Abstract
Global warming implies the risk of a changing oxygen regime in the seas and oceans of our planet. The mitochondrial complex of nuclear erythrocytes of cartilaginous fish, as the energy basis of blood cells, has repeatedly encountered such climatic fluctuations throughout their evolutionary history. In this regard, the features of the adaptive strategy of the erythrocyte mitochondrial complex in the thornback ray (Raja clavata L.) are of interest from the evolutionary and ecological points of view. The rate of oxygen consumption in resuspended (Ht = 25–30%) erythrocytes taken from the Black Sea thornback ray in saline was studied by the polarographic method. A high “basal” rate of respiration in the erythrocytes of the thornback ray was shown, which ranged from 10.5 to 21.6 pmol O2 min−1·106 cells. The addition of substrates of the mitochondrial respiration activators glutamate, maleate, and succinate to the erythrocyte suspension caused a 2–6-fold increase in the respiratory activity of thornback ray erythrocytes. In cases where the rate of respiration of erythrocytes was high, protonophore–dinitrophenol caused an inhibition of the activity of mitochondrial respiration. At low respiration rates of erythrocytes, its effect was opposite and caused a stimulation of mitochondrial respiration. Oligomycin caused a significant inhibition of the respiratory activity of the red blood cell suspension of the thornback ray. This suppression of cell respiration was enhanced under conditions of exposure to the permeabilization of erythrocytes with digitonin. This can be recommended as one of the ways to block the respiratory activity of erythrocytes in cartilaginous fish. Another way of effectively blocking the respiration of the mitochondrial complex of the thornback ray’s erythrocytes was the effect of the blockers rotenone and sodium azide. The peculiarity of the mitochondria of the erythrocytes of the thornback ray was the absence of the complete inhibition of respiration by sodium azide (NaN3), which is characteristic of the mitochondria of other fish species. Our data on the activation of the “respiration” of erythrocytes in fish indicate that the potential capabilities of cold-blooded and warm-blooded vertebrates have rather similar characteristics. This may indicate the initial “laying” of the architecture of the inner membrane to support the energy potential of the mitochondria of the cell.
1. Introduction
Rays are ancient vertebrates that evolved from armored jawless fish at the end of the Silurian period, more than 420 million years ago [1]. These animals survived a succession of many events in the geological and biological evolution of our planet. They evolutionarily “witnessed” the splitting of a single land mass into modern continents and the birth of oceans. During their long history of life, cartilaginous fish adapted to significant fluctuations in oxygen in the atmosphere and water, e.g., warm climatic conditions have been replaced by ice ages more than once. In connection with the current problems of global warming and the changing oxygen regime, it is necessary to understand what molecular biochemical adaptations contributed to such a high viability in these aquatic organisms [2].
Cartilaginous species are interesting, not only from an evolutionary point of view but also because they have high morphophysiological and biochemical plasticity. One of the clearest examples of such plasticity is the nuclear erythrocytes of these animals, thanks to which it became possible to provide the energy basis of the body for the successful existence of rays in a rapidly changing world [3]. It should be noted that the contents of the nucleus and other organelles, including mitochondria, in erythrocytes is a phenomenon that applies to all vertebrates, with the exception of mammals. Therefore, the nuclear erythrocytes of vertebrates have the metabolic capacity for the aerobic and anaerobic production of nucleoside triphosphates (NTPs) [2,4]. In mammals, erythrocytes lose their nucleus and other organelles in the process of ontogenesis and thereby free up intracellular space for hemoglobin, showing the maximum level of oxygen transport function among vertebrates. Such a reduction for mammalian erythrocytes does not pass without a trace, and with the loss of mitochondria they lose the main potential for ATP resynthesis and their ability to synthesize intracellular proteins. This evolutionary change turned mammalian erythrocytes into cells with a glycolytic type of metabolism. This metabolic aspect significantly reduces their lifetimes due to the loss of the reparation capabilities that these cells need due to the large deformation loads that pursue erythrocytes throughout their entire life cycle [5]. Fish erythrocytes endowed with mitochondria belong to cells with anaerobic and anaerobic types of metabolism. The importance of this aspect was confirmed by experimental studies, which showed that, under normal oxygen conditions, Oncorhynchus mykiss erythrocytes produce 99% of the total amount of NTP aerobically through mitochondrial “respiration” [6]. Other authors [7] researching rainbow trout erythrocytes showed that the findings were not so categorical and accounted for only 70% of the total volume of cellular metabolism under the normal regime of oxygen supply. Little is known about the energy metabolism of cartilaginous fish erythrocytes. The activity of mitochondria in erythrocytes of the ray Torpedo marmorata was studied by [5]. The data presented the quantitative characteristics of the activity of mitochondrial enzymes and the rate of the respiration of erythrocytes under the influence of various activators and inhibitors. Erythrocytes of T. marmorata, under in vitro conditions, demonstrated active oxygen consumption, which indicated the aerobic orientation of the metabolism of these cells [8]. In our opinion, research on the Black Sea ray can help expand the understanding of the functional activity of mitochondria in these evolutionarily ancient organisms and the understanding of the principles of the formation of the oxidative metabolism of nuclear erythrocytes in vertebrates.
Erythrocyte respiratory activity was studied with the use of activators and inhibitors. The proton and electron transport chains in fish mitochondria are practically no different from those in more highly organized vertebrate taxa [9]. The proton flow through the inner mitochondrial membrane (ATP synthesis) and the electronic flow along the membrane (oxygen reduction on cytochrome oxidase) are interconnected and change in parallel [10]. Thus, the control of mitochondrial respiration is nothing more than the modulation of proton fluxes. This modulation can be controlled artificially using activators and inhibitors. Malate paired with glutamate has traditionally been used in in vitro studies to study the properties of mitochondrial respiration in different cells [11]. Succinate is a substrate of mitochondrial complex II [12]. These substrates increase the flow of protons and electrons in mitochondria and thus stimulate their respiratory activity. Oligomycin (Olig), digitonin (Dig), dinitrophenol (DNF), rotenone (Rot), sodium azide (NaN3), and other substrates, on the contrary, are inhibitors of proton currents and thus inhibit mitochondrial respiration [13]. The use of these substances allowed us to assess the energy potential of the mitochondrial complex of erythrocytes of the studied species.
The study of the energetic capacity of erythrocytes of the Black Sea thornback ray is also interesting because these erythrocytes are highly resistant cells after their isolation. When stored in cold conditions at (+4 °C), they did not undergo hemolysis for up to 11 days. In addition, erythrocytes of the studied thornback rays are fairly large cells. Their linear dimensions exceeded 20 µm in length and 15 µm in width. The study of their energy potential is important, in a comparative aspect, since the linear dimensions of erythrocytes of bony fish are noticeably smaller and, most often, are only half of those indicated for the red blood cells of the Black Sea thornback ray. In this regard, the study of the energy of large cartilaginous fish erythrocytes can reflect the features of their autonomy, life cycle, and behavior in the bloodstream as well as their deformation capabilities. Thus, the aim of this study was to study the activity of the mitochondrial complex of erythrocytes of the thornback ray (Raja clavata L.) as an object of interest from an evolutionary and ecological point of view.
2. Material and Methods
2.1. Experimental Design
The experimental design is presented in Figure 1. First, the activity of the mitochondrial complex of the thornback ray erythrocytes was taken and prepared from the blood of this fish (see the protocol in Section 2.2). Second, the exposure to 2,4-dinitrophenol (DNP), an effective protonophore of the inner mitochondrial membrane, was studied by the measurement of the respiration rate. In the third step, the demonstration of the action of activators and inhibitors of mitochondrial respiration of the thornback ray erythrocytes was studied against the background of an ATP synthase blocker, oligomycin A. Additionally, the acceleration of the “respiration” of the thornback ray erythrocytes with the help of (G + M) and ADP was investigated.
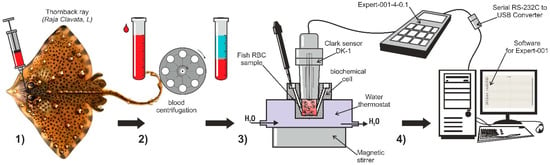
Figure 1.
Scheme of the experiment for the respiration study of the thornback ray erythrocytes (Raja clavata L.). (1) Taking blood from a stingray; (2) obtaining a suspension of erythrocytes by centrifugation; (3) polarographic cell with a Clark electrode for measuring the rate of oxygen utilization by skate erythrocytes; (4) recording part—polarograph “Expert-001 MTX” and computer.
The evolution of the effect of the inhibitors was studied in the way the chemical substances were added sequentially in order to study the inhibition of mitochondrial activities. For this study, five biological variations and four consecutive experiments were used, including two permeabilization and two nonpermeabilization experiments.
2.2. Preparation of Erythrocyte Suspensions
The rates of oxygen uptake were studied in erythrocyte suspensions of the thornback ray (Raja clavata L.). The fish were caught in the coastal waters in the area of Karadag Bay (South-eastern Crimea; 44°54′32.5″ N, 35°12′26.0″ E) using bottom gear with hooks to catch rays. The caught fish were placed in pools with sea water and artificial aeration. The thornback rays were kept in a 25,000 L seawater tank. After the catch, rays were kept for 7 days for acclimation, and the fish were kept in conditions close to the environment, with a temperature of 14–18 °C. The aeration and filtration of water was constant, and every 3 days the new fresh filtered seawater was replaced by 25% of the total volume. For the blood sampling, the rays were not anesthetized. The blood sampling was very fast. A fish was turned on its back, and blood was drawn from the heart during a 2–3 min procedure. The fish guts were kept wet during this procedure [6]. After that, rays were kept in the tank, and when they started regularly feeding processes, the fish were released to nature. Blood was obtained from the studied fish by heart puncture with a medical needle through which blood was taken with a syringe. The conditions for keeping fish and all sampling procedures were in line with the ethical standards approved by the legal acts of the Russian Federation, the principles of the Basel Declaration, and international, national, and/or institutional principles [6]. The blood was taken from individual fish in a quantity of about 10 mL. For each experiment, three fish were used to take the blood sample, and the blood was mixed in order to minimize the impact of variation. The chosen fish were females with ages of 3–5 years and weights of 1.5–2.5 kg on average. In total, 15 individual fish were used for five consecutive experiments; the individual variation did not exceed 10%. The results represented the average trend. Blood was collected in a beaker with a saline solution cooled to +4 °C at a ratio of 1:20 at pH 7.4). The isotonic saline for the erythrocytes of the thornback ray was prepared according to the following recipe: 220 mM NaCl, 300 mM CO(NH2)2 (urea), and 5 mM Tris-HCl (pH 7.4).
Erythrocytes were washed three times from plasma and leukocytes, which, after each sedimentation, were carefully aspirated from the surfaces of the precipitated cells with a micropipette [6]. Sedimentation was carried out with a K-23 refrigerated centrifuge (Janetzki, Berlin, Germany) at a speed of 1500 rpm for 10 min. The washed cells were resuspended to a hematocrit of 25–30%. The resulting suspensions were used to carry out experiments to measure the oxygen uptake rates.
2.3. Measurement of Oxygen Uptake Rates by Erythrocyte Suspension
The rates of oxygen uptake by a suspension of erythrocytes (pmol O2/min·106 cells) were measured by the polarographic method using a closed platinum Clark electrode on an Expert-001 analyzer (NPO Econics Expert, Moscow, Russia) at 26 °C in a cell with a volume of 1.2 mL. The erythrocyte suspension was poured into a cell at the bottom of which there was a magnetic stirrer, which constantly stirred the suspension to uniformly maintain the oxygen concentration in the area of the electrode membrane of the oxygen sensor. The experimental cell had two channels through which solutions of activators and inhibitors of the mitochondrial complex of the studied fish erythrocytes could be introduced into the cell using a pipette dispenser. The data of the current O2 concentration were transmitted through the ADC of the Expert-001 analyzer (NPO Econics Expert, Moscow, Russia) to a computer, where they accumulated in the form of an array of data on the current oxygen concentration. At the same time, with the help of special software, this concentration was displayed on the monitor screen, which made it possible to visually follow the progress of the experiment. The concentrations of the additions of different activators, such as a tris-glutamate, tris-malate, ADP, digitonin, oligomycin, 2,4-dinitrophenol, rotenone, tris-succinate, and NaN3 that were used are indicated in the figure captions. The original ingredient solutions were 120 times more concentrated than the final ingredient concentrations. This was due to the fact that 10 µL of the added ingredient was diluted 120 times in the cell, the volume of which, as already indicated, was only 1200 µL.
We used 2,4-dinitrophenol (DNF) and urea of an analytical grade. The succinate, NaN3, NaCl, and Tris-HCl were of a chemically pure grade. The oligomycin (CAS number 579-13-5), ADP (CAS number 58-64-0), glutamate (CAS number 6106-04-3), malate (CAS number 7554-12-3), and digitonin (CAS number 11024-24-1) were obtained from Sigma (www.sigmaaldrich.com, accessed on 12 November 2021).
2.4. Statistics
The measurement results are presented as typical curves that were obtained for five independent experiments. The data were subjected to statistical processing and are presented as arithmetic means ± standard deviations (x ± S). The collected data were examined for their homogeneity and normal distribution prior to data analysis. Significant differences in the compared results were determined using Student’s t-test. Differences were considered significant at p < 0.05.
3. Results
The results of the activity of the mitochondrial complex of the thornback ray erythrocytes are shown in Figure 2, Figure 3, Figure 4 and Figure 5. Polarographic measurements of the respiration rates of suspensions of washed erythrocytes of the thornback ray (Figure 1 and Figure 2) showed a high “basal” respiration rate of red blood cells in different series of experiments, which ranged from 10.5 to 21.6 pmol O2/min·106 cells. The calculated average value of the respiratory activity of the thornback ray erythrocytes was 14.9 ± 2.6 pmol O2/min·106 cells, which indicated a relatively high rate of “respiration” of the erythrocyte suspension of this fish species. The addition of a mixture of salts of glutamic and maleic acids (G + M) to the erythrocyte suspension of mitochondrial respiration substrates caused an almost instantaneous response, expressed as a six-fold increase in the respiration rate of the red blood cells (Figure 2). In many cases, the influence of the inhibitors was reversible. However, the sequential addition of inhibitors and activators demonstrated increases and decreases in mitochondrial respiratory activity. The experiment was continuous to demonstrate the increased hypoxia in the experimental cell. During this experiment, the activity of the inhibitors was reversible. Surprisingly, the respiratory activity of erythrocytes increased during the experiment, which illustrated the high level of tolerant behavior of the erythrocytes.
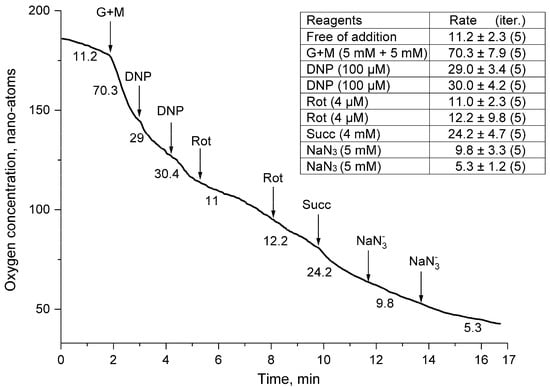
Figure 2.
Oxygen uptake rates by thornback ray erythrocyte suspension. Erythrocytes (25–30% hematocrit) were added to a medium containing 220 mM NaCl, 300 mM urea, and 5 mM Tris-HCl (pH 7.4). Arrows, respectively, indicate additions of reagents to the concentrations in the medium: 5 mM tris-glutamate and 5 mM tris-malate (G + M), 100 µM 2,4-dinitrophenol (DNP), 4 µM rotenone (Rot), 5 mM tris-succinate (Succ), and 5 mM NaN3 (N3−). The numbers above the curves indicate the rates of oxygen uptake by erythrocytes (pmol O2/min·106 cells).
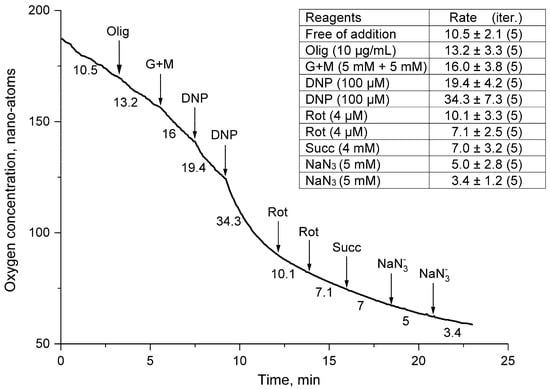
Figure 3.
Rates of oxygen uptake by a suspension of thornback ray erythrocytes in the presence of oligomycin. The medium, additives, and designations are the same as in Figure 2. The oligomycin supplement (Olig) was 10 µg/mL.
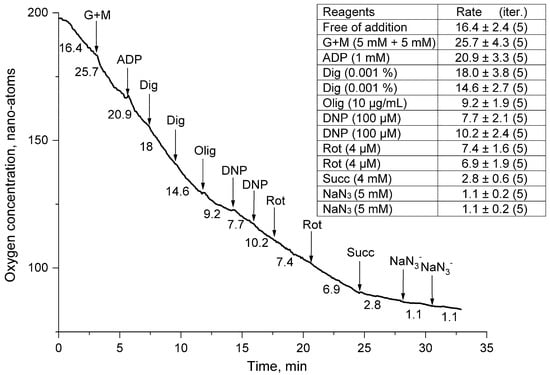
Figure 4.
Rates of oxygen uptake by a suspension of thornback ray erythrocytes permeabilized with digitonin. The medium, additives, and designations are the same as in Figure 3. The concentration of ADP introduced into the medium was 1 mM; digitonin (Dig) was 0.01.
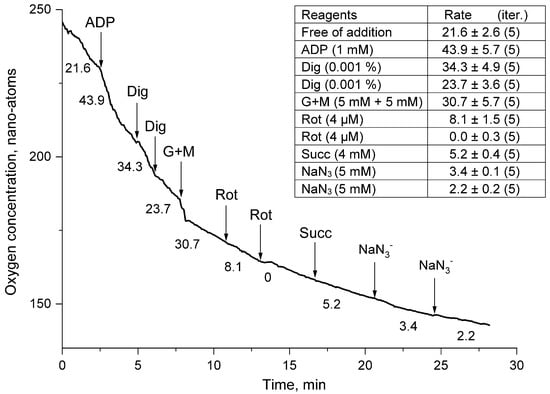
Figure 5.
Rates of oxygen uptake by a suspension of thornback ray erythrocytes permeabilized with digitonin in the presence of ADP (1 mM). The medium, additives, and designations are the same as in Figure 4.
Figure 2, Figure 3, Figure 4 and Figure 5 show typical curves for five series of independent experiments. The table in the figure shows the arithmetic mean rate of mitochondrial “respiration” of the sea fox erythrocyte suspension from five series of experiments and the error of the mean (x ± S).
Exposure to 2,4-dinitrophenol (DNP), an effective protonophore of the inner mitochondrial membrane, resulted in a 60% drop in the mitochondrial respiration rate. After the second addition of DNP, the respiration rate of the thornback ray erythrocyte suspension did not change, which indicated the absence of a deficiency in the uncoupler of mitochondrial respiration and the high efficiency of the initially added concentration (100 µM) of this protonophore (Figure 2). The addition of rotenone (Rot), a blocker of the I-th complex of the electron transport chain of the mitochondrial complex of ray erythrocytes, reduced the respiratory activity of the cell suspension of fish erythrocytes by another 64%, which practically did not change after the second addition (4 µM) of this ingredient. Against the background of the action of the inhibitor of the first complex, rotenone, the addition of succinate (Succ), a substrate of the second complex of the mitochondrial respiratory chain, to the suspension caused a two-fold increase in the respiratory activity of the thornback ray erythrocytes, which indicated the restoration of the electron flow along the respiratory chain (Figure 2). This burst of erythrocyte respiratory activity was effectively blocked by a double addition of 5 mM sodium azide (NaN3), which is an inhibitor of mitochondrial cytochrome C oxidase, the fourth complex of the respiratory chain (Figure 2).
Erythrocytes (with 25–30% hematocrit) were added to a medium containing 220 mM NaCl, 300 mM urea, and 5 mM Tris-HCl (pH 7.4). The arrows, respectively, indicate the additions of reagents to the concentrations in the medium: 5 mM tris-glutamate and 5 mM tris-malate (G + M), 100 μM 2,4-dinitrophenol (DNP), 4 μM rotenone (Rot), 5 mM tris-succinate (Succ), and 5 mM NaN3 (N3−). The numbers above the curves indicate the rates of oxygen uptake by erythrocytes (pmol O2/min·106 cells). In Figure 1, Figure 2, Figure 3 and Figure 4, typical curves are demonstrated for a series of four independent experiments. The standard deviation of the velocities was within 5% (p < 0.05).
Another experiment (Figure 3) was aimed to demonstrate the actions of activators and inhibitors of the mitochondrial respiration of thornback ray erythrocytes against the background of an ATP synthase blocker, oligomycin A. Oligomycin caused a significant inhibition of the respiratory activity of a sea ray erythrocyte suspension. Against its background, there was practically no activation of erythrocyte respiration by a mixture of glutamate and malate (G + M). The addition of the first aliquot of the oxidative phosphorylation uncoupler DNP did not change the respiratory activity of the cells. However, the second aliquot stimulated the respiration of skate erythrocytes by more than one and a half times (Figure 3). Against the background of oligomycin, the rotenone (Rot) in the first supplement reduced the rate of mitochondrial respiration of the cell suspensions by a factor of three. Against the background of this inhibition, succinate (Succ), as a substrate of respiratory complex II, did not stimulate the respiratory activity of the mitochondrial complex of thornback ray erythrocytes. An interesting feature of the mitochondria of erythrocytes of the ray was the absence of the complete inhibition of respiration by sodium azide (NaN3) in both the first and second series of experiments (Figure 3).
In the second series of experiments, we tried to accelerate the “respiration” of thornback ray erythrocytes with the help of (G + M) and ADP (Figure 4). The addition of substrates of complex I (G + M) and ATP synthase (ADP 1.0 mM) to the medium caused a slight increase in the rate of oxygen uptake by the cell suspension. The permeabilization of the plasma membrane of ray erythrocytes with digitonin (Dig) inhibited the respiration of erythrocytes of this fish species. This inhibition of cellular respiration was enhanced in the presence of oligomycin, which could be partially reversed by the addition of two additions of dinitrophenol (DNP). After that, the action of the blocker of mitochondrial respiration rotenone (Rot) on the erythrocytes of the thornback ray looked quite convincing, causing a drop in the respiratory activity of the cells. Against this background, the addition of succinate (Succ) to the cell did not stimulate respiration, and it seems the effect of rotenone only increased. The experiment was completed by blocking mitochondrial activity by adding sodium azide (NaN3) (Figure 4).
In the next series of experiments (Figure 5), events developed in the same way as in the first series (Figure 4). After the stimulation of respiration of a cell suspension of thornback ray erythrocytes by the addition of ADP, the permeabilization of the plasma membrane of erythrocytes with digitonin (Dig) caused a drop in the respiration rate of the thornback ray cell suspension. The negative impact of permeabilization (Dig) was effectively eliminated by adding a mixture of the energy substrates glutamate + malate (G + M). The activation of cell respiration (G + M), as expected, was completely blocked by rotenone, against which the attempts to “resuscitate” the respiration of the mitochondrial complex of thornback ray erythrocytes with succinate (Succ) were ineffective. The experiment ended with the addition of sodium azide (NaN3), which almost completely blocked the respiration of thornback ray erythrocytes (Figure 5).
4. Discussion
Nuclear erythrocytes of ray (Raja clavata L.), washed from plasma and resuspended in physiological saline with a suspension hematocrit (Ht) of 25–30%, showed respiratory activity in the basal state equal to 14.9 ± 2.6 pmol O2/min·106 cells. This indicator was almost equal to the respiratory activity of erythrocytes of another species of Black Sea sting ray (Dasyatis pastinaca L.), which was determined by our group at the level of 14.6 ± 1.1 pmol O2/min·106 cells [14]. If we compare the basal respiration of erythrocytes of the electric ray Torpedo marmorata Risso with the respiratory activity in the basal state of erythrocytes of the Black Sea thornback ray, it was approximately 14 times lower (~1.0 pmol O2/min·106 cells). This difference is probably due to the peculiarities of the energy and lifestyle of this tropical fish species. The electric stingray is extremely inactive and can wait for its prey for a long time (several days) without moving. It stuns with an electric discharge and provides itself with food without much effort. In addition, these rays are distinguished by their ability to survive in water with an extremely poor oxygen content. The low level of metabolism and tolerance to hypoxia apparently determined the adjustment of the mitochondrial complex of erythrocytes to the “economy” of the use of oxygen for their own needs and its delivery to tissues. It should be noted that the blood of the electric skate has a low hematocrit (Ht ≈ 25%) and erythrocytes endowed with impressive dimensions (35 × 15 µm) and huge nuclei, which occupy most of the cells [8]. Such erythrocytes are naturally not able to quickly pass through capillaries, and the overall level of oxygen transport potential in this species of fish is probably very modest.
In works devoted to the respiration of erythrocytes, most authors calculated the consumption of O2 per microliter (µL) of cells. Due to the fact that fish erythrocytes have different volumes, one μL of erythrocytes can contain a different number of cells. The respiratory activity of erythrocytes, calculated per µL of cells, differs significantly from the activity calculated per million cells (1 × 106). Thus, the volume of erythrocytes of a studied thornback ray is 900 μm3, and that of a common stingray is 800 μm3. One μL of erythrocytes of these fish species contains 1.1 million or 1.25 million cells, respectively. The rate of oxygen consumption in this case is equal to 16.4 ± 2.9 pmol O2 μL−1 erythrocytes min−1 in the thornback ray and 18.3 ± 1.2 pmol O2 μL−1 erythrocytes min−1 in the common thornback ray. Basic respiration in scorpion fish [14] was 6.0 ± 0.7 pmol O2/min·106 cells, and when converted to µL (cell volume = 300 µm3) it was 19.8 ± 2.3 pmol O2 µL−1 erythrocytes min−1. Using the scorpion fish as an example, these calculations showed that a comparison of erythrocyte respiration in different units can lead to completely opposite results. Thus, in fish with small erythrocytes, the calculation of respiration per μL of erythrocytes greatly overestimates their real aerobic potential and leads to incorrect conclusions.
We also noted a low basic respiration in other teleost fish. Thus, in the horse mackerel, the oxygen consumption by erythrocytes was 2.5 ± 0.9 pmol O2/min·106 cells (n = 7), in the annular seabream it was 2.0 ± 0.7 pmol O2/min·106 cells (n = 9), and in Spicara flexuosa it was 7, 4 ± 1.5 pmol O2/min·106 cells (n = 4) (unpublished data of the authors). The values of oxygen consumption by erythrocytes of bony fish were 2–7.5 times lower than those of the Black Sea thornback ray. The obtained results allow us to conclude that the erythrocytes of teleost fish are better than the erythrocytes of the Black Sea thornback ray in “saving” oxygen, and due to this they are capable of more efficient transport of this most important electron acceptor for mitochondrial cell respiration.
The addition of glutamate and malate (G + M) substrates of the first respiratory complex to the thornback ray erythrocyte suspension increased the respiration rate the of mitochondria of red blood cells of this fish by more than six times. Glutamate and malate have traditionally been used in in vitro studies to study the properties of mitochondrial respiration in different cells. The use of this pair of substrates was because malate in mitochondria is a substrate of NAD+-dependent dehydrogenases, the oxidation of which, in the matrix, leads to the appearance of NADH, a substrate of the respiratory chain [10]. The equilibrium of the malate dehydrogenase reaction is strongly shifted towards the formation of malate and oxidized NAD+. In view of this, to transaminate the resulting oxaloacetate and shift the equilibrium, it is necessary to add glutamate to the incubation medium, with the help of which the respiratory chain of mitochondrial cells is activated [15]. Fish erythrocytes have demonstrated this activation of mitochondrial respiration in our previous studies. In these experiments, we used G + M to activate the “respiration” of erythrocytes of the sea cat stingray (Dasyatis pastinaca L.) and bony scorpionfish (Scorpaena porcus L.). As shown by these studies, the substrates of the first mitochondrial complex, 5 mM glutamate and 5 mM malate (G + M), in all cases caused significant increases in the rates of oxygen uptake by the erythrocyte suspensions of the studied fish. The increases in oxygen consumption in scorpion erythrocyte suspensions were especially high, which were 13–37 times higher than the baseline respiration of the cell suspensions [16] The stimulation of respiration in mitochondria energized by glutamate and malate isolated from erythrocytes of the electric skate (Torpedo marmarata Risso) was also noted in the work of Pica et al. [8]. When these substrates (G + M) were added, not only was the high rate of mitochondrial respiration of fish erythrocytes striking, so too was the rapidity of the response, which occurred immediately after the addition of a stimulus, without any lag phase. Such a rapid response suggests the possible existence of a special transporter or channel in the plasma membrane of fish erythrocytes, which ensures the transport of substrates into the cytosol and mitochondria of the cells under study. Studies have shown that the presence of two transporters, the aspartate–glutamate and glutamate transporters, has been established in the inner mitochondrial membrane [17]. Aspartate is a glutamine carrier that exchanges external protonated glutamate for aspartate from the mitochondrial matrix. However, the glutamate transported by this system is not accessible to glutamate dehydrogenase (GDH) because glutamate entering in this way is rapidly transaminated by oxalacetate to form aspartate [18]. To activate the respiratory activity of mitochondria, the glutamate carrier (GC) is more suitable, which was discovered in the mid-1970s [19]. An important feature of this transporter is that it can transport glutamate both into the mitochondria and into the cytosol of the cell. The preferred direction of transport is determined by the pH difference in the matrix and cytosol as well as the state of mitochondrial energization [17].
When G + M was added against the background of oligomycin (Olig) (Figure 2), there was no activation of respiration in the thornback ray erythrocyte suspension, which indicated a significant disruption in the work of their mitochondria. The reason for such serious violations is the high selectivity of the effect of oligomycin on ATP synthase (EC 3.6.3.14) of cell mitochondria. Oligomycin has been shown to inhibit the activity of ATP synthase in eukaryotic mitochondria and in the cytoplasmic complex of bacteria. The mechanism of action of the antibiotic is the selective suppression of proton translocation in F0F1-ATP synthase (a synonym for ATP synthase), which leads to a failure of ATP synthesis and a disruption of cellular energy metabolism in general [20]. However, in our experiments, the respiration of the erythrocyte mitochondria of some fish, after their treatment with oligomycin, was activated after the addition of G + M. For example, in oligomycin-treated thornback ray erythrocytes, their respiration in the presence of G + M increased by more than 2 times, and in scorpionfish respiration increased almost 5 times, which significantly exceeded this indicator (1.5 times) in erythrocytes of a thornback ray [14]. The reasons for the resistance of these fish erythrocyte mitochondria to the action of oligomycin have not been established.
The second substrate of complex II of the mitochondrial respiratory chain, which we used in our experiments to stimulate erythrocyte mitochondria, was succinate. We considered the features of the effect of this substrate on erythrocyte respiration after the exposure of erythrocytes to the uncoupler of oxidative phosphorylation, 2,4-dinitrophenol (DNP), and the inhibitor of the first complex of the mitochondrial respiratory chain, rotenone (Rot). It was against the background of the action of these substances in our experiments that the stimulation of the respiration of thornback ray erythrocytes took place.
2,4-Dinitrophenol (DNP) is a proton ionophore that facilitates the transport of protons across biological membranes. Dinitrophenol penetrating into the intermembrane space of mitochondria with a high concentration of protons can capture H+ and promote its transfer to the matrix, where the concentration of protons is lower. There, DNP loses its H+, and, turning into an ionized form, the intermembrane space returns again. By scattering the intermembrane gradient of protons on the inner membrane of mitochondria, DNP thereby blocks the formation of macroergic bonds of inorganic phosphate during ATP synthesis. The energy used to form these bonds is dissipated as heat, which is why DNP is also called the uncoupler of oxidative phosphorylation.
In our experiments, two additions of 100 μM DNP half-blocked the rate of oxygen uptake by thornback ray erythrocytes in the presence of glutamate and malate (G + M) substrates of mitochondrial complex I (Figure 1). This indicated that DNP rapidly disorganized the functioning of the actively “breathing” mitochondria of erythrocytes of the thornback ray, and a drop in the rate of oxygen consumption indicated a violation of the transport of protons and electrons along the electron transport chain of mitochondria and a partial loss of their oxygen acceptor (O2). On the contrary, in the presence of oligomycin, the second addition of DNP caused a two-fold stimulation of O2 consumption in a weakly respiring cell suspension (Figure 2). In this case, the protonophore, due to a real decrease in the activity of ATP synthesis in erythrocytes, caused an increase in energy metabolism, which was reflected in an increase in the rate of O2 consumption by thornback ray erythrocytes.
Rotenone (C23H22O6), being a lipophilic compound with good membrane permeability, should quickly penetrate fish erythrocytes, accumulate in mitochondria, cause a disturbance in electron transfer in the mitochondrial respiratory chain, and inhibit the activity of complex I [21]). Indeed, the addition of rotenone without and in the presence of oligomycin blocked respiration, which was stronger against the background of the antibiotic (Figure 1 and Figure 2). Rotenone (Rot), as already mentioned, inhibits complex I of the respiratory chain, but the entry of electrons into the respiratory chain is possible through complex II of this chain. In the presence of rotenone, there is no accumulation of oxaloacetate, which occurs during respiration mediated by complex II and is capable of inhibiting complex II [22]. Thus, the presence of rotenone causes mitochondria inside the cell to oxidize complex II substrates but does not cause the complete blockade of the respiratory chain. Therefore, as our experiments show, rotenone only reduces the activity of the respiratory chain and does not cause its complete blockade.
Succinate is a substrate of mitochondrial complex II (succinate dehydrogenase, succinate: ubiquinone oxidoreductase, 1.3.99.1), and its addition against the background of rotenone led to a slight increase in the respiratory activity of erythrocytes of the thornback ray (Figure 1). Against the background of oligomycin (Figure 2), the addition of succinate to the thornback ray cell suspension did not cause the activation of their mitochondrial “respiration”. As mentioned earlier, oligomycin is a potent inhibitor of ATP synthase, which leads to the blocking of proton conduction. The presence of other inhibitors (DNP and Rot) in the cell is quite possible; they can enhance the inhibitory effect of mitochondrial “respiration”. The possibility of a synergistic effect of oligomycin and other mitochondrial inhibitors has been shown in earlier studies by Mills et al. [23]. It should also be noted that complex II is localized on the inner mitochondrial membrane, and lipophilic DNP and rotenone, accumulating in the hydrophobic part of the bilayer of erythrocyte mitochondrial cristae membranes in proportion to the time of their incubation, bind to the part of the complex immersed in it and inhibit the activity of the enzyme.
Sodium azide is an inhibitor of complex IV (cytochrome c oxidase: EC 1.9.3.1) of the mitochondrial respiratory chain. The inhibitory properties of this substance are mainly due to its ability to penetrate into the catalytic center of the enzyme and form strong bonds with the copper ions that make up the center. In addition, the inhibitory effect of azide is associated with their reducing ability with respect to pyridoxal phosphate and disulfide groups of the enzymatic complex [24]. In our experiments, the action of 5 mM NaN3 on the “respiration” of mitochondria in fish erythrocytes rarely led to a complete blockade of this process. Most often, the residual respiration of thornback ray erythrocytes fluctuated in experiments in the range from 1.0 to 5.0 pmol O2/min·106 cells. The absence of a complete blockade of aerobic respiration by azide indicates the presence of nonmitochondrial pathways of oxygen utilization in the erythrocytes of the thornback ray. Most likely, part of the oxygen in the process of cellular metabolism goes to the formation of reactive oxygen species (ROS), peroxide (H2O2), etc.
Digitonin (monodesmoid saponin) (Dig) at concentrations up to 0.02 mM forms complexes with cell membrane cholesterol, which leads to the partial disintegration of the bilayer structure and the formation of small pores permeable to small molecules such as ATP [25]. Erythrocytes are no exception to this rule, and treatment with digitonin should have increased the rate of extracellular ADP entry and activated the “respiration” of the cell suspension (Figure 3 and Figure 4). However, the obtained results indicated that pre-added ADP without Dig could better activate the rate of O consumption in thornback ray erythrocytes. Moreover, against the background of G + M, this activation was small (27%) (Figure 3), while without substrates of the tricarboxylic acid cycle (TCA), this increase was two-fold (Figure 4). Such an increase in the respiratory activity of thornback ray erythrocytes upon the addition of 1 mM extracellular ADP may indicate in favor of the permeability of the cell plasma membrane for this substrate, which can be either a specific channel or a transporter. The subsequent addition of Dig to the suspension of thornback ray erythrocytes, instead of activating respiration, in all cases of the experimental study led to a decrease in the respiratory activity of erythrocytes. The reaction of the mitochondrial complex to Dig indicated that this ingredient, which forms complexes with cholesterol, could cause the disintegration of the structure and increase the proton permeability of the inner mitochondrial membrane [26]. As a result, there was a drop in the potential ∆μH+, a decrease in the rate of ATP biosynthesis, and a decrease in the rate of O2 consumption (Figure 3 and Figure 4).
Oligomycin in permeabilized thornback ray erythrocytes significantly suppressed mitochondrial respiration, which neither DNP nor succinate could activate. In fact, Rot did not add anything significant to its blockade, and NaN3 finally inhibited the process of the mitochondrial “respiration” of thornback ray erythrocytes (Figure 3). In the erythrocytes of the sea cat and bony scorpion fish, the effect of oligomycin in permeabilized fish erythrocytes was similar [14]. These facts gave reason to believe that permeabilization and an antibiotic, when they acted together, strongly inhibited the mitochondrial activity of erythrocytes, and that this technique could be used in experiments to “turn off” oxidative phosphorylation in the nuclear erythrocytes of fish.
Against the background of permeabilization, glutamate with malate (G+M) and succinate (Succ) stimulated the mitochondrial activity of thornback ray erythrocytes (Figure 4); therefore, the effect of Dig was not so “destructive” for the mitochondria of thornback ray erythrocytes. However, this stimulation was not large, which made it possible to attribute to permeabilization rather than to a negative effect on the energy complex.
The erythrocytes of the studied thornback ray, as well as the previously studied erythrocytes of the stingray (Silkin et al., 2017) [14], showed a higher rate of basal “respiration” than the erythrocytes of other fish [14]. Other bony fish, horse mackerel (Trachurus trachurus), a picarel (Spicara smaris), and an annular sea bream (Diplodus annularis) (unpublished author’s data) also demonstrated relatively low basic rates of “respiration” (2.5 ± 0.9, 7.4 ± 1.5, and 2.0 ± 0.7 pmol O2/min·106 cells, respectively), which were 2–7 times lower than those of the erythrocytes of the cartilaginous Black Sea rays.
5. Conclusions
These data of the respiratory rates of erythrocytes of the studied thornback ray reflect that the features of the energy metabolism of cartilaginous fish erythrocytes expressed a high mitochondrial activity. This individual expression of the energy of erythrocytes in rays is probably due to the peculiarities of cell sizes, the capacitive characteristics of oxygen transfer, their regenerative abilities, the long duration of “life” in the bloodstream (240 days), the composition and structure of the plasma membrane, and osmotic and other processes associated with energy costs. To “turn off” the mitochondrial respiration of fish erythrocytes, as our experiments showed, the combined effect of oligomycin and the permeabilization of cell membranes was very effective. It was this combination that led to the strongest inhibition of the rate of respiratory activity of nuclear erythrocytes in fish.
In the ecological aspect, it is also important that fish live in a natural environment that is much “poorer” in terms of oxygen, and it could be assumed that this factor is the main limiter of their mitochondrial activity. Our data on the activation of the “respiration” of erythrocytes and the data of many other researchers [2] indicate that the potential capabilities of cold-blooded and warm-blooded vertebrates have similar characteristics. This also applies to the properties of the main enzymes involved in the processes of oxidative phosphorylation and complexes of the electron transport chain of vertebrate mitochondria. The initial “laying” of the architecture of the inner membrane to support the energy potential of mitochondria was so successful that the further evolution of the energy capabilities of cells and tissues “worked” in the direction of improving the rate of oxygen delivery to the end point of their respiratory chain as an electron–proton flow acceptor. Nevertheless, the established 10–100-fold difference in the rate of respiration, external and mitochondrial and consequently in the intensity of metabolism in poikilo- and homoiothermic vertebrates required the clarification of the reasons for such significant discrepancies.
The study of the bioenergetics of poikilothermic mitochondria allowed us to assert that their mitochondria can produce energy with minimal oxygen pressures or even without it. Under conditions of different oxygen regimes with equal potential activity and capabilities of mitochondria of homoiothermic and poikilothermic organisms, the difference in the intramitochondrial content of nucleotides, which regulate the rates of electron flows along the respiratory chain, is critically important. Perhaps there are other reasons that underlie the different abilities to realize the potential of mitochondrial respiration that have yet to be discovered.
Author Contributions
Conceptualisation and methodology, Y.A.S., E.N.S. and S.M.K.; software, M.Y.S. and S.M.K.; validation, Y.A.S., E.N.S. and S.M.K.; formal analysis, investigation, resources, data curation, and writing—original draft preparation, Y.A.S., A.S. and M.Y.S.; writing—review and editing, Y.A.S., E.N.S. and A.S.; visualization, M.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the framework of the state thematic, in particular the project 075-00744-21-00 “Study of fundamental physical, physiological, biochemical, reproductive, population and behavioral characteristics of marine aquatic organisms" and Interreg Enhance Microalgae Project EAPA_338/2016.
Institutional Review Board Statement
The animal study was reviewed and approved by the commission of the Institute of Biology of the Southern Seas named after A.O. Kovalevksky (approval code: 61 2022 03 2018, approved on 4/03/22).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuraku, S. Shark and Ray Genomics for Disentangling Their Morphological Diversity and Vertebrate Evolution. Dev. Biol. 2021, 477, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Savina, M.V. Mechanisms of Adaptation of Tissue Respiration in the Evolution of Vertebrates; The Science: Saint-Petersbourg, Russia, 1992. [Google Scholar]
- Jeffree, R.A.; Markich, S.J.; Oberhaensli, F.; Teyssie, J.-L. Internal Distributions of a Radio-Element Array in Cartilaginous and Bony Marine Fishes: Different and Heterogeneous. J. Environ. Radioact. 2021, 237, 106709. [Google Scholar] [CrossRef] [PubMed]
- Windberger, U.; Pöschl, C.; Peters, S.; Huber, J.; van den Hoven, R. Measurement of whole blood of different mammalian species in the oscillating shear field: Influence of erythrocyte aggregation. J. Phys. Conf. Ser. 2017, 790, 12035. [Google Scholar] [CrossRef]
- Lipunova, E.A.; Skorkina, M.Y. “Fiziologiya krovi”, Blood Physiology; Bel GU: Belgorod, Russia, 2007. [Google Scholar]
- Ferguson, R.A.; Tufts, B.L.; Boutilier, R.G. Energy Metabolism in Trout Red Cells: Consequences of Adrenergic Stimulation in Vivo and in Vitro. J. Exp. Biol. 1989, 143, 133–147. [Google Scholar] [CrossRef]
- Walsh, P.J.; Wood, C.M.; Thomas, S.; Perry, S.F. Characterization of Red Blood Cell Metabolism in Rainbow Trout. J. Exp. Biol. 1990, 154, 475–489. [Google Scholar] [CrossRef]
- Pica, A.; Scacco, S.; Papa, F.; de Nitto, E.; Papa, S. Morphological and Biochemical Characterization of Mitochondria in Torpedo Red Blood Cells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 213–219. [Google Scholar] [CrossRef]
- Focusing on Mitochondrial Form and Function Editorial. Nat. Cell Biol. 2018, 20, 735. [CrossRef]
- Plesner, L. Ecto-ATPases: Identities and Functions. Int. Rev. Cytol. 1995, 158, 141–214. [Google Scholar] [CrossRef]
- Erich Gnaiger Mitochondrial Pathways to Complex I: Respiration with Pyruvate, Glutamate and Malate. Mitochondrial Physiol. Netw. 2011, 11, 1–9.
- Grimolizzi, F.; Arranz, L. Multiple Faces of Succinate beyond Metabolism in Blood. Haematologica 2018, 103, 1586–1592. [Google Scholar] [CrossRef]
- Harvey, J.; Hardy, S.C.; Ashford, M.L.J. Dual Actions of the Metabolic Inhibitor, Sodium Azide on K ATP Channel Currents in the Rat CRI-G1 Insulinoma Cell Line. Br. J. Pharmacol. 1999, 126, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Silkin, Y.U.A.; Silkina, E.N.; Silkin, M.Y.U. The Dynamics of Heat Production in Erythrocytes of the Scorpion Fish (Scorpaena porcus Linnaeus, 1758) In Vitro. Russ. J. Mar. Biol. 2017, 43, 164–170. [Google Scholar] [CrossRef]
- Grivennikova, V.; Vinogradov, A. Generation of Reactive Oxygen Species by Mitochondria. Adv. Biol. Chem. 2013, 53, 245–296. [Google Scholar]
- Silkin, Y.A.; Silkina, E.N. The Study of Bioenergetic Characteristics of the Red Blood Cells of Black Sea Fish the Common Stingray (Dasyatis pastinaca L.) and Black Scorpionfish (Scorpaena porcus L.). Biophysics 2017, 62, 434–439. [Google Scholar] [CrossRef]
- Frigerio, F.; Casimir, M.; Carobbio, S.; Maechler, P. Tissue Specificity of Mitochondrial Glutamate Pathways and the Control of Metabolic Homeostasis. Biochim. Biophys. Acta (BBA)—Bioenerg. 2008, 1777, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Bradford, N.M.; McGivan, J.D. Quantitative Characteristics of Glutamate Transport in Rat Liver Mitochondria. Biochem. J. 1973, 134, 1023–1029. [Google Scholar] [CrossRef]
- Meijer, A.E.F.H. Mitochondria with Defective Respiratory Control of Oxidative Phosphorylation Isolated from Muscle Tissues of Thyroidectomized Rabbits. J. Neurol. Sci. 1972, 16, 445–453. [Google Scholar] [CrossRef]
- Vatlin, A.; Danilenko, V. Bacterial F0F1-ATPase Is a Namotor for the Synthesis and Hydrolysis of ATP, the Mechanism of Interaction with the Macrolide Antibiotic Oligomycin A. Adv. Mod. Biol. 2020, 140, 231–243. [Google Scholar]
- Degli Esposti, M. Inhibitors of NADH–Ubiquinone Reductase: An Overview. Biochim. Biophys. Acta (BBA)—Bioenerg. 1998, 1364, 222–235. [Google Scholar] [CrossRef]
- Bolshakov, A.P. Study of the Mechanisms of Mitochondrial Depolarization and Calcium Dysregulation Induced by the Excitatory Mediator Glutamate in Brain Neurons; Moscow Physico-Technical Institute—Bauman: Moscow, Russia, 2007. [Google Scholar]
- Mills, K.I.; Woodgate, L.J.; Gilkes, A.F.; Walsh, V.; Sweeney, M.C.; Brown, G.; Burnett, A.K. Inhibition of Mitochondrial Function in HL60 Cells Is Associated with an Increased Apoptosis and Expression of CD14. Biochem. Biophys. Res. Commun. 1999, 263, 294–300. [Google Scholar] [CrossRef]
- Poltorak, O.M.; Chukhrai, E.S. Physical and Chemical Bases of Enzymatic Catalysis; M. “Higher School”: Moscow, Russia, 1971; Volume 134. [Google Scholar]
- Lomakina, G.Y.; Fomina, A.; Ugarova, N. Study of the Kinetics of the Interaction of Digitonin and Its Analogues with HEK293 Cells by the Bioluminescent Method. Mosc. Univ. Chem. Bull. 2020, 61, 232–242. [Google Scholar]
- Szabò, I.; Leanza, L.; Gulbins, E.; Zoratti, M. Physiology of Potassium Channels in the Inner Membrane of Mitochondria. Pflugers Arch. 2012, 463, 231–246. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).