Abstract
Previous studies have shown that Nannochloropsis gaditana can partially replace fishmeal in the diet of gilthead seabream, Sparus aurata. However, its effect on muscle growth is hardly known. This experiment was carried out with gilthead seabream adults that were fed with N. gaditana at two inclusion levels (2.5 or 5%) either raw (R2.5 and R5 groups) or cellulose-hydrolyzed (H2.5 and H5 groups) for 45 days in the final fattening phase. The body length and body weight were measured in all fish at the beginning and end of the experiment. Also, the white muscle transverse area (WM), size, number and fibrillar density of the white fibers were measured in 9 fish group−1. After 45 days, the body parameters and the WM did not show significant differences among the groups. However, muscle cellularity did show significant differences, such that the hypertrophy values were higher in the H2.5 and H5 than in the R2.5 and R5 groups. On the contrary, R2.5 and R5 showed the highest fibrillar density and hyperplasia values, which are often positively correlated with the fillet firmness and therefore could improve the final quality of the fish. No significant differences attributable to the inclusion levels of N. gaditana were observed.
Key Contribution:
The muscle cellularity was significantly influenced by the feeding regimes, with the highest muscle fibrillar density in R2.5 and R5. This seems to indicate that, at low inclusion levels in the diet, the enzymatic hydrolysis of N. gaditana is not necessary for the generation of white muscle fibers in gilthead seabream adults.
1. Introduction
Gilthead seabream, Sparus aurata L., is a fast-growing carnivorous species of great commercial value in Mediterranean aquaculture. Their diet has traditionally been based on feed containing mainly fishmeal and fish oil, which entails some problems such as high cost or conservation difficulties, amongst others. Therefore, the industry needs to reduce the percentage of fishmeal, which is usually replaced by vegetable products [1]. However, the vegetal products also present a series of drawbacks such as an unbalanced composition of amino acids, deficiency of polyunsaturated fatty acids, etc. [1,2].
Currently, there is a growing interest in cultivating microalgae for aquaculture feed, as it produces optimal results in growth and survival and reduces the dependence on fishmeal and fish oils [1,3]. They are selected accordingly with criteria such as the absence of toxic compounds, nutritional values, and digestibility [2].
Several studies have shown that some species of microalgae are able to replace fishmeal in the range of 6 to 20%. In that sense, Phaedactylum tricornutum or a combination of Nannochloropsis sp. and Isochrysis sp. in Atlantic salmon, Salmo salar [4]; Tetraselmis suecica or Isochrysis sp. in European sea bass, Dicentrarchus labrax L. [5,6]; and Scenedesmus almeriensis, Tetraselmis suecia and Isochrysis lutea in gilthead seabream and Senegalese sole [7,8] have been used. Microalgae are also currently presented as an alternative to fish oil, since they can synthesize DHA (docosahexaenoic acid), EPA (eicosapentaenoic acid) or both. Some of the microalgae that are rich in these fatty acids are Schizochytrium sp., Nannochlorepsis sp., Crypthecodinium cohnii and Chlorella sp. The oils from these microalgae have replaced fish oil without negative effects on the growth of salmon [9]. Similarly, Schizochytrium sp. or Crypthecodinium cohnii have shown similar effects to fish oil on the growth and survival of gilthead seabream larvae [10,11,12]. In sea bass, some authors [13] added Isochrysis galbana to the larval diet for 15 days and found that the larval survival improved, and it was related to the development of the ciliated edge of the intestine membranes. Likewise, the presence of Isochrysis galbana triggered the production of digestive enzymes. In gilthead seabream juveniles, the effect of replacing fishmeal protein by Chlorella vulgaris meal and fish oil by a blend of Schizochytrium sp. and Microchloropsis gaditana on growth, feed utilization, muscle fatty acid composition and liver histology for 12 weeks was evaluated [14]. The authors found that the blend of dried biomasses of Schizochytrium sp. and M. gaditana could totally replace dietary fish oil in fishmeal-based diets formulated with soybean oil, without negatively affecting the growth performance, feed efficiency, proximate composition and muscle EPA and DHA levels of gilthead seabream juveniles. Moreover, replacing fishmeal protein by C. vulgaris meal up to 30% did not adversely affect the growth performance and feed utilization of the studied specimens. In red sea bream juveniles, Pagrus major, the nutritional efficiency of Nannochloropsis, Chlorella and Schizochytrium was analyzed [15]. The experimental diets were compared with a control diet without fish meal as protein, which was replaced by soy and corn meal. The control diet did contain fish oil. The results of this study showed that the fish that were fed with Nannochloropsis and Schizochytrium obtained a/good growth performance and a considerable composition of fatty acids, without observing adverse effects. In meagre juveniles (Argyrosomus regius), four experimental diets were evaluated for 30 days [16]: a control diet was based on 5% fish oil and 7% of rapeseed oil, whereas in the other three diets, fish oil was totally replaced by either poultry oil only, or blending poultry oil and one of two commercial algal oils extracted from Schizochytrium sp. Their results showed the good potential of blending these two microalgae oils to completely replace fish oil cost-effectively in diets for meagre juveniles.
Growth muscle characteristics in fish are influenced by genetic factors but also by feeding regime and environmental conditions which in turn affect the number and size distribution of the muscle fibers [17,18,19,20,21] and the adipocytes [22] that are present in the fillet. Even though studies on the effect of dietary microalgae on fish muscle growth are still scarce, the influence of Nannochloropsis oceanica in the diet on the muscle cellularity of spotted wolffish (Anarhichas minor) muscle cellularity was analyzed by some authors [23] and no differences were found in white muscle growth or fibrillar constitution between feeding groups. In 2020, our research team [24] evaluated the short-term effect of Nannochloropsis gaditana included in the diet on gilthead seabream juveniles and observed a positive effect on muscle growth. Subsequently, a long-term effect was also observed in the muscle growth of gilthead seabream adults that had been previously fed with N. gaditana during their juvenile phase [25]. Recently, the effect of finishing enriched diets with N. gaditana on the final quality of sea bream fillet was also studied [26]. Their results indicated that microalgae-enriched diets yielded favorable, dose-dependent effects on several objective quality parameters of fillets. However, the muscle cellularity was not studied in the cited work. For this reason, in the present work we have studied the muscle cellularity of gilthead seabream adults from the same population as that studied in the cited work [26] to correlate the results from both studies.
2. Materials and Methods
2.1. Rearing Conditions
This research was carried out on adult specimens (two years old) of gilthead seabream (Sparus aurata) obtained from a broodstock breed at the Centro Oceanográfico de Murcia-Instituto Español de Oceanografía (COMU-IEO), CSIC. The feeding trial was set up with 225 fish. The initial body mean values ± SEM were 450 ± 28 g (body weight) and 30 ± 0.7 cm (total body length). The specimens were randomly classified in five feeding regime groups (45 fish group−1; 3 tanks group−1 of 2000 L capacity): four groups were fed with different experimental diets containing the microalgae Nannochloropsis gaditana and a control group was fed a microalgae-free diet (Table 1). The experiment was carried out on the final fattening phase of gilthead seabream culture and it lasted 45 days.

Table 1.
Ingredient composition and proximal analysis of the experimental diets that have been used in the present work. C: control diet. R2.5 and R5: diets including 25 and 50 g kg−1 raw microalgal biomass, respectively. H2.5 and H5: diets including 25 and 50 g kg−1 hydrolyzed microalgae, respectively. Data are expressed in g kg−1 dry matter.
The rearing conditions were the same as those described by Sáez et al. [26] since the population of the present work is the same as that used by the cited authors. Fish were kept under a 12L:12D photoperiod and natural temperature, which increased gradually from 17 °C to 21 °C through the experiment. The initial stock density was 3.4 kg m−3 and the sea water renewal rate was kept at 780 L h−1 in an open flow circuit. The salinity was 37 ‰ and the light intensity ranged from 50 to 70 lux. Tanks were equipped with aerators to maintain an adequate level of oxygenation (above 6 mg L−1). The values of ammonia and nitrites were <0.1 mg L−1.
2.2. Experimental Diets
The compositions of the diets of the present study (Table 1) are the same as those used in previous studies on this species [24,25,26]. Briefly, these diets contained the microalgae Nannochloropsis gaditana at two inclusion levels (2.5 or 5%) either raw (R2.5 and R5 groups) or cellulose-hydrolyzed (H2.5 and H5 groups), the latter in order to increase the bioavailability of the cellular inner components since the thick cell walls of Nannochloropsis can hinder the digestion of microalgae and absorption of cell internal nutrients [1].
Also, a microalgae-free group (C group) was studied as the control group. Diets were formulated and manufactured at the CEIA3-Universidad de Almería facilities (Servicio de Piensos Experimentales, http://www.ual.es/stecnicos_spe, accessed on 3 February 2022) (Almeria, Spain) using standard aquafeed extrusion processing procedures. Nannochloropsis gaditana biomass was obtained from EU-H2020 SABANA facilities of the Universidad de Almería (Spain). A commercial cellulase (22178, Sigma-Aldrich, Madrid, Spain) was used for the enzymatic hydrolysis by mixing N. gaditana meal at a final concentration of 150 g dry weight L−1 in 50 mM sodium citrate buffer solution (pH 5.5) and then incubated at 45 °C under continuous agitation for 5 h as described in other work in this species [27]. The experimental diets were offered ad libitum three times a day (9:00, 14:00 and 19:00) until a maximum of 1.2% of the tank biomass. The amount of feed ingested was daily recorded in each tank to calculate the feed conversion ratios (total feed being consumed/weight gain) in each group. The final survival percentage was also calculated on all tanks.
2.3. Sampling
Body length and body weight were measured in all specimens (225 fish) at the beginning and at the end of the experiment. To do this, fish were anesthetized with 40 µL L−1 of clove oil in sea water and then individually recorded. At the end of the experiment, nine specimens from each group were slaughtered by overdose of anesthesia (60 µL L−1 of clove oil in sea water) and then transported on ice to the Faculty of Veterinary of the University of Murcia for analysis of muscle parameters.
2.4. Analysis of Muscle Growth
For muscle analysis, nine specimens per group were transversely cut to the long body axis and then the whole cross-muscle section from each fish was photographed for further morphometric analysis (Sygma-Scan Pro_5 system, Systat Version 5.0 Inc., San Jose, CA, USA). Subsequently, 5 mm-thick whole-body slices were obtained and cut into smaller blocks that were frozen in 2-methylbutane over liquid nitrogen. Sections of 8 μm thickness were obtained from those frozen blocks in a cryostat (Leyca CM 1850, Leica Microsistemas SLU, Barcelona, Spain). These sections were stained with hematoxylin –eosin for performing morphometric studies of the muscle under light microscope.
The following muscle parameters were measured by means of the morphometric analysis cited above: total cross-sectional area of the white muscle, number of white muscles fibers, area and minor axis length of white muscle fibers and muscle fibers density (number of white fibers μm−2). The average size of the white muscle fibers of each specimen was estimated from ~600 fibers (±10 SD) located at the intermediate and the apical sectors of the epaxial quadrant of the transversal section of the myotome, according to the methodology described in previous studies in teleosts [28,29].
2.5. Statistical Analysis
The statistical package SPSS 28.0.1.1. (IBM, New York, NY, USA) was used for the statistical analysis. The data distribution and the homogeneity of variances were analyzed by the Shapiro–Wilk and the Levene’s tests, respectively, for p < 0.05. For most of the parameters, both tests showed values of p > 0.05. Hence, the analysis of variance (ANOVA) and a post-hoc Tukey test were used, for p < 0.05. In the cases with values of p < 0.05 in the Shapiro–Wilk and the Levene’s tests, nonparametric tests (U of Mann–Whitney and Z of Kolmogorov–Smirnov tests) were used. All of the data were expressed as the mean ± standard error of the mean (SEM). The distribution of fibrillar sizes of each group was also studied through the analysis of the different percentiles of the specimens (9 specimens were analyzed in each group and ~600 fibers ± 10 SD were measured in each specimen).
3. Results
3.1. Body Growth and Survival
At the beginning of the experiment, the mean ± SEM body values of the fish were 30 ± 0.7 cm (body length) and 450 ± 28 g (body weight) without significant differences among the five groups. After 45 days (1.5 months) of the experiment, the results showed some differences between groups although none of them were statistically significant (Table 2). The results found are described below.

Table 2.
Body growth parameters of adult specimens of Sparus aurata at the end of the experiment (commercial stage) in the five experimental groups: C (microalgae-free diet); R2.5 and R5 (supplemented diet with 2.5% and 5% raw N. gaditana, respectively); H2.5 and H5 (supplemented diet with 2.5% and 5% hydrolyzed N. gaditana with cellulases, respectively).
- R2.5 versus R5 groups: When comparing raw diets of N. gaditana at different concentrations, the body values were similar in both groups (p > 0.05) (Table 2);
- R2.5 versus H2.5 groups: When comparing diets at 2.5% of raw versus hydrolyzed N. gaditana, the body weight showed similar values in both groups (p > 0.05) (Table 2);
- H2.5 versus H5 groups: When comparing hydrolyzed diets at different concentrations, the highest values of the body weight were reached at highest concentration, even though it was not significant (p > 0.05) (Table 2);
- R5 versus H5 groups: H5 showed the highest values of the body weight, even though it was not significant (p > 0.05) (Table 2);
- C group: The lowest body weight values were observed in this group, but it was not significant (p > 0.05) (Table 2).
The conversion rates values (mean ± SEM) in C, R2.5, R5, H2.5 and H5 were 3.0 ± 0.09, 3.0 ± 0.25, 2.7 ± 0.33, 3.10 ± 0.26 and 2.5 ± 0.25, respectively. However, these differences were not significant (p > 0.05).
The survival rates were 100% in all experimental groups at the end of the experiment.
3.2. Muscle Growth
A morphological mosaic of fibrillar sizes typical of teleost adults was observed in the transverse section of the white muscle of all specimens, with small fibers (new generation of white fibers) interspersed among large fibers (mature white fibers) (Figure 1).
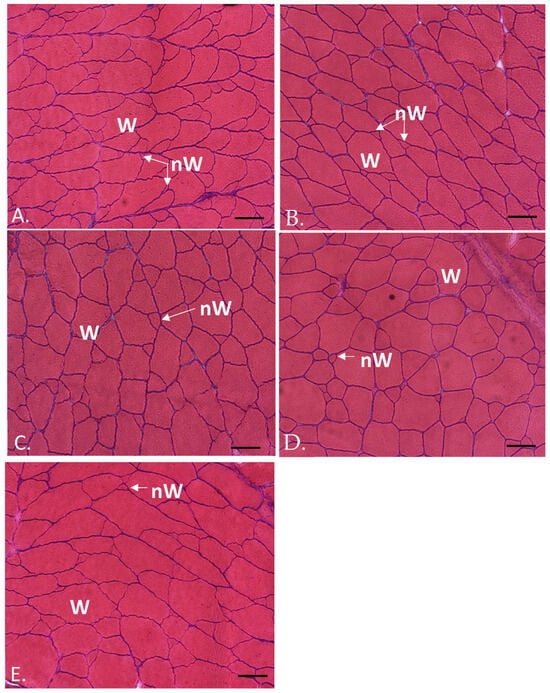
Figure 1.
Transverse sections of the white muscle of two-year-old gilthead sea breams, from R2.5 (A), R5 (B), H2.5 (C), H5 (D) and C (E) groups. Hematoxylin–eosin staining. W: white fibers; nW: new white fibers. Bars 100 µm.
Interestingly, the results of muscle parameters showed some differences between the different experimental groups, as detailed below:
R2.5 versus R5 groups: the white muscle transverse area values, as well as the hypertrophy, hyperplasia and white muscle fibers density, did not show significant differences between the R2.5 and R5 groups (p > 0.05) (Table 3; Figure 1A,B and Figure 2). The percentiles of fibrillar size distribution were also similar in both groups (Table 4).

Table 3.
Muscle growth parameters of adult specimens of Sparus aurata from the C, R2.5, R5, H2.5 and H5 groups at the end of the experiment (commercial stage).
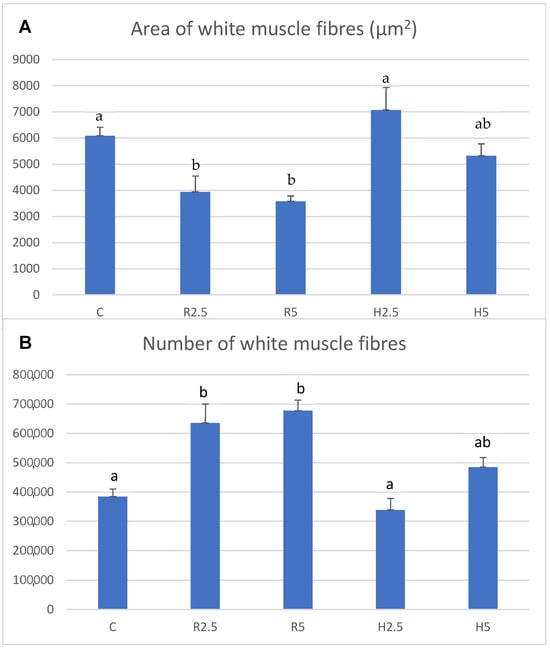
Figure 2.
Mean ± SEM values of the area of white muscle fibers (A) and number of white muscle fibers (B) of two-year-old specimens of Sparus aurata from the C, R2.5, R5, H2.5 and H5 groups at the end of the experiment (commercial stage). Different lower-case letters indicate significant differences (p < 0.05) between groups for each parameter. Mean values were obtained from nine specimens per group.

Table 4.
Fibrillar size percentiles of the white muscle fibers from C, R2.5, R5, H2.5 and H5 groups at commercial size.
R2.5 versus H2.5 groups: the white muscle transverse area values were similar in the R2.5 and H2.5 groups (p > 0.05). However, the hyperplasia and muscle fibers density values were significantly higher (p < 0.05) in R2.5 than in H2.5, whereas the hypertrophy values were significantly higher in H2.5 than in R2.5 (Table 3; Figure 1A,C and Figure 2). When comparing the percentiles of both groups, we observed that the percentage of small fibers was higher in R2.5 than in H2.5 (Table 4). Thus, the 5th and 40th percentiles of R2.5 were 7.75 and 29.82, respectively, while both percentiles were 36.38 and 3452.8, respectively, in H2.5 (Table 4).
H2.5 versus H5 groups: The white muscle transverse area values were higher in H5 than in H2.5, but it was not significant (p > 0.05). The hypertrophy values were higher in H2.5 than in H5, whereas the hyperplasia and muscle fibers density were higher in H5 than in H2.5 (Table 3; Figure 1C,D and Figure 2). However, these differences were not significant (p > 0.05). When comparing the percentiles of both groups, we observed that the percentage of small fibers was higher in H5 than in H2.5 (Table 4). Thus, the 5th and 40th percentiles of H5 were 10.7 and 258.25, respectively, while both percentiles were higher in H2.5 than in H5 (Table 4).
R5 versus H5 groups: The white muscle transverse area values were higher in H5 than in R5, but it was not significant (p > 0.05). Muscle cellularity showed higher hypertrophy values in H5 than in R5, whereas the hyperplasia and muscle fibers density values were higher in R5 (Table 3; Figure 1B,D and Figure 2). However, these differences were not significant (p > 0.05). When comparing the 5th and 40th percentiles of both groups, we observed that the 5th percentile was similar between both groups, although slightly lower in R5 than in H5 (Table 4). For its part, the 40th percentile was higher in H5 than in R5 (Table 4).
C group: The white muscle transverse area showed the lowest values in this group, even though it was not significant (p > 0.05) (Table 3). When comparing to the other groups, the hypertrophy was significantly higher in this group than in R2.5 and R5 (p < 0.05), whereas the hyperplasia showed the contrary tendency (Table 3; Figure 1E and Figure 2). When comparing to H2.5 and H5, both parameters (hypertrophy and hyperplasia) of the C group showed intermediate values to those found in H2.5 and H5, although it was not significant (p > 0.05) (Figure 2). The 5th percentile of the control group showed higher values than the other groups (Table 4).
4. Discussion
The main edible component of the fish is the fast myotome muscle. The number and size distribution of fibers (muscle cellularity) of fish vary with physiological stages and show a high plasticity dependent on intrinsic and extrinsic factors [18]. The muscle growth pattern influences the flesh quality and hence, the different rearing conditions (feeding regime, environmental factors, etc.) produce differences in the final quality of fish flesh [17,19].
The studies about the influence of dietary intake of microalgae on fish muscle growth are scarce. Knutsen et al. [23] studied the muscle growth in spotted wolffish juveniles (Anarhichas minor) that were fed with or without Nannochloropsis oceanica in the diet and found no significant differences in muscle growth between groups. Recently, our research team studied the effect of diets that were like those used in the present study. However, the cited study was carried out in the juvenile phase of gilthead sea bream [24], while the present work has been carried out during the final fattening phase of this species. The results found by Ayala et al. [24] demonstrated that after 90 days of being fed with the experimental diets, juvenile specimens fed with control and R5 diets reached the greatest growth. After the juvenile phase, once the experiment of Ayala et al. [24] ended, all the groups were transferred to a standard commercial diet up to commercial size, to study the long-term effect of the experimental juvenile diets. The results showed a long-term influence of the juvenile diets, as the highest growth was reached in adult specimens previously fed with the H5 diet during the juvenile phase [25].
However, up to now, the short-term effect of diets enriched with N. gaditana on the muscle growth of gilthead seabream adults in the final fattening phase has not been studied. Hence, the present work studies the effect of supplemented diets with N. gaditana for 1.5 months (45 days) at the final fattening phase of S. aurata. The composition of the diets and the rearing conditions were the same as those described by Ayala et al. [24,25] and Sáez et al. [26]. The results of the present work showed no significant differences in the body growth or the total transverse area of the white muscle among the different feeding groups, even though the highest values of both parameters were observed in H5. Similarly, the conversion rates values did not show significant differences among the different groups, even though the most optimal values were observed in H5.
Probably, it would be necessary to increase the feeding time with the different diets to achieve a significant effect on the growth and conversion rates of adult fish. However, muscle cellularity did show significant differences between the different feeding regimes, such that the hypertrophy was higher in the groups that were fed with hydrolyzed microalgae diets than those fed with the raw microalgae diets, whereas the hyperplasia showed the opposite trend, being higher in R2.5 and R5 than in the other groups.
The results of the hypertrophy and hyperplasia of the gilthead seabream adults of the present experiment were different to those obtained on the previous studies in this species [24,25]. Firstly, during the feeding trial of gilthead seabream juveniles, Ayala et al. [24] did not find significant differences in the muscle cellularity of the different feeding regimes groups. Secondly, when studying the long-term effect of the juvenile diets on the subsequent adult phase of gilthead seabream specimens, the diet containing prehydrolysis biomass of N. gaditana produced a positive long-term effect on the hyperplasia and fibrillar density [25]. In contrast, in the present work in which adult specimens were fed with the same diets as in the previous studies, the enzymatic hydrolysis of microalgae seemed not be necessary for the generation of fibers, as the greatest hyperplasia of white muscle fibers of the present study was observed in the R2.5 and R5 groups. These results show that, while the cellulose-hydrolysis of N. gaditana can improve the white muscle hyperplasia of seabream juveniles in long-term [21], the cellulose-hydrolysis of N. gaditana appears not to be necessary for the generation of white muscle fibers of seabream adults. This seems to indicate that the digestive tract of sea bream adults can assimilate N. gaditana in a raw state, without the need to carry out its prior hydrolysis, at least at the low inclusion levels that have been used in the present experiment (2.5 and 5%). The expression and regulation of hypertrophic and hyperplastic growth mechanisms of the musculature vary with age, genetic factors, biological cycles and environmental conditions [30,31,32]. Normally, hyperplasia predominates in juvenile and fast growth phases, while the fiber generation gradually ceases and hypertrophic growth predominates in adult phases [32,33]. However, our data showed a high plasticity of the gilthead seabream muscle of adult specimens, as has also been observed in other fish species [20,21,28,29,34]. Thus, a significant influence of enriched diets with N. gaditana was observed on the muscle cellularity of the adult specimens of the present experiment with an increase in hyperplasia in adult fish fed with R2.5 and R5 diets. Interestingly, the different muscle cellularity can influence the quality of the fillet [29,35,36,37]. Sáez et al. [26] analyzed the quality parameters of adult gilthead seabream specimens fed during the final fattening phase with the same experimental diet as those described in the present work and found the highest fillet hardness values in the R5 group. This result is correlated with the highest hyperplasia and muscle fibers density found in the R5 group of the present work. The positive correlation between muscle fibers density and fillet firmness had been demonstrated in other studies [29,35,36,37] and represents an improvement in the final quality of the product and consumer acceptance. On the contrary, the lowest fillet hardness values were observed in C and H2.5 [26], which showed the lowest hyperplasia and muscle fibers density values in the present work.
Therefore, our data suggest that short periods of time (45 days) with diets that include raw N. gaditana in the final phase of fattening are sufficient to improve muscle cellularity and, in turn, flesh quality. This is an important issue from the aquaculture sector point of view that could use these diets as a way of improving flesh quality without heavy rises in the production cost.
5. Conclusions
- Enriched diets with N. gaditana did not significantly influence body growth (length and weight) or the transverse area of the white muscle in the final phase of fattening of gilthead seabream specimens.
- The form of N. gaditana (raw versus hydrolyzed) in the diet significantly influenced the muscle cellularity of gilthead seabream adults at commercial size, in such a way that the hypertrophy was higher in fish fed with hydrolyzed microalgae than in fish fed with raw microalgae.
- The enzymatic treatment of N. gaditana did not enhance the generation of new fibers, so that the greatest hyperplasia was reached by the fish fed with raw microalgae and it was correlated with the highest fillet hardness values previously found by other authors in these specimens [26].
- No significant differences attributable to the concentration levels of N. gaditana were observed. This result, together with the fact that the greatest generation of fibers was found in the diets of raw N. gaditana, allows us to conclude that the R2.5 diet could be the most optimal for this phase in sea bream.
- The short-term effect of the microalgae-enriched diets used in the final phase of fattening of sea breams of this study should be considered by farmers to optimize culture and to improve the quality of fillet meat.
Author Contributions
M.D.A., E.C.-P., M.A., T.F.M. and F.J.A. conceived and designed the experiments. Also, all of them carried out the interpretation and discussion of the data; F.J.A., A.G., T.F.M. and M.I.S. prepared the aquafeeds.; M.A. and E.C.-P. reared the fish; M.A. and E.C.-P. performed the diet trial and the samplings. The body measurements were carried out by M.A. and E.C.-P. The fibrillar analysis and the statistical analysis were carried out by M.D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded through the research projects of MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”, (grants RTI2018-096625-B-C33 and RTI2018-096625-B-C31), and AquaTech4Feed (grant PCI2020-112204) granted by AEI within the ERA-NET BioBlue COFUND.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Committee on the Ethics of Animal Experiments of the IEO (REGA: ES300261040017) and the Ministry of Water, Agriculture and Environment of the Autonomous Community Region of Murcia (Spain; A13200101).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
This work has been carried out thanks to the collaboration between the Centro Oceanográfico de Murcia, Instituto Español de Oceanografía (COMU-IEO), CSIC, the University of Almería and the University of Murcia. The authors thank the project SABANA (grant # 727874) from the European Union’s Horizon 2020 Research and Innovation Program for the provision of N. gaditana biomass.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, F.; Leng, Y.; Lu, Q. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Ponis, E.; Robert, R.; Parisi, G. Nutritional value of fresh and concentrated algal diets for larval and juvenile Pacific oysters (Crassostrea gigas). Aquaculture 2003, 221, 491–505. [Google Scholar]
- Walker, A.; Berlinsky, D. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N. Am. J. Aquac. 2011, 73, 76–83. [Google Scholar]
- Tulli, F.; Chini Zittelli, G.; Giorgi, G.; Poli, B.M.; Tibaldi, E.; Tredici, M.R. Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization and fillet composition of European sea bass juveniles fed organic diets. J. Aquat. Food Prod. Technol. 2012, 21, 188–197. [Google Scholar] [CrossRef]
- Tibaldi, E.; ChiniZittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freez-dried Isochrysis sp. (T-ISO) biomass as a source protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–66. [Google Scholar]
- Vizcaíno, A.; López, G.; Sáez, M.; Jiménez, J.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.; Cerón-García, M.; Alarcón, F. Effects of the microalga Scenedesmus almerienses as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Saéz, M.I.; López, G.; Arizcun, M.; Abellán, E.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Tetraselmis suecia and Tisochrysis lutea meal as dietary ingredients for gilthead sea bream (Sparus aurata L.) fry. J. Appl. Phycol. 2016, 28, 2843–2855. [Google Scholar]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of fish oil with Thraustochytrid schizochytrium sp. L oil in Atlantic salmon parr (Salmo salar L.) diets. Comp. Biochem. Physiol. A 2007, 148, 382–392. [Google Scholar]
- Atalah, E.; Cruz, C.M.H.; Izquierdo, M.S.; Rosenlund, G.; Caballero, M.J.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for sea bream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar]
- Ganuza, E.; Benítez-Santana, T.; Atalah, E.; Vega-Orellana, O.; Ganga, R.; Izquierdo, M.S. Crypthecodinium cohnii and Schizochytrium sp. as a potential substitutes to fisheries-derived oils from seabream (Sparus aurata) microdiets. Aquaculture 2008, 277, 109–116. [Google Scholar]
- Eryalcin, K.M.; Roo, J.; Saleh, R.; Atalah, E.; Benítez, R.; Betancor, M.; Hernández-Cruz, C.M.; Izquierdo, M.S. Fish oil replacement by different microalgal products in microdiets for early weaning of gilthead sea bream (Sparus aurata, L.). Aquacult. Res. 2013, 44, 819–828. [Google Scholar] [CrossRef]
- Cahu, C.L.; Zambonino Infante, J.L.; Péres, A.; Quazuguel, P.; Le Gall, M.M. Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: Effect on digestive enzymes. Aquaculture 1998, 161, 479–489. [Google Scholar]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar]
- Seong, T.; Uno, Y.; Kitagima, R.; Kabeya, N.; Haga, Y.; Satoh, S. Microalgae as main ingredient for fish feed: Non-fish meal and non-fish oil diet development for red sea bream, Pagrus major, by blending of microalgae Nannochloropsis, Chlorella and Shizochytrium. Aquacult. Res. 2021, 52, 6025–6036. [Google Scholar] [CrossRef]
- Carvalho, M.; Izquierdo, M.; Valdés, M.; Montero, D.; Farías, A. Oils Combination with Microalgal Products as a Strategy for Increasing the N-3 Long-Chain Polyunsaturated Fatty Acid Content in Fish Oil-Free Diets for Meagre (Argyrosomus regius). Aquac. Nutr. 2022, 2022, 5275570. [Google Scholar] [CrossRef]
- Johnston, I.A.; Alderson, R.; Sandham, C.; Dingwall, A.; Mitchell, D.; Selkirk, C.; Nickell, D.; Baker, R.; Robertson, B.; Whyte, D.; et al. Patterns of muscle growth in early and late maturing populations of Atlantic salmon (Salmo salar L.). Aquaculture 2000, 189, 307–333. [Google Scholar]
- Johnston, I.A. Muscle development and growth: Potential implications for flesh quality in fish. Aquaculture 1999, 177, 99–115. [Google Scholar]
- Johnston, I.A.; Manthri, S.; Alderson, R.; Campbell, P.; Mitchell, D.; Whyte, D.; Dingwall, A.; Nickell, D.; Selkirk, C.; Robertson, B. Effects of dietary protein level on muscle cellularity and flesh quality in Atlantic salmon with particular reference to gaping. Aquaculture 2002, 210, 259–283. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Alderson, R.; Smart, A.; Campbell, P.; Nickell, D.; Robertson, B.; Paxton, C.G.; Burt, M.L. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar L.). J. Exp. Biol. 2003, 206, 1337–1351. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Smart, A.; Campbell, P.; Nickell, D.; Alderson, R. Plasticity of muscle fibre number in seawater stages of Atlantic salmon in response to photoperiod manipulation. J. Exp. Biol. 2003, 206, 3425–3435. [Google Scholar] [CrossRef]
- Fauconneau, B.; Andre, S.; Chmaitilly, P.Y.; Krieg, F.; Kaushik, S.J. Control of skeletal muscle fibres and adipose cells in the flesh of rainbow trout. J. Fish Biol. 1997, 50, 296–314. [Google Scholar] [CrossRef]
- Knutsen, H.R.; Johnsen, I.H.; Keizer, S.; Sorensen, M.; Roques, J.A.C.; Heden, I.; Sundell, K. Fish welfare, fast muscle cellularity, fatty acid and body-composition of juvenile spotted wolffish (Anarhichas minor) fed a combination of plant proteins and microalgae (Nannochloropsis oceanica). Aquaculture 2019, 506, 212–223. [Google Scholar] [CrossRef]
- Ayala, M.D.; Galián, C.; Fernández, V.; Chaves-Pozo, E.; García de la Serrana, D.; Sáez, M.I.; Galafat Díaz, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Influence of Low Dietary Inclusion of the Microalga Nannochloropsis gaditana (Lubián 1982) on Performance, Fish Morphology, and Muscle Growth in Juvenile Gilthead Seabream (Sparus aurata). Animals 2020, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Balsalobre, N.; Chaves-Pozo, E.; Sáez, M.I.; Galafat, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Long-term Effects of a short juvenile feeding period with diets enriched with the microalgae Nannochloropsis gaditana on the subsequent body and muscle growth of gilthead seabream, Sparus aurata L. Animals 2023, 13, 482. [Google Scholar] [CrossRef]
- Sáez, M.J.; Galafat, A.; Suárez, M.D.; Chaves-Pozo, E.; Arizcun, M.; Ayala, M.D.; Alarcón, F.J.; Martínez, T.F. Effects of raw and hydrolysed Nannochloropsis gaditana biomass included at low level in finishing diets for gilthead seabream (Sparus aurata) on fillet quality and shelf life. J. Appl. Phycol. 2023, 35, 1163–1181. [Google Scholar] [CrossRef]
- Sáez, M.I.; Galafat, A.; Vizcaíno, A.J.; Chaves-Pozo, E.; Ayala, M.D.; Arizcun, M.; Alarcón, F.J.; Suárez, M.D.; Martínez, T.F. Evaluation of Nannochloropsis gaditana raw and hydrolysed biomass at low inclusion level as dietary functional additive for gilthead seabream (Sparus aurata) juveniles. Aquaculture 2022, 556, 738288. [Google Scholar] [CrossRef]
- Ayala, M.D.; Abellán, E.; Arizcun, M.; García-Alcázar, A.; Navarro, F.; Blanco, A.; López-Albors, A. Muscle development and body growth in larvae and early postlarvae of shi drum, Umbrina cirrosa L., reared under different larval photoperiod. Muscle structural and ultrastructural study. Fish Physiol. Biochem. 2013, 39, 807–827. [Google Scholar] [CrossRef]
- Ayala, M.D.; Arizcun, M.; García-Alcázar, A.; Santaella, M.; Abellán, E. Long-term effects of the larval photoperiod on the subsequent growth of shi drum Umbrina cirrosa L. specimens and the fillet texture at commercial size. Turk. J. Fish Aquat. 2015, 15, 93–101. [Google Scholar]
- Romanello, M.G.; Scapolo, P.A.; Luprano, S.; Mascarello, F. Post-larval growth in the lateral white muscle of the eel, Anguilla anguilla. J. Fish. Biol. 1987, 30, 161–172. [Google Scholar] [CrossRef]
- Scapolo, P.A.; Veggetti, A.; Mascarello, F.; Romanello, M.G. Developmental transitions of myosin isoforms and organisation of the lateral muscle in the teleost Dicentrarchus labrax (L.). Anat. Embryol. 1988, 178, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Veggetti, A.; Mascarello, F.; Scapolo, P.A.; Rowlerson, A. Hyperplastic and hypertrophic growth of lateral muscle in Dicentrarchus labrax (L.): An ultrastructural and morphometric study. Anat. Embryol. 1990, 182, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.J.; Thorpe, J.E. Hyperplasia and hypertrophy in the growth of skeletal muscle in juvenile Atlantic salmon (Salmo salar, L.). J. Fish Biol. 1990, 37, 505–519. [Google Scholar] [CrossRef]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef] [PubMed]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Discriminative characterization of different texture profiles of various cooked fish muscles. J. Food Sci. 1984, 49, 721–726. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Role of muscle fibres in contributing firmness of cooked fish. J. Food Sci. 1990, 55, 693–696. [Google Scholar] [CrossRef]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).