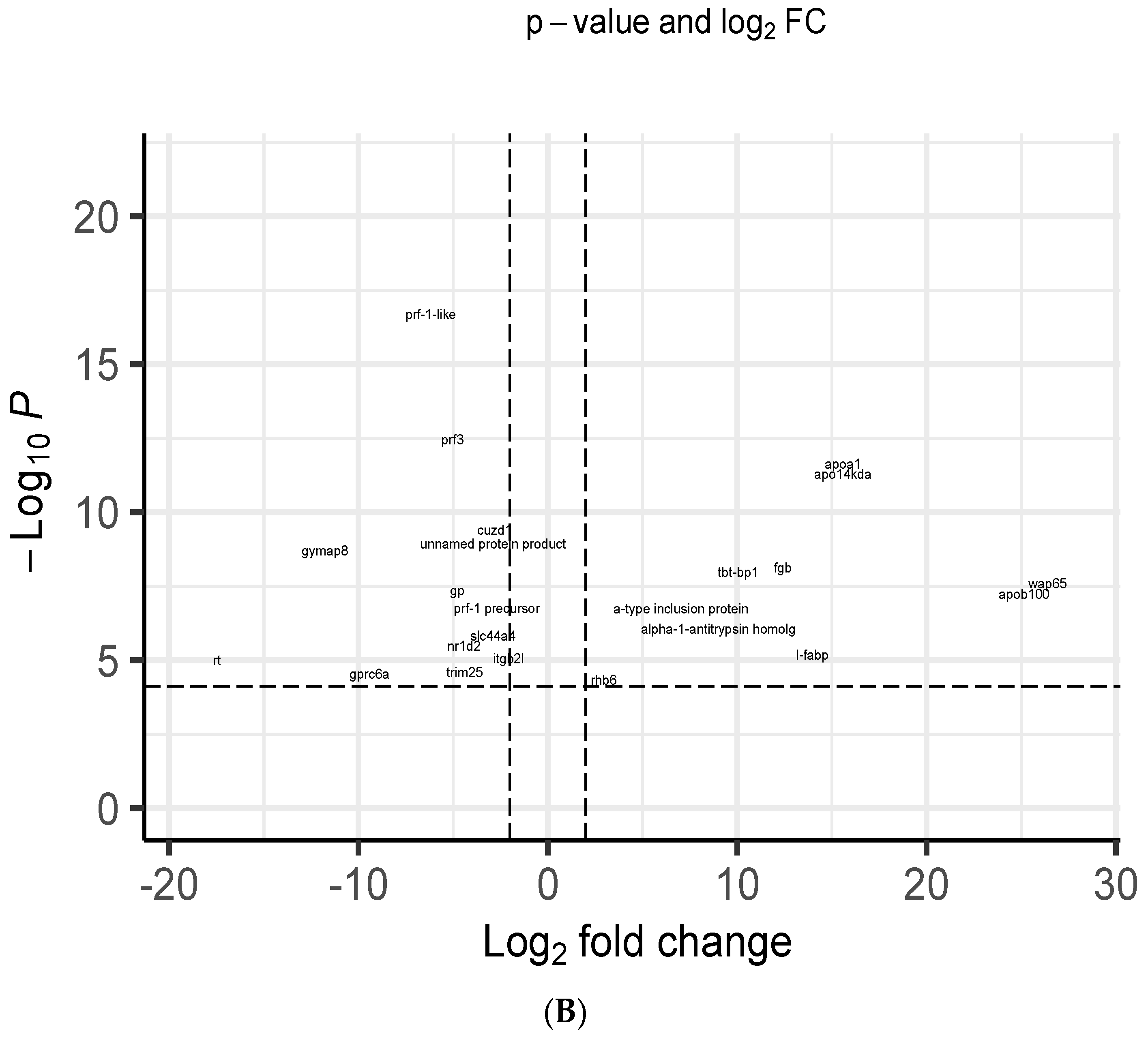
3.5. Transcriptome Analysis
4. Discussion
4.1. Malformation Types and Frequency during Early, Middle, and Late Spawning Events
4.2. Fatty Acids Profile and Deformities
4.3. Polar and Neutral Lipid Fraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal Anomalies in Reared European Fish Larvae and Juveniles. Part 1: Normal and Anomalous Skeletogenic Processes. Rev. Aquac. 2013, 5, S99–S120. [Google Scholar] [CrossRef]
- Cobcroft, J.M.; Battaglene, S.C. Skeletal Malformations in Australian Marine Finfish Hatcheries. Aquaculture 2013, 396, 51–58. [Google Scholar] [CrossRef]
- ZhenHua, M.; Tan AikYang [Tan, A.Y.D.; Qin, J.G. Jaw Deformities in the Larvae of Yellowtail Kingfish (Seriola Lalandi Valenciennes, 1833) from Two Groups of Broodstock. Indian J. Fish. 2014, 61, 138–141. [Google Scholar]
- Silbernagel, C.; Shane, M.; Drawbridge, M. Quality Considerations and Malformation Surveillance in a Marine Stocking Program. Bull. Mar. Sci. 2021, 97, 559–574. [Google Scholar] [CrossRef]
- Battaglene, S.C.; Cobcroft, J.M. Advances in the Culture of Striped Trumpeter Larvae: A Review. Aquaculture 2007, 268, 195–208. [Google Scholar] [CrossRef]
- Cobcroft, J.M.; Pankhurst, P.M.; Poortenaar, C.; Hickman, B.; Tait, M. Jaw Malformation in Cultured Yellowtail Kingfish (Seriola lalandi) Larvae. N. Z. J. Mar. Freshw. Res. 2004, 38, 67–71. [Google Scholar] [CrossRef]
- Cobcroft, J.M.; Pankhurst, P.M.; Sadler, J.; Hart, P.R. Jaw Development and Malformation in Cultured Striped Trumpeter Latris lineata. Aquaculture 2001, 199, 267–282. [Google Scholar] [CrossRef]
- Cobcroft, J.M.; Battaglene, S.C. Jaw Malformation in Striped Trumpeter Latris Lineata Larvae Linked to Walling Behaviour and Tank Colour. Aquaculture 2009, 289, 274–282. [Google Scholar] [CrossRef]
- Jara, B.; Abarca, M.; Wilson, R.; Krapivka, S.; Mercado, A.; Guiñez, R.; Marchant, L. Qualitative Analysis of Cartilaginous Jaw Element Malformation in Cultured Yellowtail Kingfish (Seriola lalandi) Larvae. Aquac. Res. 2017, 48, 4420–4428. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z.; Takeuchi, T. Nutritional Components Affecting Skeletal Development in Fish Larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of Nutrients in Skeletal Metabolism and Pathology in Fish—An Overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Sawada, Y.; Sasaki, T.; Nishio, K.; Kurata, M.; Honryo, T.; Agawa, Y. Positive Phototaxis as the Cause of Jaw Malformations in Larval Greater Amberjack, Seriola dumerili (Risso, 1810): Mitigation by Rearing in Tanks with Low-Brightness Walls. Aquac. Res. 2020, 51, 2261–2274. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Cahu, C.L.; Zambonino-Infante, J.L. Overview of Vitamin D and C Requirements in Fish and Their Influence on the Skeletal System. Aquaculture 2011, 315, 49–60. [Google Scholar] [CrossRef]
- Fernández, I.; Gisbert, E. The Effect of Vitamin A on Flatfish Development and Skeletogenesis: A Review. Aquaculture 2011, 315, 34–48. [Google Scholar] [CrossRef]
- Negm, R.K.; Cobcroft, J.M.; Brown, M.R.; Nowak, B.F.; Battaglene, S.C. Performance and Skeletal Abnormality of Striped Trumpeter Latris Lineata Larvae and Post Larvae Fed Vitamin A Enriched Artemia. Aquaculture 2014, 422, 115–123. [Google Scholar] [CrossRef]
- Dâmaso-Rodrigues, M.L.; Pousao-Ferreira, P.; Ribeiro, L.; Coutinho, J.; Bandarra, N.M.; Gavaia, P.J.; Narciso, L.; Morais, S. Lack of Essential Fatty Acids in Live Feed during Larval and Post-Larval Rearing: Effect on the Performance of Juvenile Solea senegalensis. Aquac. Int. 2010, 18, 741–757. [Google Scholar] [CrossRef]
- Gapasin, R.S.J.; Duray, M.N. Effects of DHA-Enriched Live Food on Growth, Survival and Incidence of Opercular Deformities in Milkfish (Chanos chanos). Aquaculture 2001, 193, 49–63. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the Nutritional Demand for Essential Fatty Acids by Aquaculture Species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The Role of Phospholipids in Nutrition and Metabolism of Teleost Fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Bessonart, M.; Izquierdo, M.S.; Salhi, M.; Hernandez-Cruz, C.M.; Gonzalez, M.M.; Fernandez-Palacios, H. Effect of Dietary Arachidonic Acid Levels on Growth and Survival of Gilthead Sea Bream (Sparus aurata L.) Larvae. Aquaculture 1999, 179, 265–275. [Google Scholar] [CrossRef]
- Dantagnan, P.; Hernández, A.; Borquez, A.; Mansilla, A. Inclusion of Macroalgae Meal (Macrocystis pyrifera) as Feed Ingredient for Rainbow Trout (Oncorhynchus mykiss): Effect on Flesh Fatty Acid Composition. Aquac. Res. 2009, 41, 87–94. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Socorro, J.; Arantzamendi, L.; Hernández-Cruz, C.M. Recent Advances in Lipid Nutrition in Fish Larvae. Fish Physiol. Biochem. 2000, 22, 97–107. [Google Scholar] [CrossRef]
- Koven, W.; Tandler, A.; Kissil, G.W.; Sklan, D. The Importance of N-3 Highly Unsaturated Fatty Acids for Growth in Larval Sparus Aurata and Their Effect on Survival, Lipid Composition and Size Distribution. Aquaculture 1992, 104, 91–104. [Google Scholar] [CrossRef]
- Koven, W.M.; Tandler, A.; Kissil, G.W.; Sklan, D.; Friezlander, O.; Harel, M. The Effect of Dietary (N−3) Polyunsaturated Fatty Acids on Growth, Survival and Swim Bladder Development in Sparus aurata Larvae. Aquaculture 1990, 91, 131–141. [Google Scholar] [CrossRef]
- Roo, F.J.; Hernández-Cruz, C.M.; Socorro, J.A.; Fernández-Palacios, H.; Montero, D.; Izquierdo, M.S. Effect of DHA Content in Rotifers on the Occurrence of Skeletal Deformities in Red Porgy Pagrus pagrus (Linnaeus, 1758). Aquaculture 2009, 287, 84–93. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Scolamacchia, M.; Betancor, M.; Roo, J.; Caballero, M.J.; Terova, G.; Witten, P.E. Effects of Dietary DHA and α-Tocopherol on Bone Development, Early Mineralisation and Oxidative Stress in Sparus aurata (Linnaeus, 1758) Larvae. Br. J. Nutr. 2013, 109, 1796–1805. [Google Scholar] [CrossRef]
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. Lipid Nutrition of Marine Fish during Early Development: Current Status and Future Directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Dupin, E. Multipotentiality of the Neural Crest. Curr. Opin. Genet. Dev. 2003, 13, 529–536. [Google Scholar] [CrossRef]
- Abi-ayad, S.M.E.A.; Melard, C.; Kestemont, P. Effects of N-3 Fatty Acids in Eurasian Perch Broodstock Diet on Egg Fatty Acid Composition and Larvae Stress Resistance. Aquac. Int. 1997, 5, 161–168. [Google Scholar]
- Li, Y.; Chen, W.; Sun, Z.; Chen, J.; Wu, K. Effects of N-3 HUFA Content in Broodstock Diet on Spawning Performance and Fatty Acid Composition of Eggs and Larvae in Plectorhynchus cinctus. Aquaculture 2005, 245, 263–272. [Google Scholar] [CrossRef]
- Vassallo-Agius, R.; Watanabe, T.; Yoshizaki, G.; Satoh, S.; Takeuchi, Y. Quality of Eggs and Spermatozoa of Rainbow Trout Fed an N-3 Essential Fatty Aciddeficient Diet and Its Effects on the Lipid and Fatty Acid Components of Eggs, Semen and Livers. Fish. Sci. 2001, 67, 818–827. [Google Scholar] [CrossRef]
- Villeneuve, L.; Gisbert, E.; Zambonino-Infante, J.L.; Quazuguel, P.; Cahu, C.L. Effect of Nature of Dietary Lipids on European Sea Bass Morphogenesis: Implication of Retinoid Receptors. Br. J. Nutr. 2005, 94, 877–884. [Google Scholar] [CrossRef]
- Dettleff, P.; Hernandez, E.; Partridge, G.; Lafarga-De la Cruz, F.; Martinez, V. Understanding the Population Structure and Reproductive Behavior of Hatchery-Produced Yellowtail Kingfish (Seriola lalandi). Aquaculture 2020, 522, 734948. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Taylor, W.R.; Van Dyke, G.C. Revised Procedures for Staining and Clearing Small Fishes and other Vertebrates for Bone and Cartilage Study. Cybium 1985, 9, 107–119. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride--Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Patel, A.; Dettleff, P.; Hernandez, E.; Martinez, V. A Comprehensive Transcriptome of Early Development in Yellowtail Kingfish (Seriola lalandi). Mol. Ecol. Resour. 2016, 16, 364–376. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Moran, D.; Smith, C.K.; Gara, B.; Poortenaar, C.W. Reproductive Behaviour and Early Development in Yellowtail Kingfish (Seriola lalandi Valenciennes 1833). Aquaculture 2007, 262, 95–104. [Google Scholar] [CrossRef]
- Roo, J.; Hernández-Cruz, C.M.; Mesa-Rodriguez, A.; Fernández-Palacios, H.; Izquierdo, M.S. Effect of Increasing N-3 HUFA Content in Enriched Artemia on Growth, Survival and Skeleton Anomalies Occurrence of Greater Amberjack Seriola dumerili Larvae. Aquaculture 2019, 500, 651–659. [Google Scholar] [CrossRef]
- Mesa-Rodriguez, A.; Hernández-Cruz, C.M.; Betancor, M.B.; Fernández-Palacios, H.; Izquierdo, M.S.; Roo, J. Effect of Increasing Docosahexaenoic Acid Content in Weaning Diets on Survival, Growth and Skeletal Anomalies of Longfin Yellowtail (Seriola rivoliana, Valenciennes 1833). Aquac. Res. 2018, 49, 1200–1209. [Google Scholar] [CrossRef]
- Hilton, Z.; Poortenaar, C.W.; Sewell, M.A. Lipid and Protein Utilisation during Early Development of Yellowtail Kingfish (Seriola lalandi). Mar. Biol. 2008, 154, 855–865. [Google Scholar] [CrossRef]
- Chen, B.N.; Qin, J.G.; Kumar, M.S.; Hutchinson, W.G.; Clarke, S.M. Ontogenetic Development of Digestive Enzymes in Yellowtail Kingfish Seriola Lalandi Larvae. Aquaculture 2006, 260, 264–271. [Google Scholar] [CrossRef]
- Olsen, Y.; Evjemo, J.O.; Kjørsvik, E.; Larssen, H.; Li, K.; Overrein, I.; Rainuzzo, J. DHA Content in Dietary Phospholipids Affects DHA Content in Phospholipids of Cod Larvae and Larval Performance. Aquaculture 2014, 428–429, 203–214. [Google Scholar] [CrossRef]




| Feeding Scheme of Spawning Period | ||||||
|---|---|---|---|---|---|---|
| July | August | September | October | November | December | |
| Broodstock food (%) * | ||||||
| Diet A | 100 | 50 | 71.8 | 50 | 13.2 | 0 |
| Diet B | 0 | 0 | 0 | 0 | 40.8 | 0 |
| Commercial pellet | 0 | 0 | 0 | 0 | 46 | 100 |
| Fresh fish | 0 | 50 | 28.2 | 50 | 0 | 0 |
| Nutrients supplied ** | ||||||
| Protein | 33.2 | 25.9 | 29.1 | 25.9 | 37.3 | 44.4 |
| Lipid | 4.7 | 3.3 | 3.9 | 3.3 | 12.7 | 23.3 |
| Ash | 4.1 | 3.8 | 4.0 | 3.8 | 5.4 | 8.2 |
| Spawnings analyzed | 3 | 3 | 4 | |||
| Diet A | Diet B | Fish | Commercial Pellet | |
|---|---|---|---|---|
| Proximal (%) | ||||
| Dry matter | 54 | 47 | 24.5 | 91.8 |
| Protein | 61.52 | 65.28 | 18.54 | 48.21 |
| Lipid | 8.71 | 7.28 | 1.82 | 25.36 |
| Ash | 7.62 | 5.76 | 3.57 | 8.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, C.; Rubilar, E.; Marchant, L.; Dörner, J.; Márquez, L.; Martínez, V.; Dantagnan, P. Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery. Fishes 2023, 8, 200. https://doi.org/10.3390/fishes8040200
Toledo C, Rubilar E, Marchant L, Dörner J, Márquez L, Martínez V, Dantagnan P. Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery. Fishes. 2023; 8(4):200. https://doi.org/10.3390/fishes8040200
Chicago/Turabian StyleToledo, Cesar, Eduardo Rubilar, Lorena Marchant, Jessica Dörner, Lorenzo Márquez, Víctor Martínez, and Patricio Dantagnan. 2023. "Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery" Fishes 8, no. 4: 200. https://doi.org/10.3390/fishes8040200
APA StyleToledo, C., Rubilar, E., Marchant, L., Dörner, J., Márquez, L., Martínez, V., & Dantagnan, P. (2023). Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery. Fishes, 8(4), 200. https://doi.org/10.3390/fishes8040200






