Successful Cryopreservation of Spermatogonia Stem Cells of Neotropical Catfish (Rhamdia quelen) and Enriched Germ Cell Transplantation into Common Carp (Cyprinus carpio) Testes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Maintenance
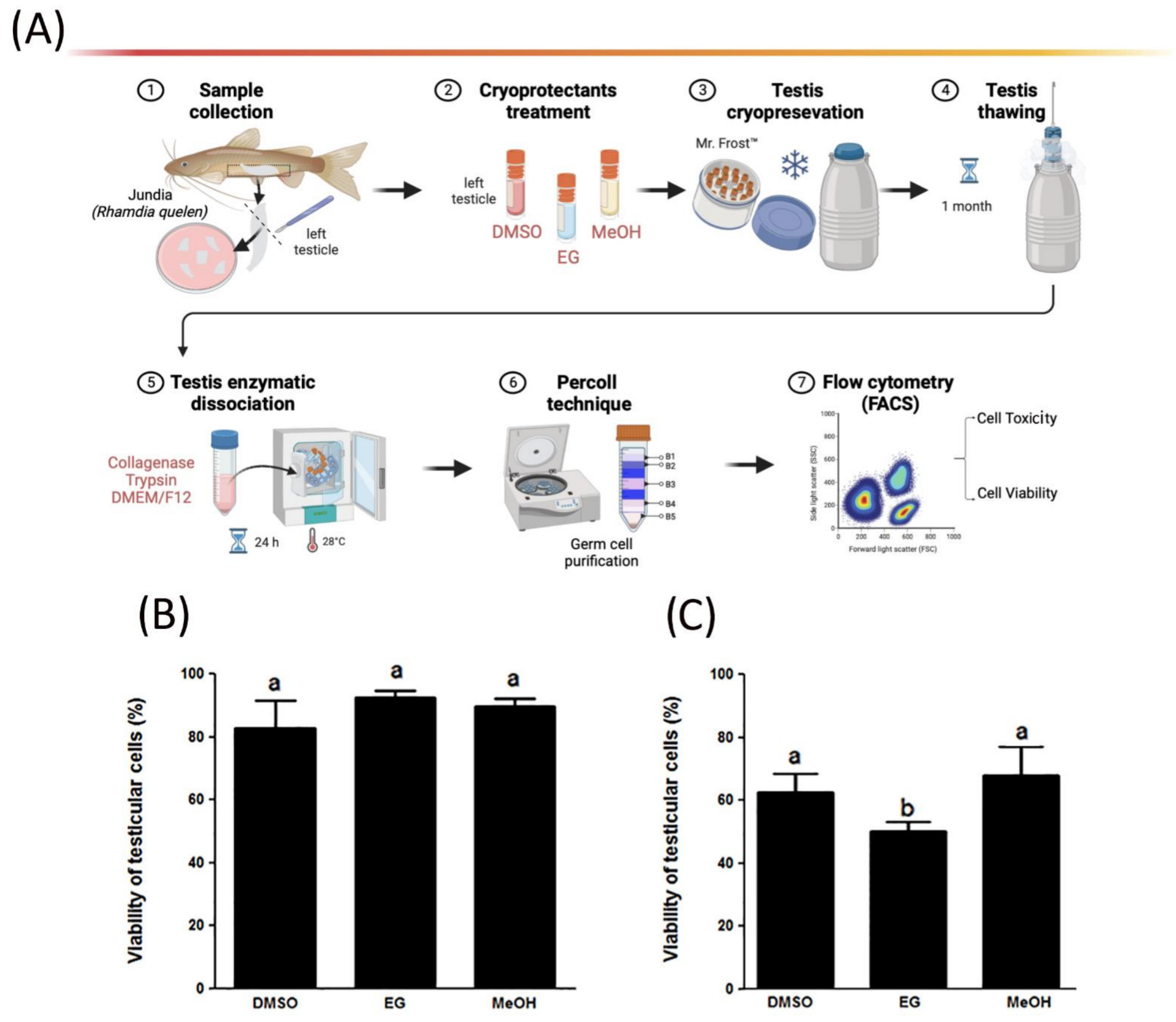
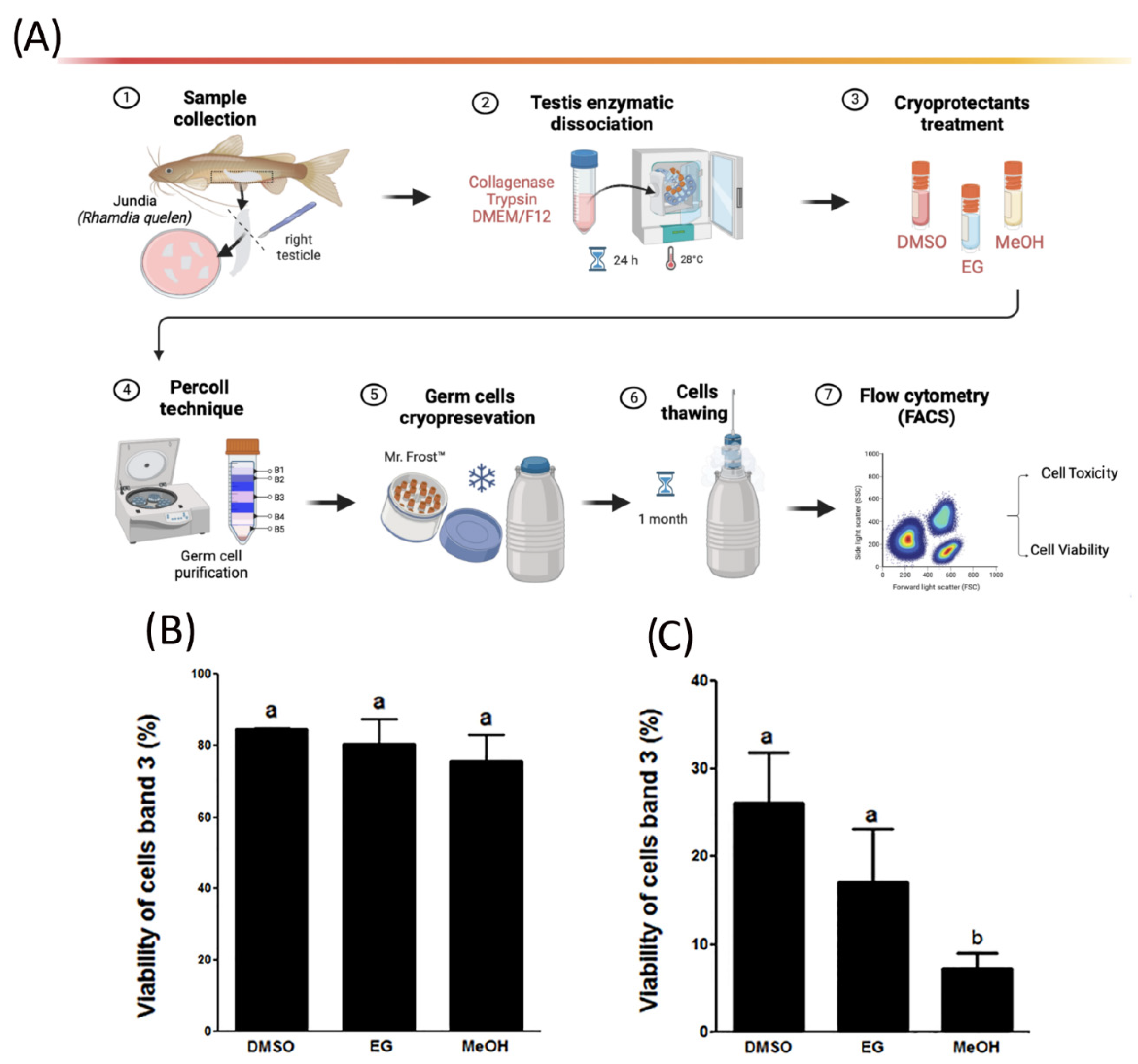
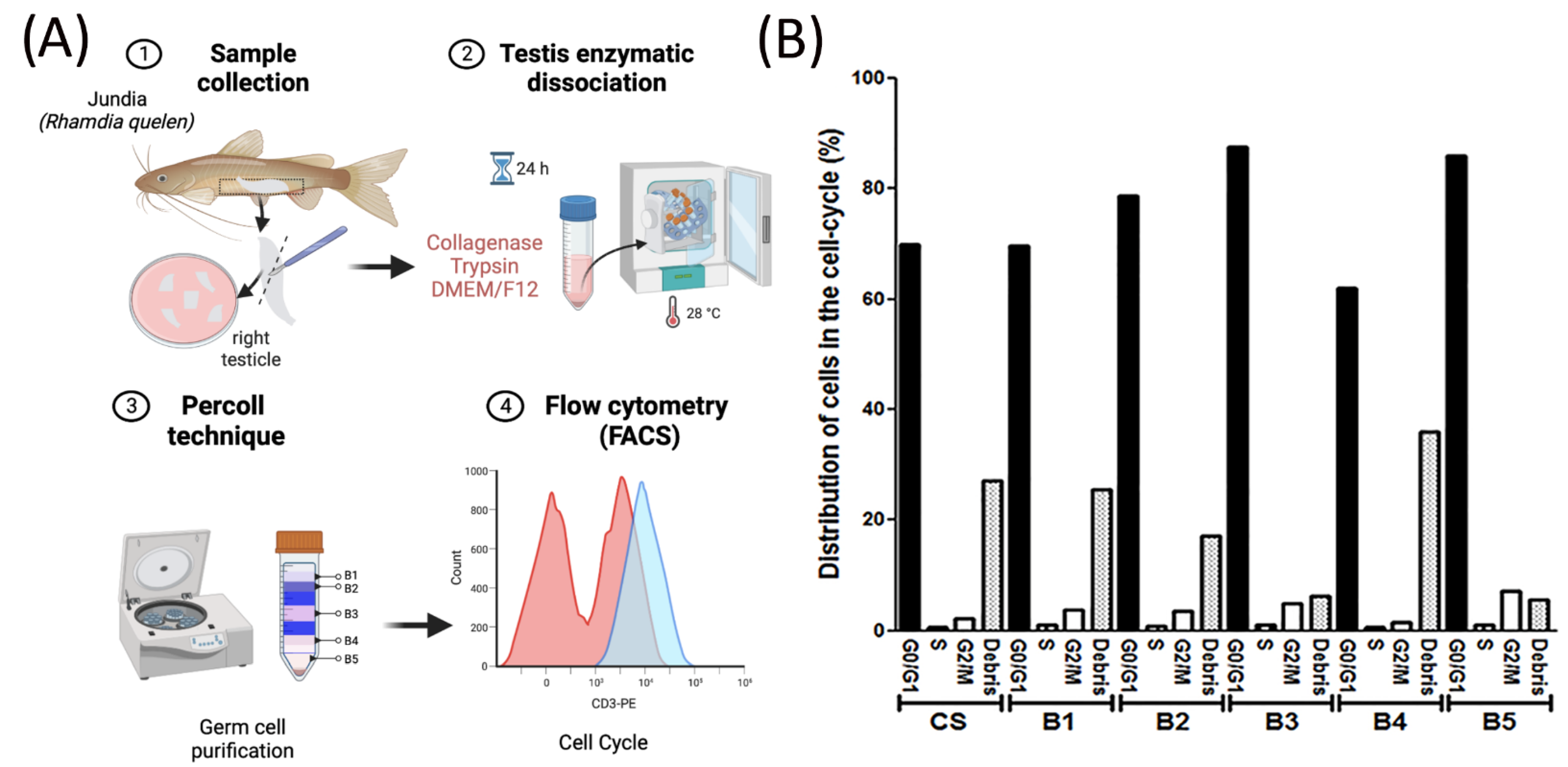
2.2. Enzymatic Dissociation of Testes
2.3. Testes and Germ Cells’ Cryopreservation Protocol
2.4. Toxicity, Viability, and Cell DNA Assessment Using Flow Cytometry
2.5. Light Microscopy for Percoll Bands
2.6. Spermatogenesis Depletion
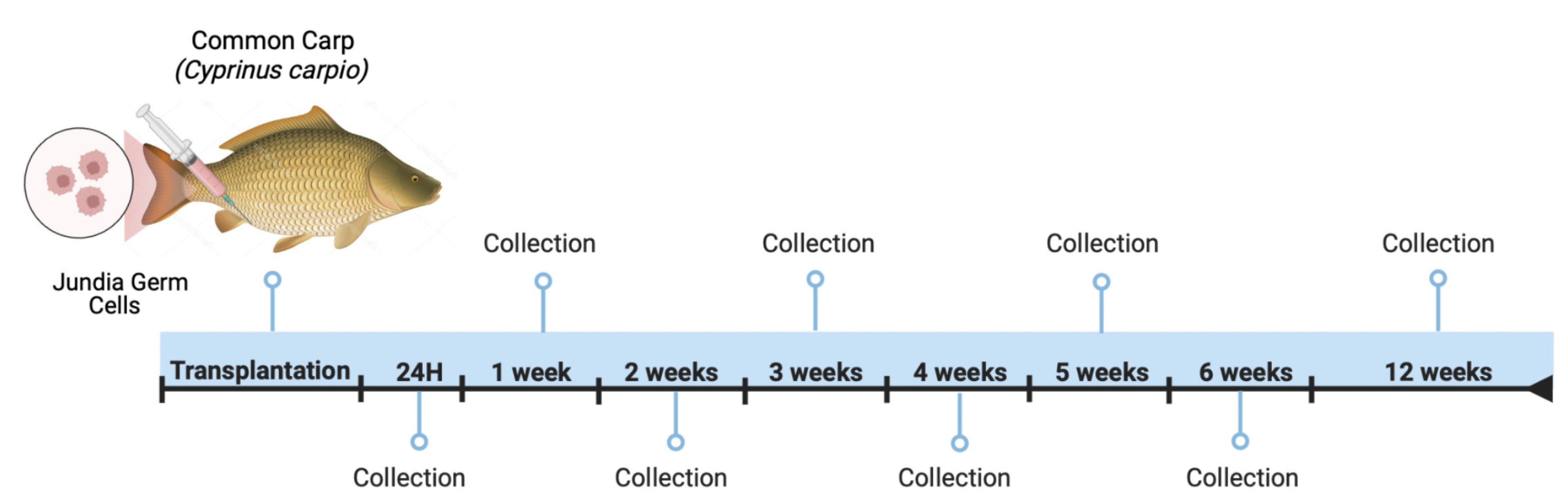
2.7. Donor Germ Cell Labeling with PKH26 and Transplantation
2.8. Microscopic Observation of Donor-Derived Germ Cells in Recipient Common Carp
2.9. Statistical Analyses
3. Results
3.1. Cell Viability of Cryopreserved Testicular Germ Cells and Toxicity of Cryoprotectants
3.2. Cell Cycle Analysis
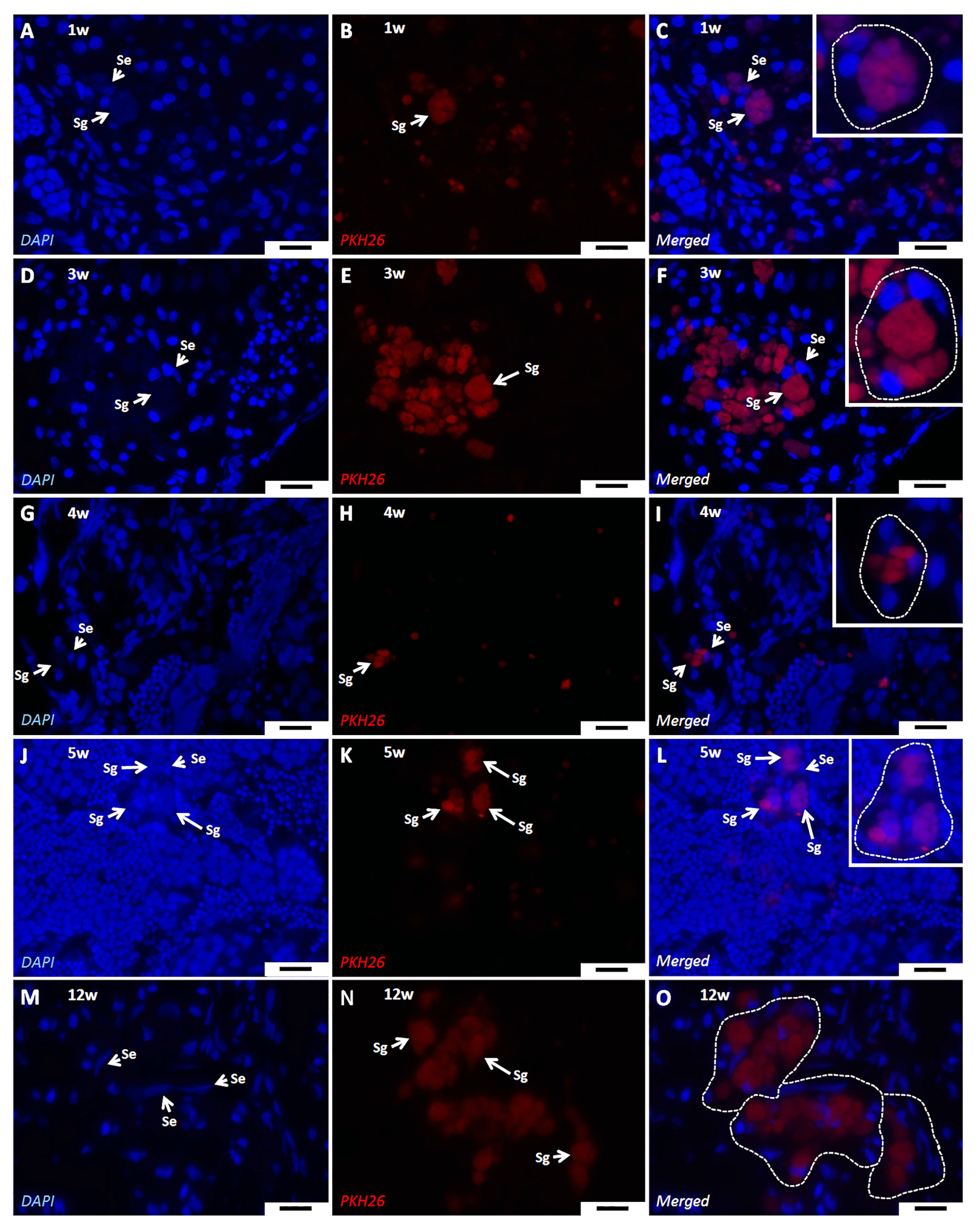
3.3. Jundia PKH26-Labeled Germ Cells in the Common Carp Testes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majhi, S.K.; Hattori, R.S.; Yokota, M.; Watanabe, S.; Strüssmann, C.A. Germ cell transplantation using sexually competent fish: An approach for rapid propagation of endangered and valuable germlines. PLoS ONE 2009, 4, e6132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Rawson, D.M.; Pekarsky, I.; Blais, I.; Lubzens, E. Low-temperature preservation of fish gonad cells and oocytes. In The Fish Oocyte: From Basic Studies to Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 411–436. [Google Scholar]
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod. Fertil. Dev. 2005, 18, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.K.; Hattori, R.S.; Rahman, S.M.; Strüssmann, C.A. Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS ONE 2014, 9, e95294. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Shikina, S.; Yoshizaki, G. Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. USA 2013, 110, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoshizaki, G. Successful cryopreservation of spermatogonia in critically endangered Manchurian trout (Brachymystax lenok). Cryobiology 2016, 72, 165–168. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Yoshizaki, G. Long-term (5 years) cryopreserved spermatogonia have high capacity to generate functional gametes via interspecies transplantation in salmonids. Cryobiology 2016, 73, 286–290. [Google Scholar] [CrossRef]
- Pšenička, M.; Saito, T.; Rodina, M.; Dzyuba, B. Cryopreservation of early stage Siberian sturgeon Acipenser baerii germ cells, comparison of whole tissue and dissociated cells. Cryobiology 2016, 72, 119–122. [Google Scholar] [CrossRef]
- Marinović, Z.; Lujić, J.; Kása, E.; Bernáth, G.; Urbányi, B.; Horváth, Á. Cryosurvival of isolated testicular cells and testicular tissue of tench Tinca tinca and goldfish Carassius auratus following slow-rate freezing. Gen. Comp. Endocrinol. 2017, 245, 77–83. [Google Scholar] [CrossRef]
- Marinović, Z.; Li, Q.; Lujić, J.; Iwasaki, Y.; Csenki, Z.; Urbányi, B.; Yoshizaki, G.; Horváth, Á. Preservation of zebrafish genetic resources through testis cryopreservation and spermatogonia transplantation. Sci. Rep. 2019, 9, 13861. [Google Scholar] [CrossRef]
- Seki, S.; Kusano, K.; Lee, S.; Iwasaki, Y.; Yagisawa, M.; Ishida, M.; Hiratsuka, T.; Sasado, T.; Naruse, K.; Yoshizaki, G. Production of the medaka derived from vitrified whole testes by germ cell transplantation. Sci. Rep. 2017, 7, 43185. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ino, Y.; Shigenaga, K.; Katayama, T.; Kuroyanagi, M.; Yoshiura, Y. Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 2018, 493, 302–313. [Google Scholar] [CrossRef]
- Hagedorn, M.M.; Daly, J.P.; Carter, V.L.; Cole, K.S.; Jaafar, Z.; Lager, C.V.; Parenti, L.R. Cryopreservation of fish spermatogonial cells: The future of natural history collections. Sci. Rep. 2018, 8, 6149. [Google Scholar] [CrossRef] [PubMed]
- RFraněk, R.; Marinović, Z.; Lujić, J.; Urbányi, B.; Fučíková, M.; Kašpar, V.; Pšenička, M.; Horváth, Á. Cryopreservation and transplantation of common carp spermatogonia. PLoS ONE 2019, 14, e0205481. [Google Scholar]
- Abualreesh, M.; Myers, J.N.; Gurbatow, J.; Johnson, A.; Xing, D.; Wang, J.; Li, S.; Coogan, M.; Vo, K.; El Husseini, N.; et al. Development of a spermatogonia cryopreservation protocol for blue catfish, Ictalurus furcatus. Cryobiology 2020, 97, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Octavera, A.; Yoshizaki, G. Production of Chinese rosy bitterling offspring derived from frozen and vitrified whole testis by spermatogonial transplantation. Fish Physiol. Biochem. 2020, 46, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Rivers, N.; Daly, J.; Jones, R.; Temple-Smith, P. Cryopreservation of testicular tissue from murray river rainbowfish, Melanotaenia fluviatilis. Sci. Rep. 2020, 10, 19355. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Silva, M.A.; Costa, G.M.; Lacerda, S.M.S.N.; Brandão-Dias, P.F.P.; Kalapothakis, E.; Júnior, A.S.; Alvarenga, E.R.; França, L.R.D. Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes. Gen. Comp. Endocrinol. 2016, 230, 48–56. [Google Scholar] [CrossRef]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yoshizaki, G.; Takeuchi, T. Surrogate broodstock produces salmonids. Nature 2004, 430, 629–630. [Google Scholar] [CrossRef]
- Okutsu, T.; Shikina, S.; Kanno, M.; Takeuchi, Y.; Yoshizaki, G. Production of trout offspring from triploid salmon parents. Science 2007, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.M.S.N.; Batlouni, S.R.; Silva, S.B.G.; Homem, C.S.P.; França, L.R. Germ cells transplantation in fish: The Nile-tilapia model. Anim. Reprod. (AR) 2018, 3, 146–159. [Google Scholar]
- Nobrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de Franca, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef]
- Hettiarachchi, D.U.; Alston, V.N.; Bern, L.; Shang, M.; Wang, J.; Xing, D.; Li, S.; Su, B.; Coogan, M.P.; Johnson, A.; et al. Producing xenogenic channel catfish, Ictalurus punctatus with cryopreserved testes and ovarian tissues of blue catfish, I. furcatus. Aquaculture 2022, 561, 738691. [Google Scholar] [CrossRef]
- Morita, T.; Miwa, M.; Kumakura, N.; Morishima, K.; Miki, T.; Takeuchi, Y.; Yoshizaki, G. Production of functional sperm from cryopreserved testicular germ cells following intraperitoneal transplantation into allogeneic surrogate in yellowtail (Seriola quinqueradiata). Cryobiology 2021, 100, 32–39. [Google Scholar] [CrossRef]
- Carneiro, P.C.F.; Bendhack, F.; Mikos, J.D.; Schorer, M.; Oliveira Filho, P.R.C.; Baldisserotto, B.; Golombieski, J.I. Jundiá: Um grande peixe para a região sul. Panor. Aqüicultura 2002, 12, 41–46. [Google Scholar]
- Fracalossi, D.M.; Meyer, G.; Santamaria, F.M.; Weingartner, M.; Zaniboni Filho, E. Performance of Jundiá, Rhamdia quelen, and Dourado, Salminus brasiliensis, in earth ponds of southern Brazil. Acta Sci.-Anim. Sci. 2004, 26, 345–352. [Google Scholar]
- Sampaio, E.V.; Sato, Y. Biologia reprodutiva e desova induzida de duas espécies de bagres (Osteichthyes: Siluriformes) da bacia rio São Francisco. Acta Sci. Biol. Sci. 2006, 28, 263–268. [Google Scholar] [CrossRef]
- Signor, A.; Feiden, A.; Boscolo, W.R.; Signor, A.A.; Gonçalves, G.S.; Sary, C.; Klein, S. Eventos reprodutivos do jundiá Rhamdia voulezi cultivado em tanques-rede. Rev. Bras. Reprodução Anim. 2013, 37, 272–277. [Google Scholar]
- Montes-Girao, P.J.; Fracalossi, D.M. Dietary lysine requirement as basis to estimate the essential dietary amino acid profile for jundiá, Rhamdia quelen. J. World Aquac. Soc. 2006, 37, 388–396. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Bombardelli, R.A.; de Oliveira, E.J.; Syperreck, M.A.; de Oliveira Pedreira, A.C.; de Freitas, J.M.A.; Marques, A.E.M.L.; Meurer, F. Silver catfish (Rhamdia quelen) breeders fed on crude glycerin-containing diets exhibited metabolic alterations and increased sperm concentration. Aquaculture 2021, 530, 735724. [Google Scholar] [CrossRef]
- Gomes, R.L.M.; Moro, E.B.; Bruno dos Santos, S.O.S.A.; Damasceno, D.Z.; Rodrigues, M.L.; Bittencourt, F. Sources of lipids in diets for silver catfish (Rhamdia quelen) juveniles. Bol. Inst. Pesca 2019, 45, e465. [Google Scholar] [CrossRef]
- Prois Flores, D.; Pianesso, D.; Kelm Battisti, E.; Ghedini Martinelli, S.; de Freitas, I.L.; Radünz Neto, J.; Picolli da Silva, L.; Aldrighi Tavares, R.; Lazzari, R. L-threonine requirement of silver catfish (Rhamdia quelen): Growth performance, body composition, plasma and liver metabolites. J. Appl. Anim. Res. 2023, 51, 358–365. [Google Scholar] [CrossRef]
- Barcellos, L.J.; Wassermann, G.F.; Scott, A.P.; Woehl, V.M.; Quevedo, R.M.; Ittzés, I.; Krieger, M.H.; Lulhier, F. Steroid profiles in cultured female jundia, the siluridae Rhamdia quelen (Quoy and Gaimard, Pisces Teleostei), during the first reproductive cycle. Gen. Comp. Endocrinol. 2001, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- de Souza França, T.; Gomes, I.C.; Sanches, E.A.; Atehortúa, M.P.; Teixeira, N.S.; Rodrigues, R.B.; de Freitas, T.R.; Galuppo, A.G.; Quirino, M.; Benato, J.L.; et al. Post-thaw dilution of Rhamdia quelen sperm improves the reproductive success. Anim. Reprod. Sci. 2022, 243, 107018. [Google Scholar] [CrossRef]
- Morón-Alcain, E.; Mendia, A.C.; Muñoz, L.H.; Boaglio, A.C.; Cerutti, P.A.; Hernández, D.R.; López, P.A.; Vigliano, F.A. Effects of heat and cold shock-induced triploidy on productive parameters of silver catfish (Rhamdia quelen) late-hatched in the reproductive season. Aquaculture 2017, 473, 303–309. [Google Scholar] [CrossRef]
- da Costa, B.B.; Marques, L.S.; Lassen, P.G.; Rodrigues, R.B.; Streit Jr, D.P. Cryopreservation-induced morphological changes in the sperm of South American silver catfish (Rhamdia quelen). J. Appl. Ichthyol. 2019, 35, 987–993. [Google Scholar] [CrossRef]
- Pérez-Atehortúa, M.; Galuppo, A.G.; Rodrigues, R.B.; de Souza França, T.; dos Santos Teixeira, N.; de Freitas, T.R.; Marques, L.S.; Gomes, I.C.; Benato, J.L.; Flores, T.; et al. The use of differential separation and density gradient with AllGrad 90% after thawing improves the sperm quality of South American catfish (Rhamdia quelen). Aquaculture 2022, 553, 738072. [Google Scholar] [CrossRef]
- de Oliveira Pedreira, A.C.; Malacarne, A.M.; Dalmaso, A.C.S.; Carvalho, K.I.F.S.; Chagas, T.V.; da Silva Gambetta, M.I.R.; Chiella, R.J.; Bombardelli, R.A. L-carnitine solution used on Rhamdia quelen thawed sperm activation boosts sperm movement, maintains larval quality, and permits to optimize the sperm use. Anim. Reprod. Sci. 2022, 245, 107054. [Google Scholar] [CrossRef]
- Malabarba, L.R.; Malabarba, M.C. Phylogeny and classification of Neotropical fish. In Biology and Physiology of Freshwater Neotropical Fish; Academic Press: Cambridge, MA, USA, 2020; pp. 1–19. [Google Scholar]
- Ye, X.; Lv, Y.; Wei, L.; Huang, J.; Wen, Y.; Zhang, G.; Zhang, S.; Yang, Z.; Liu, K. The complete mitochondrial genome of Jinbian carp Cyprinus carpio (Cypriniformes: Cyprinidae). Mitochondrial DNA Part B 2018, 3, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.M.; Batlouni, S.R.; Costa, G.M.; Segatelli, T.M.; Quirino, B.R.; Queiroz, B.M.; Kalapothakis, E.; França, L.R. A new and fast technique to generate offspring after germ cells transplantation in adult fish: The nile tilapia (Oreochromis niloticus) model. PLoS ONE 2010, 5, e10740. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.M.S.N.; Batlouni, S.R.; Assis, L.H.C.; Resende, S.M.; Campos-Silva, S.M.; França, L.R. Spermatogonial transplantation in the Nile-tilapia. In Proceedings of the International Symposium on Animal Biology of Reproduction, Belo Horizonte, Brazil, 15–18 November 2006; Volume 3, p. 183. [Google Scholar]
- Yoshizaki, G.; Fujinuma, K.; Iwasaki, Y.; Okutsu, T.; Shikina, S.; Yazawa, R.; Takeuchi, Y. Spermatogonial transplantation in fish: A novel method for the preservation of genetic resources. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 55–61. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Martinez, E.R.M.; Mura, I.L.D.D.; Doretto, L.B.; Costa, G.M.; Silva, M.A.; Digmayer, M.; Nóbrega, R.H.; França, L.R. Duration of spermatogenesis and identification of spermatogonial stem cell markers in a Neotropical catfish, Jundiá (Rhamdia quelen). Gen. Comp. Endocrinol. 2019, 273, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Crissman, H.A.; Mullaney, P.F.; Steinkamp, J.A. Chapter 12 methods and applications of flow systems for analysis and sorting of mammalian cells. Meth. Cell Biol. 1975, 9, 179–246. [Google Scholar]
- Caires, K.; Broady, J.; McLean, D. Maintaining the male germline: Regulation of spermatogonial stem cells. J. Endocrinol. 2010, 205, 133. [Google Scholar] [CrossRef]
- Corrales, C.; Leliaert, F.; Forrest, L.; Martín, M.P.; Vandelook, F.; Thines, M.; Poczai, P.; Kahila, G.; Mulcahy, D.; Haring, E.; et al. Cryopreservation. In Biodiversity Biobanking—A Handbook on Protocols and Practices; Pensoft Publishers, University of Helsinki: Helsinki, Finland, 2023; Available online: https://helda.helsinki.fi/server/api/core/bitstreams/fe66dc36-885f-4f7a-b3b4-4b98e57ac763/content (accessed on 12 September 2023).
- Saleedang, A.; Chotigeat, W.; Musikarun, P.; Sakunrang, C.; Wonglapsuwan, M. Production of semah mahseer (Tor douronensis) donor-derived offspring through xenogeneic germ cell transplantation. Aquaculture 2022, 560, 738528. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Sreebun, S.; Booncherd, K.; Khaosa-art, P.; Sooksawat, T.; Ichida, K.; Pirarat, N.; Yazawa, R. Cryopreservation of testicular cell in striped catfish (Pangasianodon hypophthalmus) and its effects on apoptosis, germ-cell specific gene expression and germ cell transplantability. Aquaculture 2023, 570, 739370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, I.F.; Martinez, E.R.M.; Digmayer, M.; Doretto, L.B.; Nóbrega, R.H. Successful Cryopreservation of Spermatogonia Stem Cells of Neotropical Catfish (Rhamdia quelen) and Enriched Germ Cell Transplantation into Common Carp (Cyprinus carpio) Testes. Fishes 2023, 8, 478. https://doi.org/10.3390/fishes8100478
Rosa IF, Martinez ERM, Digmayer M, Doretto LB, Nóbrega RH. Successful Cryopreservation of Spermatogonia Stem Cells of Neotropical Catfish (Rhamdia quelen) and Enriched Germ Cell Transplantation into Common Carp (Cyprinus carpio) Testes. Fishes. 2023; 8(10):478. https://doi.org/10.3390/fishes8100478
Chicago/Turabian StyleRosa, Ivana F., Emanuel R. M. Martinez, Melanie Digmayer, Lucas B. Doretto, and Rafael H. Nóbrega. 2023. "Successful Cryopreservation of Spermatogonia Stem Cells of Neotropical Catfish (Rhamdia quelen) and Enriched Germ Cell Transplantation into Common Carp (Cyprinus carpio) Testes" Fishes 8, no. 10: 478. https://doi.org/10.3390/fishes8100478
APA StyleRosa, I. F., Martinez, E. R. M., Digmayer, M., Doretto, L. B., & Nóbrega, R. H. (2023). Successful Cryopreservation of Spermatogonia Stem Cells of Neotropical Catfish (Rhamdia quelen) and Enriched Germ Cell Transplantation into Common Carp (Cyprinus carpio) Testes. Fishes, 8(10), 478. https://doi.org/10.3390/fishes8100478







