1. Introduction
Common carp, as the third most farmed freshwater fish, is commercially valuable [
1,
2]. Common carp is an economically important fish with the characteristics of delicious meat, high nutritional value, strong stress resistance, and environmental adaptability [
3,
4,
5,
6]. Geographical distribution differences have resulted in long-term natural and artificial selection, so some common carp species with excellent traits have been raised and selectively bred [
7]. Heilongjiang wild carp (HLJ;
Cyprinus carpio haematopirus), cold-resistant strain of purse red carp (CPR;
Cyprinus carpio ‘Red purse cold-resistant’), Songhe carp (SH;
Cyprinus carpio ‘Songhe’), and Songpu carp (SP;
Cyprinus carpio Songpu), all of which have passed by the national aquatic stock and improved breeding approval committee from the ministry of agriculture and rural of the people’s republic of china. Different indicators in tissues, such as muscle, liver, intestine, and serum, reflect the edibility and breeding value of common carp. Muscle is the tissue that determines the main nutritional components of common carp because it is rich in a variety of essential amino acids and polyunsaturated fatty acids [
8,
9]. Biochemical indicators in serum could determine metabolic and physiological status and reflect stress resistance and environmental adaptability in common carp [
10]. Digestive enzyme activity is an important indicator reflecting the absorption and digestion function, which is crucial to the healthy and rapid growth of common carp [
11]. Antioxidant enzyme activity can reflect the antioxidant capacity in common carp [
12,
13]. The expression of growth-related genes in muscle is critical for growth in common carp [
14]. Therefore, it is necessary to determine the quality and expression of growth factors in muscle, antioxidant capacity, digestion and absorption capacity, and serum biochemical indicators in common carp. These indicators play an important role in the comprehensive evaluation of edibility and breeding values and have important effects on the development and utilization of common carp.
SPM, HLJ, CPR, SH, and SP belong to the family Cyprinidae and genus Cyprinus and are common and excellent breeding species for breeding materials. The muscle quality of the Yellow River carp (Cyprinus carpio haematopterus) has been proven to be better than that of the SMP [
15]. Muscle nutrients have been analyzed in HLJ, SH, and purse red carp [
16,
17,
18]. However, a comprehensive comparative evaluation and analysis of the muscle quality, growth, stress resistance, digestion, and absorption capacity of these five common carp species have not yet been reported. In this study, the muscle nutrients, digestive enzyme and lipase activities, serum biochemical indices, and relative expression of growth-related genes of five common carp strains reared in the same rearing environment were analyzed and compared, and we determined the growth, edible nutritional value and antioxidant capacity, digestion and absorption capacity and blood metabolism capacity of these species. This study provides the theoretical basis for further breeding and artificial breeding of fine carp varieties, provides information to help meet the vast consumer demand, and provides the basis for carp production and further processing.
2. Materials and Methods
2.1. Experimental Animals and Animal Care
The experimental fish used in this study were all two-year-old carp of the SPM, HLJ, CPR, SH, and SP species, which were kept under the same feeding conditions, and came from the Hulan Fisheries Experiment Station of Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences. The initial weights of five common carp species (150.0 ± 2 g) were consistently selected. A total of 400 fish of each species were raised, and the breeding density was 500 fish/mu. After electronic marking, they were placed in the same breeding area for breeding. The feed formulation was designed according to formula feed for common carp (GB/T 36782-2018, 2019) and National Research Council (NRC) (2011) guidelines, and the proximate composition of the feed ingredient is shown in
Table 1. The common carp were fed two times per day (08:00 and 17:00) for 15 months with a pellet diet, according to a 3% body weight for 6 months, then a 2.5% body weight for 6 months, and 2% body weight for the last 3 months. Feed ingredients purchased from Hehe Feed co. LTD.
2.2. Sample Collection
Feeding was stopped for 1 day before the start of the experiment, and the body weight, total length, body length, body height, body width, tail handle length, tail handle height, head length, length of proboscis, and eye interval of 100 carp of each species were measured. Fish Anle (MS-222, 100 mg/L, Beijing Green Hengxing Biological Technology Co., Beijing, China)) was used to anesthetize the experimental fish, 9 of which were selected from each common carp species for tissue collection. Blood was collected from the tail vein and placed in a premade heparin anticoagulant tube, kept at 4 °C for 1–2 h, and centrifuged at 3500 r/min for 10 min. The upper serum was drawn and dispensed into centrifuge tubes and placed at −20 °C for use in the determination of serum biochemical indicators. The liver, intestine, and back muscles (at the same position) were collected from the fish, mixed with samples, and placed in a −80 °C freezer for the determination of corresponding indicators.
2.3. Indicator Determination
The approximate composition of the experimental fish pellet diet and muscle was assessed according to the standard procedure of AOAC (2005). A vacuum freeze dryer (FD-1A-50, Yuming, Zhengzhou, China) was used to determine the muscle moisture content in the experimental fish. The crude protein content in the muscle of the experimental fish was determined using the Kjeldahl method (GB 5009.5-2016). The Soxhlet extraction method (GB5009.6-2016) was used to determine the crude fat of muscle in the experimental fish. Chromatography (1260 and 7890 A, Agilent, Santa Clara, CA, USA) was used to determine the amino acid composition in muscle with tryptophan determined by alkaline hydrolysis (laboratory method) and other amino acids by acid hydrolysis (GB5009.124). Fatty acids were determined by gas chromatography-mass spectrometry (GC-MS). After the intestinal and liver tissues were statically ground and mixed with normal saline (1:9) in a low-temperature environment, the supernatants were assayed for intestinal digestive enzyme activities and liver antioxidant indicators. The crude ash was determined by burning the sample to constant weight at 550 °C. Total protein (TP, A045-2, coomassie brilliant blue method), trypsin (TRS, A080-2, N-benzoyl-L-arginine-ethylester method), α-amylase (α-AMS, C016-1-1, starch-iodine colorimetric method), and lipase (LPS, A054-2-1, methyl halide substrate method) were all detected using enzyme activity detection kits (Jiancheng, Nanjing, China). Liver antioxidant indices, including total superoxide dismutase (SOD, A001-3, xanthine oxidase method), catalase from Micrococcus lysodeikticus (CAT, A007-1-1, ammonium molybdate method) activity, and malondialdehyde (MDA, A003-1, thiobarbituric acid method) content, were determined by application of enzyme activity detection kits (Jiancheng, China). Serum biochemical indicators were determined by immunoturbidimetry method, including TP (105-000451-00), albumin (ALB, 105-000450-00), alanine aminotransferase (ALT, 105-000442-00), aspartate aminotransferase (AST, 105-000443-00), alkaline phosphatase (ALP, 105-000444-00), total cholesterol (T-CHO, 105-000448-00), triglyceride (TG, 105-000449-00), high-density lipoprotein (HDL, 105-000463-00), low-density lipoprotein (LDL, 105-000464-00), urea (105-000452-00), uric acid (UA, 105-000476-00) and total bile acid (TBA, 105-000456-00) purchased from Mindray of China, and all indicators were measured using a biochemical analyzer (BS350E, Mindray, Shenzhen, China). There were 9 fish in each experimental group in this study.
2.4. Nutritional Value Assessment
According to the FAO/WHO (1973) recommended standard model of nitrogen amino acid score and egg protein model for nutritional value evaluation, the amino acid score (AAS), chemical score (CS), and essential amino acid index (EAAI) were calculated as follows:
In the formula, aan refers to the percentage of a certain amino acid in the total amount of amino acids, AAn is the amino acid ratio of this amino acid in the reference protein, and N is the number of amino acid species.
2.5. RNA Extraction and RT-qPCR
RNA extraction was performed on the muscles collected from all experimental fish, which were from the same part of the back muscles. According to the manufacturer’s instructions, total RNA was extracted from common carp tissues using the RNeasy Mini Kit (Qiagen, Dusseldorf, Germany). The integrity and quality of the RNA were analyzed using 1.5% agarose gel electrophoresis. The purity of the RNA was determined by UV spectrophotometry. The OD260:280 ratio for all RNA samples was between 1.8 and 2.0. According to the instructions, each cDNA was synthesized from 1 µg of total RNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa, Beijing, China). Specific primers (
Table 2) were obtained using Primer Premier 5.0. RT-qPCR was performed according to the TB Green™ Premix Ex Taq™ II (TaKaRa, China) instructions using an ABI7500 system (Life Technologies, Carlsbad, CA, USA). The primer specificity was confirmed by dissociation curve analysis. Beta-actin (
β-actin) was used as an internal reference gene. Double-distilled water was used instead of the template as the negative control. The relative expression levels of
MSTN,
GH,
GHR,
IGF1,
IGF1R, and
MyoD were determined using the 2 (
−ΔΔCt) method [
19]. The primers used in this study are shown in
Table 2. At least three replicates per experimental group.
2.6. Data Analysis
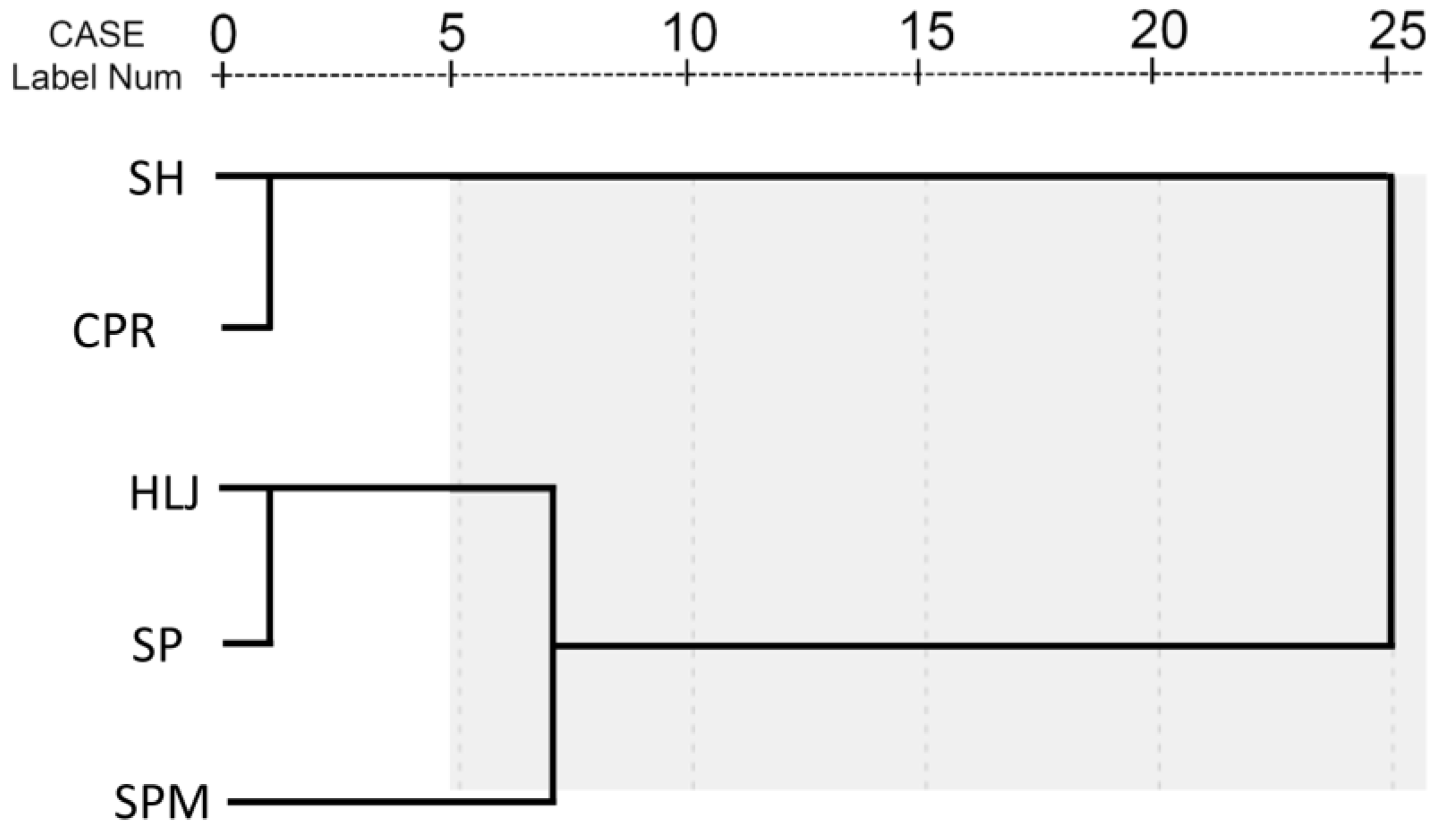
The calculation formulas of the condition factor (CF) and coefficient of variation (CV) are CF(g/cm3) = 100 * W/L3 and CV(%) = SD/Mean * 100, where W and L are the weight and body length of the fish, respectively, SD is the standard deviation, and mean is the average value of the morphological traits. Statistical significance was assessed by a one-way analysis of (ANOVA) followed by LSD multiple comparisons in SPSS statistical software version 22.0 (IBM Corp., Armonk, NY, USA). Cluster analysis by using the system clustering method in SPSS statistical software version 22.0. Statistical significance was considered at p < 0.05. All data are shown as the mean ± SD of at least three replicates.
4. Discussion
Currently, common carp accounts for 11.5% of China’s freshwater aquaculture fish [
20]. Furthermore, because of its strong environmental adaptability, common carp has become a potential candidate species for aquaculture in Asia and some European countries [
21,
22,
23]. In this study, five species of common carp were used as experimental materials, and their differences in nutritional value, blood metabolism, antioxidant capacity, digestion and absorption capacity, and growth in the same feeding environment were determined. The new species of the five common carp have been approved by the national aquatic stock and improved breeding approval committee from the ministry of agriculture and rural of the people’s republic of china. The results of this study showed that they had significant differences in morphological traits and had distant relationships. The CV of body mass was highest in the HLJ species. The CV was used as an index of the dispersion degree of population growth, indicating that HLJ could have a high selection potential for body mass, which could provide possibilities for breeding and germplasm protection among different populations. The CF of SP and CPR was the highest; CF is an index reflecting the degree of fatness and growth of fish [
24]. Because SP and CPR are both short-bodied, it is speculated that the high CF might be related to their morphological characteristics [
25,
26]. In addition, muscle nutrition and texture characteristics are important indicators reflecting the quality of fish, in which hardness and shear force are positively correlated with taste [
27], and nutritional composition is an important factor affecting the nutritional value of fish [
28]. The results of this study showed no significant difference in crude protein content among the five types of common carp species. The muscle of HLJ not only had greater hardness and shear force values but also the lowest crude fat content (
p < 0.05). However, the high fat content in fish muscle is easily affected by oxidation, which would greatly reduce the sensory quality and taste [
29], suggesting that HLJ is the better protein source. Greater hardness and shear force can also overcome the problems of difficult segmentation and molding of fish muscle in a later stage. Therefore, the meat quality of HLJ is greater than that of the other common carp species in terms of nutritional value, production, and processing.
The composition and content of amino acids in protein, especially the composition and content of essential amino acids, are important indicators for evaluating the quality and nutritional value of fish meat [
30,
31]. Amino acids in fish currently play a key role in maintaining human health. Lys and Leu have antioxidant and anticancer effects [
32]. Asp and Glu both promote wound healing and antitumor cell proliferation in humans and affect the taste of fish [
33,
34,
35,
36]. In this study, the Lys, Glu, and Asp contents of CPR muscle were significantly higher than those in the other common carp (
p < 0.05), suggesting that CPR plays an important role in human health. Furthermore, the trends in E/T and F/T were the opposite in HLJ, revealing that the nutritional value of muscle components in HLJ was higher, but its meat flavor may be poor. Currently, the FAO/WHO defines high-quality proteins as having an EAA/NEAA greater than 60% in the amino acid composition [
37]. The AAS (>1.0) and CS (>0.6) values can be used to assess the nutritional value of proteins [
38]. The results of this study showed that the EAA/NEAA values were all greater than 0.6, and the AAS and CS scores were greater than 1.0 and 0.8 (both were the highest in Lys) in the five species of common carp, respectively. The five species of common carp all produced high-quality proteins, a suitable amino acid balance, and a uniform composition. These fish could compensate for the lack of Lys in cereal foods, thereby improving the utilization rate of protein by the human body, which is of great significance for people who have a grain-based diet. The first limiting amino acid in the five common carp was Met + Cys, and the results of this study are similar to those of previous studies [
15,
17]. The SFA C16:0, a key metabolite in fish, was abundant in the five species of common carp in this study, and its level was not affected by diet [
39]. This finding was consistent with the fatty acid content in the muscle of other freshwater fishes, such as crucian carp (
Carassius carassius), Chinese perch (
Siniperca chuatsi), snakehead (
Channa argus), grass carp (
Ctenopharyngodon idella), common carp, black carp (
Mylopharyngodon piceus), silver carp (
Hypophthalmichthys molitrix), swamp eel (
Monopterus albus), and oriental weatherfish (
Misgurnus anguillicaudatus) [
40]. Fish meat is the main source of unsaturated fatty acids for humans. The EPA and DHA in PUFAs have antioxidative and antiaging effects, which can prevent the occurrence of cardiovascular and other diseases and promote brain development in mammals [
41,
42,
43,
44]. In this study, it was found that the content of DHA + EPA in HLJ muscle was the highest, and the contents of SFA and TFA were the lowest (
p < 0.05), indicating that the nutritional value of HLJ muscle might be higher and that it has a positive effect on the health of mammals.
Analysis of blood indicators can provide valuable information on the physiology and health of fish [
45]. Analysis of blood indicators can provide valuable information on the physiology and health of fish [
45]. The concentrations of TG and T-CHO in the serum rise sharply in response to environmental stress or viral infection [
45,
46]. Elevated serum LDL levels are more harmful to the heart in common carp [
47]. The results showed that the TP, TC, TG, and LDL levels were significantly lower in SPW than in the other four carp species (
p < 0.05), suggesting that SPW had stronger environmental adaptability than the other common carp. The liver is the main organ involved in the stress response and can reflect the antioxidant capacity of fish [
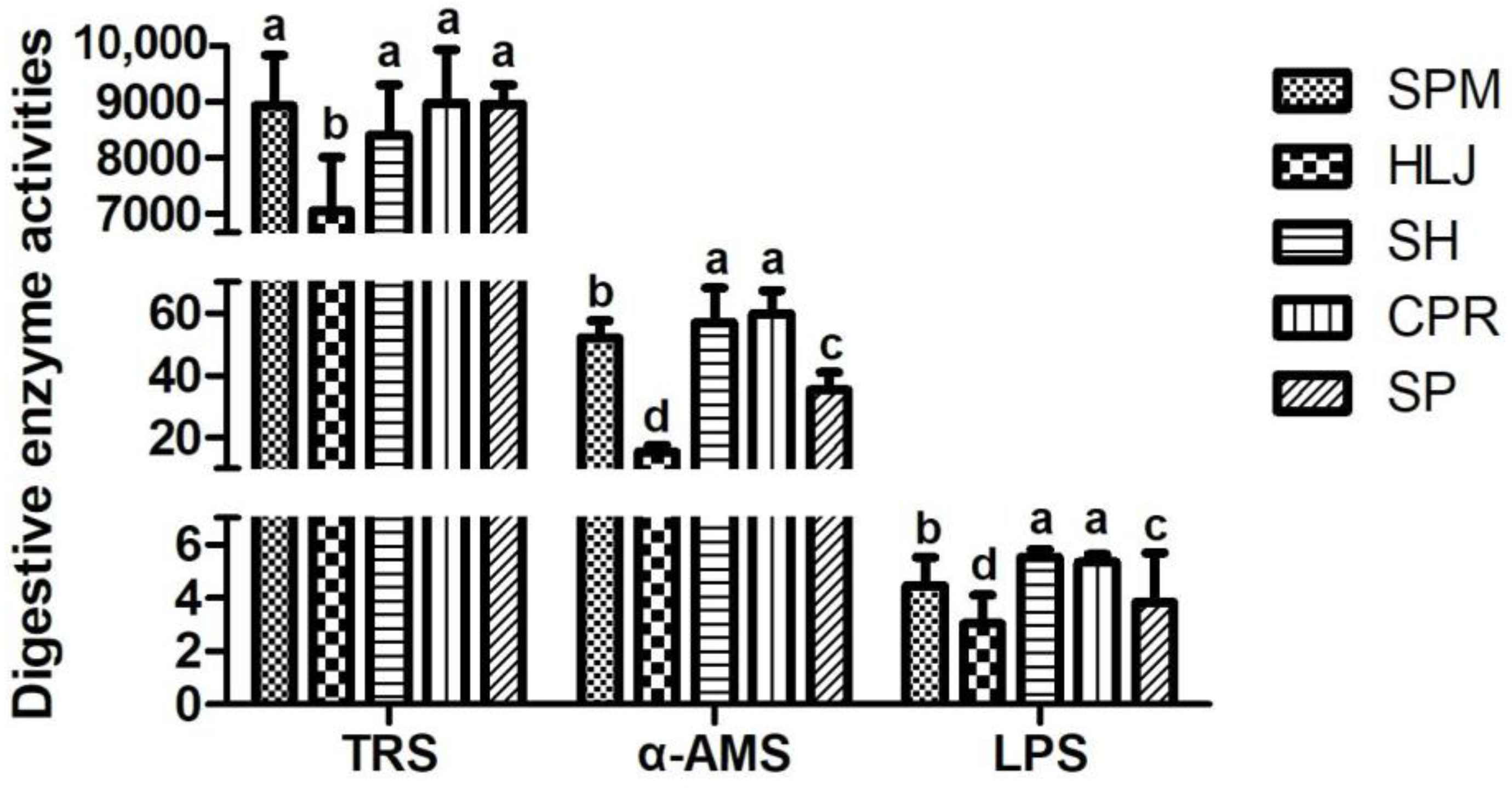
12]. The intestine is the organ of digestion and absorption in fish, and the activity of digestive enzymes in the intestine is the key indicator reflecting the absorption and digestion function in these animals [
48]. The effects of reactive oxygen species (ROS) on cellular function involved in lipid peroxidation and cellular dysfunction can be mitigated by the secretion of SOD and CAT in the liver [
49]. The level of MDA was positively correlated with the accumulation of ROS in vivo [
50]. On the other hand, LPS plays an important role in breaking down dietary fat [
51]. α-AMS is clearly involved in the breakdown of dietary carbohydrates, and its activity depends on the diet of the fish [
52]. TRS has important effects on growth in the juvenile/preadult stages [
53,
54]. The activities of SOD and CAT were significantly higher in HLJ than those in the other four common carp (
p < 0.05), and MDA was relatively low, so it is speculated that the liver’s antioxidant capacity was strongest in HLJ. In contrast, the activities of the LPS, TRS, and α-AMS intestinal digestive enzymes in HLJ were significantly lower than those in the other common carp species (
p < 0.05), which might be one of the reasons for the lower body mass of HLJ.
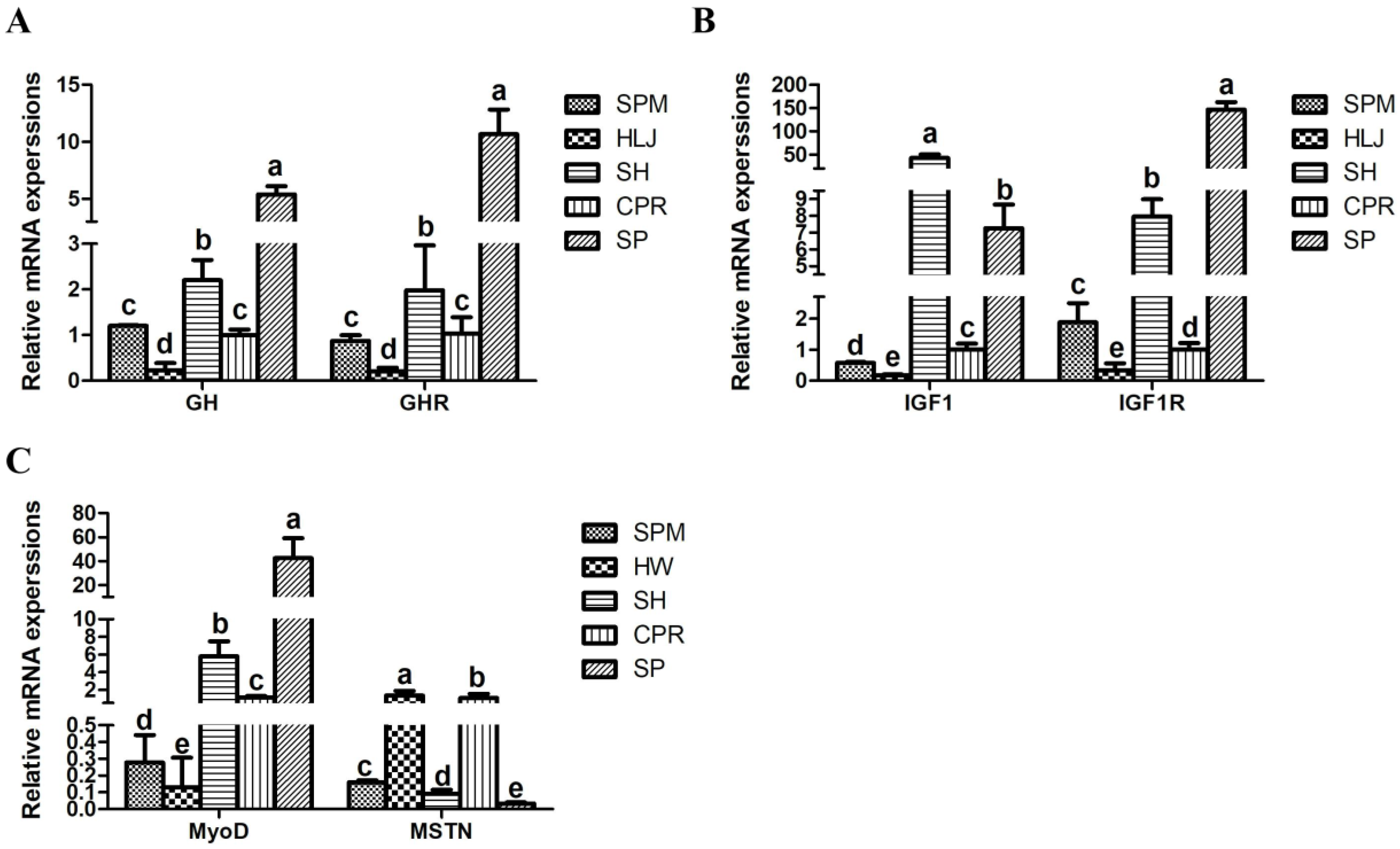
The growth of fish mainly depends on the growth of muscle. Muscle is the main edible part for consumers, and its growth is regulated by a variety of genes [
14].
GH plays a vital role in the regulation of growth hormones in vertebrates and is located at a key position along the
GH-IGF1 axis [
55]. The
GH secreted by the pituitary binds to
GHR on the surface of hepatocytes to stimulate the synthesis and release of
IGF, and
IGF and insulin-like growth factor-binding proteins (
IGFBPs) then act on
IGFR on the surface of target cells to further promote cell proliferation and growth [
56,
57].
MyoD has a crucial effect on the initiation and maintenance of skeletal muscle differentiation and development during myogenesis [
58]. The relative expression levels of
GH,
GHR,
IGF1,
IRF1R, and
MyoD were significantly higher in SP and SH than in the other species, while the expression level in HLJ was significantly lower (
p < 0.05). SP and SH might have more growth potential than the other three common carp. Moreover, previous studies have shown that
MSTN is a negative regulator of skeletal muscle growth [
59]. Interestingly, the relative expression of the
MSTN was the highest in Heilongjiang wild carp (
p < 0.05), indicating that muscle growth might be negatively regulated by MSTN in HLJ, resulting in its lower body mass.