Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Star Collection and Husbandry
2.2. Coelomic Fluid Sampling Procedure
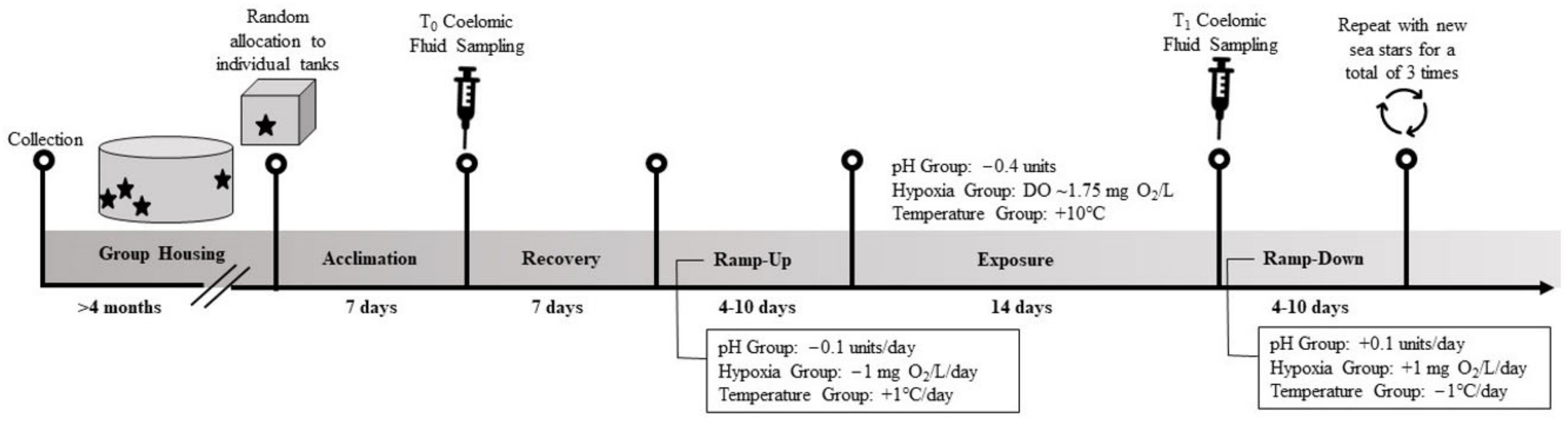
2.3. Experimental Exposures
2.4. Statistical Analyses
3. Results
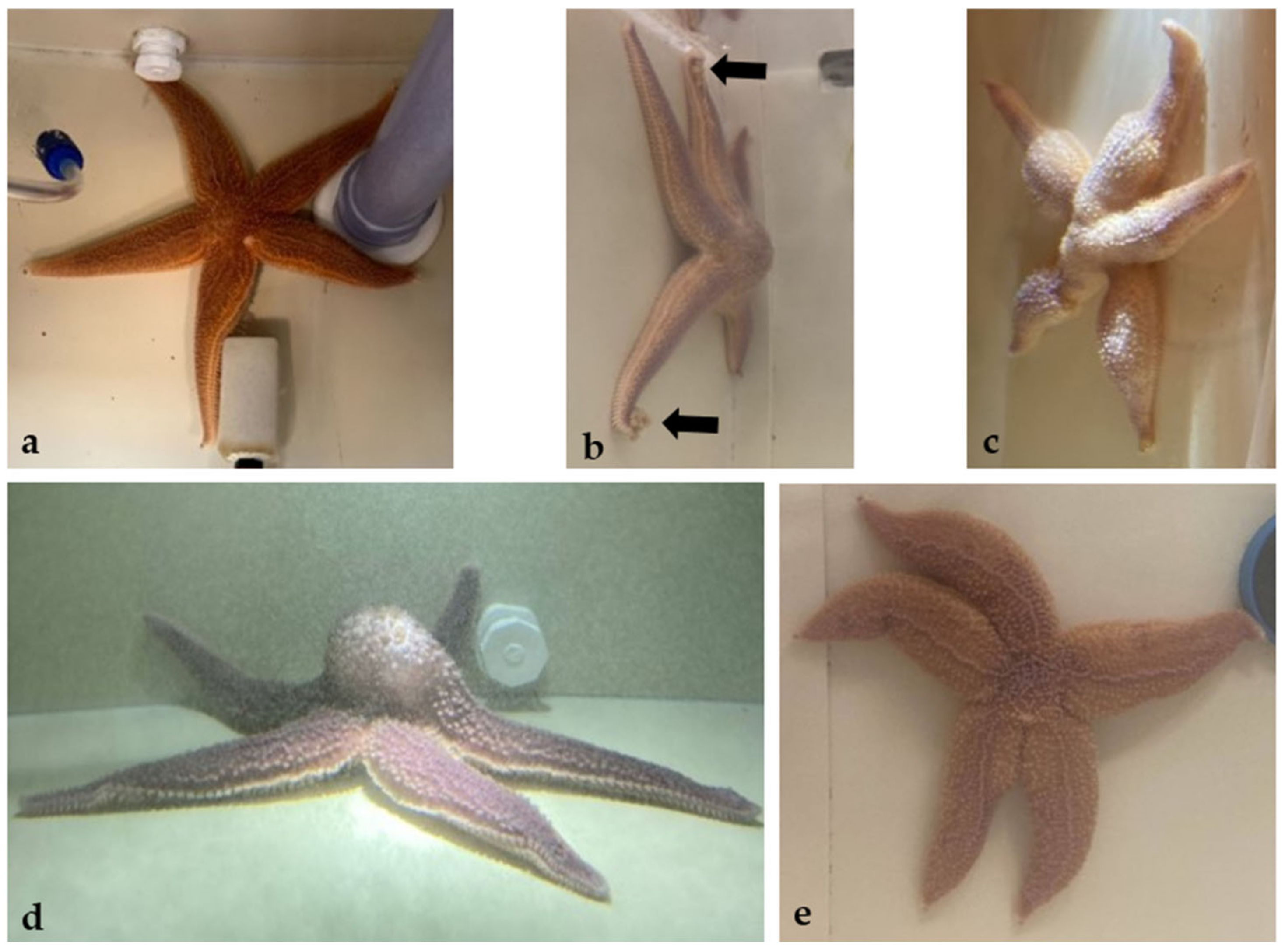
3.1. Animals
3.2. Water Quality
3.3. Blood Gas Comparison by Exposure Group
3.4. Electrolytes and Osmolality Comparison by Exposure Group
3.5. Coelomocyte Count Comparison by Exposure Group
3.6. Comparison of Coelomic Fluid Analytes between Each Exposure Group and Control Group
3.7. Coelomic Fluid Electrolytes and Osmolality Compared to Tank Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budd, G. Common starfish (Asterias rubens). In Marine Life Information Network: Biology and Sensitivity Key Information Reviews; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Assoication of the United Kingdom: Plymouth, UK, 2008. [Google Scholar]
- Vevers, H.G. The biology of Asterias rubens L.: Growth and reproduction. J. Mar. Biol. Assoc. UK 1949, 28, 165–187. [Google Scholar] [CrossRef]
- Gaymer, C.F.; Himmelman, J.H.; Johnson, L.E. Distribution and feeding ecology of the seastars Leptasterias polaris and Asterias vulgaris in the northern Gulf of St. Lawrence, Canada. J. Mar. Biol. Assoc. UK 2001, 81, 827–843. [Google Scholar] [CrossRef]
- Saier, B. Direct and indirect effects of seastars Asterias rubens on mussel beds (Mytilus edulis) in the Wadden Sea. J. Sea Res. 2001, 46, 29–42. [Google Scholar] [CrossRef]
- Dare, P.J. Notes on the swarming behaviour and population density of Asterias rubens L. (Echinodermata: Asteroidea) feeding on the mussel, Mytilus Edulis. ICES J. Mar. Sci. 1982, 40, 112–118. [Google Scholar] [CrossRef]
- Hulbert, A.W. The ecological role of Asterias vulgaris in three subtidal communities. In Echinoderms: Present and Past; Jangoux, M., Ed.; A.A. Balkema: Rotterdam, UK, 1980; pp. 191–196. [Google Scholar]
- Bucci, C.; Francoeur, M.; McGreal, J.; Smolowitz, R.; Zazueta-Novoa, V.; Wessel, G.M.; Gomez-Chiarri, M. Sea star wasting disease in Asterias forbesi along the Atlantic coast of North America. PLoS ONE 2017, 12, e0188523. [Google Scholar] [CrossRef] [PubMed]
- Oulhen, N.; Byrne, M.; Duffin, P.; Gomez-Chiarri, M.; Hewson, I.; Hodin, J.; Konar, B.; Lipp, E.K.; Miner, B.G.; Newton, A.L.; et al. A Review of Asteroid Biology in the Context of Sea Star Wasting: Possible Causes and Consequences. Biol. Bull. 2022, 243, 50–75. [Google Scholar] [CrossRef]
- Hewson, I.; Sullivan, B.; Jackson, E.W.; Xu, Q.; Long, H.; Lin, C.; Quijano Cardé, E.M.; Seymour, J.; Siboni, N.; Jones, M.R.L.; et al. Perspective: Something old, something new? Review of wasting and other mortality in Asteroidea (Echinodermata). Front. Mar. Sci. 2019, 6, 406. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Reisinger, A. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2007: Synthesis Report; IPCC: Geneva, Switzerland, 2008; pp. 1–104. [Google Scholar]
- Collard, M.; Catarino, A.I.; Bonnet, S.; Flammang, P.; Dubois, P. Effects of CO2-induced ocean acidification on physiological and mechanical properties of the starfish Asterias rubens. J. Exp. Mar. Bio. Ecol. 2013, 446, 355–362. [Google Scholar] [CrossRef]
- Hernroth, B.; Baden, S.; Thorndyke, M.; Dupont, S. Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat. Toxicol. 2011, 103, 222–224. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cells Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef]
- Hrycik, A.R.; Almeida, L.Z.; Höök, T.O. Sub-lethal effects on fish provide insight into a biologically-relevant threshold of hypoxia. Oikos 2017, 126, 307–317. [Google Scholar] [CrossRef]
- Vaquer-Sunyer, R.; Duarte, C.M. Threholds for hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E.; Fox, R.S.; Barnes, R.D. Echinodermata. In Invertbrate Zoology; Brooks/Cole-Thomson Learning: Belmont, CA, USA, 2004; pp. 872–929. [Google Scholar]
- Farmanfarmaian, A. The respiratory physiology of echinoderms. In Physiology of Echinodermata; Boolootian, R.A., Ed.; Interscience Publishers: New York, NY, USA, 1966; pp. 245–265. [Google Scholar]
- Diehl, W.J.; McEdward, L.; Proffitt, E.; Rosenberg, V.; Lawrence, J.M. The response of Luidia clathrata (Echinodermata: Asteroidea) to hypoxia. Comp. Biochem. Physiol. Part A Physiol. 1979, 62, 669–671. [Google Scholar] [CrossRef]
- Theede, H.; Ponat, A.; Hiroki, K.; Schlieper, C. Studies on the resistance of marine bottom invertebrates to oxygen-deficiency and hydrogen sulphide. Mar. Biol. 1969, 2, 325–337. [Google Scholar] [CrossRef]
- Holm, K.; Hernroth, B.; Thorndyke, M. Coelomocyte numbers and expression of HSP70 in wounded sea stars during hypoxia. Cell Tissue Res. 2008, 334, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.A.; Besemer, R.M.; DeRito, C.M.; Kocian, J.; Porter, I.R.; Raimondi, P.T.; Rede, J.E.; Schiebelhut, L.M.; Sparks, J.P.; Wares, J.P.; et al. Evidence that microorganisms at the animal-water interface drive sea star wasting disease. Front. Microbiol. 2021, 11, 3278. [Google Scholar] [CrossRef]
- Gruber, N. Warming up, turning sour, losing breath: Ocean biogeochemistry under global change. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 1980–1996. [Google Scholar] [CrossRef]
- Alexander, M.A.; Scott, J.D.; Friedland, K.D.; Mills, K.E.; Nye, J.A.; Pershing, A.J.; Thomas, A.C. Projected sea surface temperatures over the 21st century: Changes in the mean, variability and extremes for large marine ecosystem regions of Northern Oceans. Elem. Sci. Anth. 2018, 6, 9. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Peck, L.S.; Webb, K.E.; Miller, A.; Clark, M.S.; Hill, T. Temperature limits to activity, feeding and metabolism in the Antarctic starfish Odontaster validus. Mar. Ecol. Prog. Ser. 2008, 358, 181–189. [Google Scholar] [CrossRef]
- Lang, B.J.; Donelson, J.M.; Caballes, C.F.; Doll, P.C.; Pratchett, M.S. Metabolic Responses of Pacific Crown-of-Thorns Sea Stars (Acanthaster sp.) to Acute Warming. Biol. Bull. 2021, 241, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.P.; Alfaya, J.E.F.; Palomo, M.G.; Giulianelli, S.; Nieto Vilela, R.A.; Bigatti, G. Ocean warming lead to heat shock protein expression and decrease in the feeding rate of the Patagonian sea star Anasterias minuta. J. Exp. Mar. Bio. Ecol. 2022, 546, 151661. [Google Scholar] [CrossRef]
- Morón Lugo, S.C.; Baumeister, M.; Nour, O.M.; Wolf, F.; Stumpp, M.; Pansch, C. Warming and temperature variability determine the performance of two invertebrate predators. Sci. Rep. 2020, 10, 6780. [Google Scholar] [CrossRef] [PubMed]
- Staehli, A.; Schaerer, R.; Hoelzle, K.; Ribi, G. Temperature induced disease in the starfish Astropecten jonstoni. Mar. Biodivers. Rec. 2009, 2, e78. [Google Scholar] [CrossRef]
- Bates, A.E.; Hilton, B.J.; Harley, C.D.G. Effects of temperature, season and locality on wasting disease in the keystone predatory sea star Pisaster ochraceus. Dis. Aquat. Organ. 2009, 86, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Harvell, C.D.; Montecino-Latorre, D.; Caldwell, J.M.; Burt, J.M.; Bosley, K.; Keller, A.; Heron, S.F.; Salomon, A.K.; Lee, L.; Pontier, O.; et al. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 2019, 5, eaau7042. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, K. The coelomocytes of asteroid echinoderms. In Invertebrate Blood; Cheng, T.C., Ed.; Springer: New York, NY, USA, 1984; pp. 7–39. [Google Scholar]
- Chia, F.S.; Xing, J. Echinoderm coelomocytes. Zool. Stud. 1996, 35, 231–254. [Google Scholar]
- National Research Council Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2010.
- Giese, A.G. On the biochemical constitution of some echinoderms. In Physiology of Echinodermata; Boolootian, R.A., Ed.; Interscience Publishers: New York, NY, USA, 1966; pp. 757–796. [Google Scholar]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Redrobe, S.; MacDonald, J. Sample collection and clinical pathology of reptiles. Vet. Clin. North Am. Exot. Anim. Pract. 1999, 2, 709–730. [Google Scholar] [CrossRef]
- Wahltinez, S.J.; Stacy, N.I.; Lahner, L.L.; Newton, A.L. Coelomic fluid evaluation in clinically normal ochre sea stars Pisaster ochraceus: Cell counts, cytology, and biochemistry reference intervals. J. Aquat. Anim. Health 2019, 31, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.J.; Tlusty, M.; Merigo, C.; Weber, E.S. Metabolic and respiratory status of cold-stunned Kemp’s ridley sea turtles (Lepidochelys kempii). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007, 177, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Mallo, K.M.; Ross, P.M.; Segars, A. Venous blood gases and lactates of wild loggerhead sea turtles (Caretta caretta) following two capture techniques. J. Wildl. Dis. 2003, 39, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.E.; Matsche, M.A.; Rosemary, K. Preserving whole blood in formalin extends the specimen stability period for manual cell counts for fish. Vet. Clin. Pathol. 2014, 43, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.-P. Guide to Best Practices for Ocean Acidification Research and Data Reporting; Publications Office of the European Union: Luxembourg, The Netherlands, 2011; ISBN 9789279206504. [Google Scholar]
- Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 2320 alkalinity. In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter , T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Lavigne, H. Technical Note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 2009, 6, 2121–2133. [Google Scholar] [CrossRef]
- Wahltinez, S.J.; Kroll, K.J.; Nunamaker, E.A.; Denslow, N.D.; Stacy, N.I. Practical euthanasia method for common sea stars (Asterias rubens) that allows for high-quality RNA sampling. Animals 2021, 11, 1847. [Google Scholar] [CrossRef]
- Kaneshiro, E.S.; Karp, R.D. The ultrastructure of coelomocytes of the sea star Dermasterias imbricata. Biol. Bull. 1980, 159, 295–310. [Google Scholar] [CrossRef]
- Catarino, A.I.; Bauwens, M.; Dubois, P. Acid-base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ. Sci. Pollut. Res. 2012, 19, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, Y.S.; Thomsen, J.; Pansch, C.; Melzner, F.; Wahl, M. Sour times: Seawater acidification effects on growth, feeding behaviour and acid-base status of Asterias rubens and Carcinus maenas. Mar. Ecol. Prog. Ser. 2012, 459, 85–97. [Google Scholar] [CrossRef]
- McElroy, D.J.; Nguyen, H.D.; Byrne, M. Respiratory response of the intertidal seastar Parvulastra exigua to contemporary and near-future pulses of warming and hypercapnia. J. Exp. Mar. Bio. Ecol. 2012, 416, 1–7. [Google Scholar] [CrossRef]
- Collard, M.; Laitat, K.; Moulin, L.; Catarino, A.I.; Grosjean, P.; Dubois, P. Buffer capacity of the coelomic fluid in echinoderms. Comp. Biochem. Physiol. Part A 2013, 166, 199–206. [Google Scholar] [CrossRef]
- Spicer, J.I. Oxygen and acid-base status of the sea urchin Psammechinus miliaris during environmental hypoxia. Mar. Biol. 1995, 124, 71–76. [Google Scholar] [CrossRef]
- Portner, H.O.; Grieshaber, M.K.; Heisler, N. Anaerobiosis and acid-base status in marine invertebrates: Effect of environmental hypoxia on extracellular and intracellular pH in Sipunculus nudus L. J. Comp. Physiol. B 1984, 155, 13–20. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Lane, J.M. The utilization of nutrients by post-metamorphic echinoderms. In Echinoderm Nutrition; Jangoux, M., Lawrence, J.M., Eds.; A.A. Balkema: Amsterdam, The Netherlands, 1982; pp. 331–371. [Google Scholar]
- Low, N.H.N.; Micheli, F. Short- and long-term impacts of variable hypoxia exposures on kelp forest sea urchins. Sci. Rep. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Garcia, H.E.; Boyer, T.P.; Levitus, S.; Locarnini, R.A.; Antonov, J. On the variability of dissolved oxygen and apparent oxygen utilization content for the upper world ocean: 1955 to 1998. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Binyon, J. Ionic regulation and mode of adjustment to reduced salinity of the starfish Asterias rubens L. J. Mar. Biol. Assoc. UK 1962, 42, 49. [Google Scholar] [CrossRef]
- Diehl, W.J.; Lawrence, J.M. The effect of salinity on coelomic fluid osmolyte concentration and intracellular water content in Luidia clathrata (Say) (Echinodermata: Asteroidea). Comp. Biochem. Physiol. Part A Physiol. 1984, 79, 119–126. [Google Scholar] [CrossRef]
- Orr, J.C. Recent and Future Changes in Ocean Carbonate Chemistry. In Ocean Acidification; Gattuso, J.-P., Hansson, L., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 41–66. [Google Scholar]
- Andersson, A.J.; Mackenzie, F.T. Effects of Ocean Acidification on Benthic Processes, Organisms, and Ecosystems. In Ocean Acidification; Gattuso, J.-P., Hansson, L., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 122–153. ISBN 9780199591091. [Google Scholar]
- Sweadner, K.J.; Goldin, S.M. Active Transport of Sodium and Potassium Ions: Mechanism, Function, and Regulation. N. Engl. J. Med. 1980, 302, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, K. In vitro studies on the effects of cell-free coelomic fluid, calcium, and/or magnesium on clumping of coelomocytes of the sea star Asterias forbesi (Echinodermata: Asteroidea). Biol. Bull. 1982, 163, 438–452. [Google Scholar] [CrossRef]
- Boolootian, R.A.; Giese, A.C. Clotting of echinoderm coelomic fluid. J. Exp. Zool. 1959, 140, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Oweson, C.; Li, C.; Söderhäll, I.; Hernroth, B. Effects of manganese and hypoxia on coelomocyte renewal in the echinoderm, Asterias rubens (L.). Aquat. Toxicol. 2010, 100, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Coteur, G.; Corriere, N.; Dubois, P. Environmental factors influencing the immune responses of the common European starfish (Asterias rubens). Fish Shellfish. Immunol. 2004, 16, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Weatherby, T.M.; DeRito, C.M.; Besemer, R.M.; Hewson, I. Sea star wasting disease pathology in Pisaster ochraceus shows a basal-to-surface process affecting color phenotypes differently. Dis. Aquat. Organ. 2021, 145, 21–33. [Google Scholar] [CrossRef]
- Cole, W.H. The composition of fluids and sera of some marine animals and of the sea water in which they live. J. Gen. Physiol. 1940, 23, 575–584. [Google Scholar] [CrossRef]
- Binyon, J. Salinity Tolerance and Permeability to Water of the Starfish Asterias rubens L. J. Mar. Biol. Assoc. UK 1961, 41, 161–174. [Google Scholar] [CrossRef]
- Ferguson, J.C. Hyperosmotic properties of the fluids of the perivisceral coelom and water vascular system of starfish kept under stable conditions. Comp. Biochem. Physiol. Part A Physiol. 1990, 95, 245–248. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
| A Priori Testing | Post Hoc Testing | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter (Unit) | Treatment Group | Mean | Range | SD | p | Group Pairing | Difference in Means | p |
| pH | pH | 7.37 | 7.20–7.56 | 0.07 | <0.001 * | pH–Hypoxia | 0.56 | <0.001 * |
| Hypoxia | 7.94 | 7.74–8.87 | 0.12 | pH–Temperature | 0.48 | <0.001 * | ||
| Temperature | 7.85 | 7.68–8.03 | 0.07 | Temperature–Hypoxia | 0.09 | <0.001 * | ||
| Control | 7.80 | 7.68–7.95 | 0.06 | Control–pH | 0.44 | <0.001 * | ||
| Control–Hypoxia | 0.13 | <0.001 * | ||||||
| Control–Temperature | 0.04 | <0.001 * | ||||||
| Dissolved Oxygen (mg O2/L) | pH | 9.41 | 9.05–9.73 | 0.12 | <0.001 * | pH–Hypoxia | 7.62 | <0.001 * |
| Hypoxia | 1.80 | 1.12–5.25 | 0.34 | pH–Temperature | 1.60 | <0.001 * | ||
| Temperature | 7.81 | 7.56–8.04 | 0.09 | Temperature–Hypoxia | 6.01 | <0.001 * | ||
| Control | 9.49 | 8.13–9.74 | 0.15 | Control–pH | 0.09 | <0.001 * | ||
| Control–Hypoxia | 7.70 | <0.001 * | ||||||
| Control–Temperature | 1.69 | <0.001 * | ||||||
| Temperature (℃) | pH | 7.7 | 7.3–8.0 | 0.2 | <0.001 * | pH–Hypoxia | 0.2 | <0.001 * |
| Hypoxia | 7.5 | 6.9–7.7 | 0.2 | pH–Temperature | 9.5 | <0.001 * | ||
| Temperature | 17.2 | 16.5–17.5 | 0.2 | Temperature–Hypoxia | 9.7 | <0.001 * | ||
| Control | 7.6 | 6.8–8.0 | 0.2 | Control–pH | 0.1 | <0.001 * | ||
| Control–Hypoxia | 0.2 | <0.001 * | ||||||
| Control–Temperature | 9.5 | <0.001 * | ||||||
| Tank | Mean Temperature (°C) | Temperature Range (°C) | SD |
|---|---|---|---|
| Temp 1 | 17.55 | 17.20–18.74 | 0.08 |
| Temp 2 | 17.82 | 17.54–18.66 | 0.10 |
| Temp 3 | 17.77 | 17.41–19.04 | 0.15 |
| Temp 4 | 17.76 | 13.74–18.10 | 0.23 |
| Temp 5 | 17.73 | 17.50–18.79 | 0.06 |
| Temp 6 | 17.88 | 17.67–18.70 | 0.05 |
| Control 1 | 8.06 | 7.68–8.960 | 0.14 |
| Control 2 | 8.13 | 7.81–8.88 | 0.15 |
| Control 3 | 7.95 | 6.78–8.960 | 0.28 |
| Timepoint | Group | pH | Alkalinity (mmol/kg) | DIC (mmol/kg) | pCO2 (μatm) | HCO3− (mmol/kg) | CO32− (mmol/kg) | ΩAragonite | ΩCalcite |
|---|---|---|---|---|---|---|---|---|---|
| Before Ramp-Up | pH | 7.78 ± 0.09 | 1.64 ± 0.44 | 2.18 ± 0.10 | 783.5 ± 177.7 | 2.07 ± 0.10 | 0.07 ± 0.01 | 1.03 ± 0.17 | 1.63 ± 0.26 |
| Control | 7.85 ± 0.04 | 1.66 ± 0.38 | 2.18 ± 0.12 | 655.6 ± 88.0 | 2.07 ± 0.11 | 0.08 ± 0.01 | 1.19 ± 0.11 | 1.89 ± 0.17 | |
| After Ramp-Up | pH | 7.45 ± 0.05 | 1.50 ± 0.49 | 2.22 ± 0.12 | 1653.9 ± 181.3 | 2.11 ± 0.12 | 0.03 ± 0.01 | 0.49 ± 0.07 | 0.78 ± 0.11 |
| Control | 7.82 ± 0.10 | 1.34 ± 0.47 | 2.14 ± 0.10 | 704.6 ± 172.9 | 2.03 ± 0.10 | 0.07 ± 0.01 | 1.10 ± 0.20 | 1.74 ± 0.32 | |
| 7 days of Exposure | pH | 7.33 ± 0.06 | 1.35 ± 0.38 | 2.17 ± 0.12 | 2158.6 ± 206.9 | 2.05 ± 0.12 | 0.02 ± 0.004 | 0.36 ± 0.07 | 0.56 ± 0.11 |
| Control | 7.79 ± 0.03 | 1.66 ± 0.63 | 2.05 ± 0.15 | 714.0 ± 87.9 | 1.95 ± 0.14 | 0.06 ± 0.01 | 0.97 ± 0.07 | 1.54 ± 0.11 | |
| 14 days of Exposure | pH | 7.45 ± 0.05 | 1.39 ± 0.48 | 2.06 ± 0.17 | 1548.2 ± 273.2 | 1.96 ± 0.16 | 0.03 ± 0.003 | 0.45 ± 0.04 | 0.72 ± 0.06 |
| Control | 7.82 ± 0.06 | 1.21 ± 0.40 | 2.00 ± 0.14 | 655.0 ± 107.0 | 1.90 ± 0.13 | 0.07 ± 0.01 | 1.02 ± 0.15 | 1.61 ± 0.23 |
| Analyte | Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Hypoxia | Temperature | Control | |||||||||||||
| T0 Mean | T1 Mean | p | D | T0 Mean | T1 Mean | p | D | T0 Mean | T1 Mean | p | D | T0 Mean | T1 Mean | p | D | |
| pH(TC) | 7.42 ± 0.08 | 7.31 ± 0.08 | 0.011 * | G | 7.45 ± 0.10 | 7.65 ± 0.07 | <0.001 * | G | 7.56 ± 0.06 | 7.35 ± 0.08 | <0.001 * | G | 7.54 ± 0.05 | 7.51 ± 0.11 | 0.426 | G |
| pCO2(TC) (mmHg) | 2.2 ± 0.2 | 2.4 ± 0.2 | 0.059 | NG | 2.1 ± 0.2 | 1.8 ± 0.2 | <0.001 * | G | 2.1 ± 0.2 | 3.0 ± 0.2 | <0.001 * | G | 2.0 ± 0.1 | 2.3 ± 0.2 | 0.019 * | G |
| pO2(TC) (mmHg) | 103 ± 9 | 113 ± 6 | 0.013 * | G | 107 ± 6 | 55 ± 2 | 0.008 * | NG | 114 ± 8 | 98 ± 7 | <0.001 * | G | 113 ± 6 | 112 ± 8 | 0.455 | G |
| HCO3 (mmol/L) | 1.9 ± 0.4 | 1.6 ± 0.3 | 0.103 | NG | 1.9 ±0.3 | 2.7 ± 0.4 | <0.001 * | G | 2.4 ± 0.2 | 2.0 ± 0.3 | <0.001 * | G | 2.2 ± 0.3 | 2.4 ± 0.5 | 0.231 | G |
| TCO2 (mmol/L) | BLD | BLD | - | - | BLD | BLD | - | - | BLD | BLD | - | - | BLD | BLD | - | - |
| Lactate (mmol/L) | BLD | BLD | - | - | BLD | BLD | - | - | BLD | BLD | - | - | BLD | BLD | - | - |
| Magnesium (mmol/L) | 52 ± 3 | 54 ±7 | 0.690 | G | 51 ± 6 | 47 ± 6 | 0.201 | G | 65 ± 13 | 54 ± 4 | 0.068 | NG | 51 ± 7 | 54 ± 2 | 0.564 | G |
| Sodium (mmol/L) | 427 ± 6 | 416 ± 10 | 0.148 | G | 425 ± 3 | 402 ± 57 | 0.715 | NG | 451 ± 8 | 435 ± 4 | 0.009 * | G | 442 ± 4 | 432 ± 4 | 0.111 | G |
| Potassium (mmol/L) | 9.4 ± 0.5 | 9.2 ± 0.3 | 0.294 | G | 9.4 ± 0.1 | 9.5 ± 1.0 | 0.886 | G | 10.0 ± 0.1 | 9.6 ± 0.3 | 0.023 * | G | 9.8 ± 0.1 | 9.4 ± 0.1 | 0.004 * | G |
| Chloride (mmol/L) | 495 ± 7 | 482 ± 12 | 0.118 | G | 492 ± 4 | 468 ± 64 | 0.715 | NG | 521 ± 8 | 505 ± 4 | 0.015 * | G | 511 ± 5 | 501 ± 7 | 0.144 | G |
| Calcium (mmol/L) | 9.7 ± 0.1 | 9.4 ± 0.2 | 0.033 * | G | 9.5 ± 0.2 | 9.2 ± 1.1 | 0.853 | NG | 10.0 ± 0.1 | 9.8 ± 0.1 | 0.037 * | G | 9.9 ± 0.3 | 9.6 ± 0.2 | 0.199 | G |
| Osmolality (mmol/kg) | 959 ± 8 | 941 ± 4 | 0.004 * | G | 944 ± 4 | 939 ± 6 | 0.138 | G | 984 ± 5 | 968 ± 25 | 0.232 | G | 986 ± 6.6 | 944 ± 3.4 | 0.066 | G |
| Coelomocyte Counts (× 103 cells/Μl) | 15.59 ± 15.88 | 5.78 ± 7.07 | 0.068 | NG | 8.90 ± 13.60 | 14.10 ± 22.15 | 0.144 | NG | 8.58 ± 12.43 | 11.78 ± 14.66 | 0.068 | NG | 1.14 ± 0.77 | 1.13 ± 0.37 | 0.971 | NG |
| Analyte | Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH vs. Control | Hypoxia vs. Control | Temperature vs. Control | |||||||||
| Control Group T1 Mean ± SD | D | pH Group T1 Mean ± SD | p | D | Hypoxia Group T1 Mean ± SD | p | D | Temperature Group T1 Mean ± SD | p | D | |
| pH(TC) | 7.51 ± 0.11 | G | 7.31 ± 0.08 | <0.001 * | G | 7.65 ± 0.07 | 0.004 * | G | 7.35 ± 0.07 | 0.002 * | G |
| pCO2(TC) (mmHg) | 2.3 ± 0.2 | G | 2.4 ± 0.2 | 0.540 | NG | 1.8 ± 0.2 | <0.001 * | G | 3.0 ± 0.2 | <0.001 * | G |
| pO2(TC) (mmHg) | 112 ± 8 | G | 113 ± 6 | 0.638 | G | 55 ± 2 | <0.001 * | NG | 98 ± 7 | <0.001 * | G |
| HCO3 (mmol/L) | 2.4 ± 0.5 | G | 1.6 ± 0.3 | 0.001 * | NG | 2.7 ± 0.4 | 0.182 | G | 2.0 ± 0.3 | 0.066 | G |
| TCO2 (mmol/L) | BLD | - | BLD | BLD | - | BLD | BLD | - | BLD | BLD | - |
| Lactate (mmol/L) | BLD | - | BLD | BLD | - | BLD | BLD | - | BLD | BLD | - |
| Magnesium (mmol/L) | 56 ± 4 | G | 54 ± 1 | 0.311 | G | 52 ± 9 | 0.293 | G | 54 ± 5 | 0.339 | G |
| Sodium (mmol/L) | 438 ± 10 | G | 426 ± 16 | 0.109 | G | 419 ± 46 | 0.443 | NG | 438 ± 10 | 0.919 | G |
| Potassium (mmol/L) | 9.6 ± 0.3 | G | 9.4 ± 0.3 | 0.230 | G | 9.5 ± 0.7 | 0.756 | G | 9.9 ± 0.6 | 0.193 | G |
| Chloride (mmol/L) | 508 ± 11 | G | 493 ± 18 | 0.087 | G | 486 ± 51 | 0.609 | NG | 508 ± 10 | 0.982 | G |
| Calcium (mmol/L) | 9.8 ± 0.3 | G | 9.5 ± 0.3 | 0.072 | G | 9.5 ± 0.9 | 0.949 | NG | 9.8 ± 0.2 | 0.999 | G |
| Osmolality (mmol/kg) | 962 ± 23 | G | 955 ± 19 | 0.566 | G | 956 ± 22 | 0.686 | G | 983 ± 25 | 0.130 | G |
| Coelomocyte Counts (×103 cells/μL) | 1.04 ± 0.29 | G | 3.96 ± 5.49 | 0.011 * | NG | 9.41 ± 16.72 | 0.002 * | NG | 7.99 ± 11.41 | 0.002 * | NG |
| Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Hypoxia | Temperature | Control | ||||||||
| Analyte (Unit) | Sample Type | T0 Mean (n = 14) | p | T1 Mean (n = 3) | p | T1 Mean (n = 4) | p | T1 Mean (n = 3) | p | T1 Mean (n = 3) | p |
| Magnesium (mmol/L) | SeaStar | 54 | 0.311 | 51 | 0.200 | 49 | 0.715 | 51 | 0.166 | 57 | 0.100 |
| Tank | 46 | 42 | 47 | 47 | 50 | ||||||
| Sodium (mmol/L) | SeaStar | 338 | 0.535 | 428 | 0.100 | 435 | 0.200 | 441 | 0.700 | 440 | 0.100 |
| Tank | 394 | 337 | 409 | 410 | 401 | ||||||
| Potassium (mmol/L) | SeaStar | 9.6 | 0.017 * | 9.4 | 0.100 | 9.6 | 0.028 * | 10.0 | 0.109 | 9.8 | 0.100 |
| Tank | 8.2 | 7.0 | 8.6 | 8.6 | 8.4 | ||||||
| Chloride (mmol/L) | SeaStar | 506 | 0.445 | 496 | 0.100 | 504 | 0.057 | 512 | 0.593 | 509 | 0.100 |
| Tank | 452 | 391 | 452 | 476 | 471 | ||||||
| Calcium (mmol/L) | SeaStar | 9.8 | 0.203 | 9.6 | 0.200 | 9.9 | 0.068 | 9.9 | 0.100 | 9.9 | 0.166 |
| Tank | 8.9 | 7.8 | 8.8 | 9.2 | 9.1 | ||||||
| Osmolality (mmol/kg) | SeaStar | 969 | 0.018 * | 963 | 0.200 | 962 | 0.068 | 975 | 0.400 | 969 | 1.00 |
| Tank | 866 | 824 | 911 | 944 | 967 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahltinez, S.J.; Kroll, K.J.; Behringer, D.C.; Arnold, J.E.; Whitaker, B.; Newton, A.L.; Edmiston, K.; Hewson, I.; Stacy, N.I. Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes 2023, 8, 51. https://doi.org/10.3390/fishes8010051
Wahltinez SJ, Kroll KJ, Behringer DC, Arnold JE, Whitaker B, Newton AL, Edmiston K, Hewson I, Stacy NI. Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes. 2023; 8(1):51. https://doi.org/10.3390/fishes8010051
Chicago/Turabian StyleWahltinez, Sarah J., Kevin J. Kroll, Donald C. Behringer, Jill E. Arnold, Brent Whitaker, Alisa L. Newton, Kristina Edmiston, Ian Hewson, and Nicole I. Stacy. 2023. "Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors" Fishes 8, no. 1: 51. https://doi.org/10.3390/fishes8010051
APA StyleWahltinez, S. J., Kroll, K. J., Behringer, D. C., Arnold, J. E., Whitaker, B., Newton, A. L., Edmiston, K., Hewson, I., & Stacy, N. I. (2023). Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes, 8(1), 51. https://doi.org/10.3390/fishes8010051






