A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nitrite Exposure and Sampling
2.3. Biochemical Analysis of Serum
2.4. Light Microscopy of Gill Tissues
2.5. SEM of Gill Tissues
2.6. FAAs Assay
2.7. Statistical Analysis
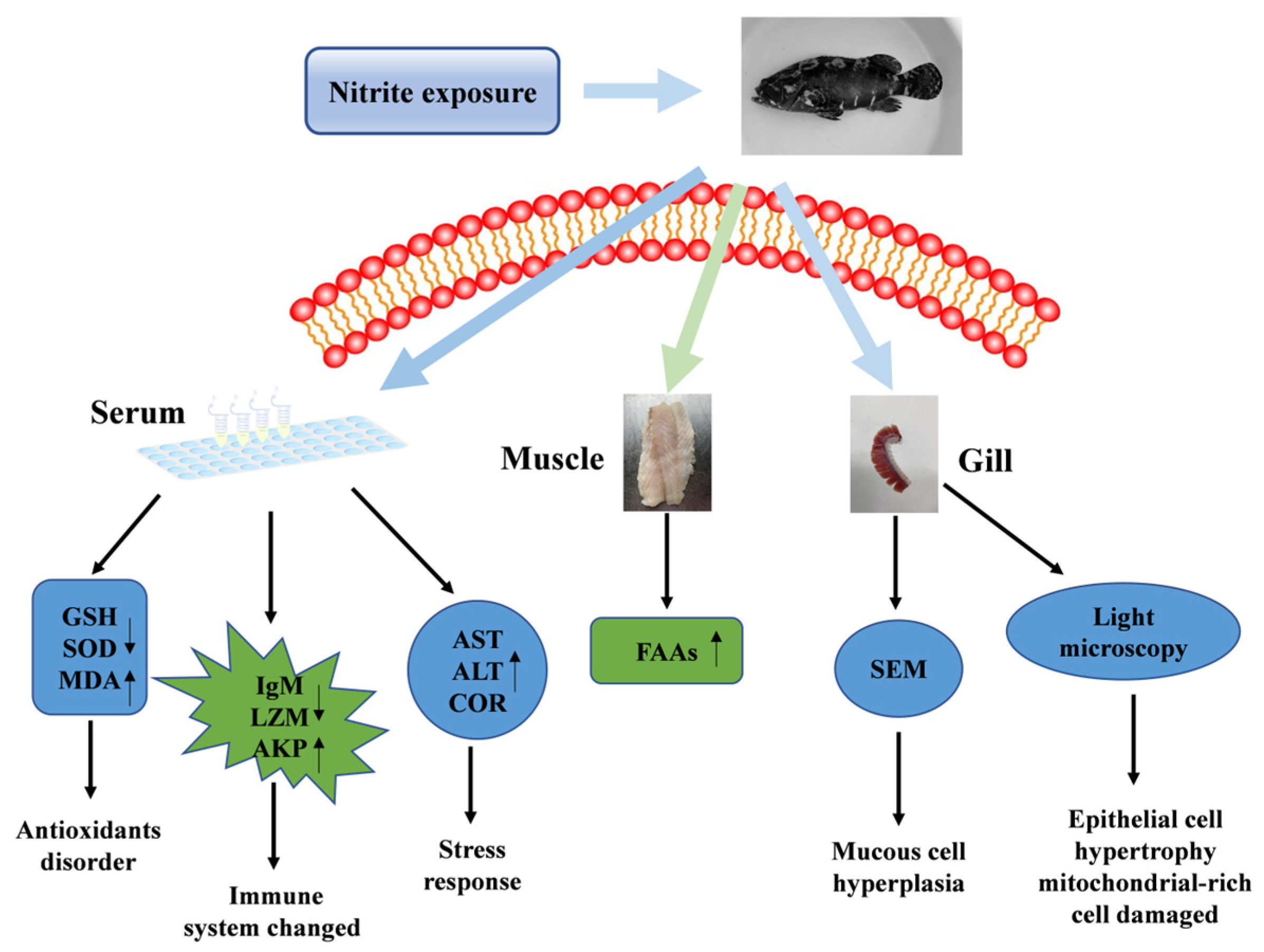
3. Results
3.1. The Effects of Nitrite Exposure on the Survival of Grouper
3.2. Effects of Nitrite Exposure on Serum Biochemical Index
3.3. Effects of Nitrite Exposure on Immunity in Grouper
3.4. Effects of Nitrite Exposure on Oxidative Stress in Grouper
3.5. Effects of Nitrite Exposure on FAAs in Grouper
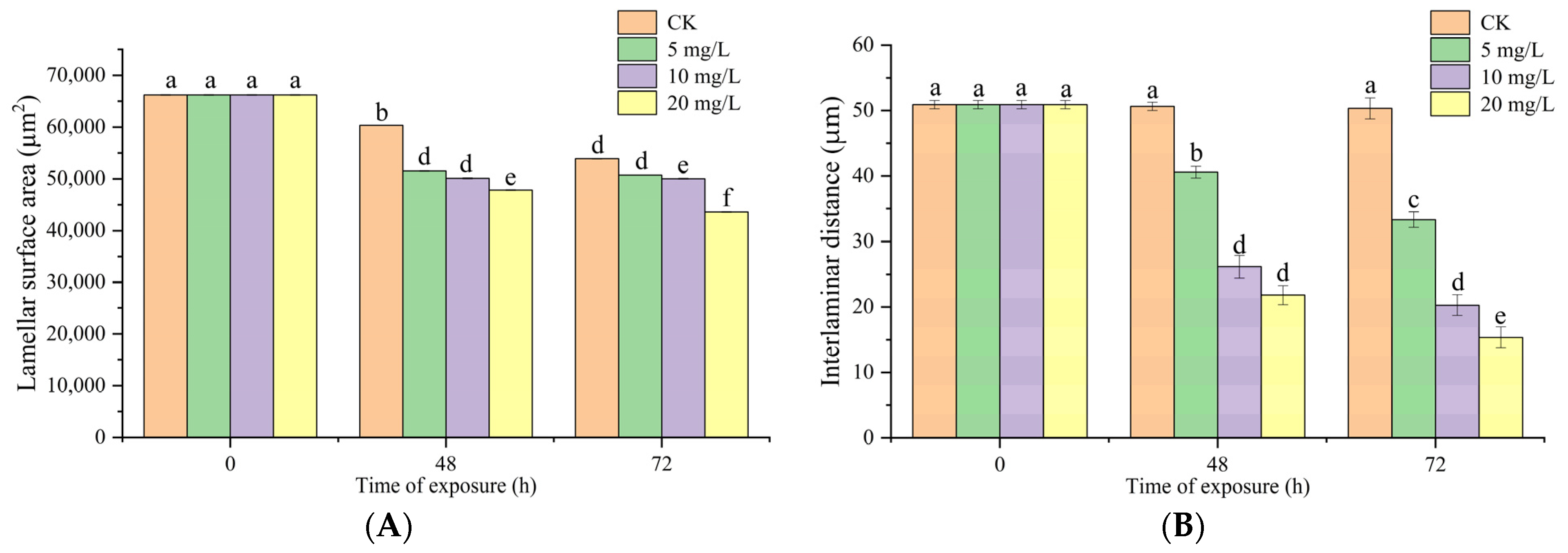
3.6. Effects of Nitrite Exposure on Light Microscopy of Gill Tissues
3.7. Effects of Nitrite Exposure on SEM of Gill Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hilmy, A.; El-Domiaty, N.; Wershana, K. Acute and chronic toxicity of nitrite to Clarias lazera. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 86, 247–253. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr.; Morris, D.P. Toxicity of nitrite to fish: A review. Trans. Am. Fish. Soc. 1986, 115, 183–195. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Nitrogen biogeochemistry of aquaculture ponds. Aquaculture 1998, 166, 181–212. [Google Scholar] [CrossRef]
- Mazik, P.M.; Hinman, M.L.; Winkelmann, D.A.; Klaine, S.J.; Simco, B.A.; Parker, N.C. Influence of nitrite and chloride concentrations on survival and hematological profiles of striped bass. Trans. Am. Fish. Soc. 1991, 120, 247–254. [Google Scholar] [CrossRef]
- Das, P.; Ayyappan, S.; Das, B.; Jena, J. Nitrite toxicity in Indian major carps: Sublethal effect on selected enzymes in fingerlings of Catla catla, Labeo rohita and Cirrhinus mrigala. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 3–10. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Dietary L-tryptophan modulates growth and immuno-metabolic status of L abeo rohita juveniles exposed to nitrite. Aquac. Res. 2015, 46, 2013–2024. [Google Scholar] [CrossRef]
- Sun, S.; Ge, X.; Xuan, F.; Zhu, J.; Yu, N. Nitrite-induced hepatotoxicity in bluntsnout bream (Megalobrama amblycephala): The mechanistic insight from transcriptome to physiology analysis. Environ. Toxicol. Pharmacol. 2014, 37, 55–65. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-Y.; Lim, L.-J.; Kim, S.K.; Choi, H.S.; Hur, Y.B. Effects of waterborne nitrite on hematological parameters and stress indicators in olive flounders, Paralichthys olivaceus, raised in bio-floc and seawater. Chemosphere 2018, 209, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Wang, A.-L.; Zhang, Y.-J.; Li, Z.-H.; Wang, J.-X.; Sun, R.-Y. Effects of nitrite on lethal and immune response of Macrobrachium nipponense. Aquaculture 2004, 232, 679–686. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, Y.J.; Lee, K.M. Effects of Nitrite Exposure on the Hematological Properties, Antioxidant and Stress Responses of Juvenile Hybrid Groupers, Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀. Antioxidants 2022, 11, 545. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, X.; Li, M.; Wang, R.; Qian, Y.; Hong, M. Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108867. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, Y.; Wang, Y.; Xu, S.; Zhou, L.; Li, J.; Li, X. Chronic nitrate exposure cause alteration of blood physiological parameters, redox status and apoptosis of juvenile turbot (Scophthalmus maximus). Environ. Pollut. 2021, 283, 117103. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 9–24. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Chen, Y.; Si, Q.; Tian, J.; Jiang, Q.; Yang, J. Exposure time relevance of response to nitrite exposure: Insight from transcriptional responses of immune and antioxidant defense in the crayfish, Procambarus clarkii. Aquat. Toxicol. 2019, 214, 105262. [Google Scholar] [CrossRef]
- Doblander, C.; Lackner, R. Metabolism and detoxification of nitrite by trout hepatocytes. Biochim. Et Biophys. Acta BBA-Gen. Subj. 1996, 1289, 270–274. [Google Scholar] [CrossRef]
- Tomasso, J. Environmental nitrite and aquaculture: A perspective. Aquac. Int. 2012, 20, 1107–1116. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.-L.; Han, C.; Huang, B.; Lei, J.-L. The physiological performance and immune response of juvenile turbot (Scophthalmus maximus) to nitrite exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Zeng, C. Subchronic exposure to nitrite, potassium and their combination on survival, growth, total haemocyte count and gill structure of juvenile blue swimmer crabs, Portunus pelagicus. Ecotoxicol. Environ. Saf. 2009, 72, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Z.; Ding, N.; Xiong, W.; Zheng, G.; Lin, Q.; Zhang, G. Effects of temperature on the survival, feeding, and growth of pearl gentian grouper (female Epinephelus fuscoguttatus× male Epinephelus lanceolatus). Fish. Sci. 2018, 84, 399–404. [Google Scholar] [CrossRef]
- Miao, B.-B.; Niu, S.-F.; Wu, R.-X.; Liang, Z.-B.; Zhai, Y. The MicroRNAs-Transcription Factors-mRNA Regulatory Network Plays an Important Role in Resistance to Cold Stress in the Pearl Gentian Grouper. Front. Mar. Sci. 2022, 8, 824533. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Fei, F.; Huang, B.; Meng, X.S.; Zhang, T.; Zhao, K.-F.; Chen, H.-B.; Xing, R.; Liu, B.-L. Alterations in hematological and biochemical parameters, oxidative stress, and immune response in Takifugu rubripes under acute ammonia exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 243, 108978. [Google Scholar] [CrossRef]
- Lin, Y.; Miao, L.-H.; Pan, W.-J.; Huang, X.; Dengu, J.M.; Zhang, W.-X.; Ge, X.-P.; Liu, B.; Ren, M.-C.; Zhou, Q.-L. Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish Shellfish Immunol. 2018, 76, 126–132. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Xie, J. The Effects of Lemon Balm (Melissa officinalis L.) Essential Oil on the Stress Response, Anti-Oxidative Ability, and Kidney Metabolism of Sea Bass during Live Transport. Animals 2022, 12, 339. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, F.; Wang, T.; Zheng, X.; Li, Y.; Huang, B.; Zhang, C. Physiological and morphological changes in Turbot (Psetta maxima) gill tissue during waterless storage. Aquaculture 2019, 508, 30–35. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology 2021, 11, 11. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Guo, M.; Fang, D.; Mei, J.; Xie, J. Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea). Animals 2022, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qi, C.; Lin, C.; Tang, R. Nitrite Stress Induces Oxidative Stress and Leads to Muscle Quality Decreased in Wuchang Bream (Megalobrama amblycephala Yih) Juveniles. Water 2022, 14, 160. [Google Scholar] [CrossRef]
- Lin, W.; Li, L.; Chen, J.; Li, D.; Hou, J.; Guo, H.; Shen, J. Long-term crowding stress causes compromised nonspecific immunity and increases apoptosis of spleen in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 80, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.-L. Effect of acute exposure to nitrite on physiological parameters, oxidative stress, and apoptosis in Takifugu rubripes. Ecotoxicol. Environ. Saf. 2020, 188, 109878. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Rai, U. Dose and time-related in vitro effects of glucocorticoid on phagocytosis and nitrite release by splenic macrophages of wall lizard Hemidactylus flaviviridis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 461–470. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Watts, M.; Munday, B.; Burke, C. Immune responses of teleost fish. Aust. Vet. J. 2001, 79, 570–574. [Google Scholar] [CrossRef]
- Hamed, S.B.; Guardiola, F.; Cuesta, A.; Martínez, S.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; Esteban, M.Á. Head kidney, liver and skin histopathology and gene expression in gilthead seabream (Sparus aurata L.) exposed to highly polluted marine sediments from Portman Bay (Spain). Chemosphere 2017, 174, 563–571. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Y.-G.; Feng, Y.-H.; Li, M.-Y.; Wang, G.-Q.; Ma, Y.-F. Toxic effects of waterborne lead (Pb) on bioaccumulation, serum biochemistry, oxidative stress and heat shock protein-related genes expression in Channa argus. Chemosphere 2020, 261, 127714. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Morales, A.E.; Palma, J.M.; de la Higuera, M. Blood antioxidant defenses and hematological adjustments in crowded/uncrowded rainbow trout (Oncorhynchus mykiss) fed on diets with different levels of antioxidant vitamins and HUFA. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 440–447. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Han, C.; Huang, B.; Lei, J.L. Effects of ammonia exposure on stress and immune response in juvenile turbot (Scophthalmus maximus). Aquac. Res. 2017, 48, 3149–3162. [Google Scholar] [CrossRef]
- Dutra, F.M.; Rönnau, M.; Sponchiado, D.; Forneck, S.C.; Freire, C.A.; Ballester, E.L.C. Histological alterations in gills of Macrobrachium amazonicum juveniles exposed to ammonia and nitrite. Aquat. Toxicol. 2017, 187, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, S.; Schulze, S.; Eberhardt, U.; Schulz, C.; Schroeder, J. Acute and chronic nitrite toxicity in juvenile pike-perch (Sander lucioperca) and its compensation by chloride. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 157, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Frances, J.; Allan, G.L.; Nowak, B.F. The effects of nitrite on the short-term growth of silver perch (Bidyanus bidyanus). Aquaculture 1998, 163, 63–72. [Google Scholar] [CrossRef]
- Benli, A.Ç.K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Shimura, R.; Ma, Y.X.; Ijiri, K.; Nagaoka, S.; Uchiyama, M. Nitrate toxicity on visceral organs of medaka fish, Oryzias latipes: Aiming to raise fish from egg to egg in space. Biol. Sci. Space 2004, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Yashpal, M.; Mittal, S.; Mittal, A.K. Morphology of the pharyngeal cavity, especially the surface ultrastructure of gill arches and gill rakers in relation to the feeding ecology of the catfish Rita rita (Siluriformes, Bagridae). J. Morphol. 2005, 265, 197–208. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Cao, J.; Mei, J.; Xie, J. Effects of ascorbic acid and β-1, 3-glucan on survival, physiological response and flesh quality of cultured tiger grouper (Epinephelus fuscoguttatus) during simulated transport in water. Biology 2020, 9, 37. [Google Scholar] [CrossRef]
- Alcaraz, G.; Espina, S. Scope for growth of juvenile grass carp Ctenopharyngodon idella exposed to nitrite. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1997, 116, 85–88. [Google Scholar] [CrossRef]
- Reddy, P.P.; Devi, G.S. An evaluation of zinc sulphate toxicity on protein, amino acid and transaminase levels in freshwater fish, Channa striata (bloch). Indian J. Anim. Res. 2021, 55, 1342–1346. [Google Scholar] [CrossRef]
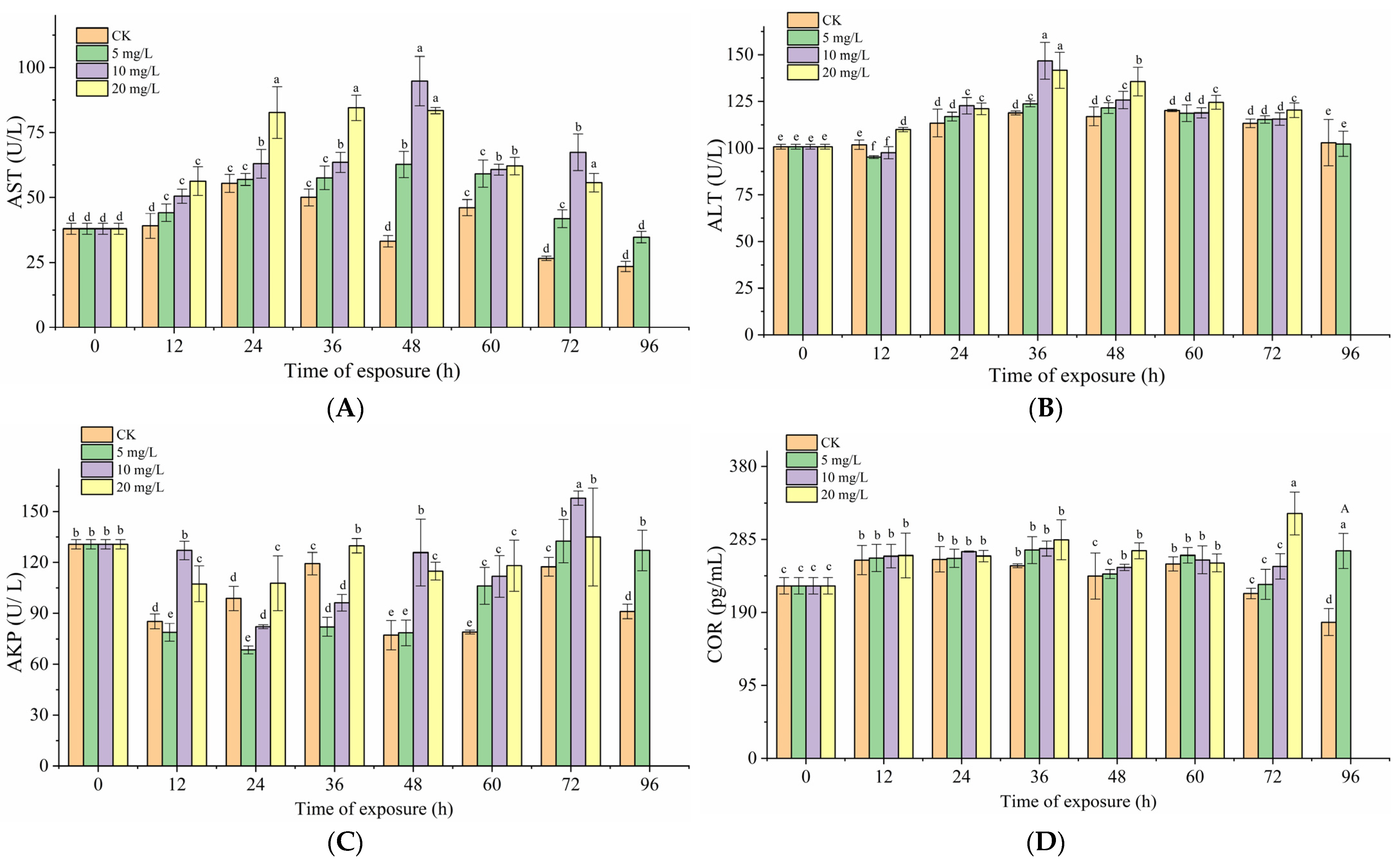

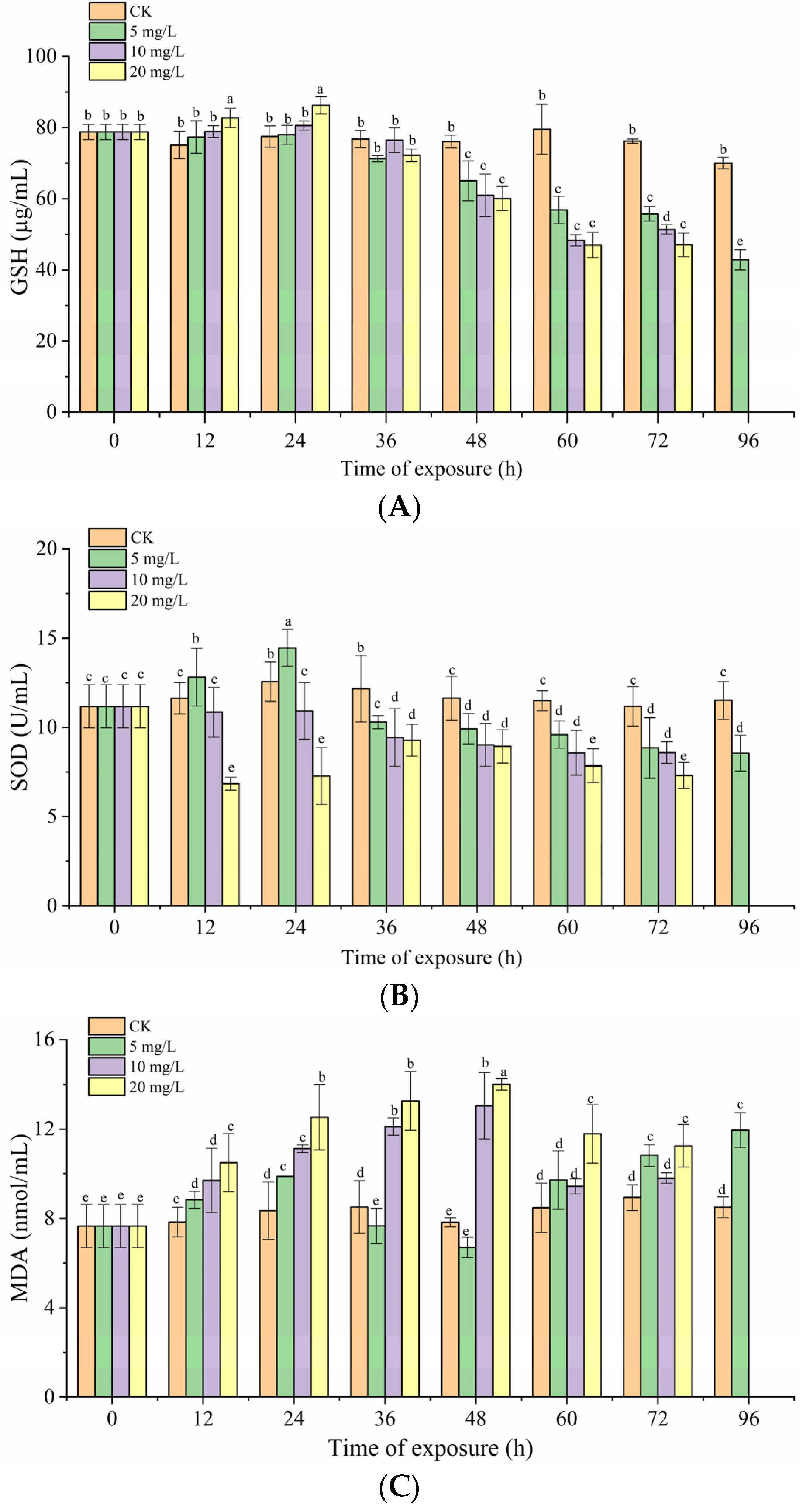
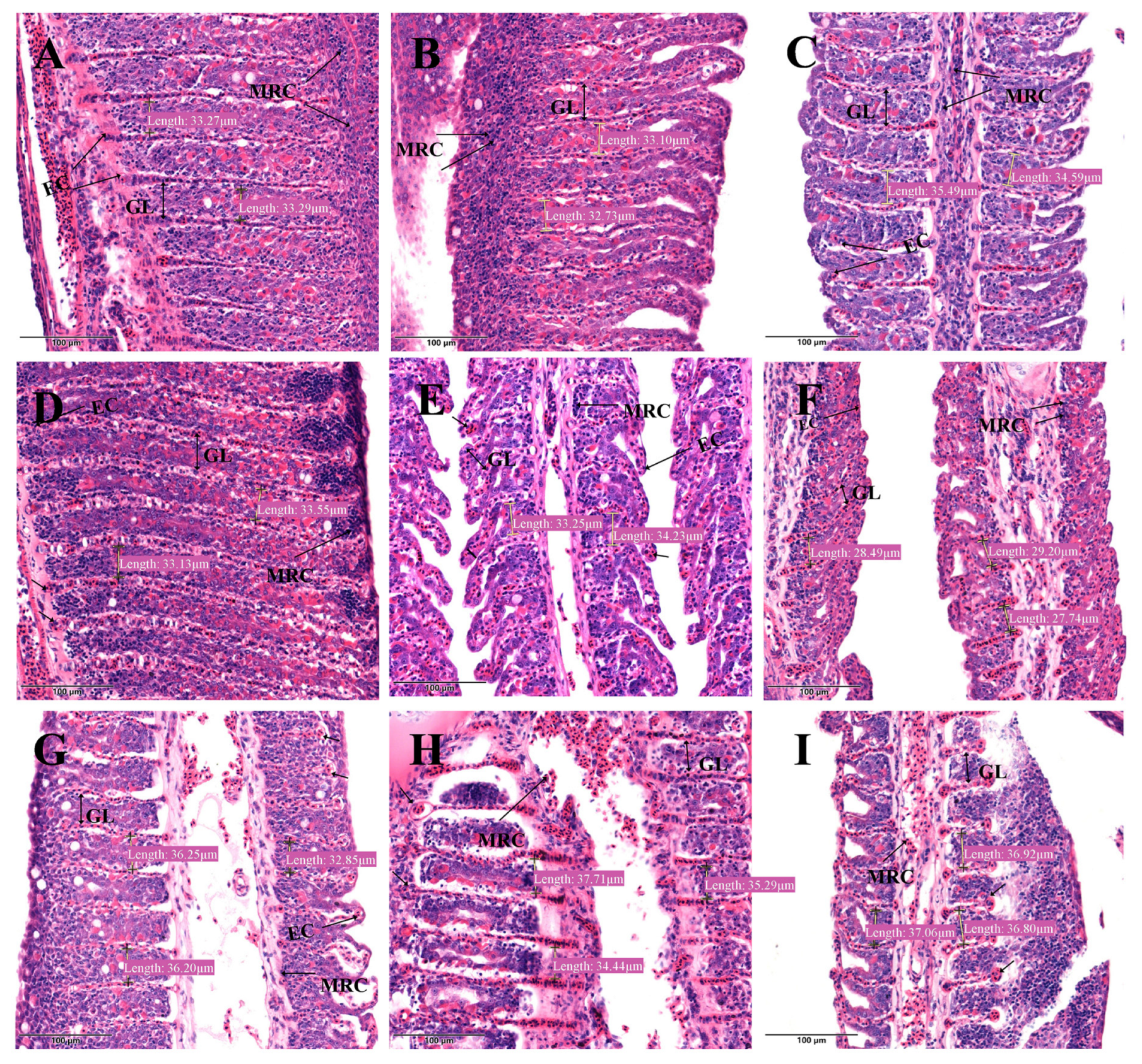
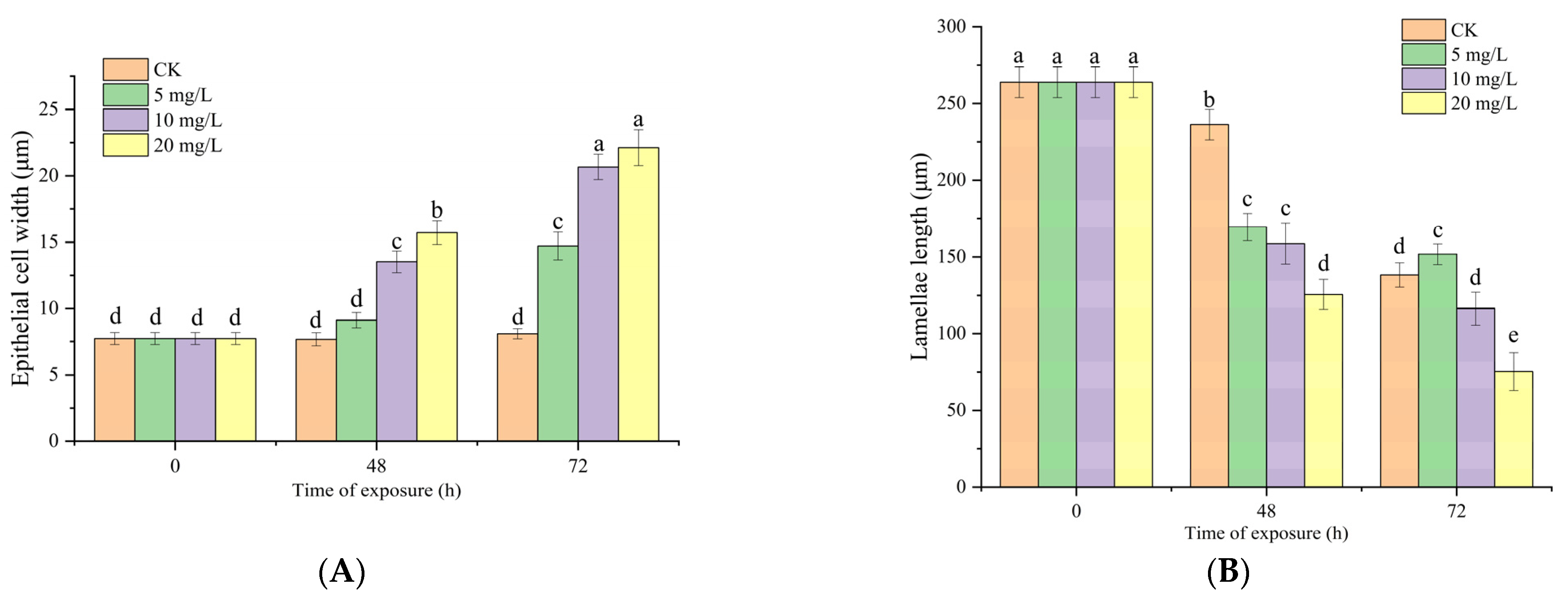

| Parameters | Source of Variation | df | F | p |
|---|---|---|---|---|
| AST | Time | 7 | 80.372 | <0.01 |
| Nitrite exposure | 3 | 145.538 | <0.01 | |
| Time × Nitrite exposure | 21 | 15.991 | <0.01 | |
| ALT | Time | 7 | 64.960 | <0.01 |
| Nitrite exposure | 3 | 18.887 | <0.01 | |
| Time × Nitrite exposure | 21 | 3.571 | <0.01 | |
| AKP | Time | 7 | 83.231 | <0.01 |
| Nitrite exposure | 3 | 1.892 | <0.01 | |
| Time × Nitrite exposure | 21 | 30.997 | <0.01 | |
| COR | Time | 7 | 9.960 | <0.01 |
| Nitrite exposure | 3 | 14.020 | <0.01 | |
| Time × Nitrite exposure | 21 | 5.207 | <0.01 | |
| IgM | Time | 7 | 8.967 | <0.01 |
| Nitrite exposure | 3 | 21.576 | <0.01 | |
| Time × Nitrite exposure | 21 | 8.320 | <0.01 | |
| LZM | Time | 7 | 5.420 | <0.01 |
| Nitrite exposure | 3 | 22.268 | <0.01 | |
| Time × Nitrite exposure | 21 | 1.910 | <0.01 | |
| GSH | Time | 7 | 41.299 | <0.01 |
| Nitrite exposure | 3 | 31.318 | <0.01 | |
| Time × Nitrite exposure | 21 | 7.401 | <0.01 | |
| SOD | Time | 7 | 6.716 | <0.01 |
| Nitrite exposure | 3 | 34.429 | <0.01 | |
| Time × Nitrite exposure | 21 | 3.623 | <0.01 | |
| MDA | Time | 7 | 8.967 | <0.01 |
| Nitrite exposure | 3 | 21.576 | <0.01 | |
| Time × Nitrite exposure | 21 | 8.320 | <0.01 | |
| Asp | Time | 7 | 2591.511 | <0.01 |
| Nitrite exposure | 3 | 437.998 | <0.01 | |
| Time × Nitrite exposure | 21 | 232.758 | <0.01 | |
| Thr | Time | 7 | 3140.926 | <0.01 |
| Nitrite exposure | 3 | 926.126 | <0.01 | |
| Time × Nitrite exposure | 21 | 404.840 | <0.01 | |
| Ser | Time | 7 | 8349.712 | <0.01 |
| Nitrite exposure | 3 | 5801.992 | <0.01 | |
| Time × Nitrite exposure | 21 | 1601.050 | <0.01 | |
| Glu | Time | 7 | 29,835.143 | <0.01 |
| Nitrite exposure | 3 | 5147.610 | <0.01 | |
| Time × Nitrite exposure | 21 | 4510.256 | <0.01 | |
| Gly | Time | 7 | 656,602.897 | <0.01 |
| Nitrite exposure | 3 | 100,475.191 | <0.01 | |
| Time × Nitrite exposure | 21 | 60,079.333 | <0.01 | |
| Ala | Time | 7 | 38,253.514 | <0.01 |
| Nitrite exposure | 3 | 19,294.190 | <0.01 | |
| Time × Nitrite exposure | 21 | 7223.457 | <0.01 | |
| Cys | Time | 7 | 1561.556 | <0.01 |
| Nitrite exposure | 3 | 138.543 | <0.01 | |
| Time × Nitrite exposure | 21 | 68.667 | <0.01 | |
| Val | Time | 7 | 414.019 | <0.01 |
| Nitrite exposure | 3 | 1882.457 | <0.01 | |
| Time × Nitrite exposure | 21 | 577.514 | <0.01 | |
| Met | Time | 7 | 317.313 | <0.01 |
| Nitrite exposure | 3 | 1050.469 | <0.01 | |
| Time × Nitrite exposure | 21 | 252.804 | <0.01 | |
| Ile | Time | 7 | 1241.423 | <0.01 |
| Nitrite exposure | 3 | 3313.531 | <0.01 | |
| Time × Nitrite exposure | 21 | 864.202 | <0.01 | |
| Leu | Time | 7 | 8808.686 | <0.01 |
| Nitrite exposure | 3 | 30,658.972 | <0.01 | |
| Time × Nitrite exposure | 21 | 7723.441 | <0.01 | |
| Tyr | Time | 7 | 1346.143 | <0.01 |
| Nitrite exposure | 3 | 4115.105 | <0.01 | |
| Time × Nitrite exposure | 21 | 1067.422 | <0.01 | |
| Phe | Time | 7 | 7631.201 | <0.01 |
| Nitrite exposure | 3 | 11,812.878 | <0.01 | |
| Time × Nitrite exposure | 21 | 2839.133 | <0.01 | |
| Lys | Time | 7 | 72,215.842 | <0.01 |
| Nitrite exposure | 3 | 51,218.828 | <0.01 | |
| Time × Nitrite exposure | 21 | 28,975.977 | <0.01 | |
| Nh3 | Time | 7 | 3178.977 | <0.01 |
| Nitrite exposure | 3 | 3152.285 | <0.01 | |
| Time × Nitrite exposure | 21 | 1027.451 | <0.01 | |
| His | Time | 7 | 658.735 | <0.01 |
| Nitrite exposure | 3 | 3949.875 | <0.01 | |
| Time × Nitrite exposure | 21 | 1337.317 | <0.01 | |
| Arg | Time | 7 | 1492.122 | <0.01 |
| Nitrite exposure | 3 | 1178.105 | <0.01 | |
| Time × Nitrite exposure | 21 | 903.914 | <0.01 |
| Nitrite Concentration (mg/L) | Time of Exposure (h) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 96 h | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - |
| 20 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - |
| Nitrite Concentration | Time of Exposure (h) | Free Amino Acids | ||||||
| Asp & | Thr # | Ser # | Glu & | Gly # | Ala # | Cys | ||
| 0 mg/L | 0 h | 5.15 ± 0.04 a | 26.53 ± 0.03 a | 27.37 ± 0.03 a | 60.79 ± 0.03 a | 169.89 ± 0.16 a | 100.23 ± 0.14 a | 3.28 ± 0.03 a |
| 12 h | 3.09 ± 0.07 c | 27.53 ± 0.04 a | 24.59 ± 0.00 b | 47.63 ± 0.04 c | 188.94 ± 0.33 a | 84.43 ± 0.07 b | 1.55 ± 0.06 c | |
| 24 h | 2.423 ± 0.04 d | 21.35 ± 0.30 b | 17.86 ± 0.43 c | 46.01 ± 0.61 c | 117.21 ± 0.35 b | 51.46 ± 0.92 d | 2.58 ± 0.05 b | |
| 36 h | 2.25 ± 0.01 d | 20.77 ± 0.04 b | 16.40 ± 0.02 c | 26.73 ± 0.01 e | 121.66 ± 0.12 b | 49.73 ± 0.14 d | 0.49 ± 0.01 d | |
| 48 h | 1.87 ± 0.01 e | 13.50 ± 0.13 d | 14.37 ± 0.10 d | 32.59 ± 0.05 d | 61.04 ± 0.03 c | 47.10 ± 0.02 d | 0.38 ± 0.06 d | |
| 60 h | 1.38 ± 0.06 e | 17.76 ± 0.35 c | 13.15 ± 0.02 d | 31.79 ± 0.12 d | 83.88 ± 0.05 c | 40.56 ± 0.32 d | 0.40 ± 0.00 d | |
| 72 h | 1.59 ± 0.01 e | 16.14 ± 0.03 c | 13.63 ± 0.01 d | 26.94 ± 0.01 e | 48.34 ± 0.01 d | 47.45 ± 0.04 d | 0.41 ± 0.04 d | |
| 96 h | 1.68 ± 0.07 e | 9.62 ± 0.41 e | 13.06 ± 0.20 d | 23.86 ± 0.05 e | 21.35 ± 0.18 d | 44.54 ± 0.15 d | 0.28 ± 0.02 d | |
| 5 mg/L | 0 h | 5.15 ± 0.04 a | 26.53 ± 0.03 a | 27.37 ± 0.03 a | 60.79 ± 0.03 a | 169.89 ± 0.16 a | 100.23 ± 0.14 a | 3.28 ± 0.03 a |
| 12 h | 3.37 ± 0.05 c | 20.46 ± 0.10 b | 24.54 ± 0.11 b | 43.69 ± 0.01 b | 171.10 ± 0.24 a | 72.16 ± 0.10 c | 1.05 ± 0.03 c | |
| 24 h | 2.17 ± 0.01 d | 16.35 ± 0.01 c | 19.54 ± 0.03 c | 38.88 ± 0.01 d | 127.74 ± 0.06 b | 62.38 ± 0.06 c | 1.12 ± 0.01 c | |
| 36 h | 2.17 ± 0.03 d | 19.70 ± 0.04 c | 23.50 ± 0.03 b | 42.59 ± 0.02 c | 112.92 ± 0.22 b | 80.43 ± 0.08 b | 0.50 ± 0.06 d | |
| 48 h | 2.00 ± 0.02 d | 14.13 ± 0.31 d | 15.90 ± 0.13 c | 38.41 ± 0.04 d | 57.42 ± 0.00 c | 53.05 ± 0.10 d | 0.63 ± 0.00 d | |
| 60 h | 4.08 ± 0.06 b | 20.20 ± 0.11 b | 24.61 ± 0.11 b | 50.84 ± 0.09 b | 130.15 ± 0.03 b | 82.42 ± 0.16 b | 0.58 ± 0.01 d | |
| 72 h | 2.22 ± 0.05 d | 7.16 ± 0.03 e | 8.37 ± 0.00 e | 22.33 ± 0.03 e | 40.10 ± 0.03 d | 43.42 ± 0.06 d | 1.00 ± 0.05 d | |
| 96 h | 3.57 ± 0.12 c | 12.59 ± 0.07 d | 16.24 ± 0.11 c | 34.97 ± 0.34 d | 81.19 ± 0.24 c | 79.85 ± 0.17 c | 0.44 ± 0.03 d | |
| 10 mg/L | 0 h | 5.15 ± 0.04 a | 26.53 ± 0.03 a | 27.37 ± 0.03 a | 60.79 ± 0.03 a | 169.89 ± 0.16 a | 100.23 ± 0.14a | 3.28 ± 0.03 a |
| 12 h | 2.6 ± 0.25 d | 17.34 ± 0.97 c | 24.10 ± 0.53 b | 44.20 ±0.17 c | 146.93 ± 0.42 b | 78.90 ± 0.98 c | 1.13 ± 0.43 c | |
| 24 h | 2.05 ± 0.02 d | 14.87 ± 0.27 d | 22.10 ± 0.15 b | 32.84 ± 0.13 d | 123.02 ± 0.51 b | 65.50 ± 0.16 c | 0.66 ± 0.02 d | |
| 36 h | 1.51 ± 0.02 e | 13.50 ± 0.33 d | 24.77 ± 0.15 b | 39.35 ± 0.02 d | 109.86 ± 0.09 b | 71.23 ± 0.12 c | 0.44 ± 0.11 d | |
| 48 h | 1.25 ± 0.04 e | 7.172 ± 0.09 e | 14.57 ± 0.02 d | 27.71 ± 0.05 e | 40.23 ± 0.11 d | 48.28 ± 0.05 d | 0.07 ± 0.05 d | |
| 60 h | 1.66 ± 0.00 e | 12.66 ± 0.11 d | 17.77 ± 0.08 c | 32.08 ± 0.04 d | 71.84 ± 0.06 c | 58.13 ± 0.02 d | 0.19 ± 0.02 d | |
| 72 h | 3.14 ± 0.13 c | 19.44 ± 0.08 c | 24.41 ± 0.10 b | 49.57 ± 0.58 c | 68.35 ± 0.11 c | 92.57 ± 0.09 b | 0.73 ± 0.04 d | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 20 mg/L | 0 h | 5.15 ± 0.04 a | 26.53 ± 0.03 a | 27.37 ± 0.03 a | 60.79 ± 0.03 a | 169.89 ± 0.16 a | 100.23 ± 0.14 a | 3.28 ± 0.03 a |
| 12 h | 2.00 ± 0.08 d | 17.29 ± 0.95 c | 13.35 ± 0.33 d | 31.95 ± 0.12 d | 80.04 ± 0.06 c | 42.45 ± 0.05 d | 0.38 ± 0.02 d | |
| 24 h | 2.38 ± 0.00 d | 15.03 ± 0.37c | 14.34 ± 0.14 d | 34.72 ± 0.11 d | 53.63 ± 0.04c | 51.04 ± 0.03 d | 0.32 ± 0.01 d | |
| 36 h | 2.19 ± 0.01 d | 13.45 ± 0.33 d | 19.64 ± 0.02 c | 34.39 ± 0.02 d | 158.47 ± 0.23 a | 66.48 ± 0.18 c | 0.62 ± 0.00 d | |
| 48 h | 2.20 ± 0.01 d | 7.11 ± 0.12 e | 13.52 ± 0.20 d | 28.41 ± 0.41 e | 57.45 ± 0.03 c | 42.94 ± 0.18 d | 0.04 ± 0.01 d | |
| 60 h | 2.67 ± 0.09 d | 12.62 ± 0.14 d | 9.55 ± 0.03 e | 29.44 ± 0.01 e | 51.56 ± 0.05 c | 46.50 ± 0.10 d | 0.55 ± 0.01 d | |
| 72 h | 2.62 ± 0.09 d | 19.36 ± 0.15 c | 13.89 ± 0.10 d | 34.12 ± 0.06 d | 41.87 ± 0.15 d | 65.07 ± 0.10 c | 0.45 ± 0.02 d | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Nitrite Concentration | Time of Exposure (h) | Free Amino Acids | ||||||
| Val* | Met | Ile* | Leu* | Tyr* | Phe* | Lys | ||
| 0 mg/L | 0 h | 13.52 ± 0.05 c | 6.92 ± 0.06 c | 10.68 ± 0.05 d | 17.17 ± 0.05 c | 7.56 ± 0.14 c | 4.52 ± 0.11 e | 69.36 ± 013 d |
| 12 h | 12.96 ± 0.04 c | 7.32 ± 0.03 c | 10.55 ± 0.04 d | 16.31 ± 0.07 c | 7.65 ± 0.08 c | 4.73 ± 0.04 e | 89.34 ± 0.04 b | |
| 24 h | 12.65 ± 0.37 c | 7.45 ± 0.58c | 15.57 ± 0.03 b | 19.34 ± 0.20 c | 9.74 ± 0.21 b | 8.18 ± 0.01 c | 69.84 ± 0.47 d | |
| 36 h | 13.69 ± 0.03 c | 7.85 ± 0.04 c | 11.83 ± 0.02 d | 17.55 ± 0.00 c | 7.08 ± 0.07 c | 6.80 ± 0.14 d | 68.40 ± 0.04 d | |
| 48 h | 13.83 ± 0.02 c | 8.95 ± 0.03 b | 12.73 ± 0.01 c | 19.52 ± 0.01 c | 9.34 ± 0.01 b | 9.73 ± 0.02 c | 46.34 ± 0.06 f | |
| 60 h | 12.36 ± 0.18 c | 7.94 ± 0.02 c | 10.78 ± 0.13 d | 16.49 ± 0.12 c | 6.74 ± 0.04 c | 6.37 ± 0.01 d | 49.71 ± 0.14 f | |
| 72 h | 14.07 ± 0.03 c | 9.09 ± 0.05 b | 12.28 ± 0.02 c | 19.25 ± 0.04 c | 9.22 ± 0.14 b | 8.72 ± 0.01 c | 47.58 ± 0.25 f | |
| 96 h | 14.72 ± 0.11 c | 8.97 ± 0.13 b | 13.24 ± 0.10 c | 20.57 ± 0.17 b | 9.19 ± 0.19 b | 9.83 ± 0.01 c | 50.39 ± 0.18 e | |
| 5 mg/L | 0 h | 13.52 ± 0.05 c | 6.92 ± 0.06 c | 10.68 ± 0.05 d | 17.17 ± 0.05 c | 7.56 ± 0.14 c | 4.52 ± 0.11 e | 69.36 ± 0.13 d |
| 12 h | 15.38 ± 0.01 b | 8.84 ± 0.02 b | 11.87 ± 0.01 d | 18.68 ± 0.01 c | 7.56 ± 0.00 c | 7.02 ± 0.06 e | 73.37 ± 0.08 c | |
| 24 h | 16.93 ± 0.01 b | 10.85 ± 0.03 a | 13.96 ± 0.01 c | 22.72 ± 0.02 b | 10.36 ± 0.02 a | 9.76 ± 0.03 c | 96.74 ± 0.01 a | |
| 36 h | 18.88 ± 0.03 b | 11.41 ± 0.02 a | 16.57 ± 0.00 a | 25.96 ± 0.04 a | 11.45 ± 0.01 a | 11.99 ± 0.03 b | 74.74 ± 0.05 c | |
| 48 h | 15.18 ± 0.04 b | 9.10 ± 0.05 b | 13.51 ± 0.00 c | 21.08 ± 0.02 b | 8.67 ± 0.01 b | 9.00 ± 0.06 c | 70.16 ± 0.04 c | |
| 60 h | 17.25 ± 0.04 b | 9.32 ± 0.01 b | 13.58 ± 0.00 c | 21.06 ± 0.04 b | 7.86 ± 0.02 c | 7.38 ± 0.08 d | 83.65 ± 0.15 b | |
| 72 h | 8.75 ± 0.09 d | 5.44 ± 0.13 d | 6.98 ± 0.01 f | 10.57 ± 0.02 d | 4.76 ± 0.04 d | 3.65 ± 0.01 a | 87.13 ± 0.02 b | |
| 96 h | 18.85 ± 0.07 b | 10.19 ± 0.10 a | 15.27 ± 0.04 b | 25.17 ± 0.06 a | 10.49 ± 0.03 a | 7.62 ± 0.04 d | 35.78 ± 0.02 g | |
| 10 mg/L | 0 h | 13.52 ± 0.05 c | 6.92 ± 0.06 c | 10.68 ± 0.05 d | 17.17 ± 0.05 c | 7.56 ± 0.14 c | 4.52 ± 0.11 e | 69.36 ± 0.13 d |
| 12 h | 15.18 ± 0.94 b | 8.86 ± 0.47 b | 12.43 ± 0.64 c | 18.63 ± 0.10 c | 7.59 ± 0.02 c | 5.95 ± 0.07 Be | 69.11 ± 0.19 d | |
| 24 h | 16.40 ± 0.04 b | 9.70 ± 0.02 b | 14.38 ± 0.03 b | 21.83 ± 0.09 b | 9.76 ± 0.10 b | 10.33 ± 0.10 Ac | 63.51 ± 0.16 d | |
| 36 h | 17.66 ± 0.07 b | 10.75 ± 0.14 a | 15.63 ± 0.05 b | 23.93 ± 0.02 b | 11.12 ± 0.01 a | 11.76 ± 0.02 Bb | 64.56 ± 0.07 d | |
| 48 h | 13.19 ± 0.11 c | 8.50 ± 0.16 b | 12.08 ± 0.04 c | 18.53 ± 0.05 c | 9.14 ± 0.01 b | 9.62 ± 0.02 Bd | 35.79 ± 0.06 g | |
| 60 h | 15.07 ± 0.04 b | 9.36 ± 0.01 b | 13.36 ± 0.02 c | 20.67 ± 0.00 b | 9.73 ± 0.01 b | 10.28 ± 0.02 Ac | 48.98 ± 0.01 f | |
| 72 h | 22.98 ± 0.04 a | 11.90 ± 0.07 a | 17.32 ± 0.00 a | 27.89 ± 0.01 a | 11.78 ± 0.06 a | 12.17 ± 0.07 Aa | 97.18 ± 0.05 a | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 20 mg/L | 0 h | 13.52 ± 0.05 c | 6.92 ± 0.06 c | 10.68 ± 0.05 d | 17.18 ± 0.05 c | 7.56 ± 0.14 c | 4.52 ± 0.11 e | 69.36 ± 0.13 d |
| 12 h | 10.59 ± 0.01 c | 7.16±0.03 c | 8.62 ± 0.01 e | 12.95 ± 0.02 d | 5.97 ± 0.01 d | 5.84 ± 0.04 e | 69.46 ± 0.00 d | |
| 24 h | 13.13 ± 0.04 c | 7.03 ± 0.05 c | 10.87 ± 0.02 d | 16.82 ± 0.01 c | 6.78 ± 0.01 c | 6.50 ± 0.02 d | 74.10 ± 0.06 c | |
| 36 h | 13.87 ± 0.02 c | 7.54 ± 0.02 c | 10.74 ± 0.05 d | 16.42 ± 0.01 a | 6.72 ± 0.01 c | 4.99 ± 0.01 e | 88.48 ± 0.06 b | |
| 48 h | 12.79 ± 0.06 c | 6.37 ± 0.12 c | 9.99 ± 0.00 d | 15.08 ± 0.06 c | 6.05 ± 0.01 c | 6.86 ± 0.02 d | 59.47 ± 0.09 e | |
| 60 h | 8.90 ± 0.01 d | 5.17 ± 0.05 d | 6.83 ± 0.01 f | 9.81 ± 0.01 d | 4.38 ± 0.05 d | 3.35 ± 0.04 f | 76.82 ± 0.07 c | |
| 72 h | 14.29 ± 0.03 c | 8.68 ± 0.04 b | 11.99 ± 0.04 c | 18.62 ± 0.06 c | 9.13 ± 0.00 b | 6.95 ± 0.09 d | 74.07 ± 0.15 c | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Nitrite Concentration | Time of Exposure (h) | Free Amino Acids | ||||||
| Nh3 | His* | Arg | Total | Umami | Sweet | Bitter | ||
| 0 mg/L | 0 h | 19.16 ± 0.07 d | 7.79 ± 0.04 c | 11.34 ± 0.39 e | 561.26 | 65.94 | 324.02 | 61.24 |
| 12 h | 19.50 ± 0.04 d | 7.70 ± 0.00 c | 15.10 ± 0.05 c | 568.92 | 50.72 | 325.49 | 59.90 | |
| 24 h | 15.59 ± 0.14 d | 7.60 ± 0.08 c | 12.79 ± 0.10 d | 437.64 | 48.43 | 207.88 | 73.08 | |
| 36 h | 15.94 ± 0.02 d | 7.39 ± 0.01 c | 12.91 ± 0.03 d | 407.47 | 28.98 | 208.56 | 64.34 | |
| 48 h | 14.91 ± 0.08 e | 7.54 ± 0.09 c | 10.80 ± 0.02 e | 324.54 | 34.46 | 136.01 | 72.69 | |
| 60 h | 15.49 ± 0.23 d | 7.19 ± 0.07 c | 11.86 ± 0.03 e | 333.85 | 33.17 | 155.35 | 59.93 | |
| 72 h | 17.69 ± 0.07 d | 7.63 ± 0.11 c | 11.77 ± 0.02 e | 311.80 | 28.53 | 125.56 | 71.17 | |
| 96 h | 18.76 ± 0.04 d | 7.20 ± 0.03 c | 11.71 ± 0.11 e | 278.97 | 25.54 | 88.57 | 74.75 | |
| 5 mg/L | 0 h | 19.16 ± 0.07 d | 7.79 ± 0.04 c | 11.34 ± 0.39 e | 561.26 | 65.94 | 324.02 | 61.24 |
| 12 h | 18.51 ± 0.06 d | 9.19 ± 0.07 b | 13.4 ± 0.04 d | 520.19 | 47.06 | 288.26 | 69.70 | |
| 24 h | 17.71 ± 0.05 d | 8.89 ± 0.03 b | 18.35 ± 0.03 b | 494.34 | 41.05 | 226.01 | 82.56 | |
| 36 h | 17.66 ± 0.09 d | 10.47 ± 0.08 a | 19.27 ± 0.05 b | 499.99 | 44.76 | 236.55 | 95.11 | |
| 48 h | 16.11 ± 0.01 d | 7.78 ± 0.04 c | 15.66 ± 0.12 c | 367.79 | 40.41 | 140.50 | 75.22 | |
| 60 h | 23.83 ± 0.47 c | 9.57 ± 0.05 b | 17.35 ± 0.07 c | 523.73 | 54.92 | 257.38 | 76.70 | |
| 72 h | 21.48 ± 0.03 c | 4.74 ± 0.02 d | 12.42 ± 0.01 e | 290.52 | 24.55 | 99.05 | 39.45 | |
| 96 h | 29.34 ± 0.11 b | 8.52 ± 0.06 b | 9.70 ± 0.17 f | 399.60 | 38.54 | 189.87 | 85.83 | |
| 10 mg/L | 0 h | 19.16 ± 0.07 d | 7.79 ± 0.04 c | 11.34 ± 0.39 e | 561.26 | 65.94 | 324.02 | 61.24 |
| 12 h | 17.75 ± 0.11 d | 8.34 ± 0.23 b | 12.01 ± 0.03 e | 491.05 | 46.80 | 267.27 | 68.12 | |
| 24 h | 17.28 ± 0.02 d | 9.09 ± 0.00 b | 12.28 ± 0.02 e | 445.57 | 34.89 | 225.49 | 81.76 | |
| 36 h | 19.79 ± 0.30 d | 9.78 ± 0.02 b | 13.59 ± 0.03 d | 459.10 | 40.86 | 219.36 | 89.80 | |
| 48 h | 18.01 ± 0.33 d | 7.00 ± 0.02 c | 8.72 ± 0.01 f | 279.86 | 28.96 | 110.25 | 69.56 | |
| 60 h | 18.60 ± 0.08 d | 8.26 ± 0.02 b | 12.15 ± 0.04 e | 360.71 | 33.74 | 160.40 | 77.29 | |
| 72 h | 24.35 ± 0.22 c | 11.51 ± 0.02 a | 21.50 ± 0.05 a | 516.63 | 52.71 | 204.77 | 103.49 | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 20 mg/L | 0 h | 19.16 ± 0.07 d | 7.79 ± 0.04 c | 11.34 ± 0.39 e | 561.27 | 65.94 | 324.02 | 61.25 |
| 12 h | 15.47 ± 0.12 d | 6.30 ± 0.02 c | 15.09 ± 0.04 c | 344.91 | 33.95 | 153.13 | 50.27 | |
| 24 h | 17.16 ± 0.03 d | 7.08 ± 0.09 c | 15.03 ± 0.07 c | 345.96 | 37.10 | 134.04 | 61.18 | |
| 36 h | 22.54 ± 0.04 c | 7.72 ± 0.01 c | 14.59 ± 0.01 d | 488.85 | 36.58 | 258.04 | 60.46 | |
| 48 h | 16.45 ± 0.22 d | 6.74 ± 0.00 c | 11.72 ± 0.04 e | 303.19 | 30.61 | 121.02 | 57.51 | |
| 60 h | 26.57 ± 0.34 b | 4.59 ± 0.04 d | 14.41 ± 0.00 d | 313.72 | 32.11 | 120.23 | 37.86 | |
| 72 h | 34.37 ± 0.45 a | 7.77 ± 0.04 c | 16.78 ± 0.07 c | 380.03 | 36.74 | 140.19 | 68.75 | |
| 96 h | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Fang, D.; Mei, J.; Xie, J.; Qiu, W. A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper. Fishes 2022, 7, 235. https://doi.org/10.3390/fishes7050235
Zhang H, Fang D, Mei J, Xie J, Qiu W. A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper. Fishes. 2022; 7(5):235. https://doi.org/10.3390/fishes7050235
Chicago/Turabian StyleZhang, Hongzhi, Dan Fang, Jun Mei, Jing Xie, and Weiqiang Qiu. 2022. "A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper" Fishes 7, no. 5: 235. https://doi.org/10.3390/fishes7050235
APA StyleZhang, H., Fang, D., Mei, J., Xie, J., & Qiu, W. (2022). A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper. Fishes, 7(5), 235. https://doi.org/10.3390/fishes7050235











