Investigating the Applicability of Ichthyoplanktonic Indices in Better Understanding the Dynamics of the Northern Stock of the Population of Atlantic Hake Merluccius merluccius (L.)
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Samples
2.2. Eggs Identification and Standardization of Samples
2.3. Larvae Identification and Standardization of Samples
2.4. Larvae Survival Estimates
2.5. Year–Class Abundant Standardization Data
2.6. Temporal Variability in Ichthyoplankton Surveys
2.7. Statistical Analysis
3. Results
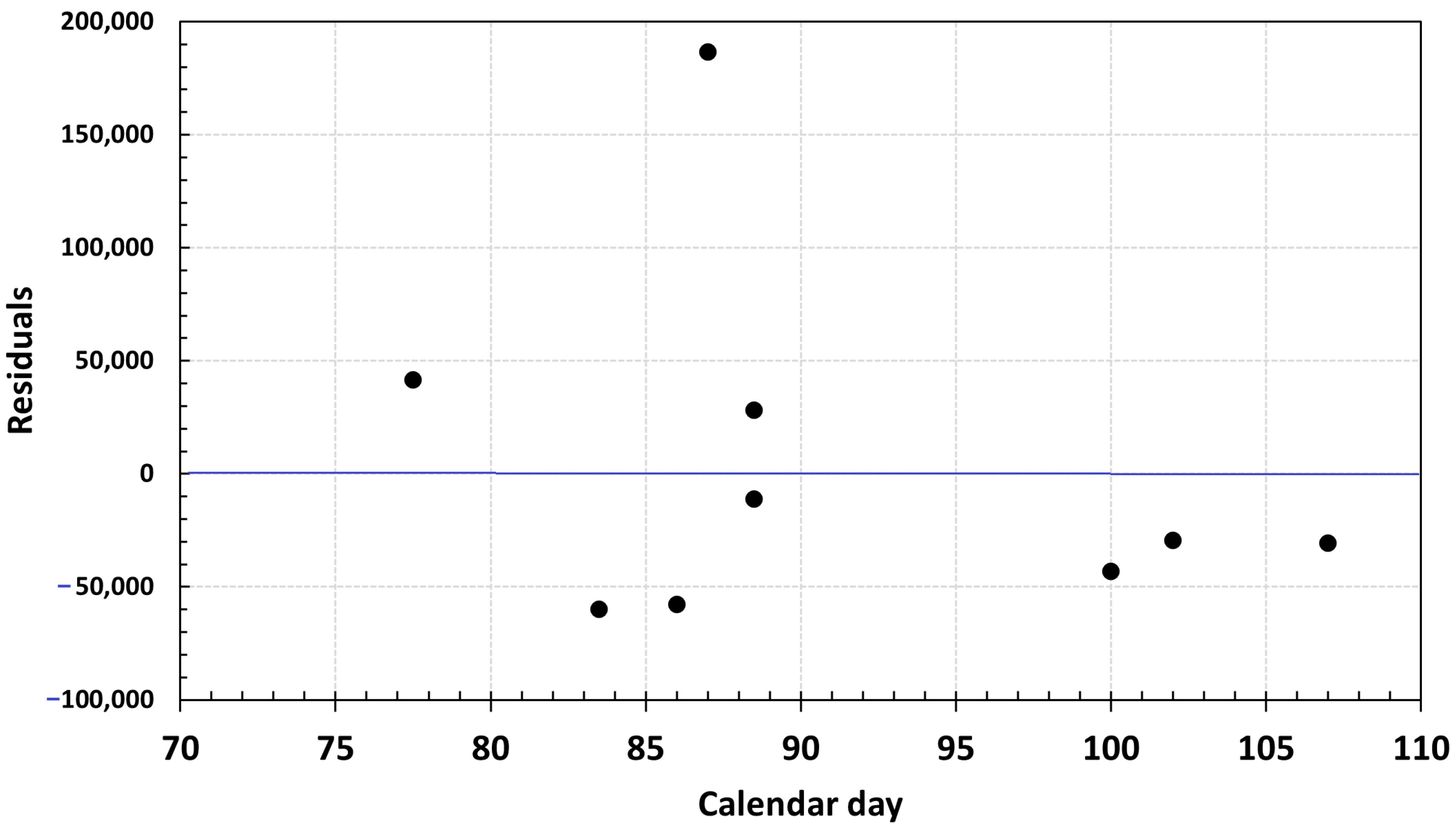
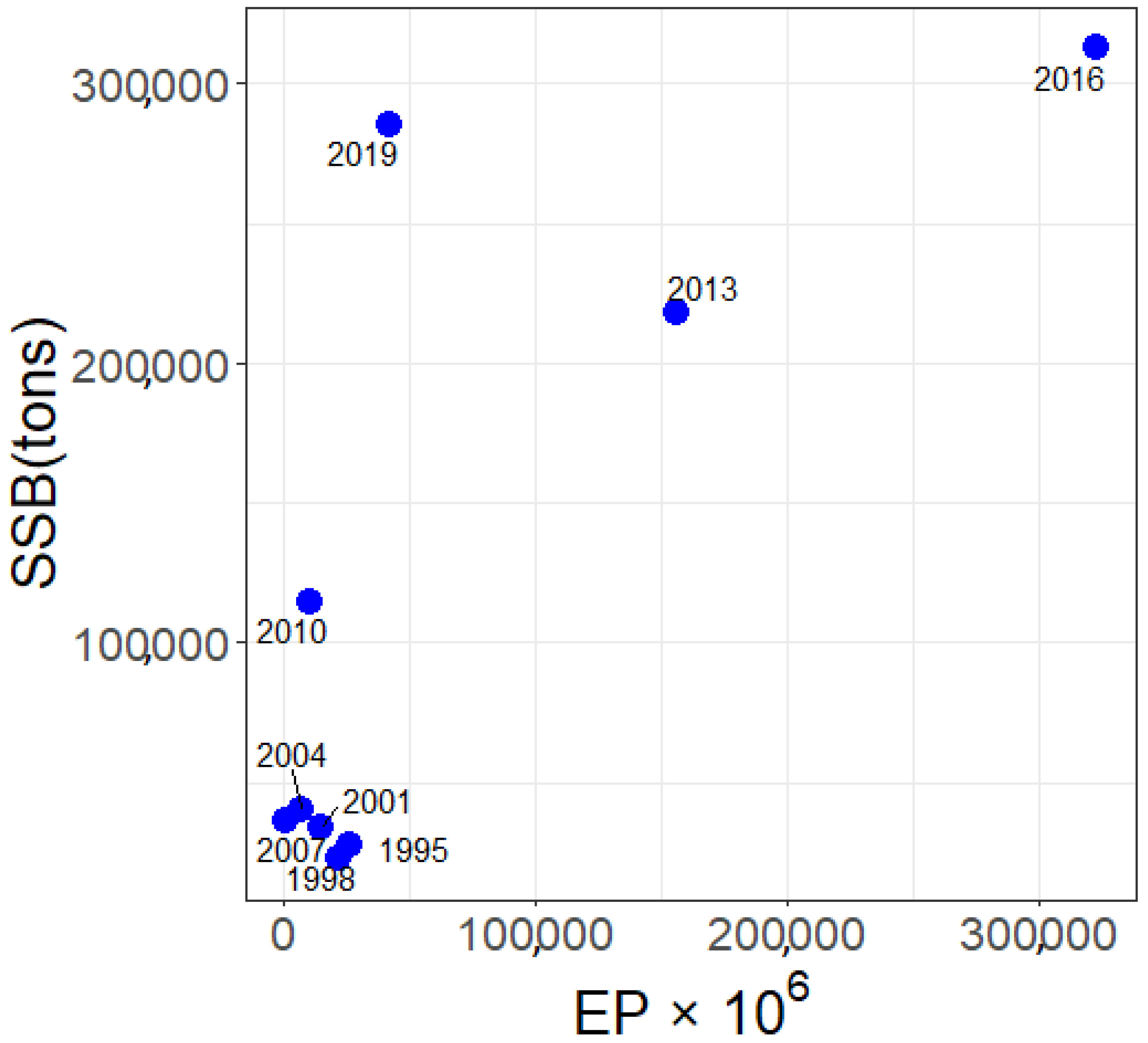
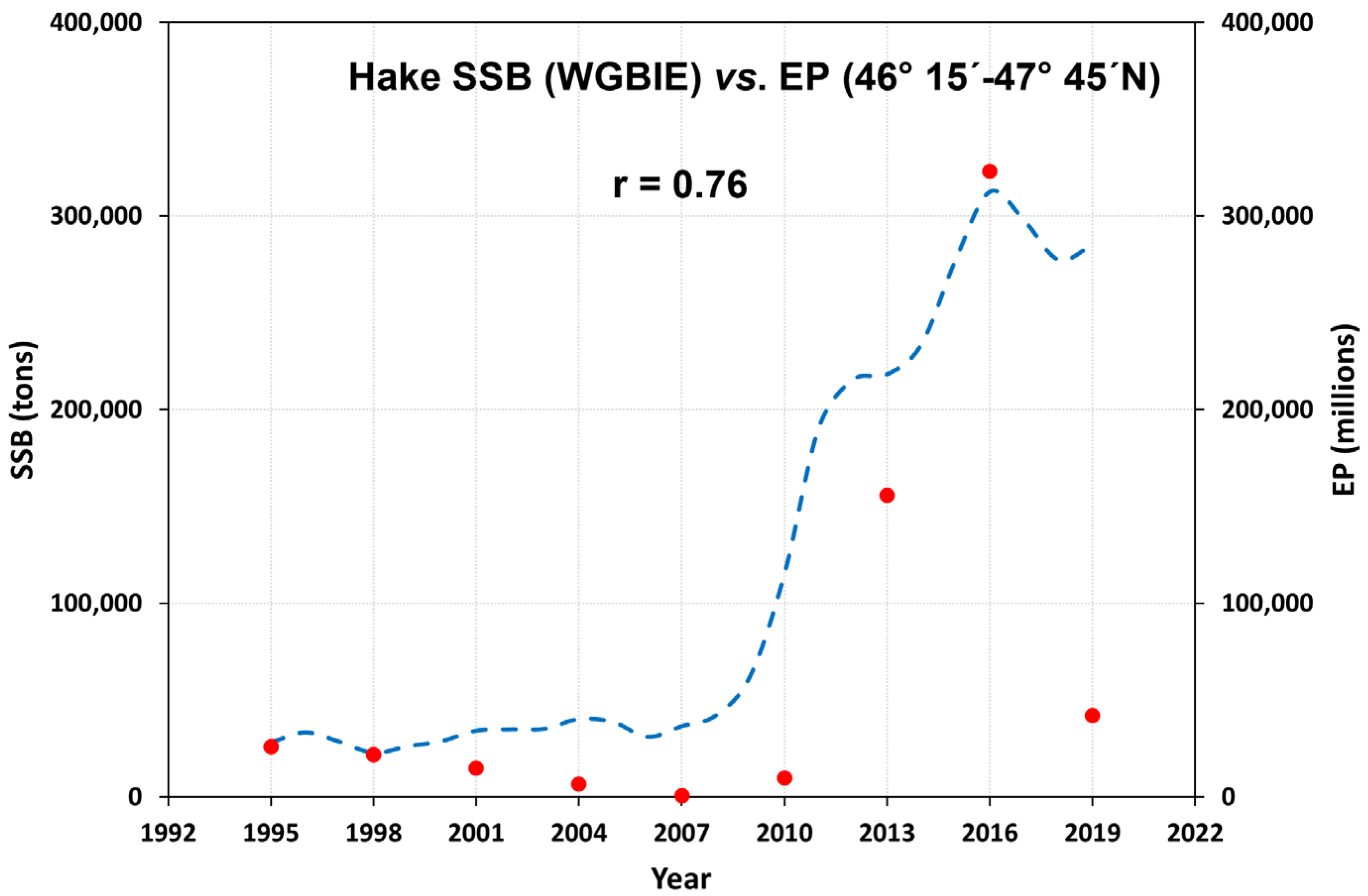
3.1. Eggs and SSB Correlation
3.2. Larval Survival Estimates
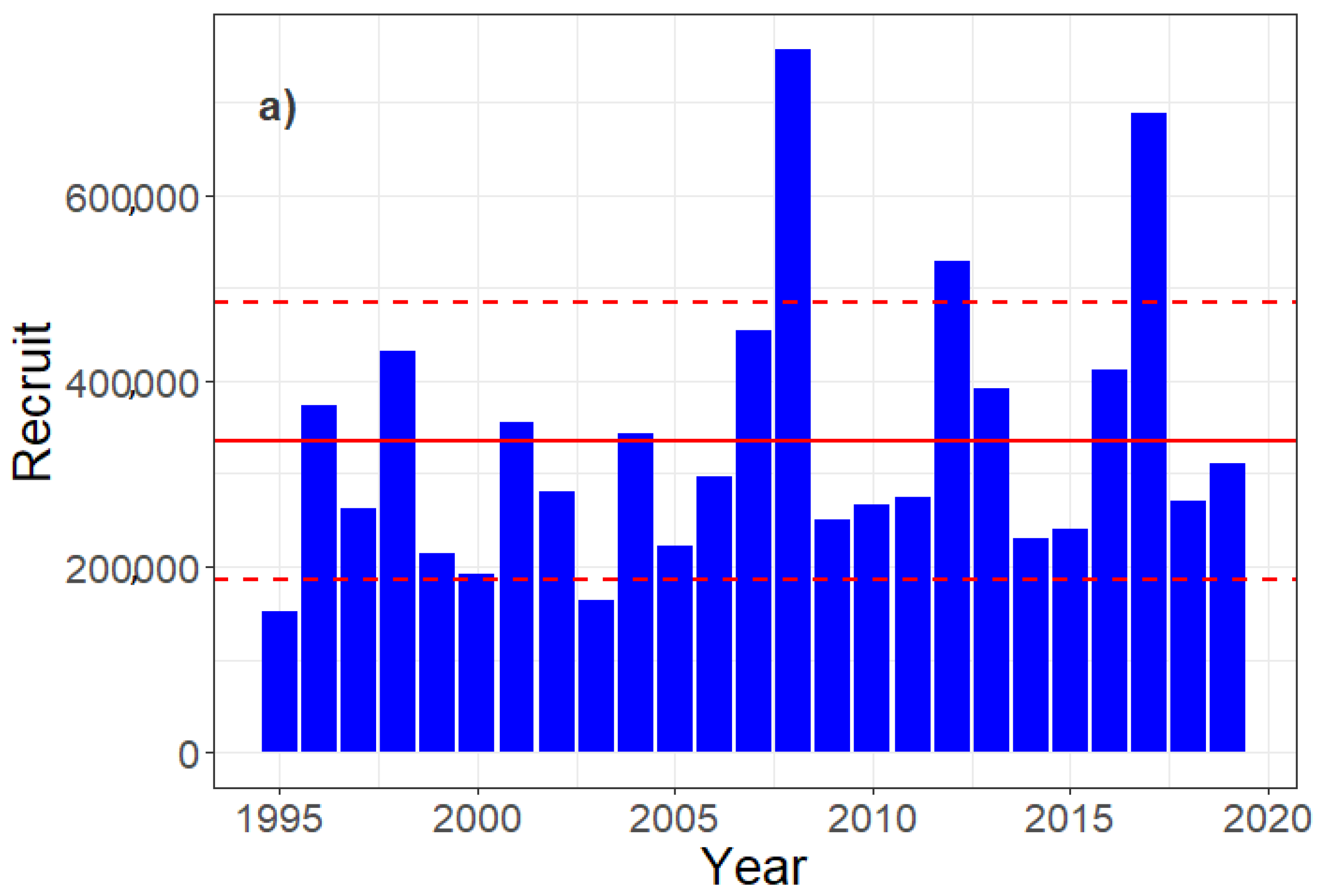
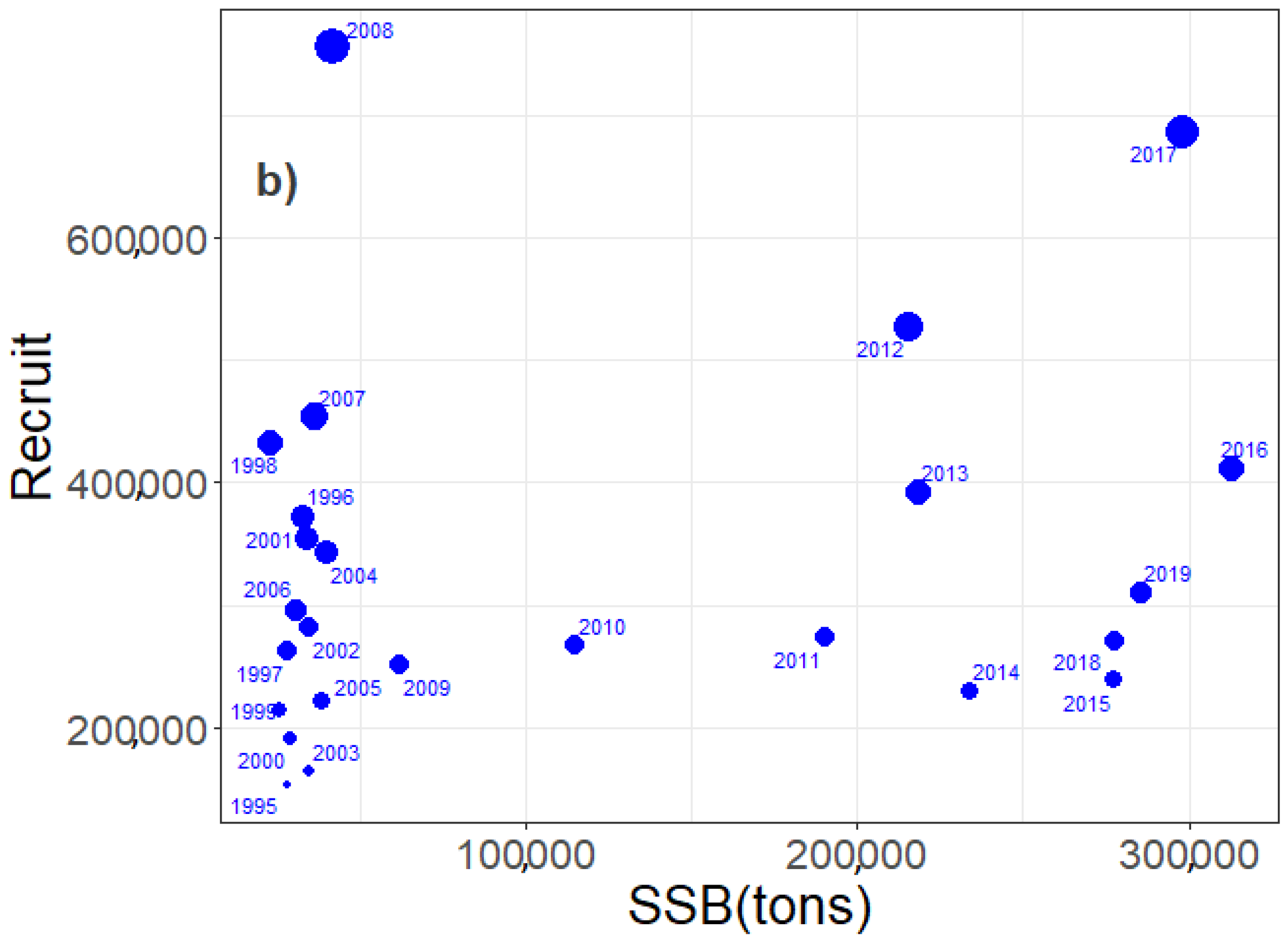
3.3. Egg, Larvae, and Recruit Correlations
4. Discussion
4.1. Hake Egg Index as a Proxy for SSB Abundance
4.2. Determination of the Year–Class Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunderson, D.R. Survey and Fisheries Resources; John Willey and Sons: New York, NY, USA, 1993; p. 248. [Google Scholar]
- Rotherham, D.; Underwood, A.J.; Chapman, M.G.; Gray, C.A. A strategy for developing scientific sampling tools for fishery-independent surveys of estuarine fish in New South Wales, Australia. ICES J. Mar. Sci. 2007, 64, 1512–1516. [Google Scholar] [CrossRef]
- Pennino, M.G.; Conesa, D.; Lopez-Quılez, A.; Muñoz, F.; Fernandez, A.; Bellido, J.M. Fishery-dependent and -independent data lead to consistent estimations of essential habitats. ICES J. Mar. Sci. 2016, 73, 2302–2310. [Google Scholar] [CrossRef]
- Murua Aurizenea, H. Reproductive fundamentals for the estimation of egg reproduction of the European hake, Merluccius merluccius, in the Bay of Biscay. Ph.D. Dissertation, Zoology and Animal Cell Biology, Euskal Herriko Univertsitatea, Leioa, Spain, 2006. [Google Scholar]
- ICES. Working Group for the Bay of Biscay and the Iberian Waters Ecoregion (WGBIE); ICES Scientific Reports: Toronto, ON, Canada, 2020; Volume 2, p. 845. [Google Scholar] [CrossRef]
- Quinn, T.J.; Deriso, I. Quantitative Fish Dynamics; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Lasker, R. An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy, Engraulis Mordax; NOAA Technical Report: Springfield, VA, USA, 1985; Volume 36, p. 99. [Google Scholar]
- Ralston, S.; Bence, J.R.; Eldridge, M.B.; Lenarz, W.H. An approach to estimating rockfish biomass based on larval production, with application to Sebastes jordani. Fish. Bull. 2003, 101, 129–146. [Google Scholar]
- Murua, H.; Ibaibarriaga, L.; Alvarez, P.; Santos, M.; Korta, M.; Santurtun, M.; Motos, L. The daily egg production method: A valid tool for application to European hake in the Bay of Biscay? Fish. Res. 2010, 104, 100–110. [Google Scholar] [CrossRef]
- Houde, E.D. Patterns and Consequences of Selective Processes in Teleost Early Life Histories; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Ibaibarriaga, L.; Irigoien, X.; Santos, M.; Motos, L.; Fives, J.; Franco, C.; Bez, N.; Eltink, G.; Farina, A.; Hammer, C.; et al. Egg and larvae distributions of seven fish species in the north-east Atlantic waters. Fish. Oceanogr. 2007, 16, 284–293. [Google Scholar] [CrossRef]
- Houde, E.D. Emerging from Hjort’s Shadow. J. Northw. Atl. Fish. Sci. 2008, 41, 53–70. [Google Scholar] [CrossRef]
- Bailey, K.M.; Houde, E.D. Predation on eggs and larvae of marine fishes and the recruitment problem. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1989; Volume 25, pp. 1–83. [Google Scholar]
- Cushing, D.H. Marine Ecology and Fisheries; Cambridge University Press: Cambridge, UK, 1975; p. 278. [Google Scholar]
- Cushing, D.H. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 250–293. [Google Scholar]
- Hjort, J. Fluctuations in the Great Fisheries of Northern Europe Viewed in the Light of Biological Research. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 1914, 20, 1–228. [Google Scholar]
- Lasker, R. The relation between oceanographic conditions, and larval anchovy food in the California Current: Identification of factors contributing to recruitment failure. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 1978, 173, 212–230. [Google Scholar]
- Van der veer, H.; Geffen, A.J.; Witte, J.I. Exceptionally strong year classes in plaice Pleuronectes platessa: Are they generated during the pelagic stage only, or also, in the juvenile stage? Mar. Ecol. Prog. Ser. 2000, 199, 255–262. [Google Scholar] [CrossRef]
- Van der veer, H.W.; Witte, J.I. Year class strength of plaice Pleuronectes platessa in the Southern Bight of the North Sea: A validation and analysis of the inverse relationship with winter sea seawater temperature. Mar. Ecol. Prog. Ser. 1999, 184, 245–257. [Google Scholar] [CrossRef]
- Zijlstra, J.J.; Witte, J.I. On the recruitment of 0-group plaice in the North Sea. Neth. J. Zool. 1985, 35, 360–376. [Google Scholar] [CrossRef]
- Watanabe, Y.; Zenitani, H.; Kimura, R. Population decline of the Japanese sardine Sardinops melanostictus owing to recruitment failure. Can. J. Fish. Aquat. Sci. 1995, 52, 1609–1616. [Google Scholar] [CrossRef]
- Nash, R.D.M.; Dickey-Collas, M. The influence of life history dynamics and environment on the determination of year class strength in North Sea herring (Clupea harengus L.). Fish. Oceanogr. 2005, 14, 279–291. [Google Scholar] [CrossRef]
- Oeberst, R.; Klenz, B.; Gröhsler, T.; Dickey-Collas, M.; Nash, R.D.M.; Zimmermann, C. When is year-class strength determined in western Baltic herring? ICES J. Mar. Sci. 2009, 66, 1667–1672. [Google Scholar] [CrossRef]
- Boyra, G.; Martínez, U.; Cotano, U.; Santos, M.; Irigoien, X.; Uriarte, A. Acoustic surveys for juvenile anchovy in the Bay of Biscay: Abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns. ICES J. Mar. Sci. 2013, 70, 1354–1368. [Google Scholar] [CrossRef]
- Murua, H.; Motos, L.; Lucio, P. Reproductive modality and batch fecundity of the European Hake (Merluccius merluccius L.) in the Bay of Biscay. Calif. Rep. 1998, 39, 196–203. [Google Scholar]
- Murua, H.; Motos, L. Reproductive strategy and spawning activity of the European hake Merluccius merluccius (L.) in the Bay of Biscay. J. Fish Biol. 2006, 69, 1288–1303. [Google Scholar] [CrossRef]
- Alvarez, P.; Motos, L.; Uriarte, A.; Egaña, J. Spatial and temporal distribution of European hake, Merluccius merluccius (L.), eggs and larvae in relation to hydrographical conditions in the Bay of Biscay. Fish. Res. 2001, 50, 111–128. [Google Scholar] [CrossRef]
- Alvarez, P.; Fives, J.; Motos, L.; Santos, M. Distribution and abundance of european hake Merluccius merluccius (L.), eggs and larvae in the Northe-east atlantic waters in 1995 and 1998 in relation to hydrographic conditions. J. Plankton Res. 2004, 26, 811–826. [Google Scholar] [CrossRef]
- Staby, A.; Skjæraasen, J.E.; Geffen, A.J.; Howell, D. Spatial and temporal dynamics of European hake (Merluccius merluccius) in the North Sea. ICES J. Mar. Sci. 2018, 75, 2033–2044. [Google Scholar] [CrossRef]
- ICES. SISP 6. Manual for the Mackerel and Horse Mackerel Egg Surveys (MEGS): Sampling at Sea; Version 2.2.; Series of ICES Survey Protocols 2019; ICES: Toronto, ON, Canada, 2019; p. 82. [Google Scholar]
- Gehringer, J.W. An all-metal plankton sampler (Model Gulf III). Spec. Scient. Rep. U. S. Fish Wildl. Servo Fish. 1952, 88, 7–12. [Google Scholar]
- Porebski, J. Application of the Surface Adhesion Test to identify the egg of the hake Merluccius spp. ICSEF Hake RC Surv. 1975, 2, 102–106. [Google Scholar]
- Coombs, S.H.; Mitchell, C.E. The development rate of eggs and larvae of the hake, Merluccius merluccius (L.) and their distribution to the west of the British Isles. J. Cons. Int. Explor. Mer. 1982, 40, 119–126. [Google Scholar] [CrossRef]
- Smith, P.E.; Richardson, S.L. Standard techniques for pelagic fish eggs and larval survey. FAO Fish. Tech. Pap. 1977, 175, 27–73. [Google Scholar]
- Fowler, G.M.; Smith, S.J. Length changes in silver hake (Merluccius bilinearis) larvae: Effects of formalin, ethanol, and freezing. Can. J. Fish. Aquat. Sci. 1983, 40, 866–870. [Google Scholar] [CrossRef]
- Alvarez, P.; Cotano, U. Growth, mortality and hatch-date distributions of European hake larvae, Merluccius merluccius (L.), in the Bay of Biscay. Fish. Res. 2005, 76, 379–391. [Google Scholar] [CrossRef]
- ICES. Working Group for the Bay of Biscay and the Iberian Waters Ecoregion (WGBIE); ICES Scientific Reports: Toronto, ON, Canada, 2019; Volume 1, p. 692. [Google Scholar] [CrossRef]
- Heath, M. An evaluation an review of the ICES herring larval surveys in the North Sea and adjacent waters. Bull. Mar. Sci. 1993, 53, 795–817. [Google Scholar]
- Methot, R.D.; Wetzel, C. Stock synthesis: A biological and statistical framework for fish stock assessment and fishery management. Fish. Res. 2013, 142, 86–99. [Google Scholar] [CrossRef]
- Shima, M.; Bailey, K.M. Comparative analysis of ichthyoplankton sampling gear for early life stages of walleye pollock (Theragra chalcogramma). Fish. Oceanogr. 1994, 3, 50–59. [Google Scholar] [CrossRef]
- Heyer, L.J.; Kruglyak, S.; Yooseph, S. Exploring Expression Data: Identification and Analysis of Coexpressed Genes. Genome Res. 1999, 9, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Köster, F.W.; Huwer, B.; Kraus, G.; Diekmann, R.; Eero, M.; Makarchouk, A.; Örey, S.; Dierking, J.; Margonski, P.; Herrmann, J.P.; et al. Egg production methods applied to Eastern Baltic cod provide indices of spawning stock dynamics. Fish. Res. 2020, 227, 105553. [Google Scholar] [CrossRef]
- ICES. ICES Working Group on Mackerel and Horse Mackerel Egg Surveys (WGMEGS: Outputs from a 2020 Meeting); ICES: Toronto, ON, Canada, 2021; Volume 3, p. 88. [Google Scholar]
- Stratoudakis, Y.; Bernal, M.; Ganias, K.; Uriarte, A. The daily egg production method: Recent advances, current applications and future challenges. Fish Fish. 2006, 7, 35–57. [Google Scholar] [CrossRef]
- Lockwood, S.J.; Nichols, J.H.; Dawson, W. The estimation of a mackerel (Scomber scombrus L.) spawning stock size by plankton survey. J. Plankton Res. 1981, 3, 217–233. [Google Scholar] [CrossRef]
- Bernal, M.; Stratoudakis, M.; Wood, Y.; Ibaibarriaga, S.; Valdés, L.; Borchers, D. A revision of daily egg production estimation methods, with application to Atlanto-Iberian sardine. 2. Spatially and an environmentally explicit estimates of egg production. ICES J. Mar. Sci. 2011, 68, 528–536. [Google Scholar] [CrossRef]
- Motos, L.; Uriarte, A.; Valencia, V. The spawning environment of the Bay of Biscay anchovy (Engraulis encrasicolus L.). Sci. Mar. 1996, 60, 117–140. [Google Scholar]
- Butler, J.L.; Jacobson, L.D.; Barnes, J.T.; Moser, H.G. Biology and population dynamics of cowcod (Sebastes levis) in the southern California Bight. Fish. Bull.-Natl. Ocean. Atmos. Adm. 2003, 101, 260–280. [Google Scholar]
- Shinohara, N.; Nishijima, S.; Ichinokawa, M.; Yukami, R. Standardizing Monthly Egg Survey Data as an Abundance Index for Spawning Stock Biomass of Chub Mackerel in the Northwest Pacific; ICES Document NPFC-2021-TWG CMSA04-WP04; ICES: Toronto, ON, Canada, 2021. [Google Scholar]
- Bruge, A.; Alvarez, P.; Fontan, A.; Cotano, G.; Chust, U. Thermal niche tracking and future distribution of Atlantic mackerel spawning in response to ocean warming. Front. Mar. Sci. 2016, 3, 86. [Google Scholar] [CrossRef]
- Brunel, T.; van Damme, C.J.G.; Samson, M.; Dickey-Collas, M. Quantifying the influence of geography and environment on the northeast Atlantic mackerel spawning distribution. Fish. Oceanogr. 2018, 27, 159–173. [Google Scholar] [CrossRef]
- Bernal, M.; Stratoudakis, Y.; Coombs, S.; Angelico, M.M.; Lago de Lanzós, A.; Porteiro, C.; Sagarminaga, Y.; Santos, M.; Uriarte, A.; Cunha, E.; et al. Sardine spawning off the European Atlantic coast: Characterization of and spatio-temporal variability in spawning habitat. Prog. Oceanogr. 2007, 74, 210–227. [Google Scholar] [CrossRef]
- Myers, R. Stock and recruitment: Generalizations about maximum reproductive rate, density dependence, and variability using meta-analytic approaches. ICES J. Mar. Sci. 2001, 58, 937–951. [Google Scholar] [CrossRef]
- Hunter, J.R. The Feeding Behaviour and Ecology of Marine Fish Larvae. In Fish Behavior and Its Use in the Capture and Culture of Fishes, Proceedings of the Conference on the Physiological and Behavioral Manipulation of Food Fish as Production and Management Tools, Bellagio, Italy, 3–8 November 1977; Bardach, J.E., Magnuson, J.J., May, R.C., Reinhart, J.M., Eds.; WorldFish: Penang, Malaysia, 1980; pp. 287–326. [Google Scholar]
- Guevara-Fletcher, C.; Alvarez, P.; Sanchez, J.; Iglesias, J. The effect of temperature on the development of yolk-sac larvae of European hake (Merluccius merluccius L.) under laboratory conditions. Aquac. Res. 2016, 48, 1392–1405. [Google Scholar] [CrossRef]
- Marrale, D.; Alvarez, P.; Motos, L. Early life development and identification of European hake, Merluccius merluccius L. Ozeanografika 1996, 1, 5–26. [Google Scholar]
- Houde, E.D. Comparative growth, mortality, and energetics of marine fish larvae: Temperature and implied latitudinal effects. Fish. Bull. 1989, 87, 471–495. [Google Scholar]
- Payne, M.R.; Hatfield, E.M.C.; Dickey-Collas, M.; Falkenhaug, T.; Gallego, A.; Gröger, J.; Licandro, P.; Llope, M.; Munk, P.; Röckmann, C.; et al. Recruitment in a changing environment: The 2000s North Sea herring recruitment failure. ICES J. Mar. Sci. 2009, 66, 272–277. [Google Scholar] [CrossRef]
- Bjelland, R.M.; Skiftesvik, A.B. Larval development in European hake (Merluccius merluccius L.) reared in a semi-intensive culture system. Aquac. Res. 2006, 37, 1117–1129. [Google Scholar] [CrossRef]
- Olivar, M.P.; Fortuño, J.M. Guide to ichthyoplankton of the Southeast Atlantic (Benguela Current region). Sci. Mar. 1991, 55, 1–383. [Google Scholar]
- Sumida, B.Y.; Moser, G. Food and feeding of pacific hake larvae, Merlucius productus, off sourthern California and Northern Baja California. Calif. Rep. 1980, XXI, 161–166. [Google Scholar]
- Pillar, S.C.; Wilkinson, I.S. The diet of Cape hake Merluccius capensis on the south coast of South Africa. S. Afr. J. Mar. Sci. 1995, 15, 225–239. [Google Scholar] [CrossRef]
- Velasco, F.; Olaso, I. Hake Food Consumption in the Southern Bay of Biscay Estimated from a Gastric Evacuation Model; ICES CM: Toronto, ON, Canada, 2000; Volume Q11, pp. 1–15. [Google Scholar]


| Year | Vessel | Survey in Bay of Biscay | Period | Gear | No. Samples |
|---|---|---|---|---|---|
| 1995 | Investigador | 22 March–1 April | March | B-60 | 62 |
| 1998 | W. Herwig | 15–31 March | March | Nackthai | 27 |
| C. Saavedra | 21–22 April | April | B-40 | 9 | |
| Tridens | 21–30 April | April | G-III | 59 | |
| 2001 | W. Herwig | 31 March–11 April | April | Nackthai | 77 |
| Investigador | 11–18 April | April | B-40 | 44 | |
| 2004 | Investigador | 24 March–11 April | March–April | B-40 | 75 |
| Endeavor | 27 April–7 May | April–May | G-VII | 44 | |
| 2007 | Itsaslagunak | 2–22 April | April | B-40 | 44 |
| 2010 | Investigador | 23 March–14 April | March–April | B-40 | 37 |
| 2013 | A. Alvariño | 22 March–6 April | March–April | B-40 | 64 |
| 2016 | R. Margalef | 19 March–7 April | March–April | B-40 | 50 |
| 2019 | R. Margalef | 19 March–6 April | March–April | B-40 | 76 |
| Index | Age Class (Days) | SL Class (mm) | ||
|---|---|---|---|---|
| Min | Max | Min | Max | |
| N < 10 | 0 | 10 | 2 | 3 |
| N > 10 | 11 | 52 | 3.1 | 13 |
| N > 15 | 15 | 52 | 4.1 | 13 |
| N > 25 | 25 | 52 | 6.1 | 13 |
| Ntot | 0 | 52 | 2 | 13 |
| Index | Comment |
|---|---|
| Recruit_BIE | SS3-derived age-0 recruitment for NSH [37] |
| EVHOE | Juveniles index based on EVHOE bottom trawl surveys |
| YCI 10 | Abundance of hake < 10 cm based on EVHOE surveys |
| YCI 20 | Abundance of hake 11–20 cm based on EVHOE surveys |
| N (age) | Larval index for individual by age (see Table 2) |
| Year | Month | Total Area | Positive Area | EP (Egg/Day) | T20m | Positive Area | SSB |
|---|---|---|---|---|---|---|---|
| km2 | km2 | × 106 | (°C) | % | ton | ||
| 1995 | 3 | 21,094 | 9261 | 25,972 | 11.9 | 44% | 57,940 |
| 1998 | 3–4 | 149,680 | 16,903 | 21,671 | 11.9 | 11% | 42,821 |
| 2001 | 3–4 | 90,549 | 10,490 | 14,931 | 12.0 | 11% | 51,936 |
| 2004 | 4 | 117,775 | 6320 | 6774 | 11.7 | 5% | 61,989 |
| 2007 | 4 | 71,523 | 2117 | 782 | 12.1 | 3% | 59,449 |
| 2010 | 3–4 | 77,982 | 10,549 | 9915 | 11.02 | 13% | 186,608 |
| 2013 | 3–4 | 103,434 | 23,117 | 155,830 | 11.28 | 22% | 259,818 |
| 2016 | 3–4 | 88,472 | 27,372 | 322,985 | 11.50 | 31% | 333,329 |
| 2019 | 3–4 | 103,079 | 25,176 | 41,969 | 11.63 | 24% | 298,571 |
| Año | SL (SD) mm | L0/m2 | Z | R2 (%) | p | SvR | T20m |
|---|---|---|---|---|---|---|---|
| 1995 | 5.3 (0.87) | 150.08 | −0.651 | 88.9 | 0.0014 | 0.56 | 12.3 |
| 1998 | 4.0 (0.66) | 3.95 | −0.3365 | 26.2 | 0.0510 | 0.71 | 12.5 |
| 2001 | 5.0 (1.12) | 52.18 | −0.4957 | 52.1 | 0.0048 | 0.61 | 12.5 |
| 2004 | 4.1 (0.79) | 5.83 | −0.3245 | 34.4 | 0.0265 | 0.72 | 11.5 |
| 2007 | 5.0 (0.55) | 3.31 | −0.2983 | 24.4 | 0.0585 | 0.74 | 12.6 |
| 2010 | 4.3 (0.89) | 77.82 | −0.5765 | 80.8 | 0.0000 | 0.56 | 11.6 |
| 2013 | 4.8 (1.14) | 371.33 | −0.5451 | 73.1 | 0.0002 | 0.58 | 11.3 |
| 2016 | 3.8 (0.65) | 69.72 | −0.5812 | 66.3 | 0.0008 | 0.56 | 11.6 |
| Recruit_BIE | EVHOE | YCI10 | YCI20 | |
|---|---|---|---|---|
| N < 10 | 0.177 | 0.700 | 0.863 | 0.328 |
| N > 10 | 0.237 | 0.000 | −0.177 | 0.158 |
| N > 15 | 0.453 | −0.058 | −0.215 | 0.096 |
| N > 25 | 0.233 | −0.058 | −0.284 | 0.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, P.; Garcia, D.; Cotano, U. Investigating the Applicability of Ichthyoplanktonic Indices in Better Understanding the Dynamics of the Northern Stock of the Population of Atlantic Hake Merluccius merluccius (L.). Fishes 2023, 8, 50. https://doi.org/10.3390/fishes8010050
Alvarez P, Garcia D, Cotano U. Investigating the Applicability of Ichthyoplanktonic Indices in Better Understanding the Dynamics of the Northern Stock of the Population of Atlantic Hake Merluccius merluccius (L.). Fishes. 2023; 8(1):50. https://doi.org/10.3390/fishes8010050
Chicago/Turabian StyleAlvarez, Paula, Dorleta Garcia, and Unai Cotano. 2023. "Investigating the Applicability of Ichthyoplanktonic Indices in Better Understanding the Dynamics of the Northern Stock of the Population of Atlantic Hake Merluccius merluccius (L.)" Fishes 8, no. 1: 50. https://doi.org/10.3390/fishes8010050
APA StyleAlvarez, P., Garcia, D., & Cotano, U. (2023). Investigating the Applicability of Ichthyoplanktonic Indices in Better Understanding the Dynamics of the Northern Stock of the Population of Atlantic Hake Merluccius merluccius (L.). Fishes, 8(1), 50. https://doi.org/10.3390/fishes8010050









