Abstract
The growth performance of Scatophagus argus (25.53 ± 0.89 g) reared in Songkhla Lake, a brackish lagoon, was evaluated after feeding isonitrogenous and isocaloric diets containing hot-water extract from a seaweed, Sargassum sp., at 0, 0.25, 0.50, 0.75 or 1 g·kg−1. Triplicate groups of fish were reared in 15 net cages (50 × 50 × 60 cm) at a density of 20 fish per cage, and the diets were fed twice a day at a rate of 5% of fish body weight per day for 8 weeks. The final body weight, weight gain (WG), average daily gain, and feed intake (FI) showed significant positive and quadratic responses to the Sargassum extract level. The highest WG (57.97%) and FI (0.34) were recorded in the fish that received the hot-water extract at 0.25–0.5 g·kg−1 of diet. The protein efficiency ratio showed significant positive linear and quadratic responses to the Sargassum extract level, while the hepatosomatic index showed a significant positive linear response to the Sargassum extract level. The body protein and ash contents showed positive, linear and quadratic responses to the Sargassum extract level, and the highest crude protein (49%) was observed in the 0.5 g·kg−1 diet group. There were no significant effects of the Sargassum extract levels on feed conversion ratio, food conversion efficiency, lipid and moisture contents, survival rate, hematocrit, and white or red blood cell counts. However, hematological data between fish in the control group and the Sargassum extract groups tended to be different. Overall, the optimum level of seaweed-extract supplementation for spotted scat under natural conditions was determined to be approximately 0.5 g·kg−1 diet.
1. Introduction
Seaweeds have a potential to be a dietary supplement ingredient of animal feeds since they are rich in useful metabolites (pigments, phenolic compound, and polysaccharides) and minerals [1] as well as an umami component, glutamic acid [2]. Hot-water extraction is an effective way to utilize the bioactive component of seaweeds. For example, a hot-water extract from gulfweed was found to stimulate immunity in white shrimp, Litopenaeus vannamei [3], black tiger shrimp, Penaeus monodon [4,5], Asian sea bass, Lates calcarifer [6,7] and rainbow trout, Oncorhynchus mykiss [8], by limiting the production of immunosuppressants. Other studies reported that the stimulation of immunity is caused by the generation of immunostimulants [9]. Zeraatpisheh et al. (2018) found the highest weight gain and specific growth rate in rainbow trout, O. mykiss, fed with a dietary hot-water extract of S. angustifolium at 400 mg·kg−1 of diet [8]. Hot-water extracts of Sargassum supplemented in diets were capable of increasing immunity and resistance to microbial infections [10,11].
It is also reported that Sargassum extracts often have a double-edged sword effect, in which they stimulate the immune systems of aquatic animals but also contain antinutritional factors such as phlorotannins. Phlorotannins are a major phenolic compound and a type of tannin found in brown algae. Tannins are complex secondary metabolites commonly present in plants, which are known to make proteins unavailable via binding. It has been classified as an antinutritional factor for monogastric animals with known negative effects on feed intake, nutrient digestibility, and production performance [12]. Seaweeds are thus often mixed with animal feed to weaken the negative impact on animals [1]. This is also the case for Sargassum extracts, and therefore it is important for aquaculture application to determine the optimal supplementation level of Sargassum hot-water extracts.
Spotted scat (Scatophagus argus Linnaeus, 1766) is a brackish water fish that is popular among fish consumers and as an ornamental fish. It is tolerant to a wide range of salinity, can be well adapted to fluctuations of environmental conditions [13], and is omnivorous [14]. Because of these characteristics, spotted scat is a candidate for coastal aquaculture with the potential to generate a high income for farmers. The supplementation of Sargassum extracts has the potential to contribute to the establishment of a sustainable and cost-effective aquaculture of S. argus, but there have been no studies on the optimum Sargassum extract level in S. argus. The present study was therefore conducted to determine the optimum Sargassum extract level in S. argus aquaculture under natural conditions in terms of growth, feed efficiency, hematological data and body composition. The information will be useful to develop the culture of this species.
2. Materials and Methods
2.1. Preparation of Sargassum sp. Extract
Sargassum sp. was collected from the coast of Bo Mao Bay (latitude 10°43′ N; longitude 99°23′ E), Pathio District, Chumphon Province, Thailand. The seaweed samples were divided into two parts. The first part was dried as herbarium specimens for classification, and the second was washed with freshwater and dried in a hot-air oven at 60 °C for 48 h. The second samples were then milled using an herb grinder and kept in air-tight plastic bottles at 4 °C until use. Upon extraction, they were milled to a particle size of less than 2 mm and stored in air-tight plastic bottles at 4 °C. The homogeneous powder was subjected to extraction at 120 °C by hot water, as reported previously [7]. The extract was then filtered through a nylon mesh (300 µm pore size), freeze-dried, and kept at 4 °C. The proximate composition of hot water extract from Sargassum polycystum was analyzed by AOAC [15] and it is presented in Table 1.

Table 1.
Proximate composition of hot-water extract from Sargassum polycystum.
2.2. Experimental Diets
Four experimental diets were formulated by supplementing 0.25, 0.50, 0.75 or 1.0 g·kg−1 of Sargassum extract. The basal diet contained 5 g·kg−1 carboxymethylcellulose (CMC), and the Sargassum extract was added as described in Table 1. A control diet without Sargassum extract was also prepared. Each diet was stored at 4 °C until use. The ingredients and proximate composition of the experimental diets were determined using a standard method [15], as presented in Table 2.

Table 2.
Formulation and proximate composition of experimental diets.
2.3. Total Phenolic Content
The total phenolic content (TPC) of the hot-water extract was measured using the Folin–Ciocalteu method, as described previously [16]. Briefly, 100 μL of the sample (5 mg·mL−1) was mixed with 0.75 mL of diluted Folin–Ciocalteu reagent (1:9 v/v; Folin–Ciocalteu reagent: distilled water) and then stood at room temperature for 5 min. Next, 0.75 mL of 10% sodium carbonate solution was added, followed by the addition of 10 mL of distilled water. After mixing, the mixture was allowed to stand at room temperature for 90 min. The TPC was determined using a spectrophotometer at 725 nm. Tannic acid was used as the standard, and the TPC was expressed in terms of mg tannic acid equivalents (TAE) per 100 g of dried sample.
2.4. Experimental Design
Spotted scat were obtained from the marine fish hatchery at the Coastal Aquaculture Research and Development Regional Centre 6, Songkhla, Thailand. The fish were conditioned in two 1000 L seawater tanks and acclimated to the local conditions for 7 days. During this acclimation period, the fish were fed a commercial diet (Fishmate Carnivorous and Herbivorous Fish Food 4641, INTEQC feed company, Kalong, Thailand). At the beginning of the experiment, the fish (initial mean body weight: 25.53 ± 0.89 g) were randomly divided into five groups in three replicates. Each replicate, containing 20 fish, was reared in a net cage (size 50 × 50 × 60 cm). The 15 cages were randomly placed in the coastal area of Songkhla lake (7°10′47.8″ N 100°32′25.4″ E).
The fish were fed at a rate of 5% weight of fish each day at 10:30 a.m. Fish immediately accepted experimental diets. The fish were weighed at the beginning of the experiment and then biweekly for 8 weeks. Before weighing, they were fasted for 24 h, allowing the gut to empty. Five fish per cage were sacrificed at the end of the trial and frozen for subsequent body composition analysis. The whole body of the five fish was pooled, dried at 70 °C for 72 h, and subjected to body composition analysis following the AOAC method [15]. All fish were handled with care throughout the study and were killed following the guidelines of the Animals for Scientific Purpose Act 2015 and the National Research Council of Thailand.
2.5. Evaluation of Growth Performance and Feed Utilization
The growth performance, feed utilization, and survival were estimated in terms of mean weight gain (WG), average daily growth (ADG), feed conversion ratio (FCR), feed intake (FI), food conversion efficiency (FCE), protein efficiency ratio (PER), hepatosomatic index (HSI), and survival rate (SR), which were calculated as follows:
| WG: [FBW (g) − IBW (g)]/IBW (g) × 100 |
| ADG: [FBW (g) − IBW (g)]/d |
| FCR: feed intake (g)/WG (g) |
| FI: feed intake (g) × 100/{[IBW(g) + FBW(g))/2] × [initial fish quantity + final fish quantity]/2 × (experimental period)} |
| FCE: {[WG (g)/feed intake (g)] × 100} |
| PER: body weight gain (g)/protein intake (g) |
| HSI: (liver weight (g) × 100)/body weight (g) |
| SR: 100 × (final fish number of fish/initial number of fish) |
2.6. Evaluation of Hematological Data
At the end of the trial period, blood samples were collected from the caudal vein of 15 fish in each treatment to conduct the hematocrit and blood-cell count. The hematocrit was measured by the microhematocrit method using a hematocrit ruler. The blood-cell count (white and red blood cells) was performed in three replications using a hemocytometer.
2.7. Statistical Analysis
The data of growth, feed efficiency, hematological data and body composition were tested for normality and homogeneity of variances using the Shapiro–Wilk test and Levene’s test, respectively. These data are reported as mean ± standard deviation (SD). One way analysis of variance (ANOVA) followed by the Duncan test was used to detect the inter-group differences. The orthogonal polynomial contrasts were calculated to find the linear and/or quadratic response to the Sargassum extract levels. These analyses were conducted using SPSS (Version 22, Chicago, IL, USA). Differences were considered significant at p < 0.05.
3. Results
3.1. Growth Performance, Feed Utilization, Hepatosomatic Index and Survival Rate
All data were analyzed by parametric methods based on their normality and homoscedasticity. The hot-water extract from Sargassum had a significant effect on several growth performance parameters, FBW, WG, and ADG (Table 3; ANOVA, p < 0.05). The FBW, WG, and ADG were quadratically (p = 0.001, 0.036, and 0.012, respectively) but not linearly (p = 0.620, 0.585, and 0.539, respectively) affected by the addition of the hot-water extract. The highest FBW (40.10 g), WG (57.97%), and ADG (0.26 g·d−1) were recorded in the fish that received the hot-water extract at 0.5 g·kg−1 of diet. The hot-water extract at 1 g·kg−1 of diet appeared to be the least appropriate since the fish in this group recorded the lowest value for FBW (34.95 g), WG (37.54%), and ADG (0.17 g·day−1), although some values did not show significant differences with those of fish that were fed control diet: FBW (36.59 g), WG (42.51%), and ADG (0.19 g·day−1).

Table 3.
Growth performance, feed utilization, and survival rate of spotted scat fed the experimental diets for 8 weeks.
The hot-water extract from Sargassum did not have significant effects on FCR or FCE (ANOVA, p = 0.123 and 0.055, respectively). There were neither linear or quadratic effects of the hot-water extract on FCR or FCE, although the association was close to the statistical significance for quadratic relationship (p = 0.201 and 0.118, respectively).
The effect of the hot-water extract on FI did not reach the statistical significance (ANOVA, p = 0.059). The FI were, however, quadratically (p = 0.018) affected by the hot-water extract from Sargassum. The highest FI was recorded in the fish that received the hot-water extract at 0.25 g·kg−1 of diet (0.34), being similar to the value of fish that received the hot-water extract at 0.50 g·kg−1 of diet (0.33). The FI values were not significantly different between the fish that received the hot-water extract at 0 (control), 0.5, 0.75, and 1 g·kg−1 of diet.
The PER value was significantly affected by the hot-water extract from Sargassum (ANOVA, p = 0.000) with significant quadratic (p = 0.011) and linear (p = 0.014) responses. Fish that received the hot-water extract at 0.50 g·kg−1 of diet showed the highest PER (7.59). The PER values in the fish that received the hot-water extract at 0 (control), 0.25, and 1 g·kg−1 of diet did not show any significant differences.
The HSI was not significantly affected by the hot-water extract from Sargassum in this study (ANOVA, p = 0.264), but a significant linear response was detected for this parameter (p = 0.044). There were no significant differences in the SR value among the treatments, which varied between 98.33 and 100%.
The optimum supplementation levels were determined for FBW, WG, ADG, FI, and PER, in which significant quadratic responses were observed (Figure 1). The calculated optimum levels were 0.46, 0.47, 0.47, 0.38, and 0.52, respectively.
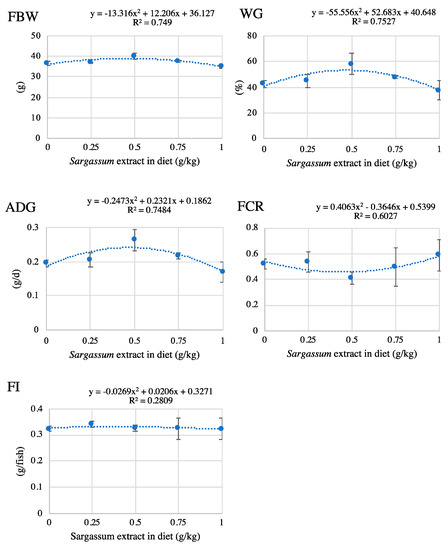
Figure 1.
Determination of the optimum supplementation levels of Sargassum extracts in terms of final body weight (FBW), weight gain (WG), average daily growth (ADG), feed intake (FI), and protein efficiency ratio (PER) in 8-week feeding trial.
3.2. Body Composition
The effect of the hot-water extract from Sargassum on the whole-body composition of the spotted scat is shown in Table 4. ANOVA detected significant effects of the hot-water extract on the crude protein and ash contents (p = 0.000 for both parameters). The hot-water extract level also had significant linear (p = 0.005, p = 0.033) and quadratic (p = 0.000, p = 0.000) effects on the crude protein and ash contents, but not on the crude lipid or moisture contents. The highest crude protein level was recorded in the fish that received the hot-water extract at 0.50 g·kg−1 of diet (49.08% dry matter). The crude protein in the fish that received the hot-water extract at 0.75 and 0.25 g·kg−1 of diet (48.04 and 47.29% dry matter) were significantly higher than those of fish that received the hot-water extract at 0 (control) and 1 g·kg−1 of diet (47.11 and 46.66% dry matter, respectively). The increase in the hot-water extract level had significant effects on the ash content, in which fish that received the hot-water extract at 1 g·kg−1 of diet represented the highest ash content (14.93% dry matter) together with fish that received the control diet (14.56% dry matter). The hot-water extract at 0.25 g·kg−1 of diet appeared to be the least appropriate since it resulted in the lowest ash content. There were no significant differences in crude lipid and moisture contents among the treatments (p > 0.05).

Table 4.
Whole-body composition of spotted scat fed the experimental diets for 8 weeks.
3.3. Hematological Data
The hematological data are shown in Table 5. The hot-water extract from Sargassum did not have significant linear or quadratic effects on hematocrit (p = 0.765 and 0.329), white blood cells (p = 0.509 and 0.399), and red blood cells (p = 0.884 and 0.659). The hematocrit and red blood cell values tended to increase with increasing levels of Sargassum extract, while the white blood cells showed the opposite trend.

Table 5.
Hematocrit, white and red blood cell counts in spotted scat fed the experimental diets for 8 weeks.
4. Discussion
In this study, the supplementation of Sargassum hot-water extracts generally had positive effects on the growth performance and feed utilization of spotted scat. The chemical composition of the seaweed extracts was characterized by a high protein content and low lipid, ash, and moisture contents, which may have contributed to the improvements. This composition was different from hot-water extracts from other algae; S. cristaefolium hot-water extracts showed crude protein, lipid, ash, and moisture contents of 2.05 ± 0.02, 0.8 ± 0.01, 63.41 ± 0.67, and 5.25 ± 0.05%, respectively [10]. Yangthong et al. (2016) demonstrated that hot-water extract from Sargassum sp. (SG-0044) contains no crude lipid, whereas the moisture, crude protein, ash, and total phenolic contents are 4.68 ± 0.21, 12.37 ± 0.21, 24.72 ± 0.13 and 327.88–370.86 mg·g−1 tannic acid equivalent, respectively [7]. Hwang et al. (2010) reported that a hot-water extract form S. hemiphyllum had 10.1% moisture, 38.5% crude protein, 8.4% lipid, 12.2% ash, and 30.8% total carbohydrate [17]. Therefore, the chemical composition of seaweed extracts changes depending on the species of seaweeds and the extraction method, which warrants further studies to establish the standard supplementation protocol.
In the present study, Sargassum hot-water extracts at 0.5 g·kg−1 of diet improved the growth of spotted scat without significantly reducing the survival rate. The optimum supplementation levels of algae hot-water extract were variable depending on the conditions and species, but 0.5 g kg−1 of diet seems to be a reasonable level from the present and previous studies. Wong et al. (2013) reported that the optimum supplementation level of S. polycystum hot-water extract for the growth of Epinephelus coiodes (35.8 ± 0.8 g) was 0.5 g·kg−1 of diet. Yangthong et al. (2016) found that supplementation of Sargassum (SG-0044) hot-water extracts at 0 (control) and 0.5 g·kg−1 of diet resulted in better feed intake than 1 and 2 g·kg−1 of diet in Asian seabass [7]. The high doses of Sargassum extracts may result in increased tannin content in diet, thus reducing the appetite of fish, as observed in this study at equal to or greater than 0.75 g·kg−1 of diet supplementation. However, it must be pointed out that this study was conducted in net cages in the open sea, and the diet fed to the fish was 5% of the body weight of the fish in each net cage. Different culture conditions, including the feeding amount, may yield different results.
Hematological parameters are a pathophysiological reflector of the entire body and indicate the health status of fish. In the current study, the use of dietary Sargassum extract resulted in no significant differences in hematocrit nor in the white and red blood cell counts of spotted scat. Yang et al. (2014) also found that dietary fucoidan supplementation at 0.05, 0.1 and 0.2%·kg−1 feed have no significant effects on the hematocrit and white and red blood cell counts in yellow catfish (2.04 ± 0.02 g) [18]. Future infection experiments may substantiate the tendency of the hematological parameters observed in this study, where Sargassum extracts slightly decreased white blood cells.
The main aim of this study is to determine the optimum supplementation level of Sargassum hot-water extract for spotted scat under natural culture conditions, which turned out to be about 0.5 g·kg−1 considering various aquaculture parameters. The next study should focus on identifying the effective components and target metabolic pathway of Sargassum hot-water extract. The Sargassum hot-water extract was found to contain four of the eight essential sugars that work at the cellular level [7], in which fucose was the main sugar component. The dietary supplementation of alginate, one of the dominant polysaccharides of Sargassum [19], is known to enhance the activity of antioxidant enzymes and expression levels of immune-related genes in grass carp [20]. Fucoidan, another dominant polysaccharide of the extract, is also a well-known immune enhancer [21]. In contrast to the immune-related functions, little is known for the relationship between these polysaccharides and fish growth. It will be interesting to conduct feeding trials focusing on growth-related genes such as growth hormone and insulin-like growth factor I [22,23,24]. Stable in-door conditions would be suitable for these studies to accurately evaluate the effect of Sargassum hot-water extract on fish physiology.
5. Conclusions
The results of this research found that hot-water extracts of Sargassum affect the accumulation of protein and ash in Scatophagus argus, which is the most important farmed fish in Songkhla lake. This study also showed that the supplementation of the Sargassum hot-water extracts at a level of approximately 0.5 g·kg−1 of diet is the most appropriate level for growth performance. It is therefore recommended to supplement feed with Sargassum extract at that level in the aquaculture of spotted scat in order to take advantage of the positive growth performance. This experiment is the first involving algae extract fed to fish in natural water/open sea conditions located in a commercial fishery area. Although the addition of algal extract may increase the production costs of S. argus, the many improvements observed in this study highlight its potential for practical applications.
Author Contributions
Conceptualization, M.Y. and J.R.; investigation, M.Y. and J.R.; data curation, M.Y., J.R. and G.K.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by King Mongkut’s Institute of Technology Ladkrabang: KMITL Research and Innovation Services (KREF016102) and the Coastal Aquaculture Research and Development Regional Center 6, Songkhla, Thailand, who provided fish rearing facilities.
Institutional Review Board Statement
Ethical review and approval were waived for this study in conformity with Thailand law. Yangthong and Ruensirikul have a license to use animals for scientific experiments (license number U1-04670-2559 and U1-05152-2559, respectively), and the study was conducted according to the guidelines of the Declaration of Helsinki.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.-T.; Lee, C.-S.; Chen, J.-C. Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2006, 20, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, G.; Sivagnanavelmurugan, M.; Balasubramanian, V.; Palavesam, A. Effect of hot water extracts of brown seaweeds Sargassum spp. on growth and resistance to white spot syndrome virus in shrimp Penaeus monodon postlarvae. Aquac. Res. 2010, 41, e545–e553. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef]
- Yangthong, M.; Thawonsuwan, J.; Towatana, N.H.; Phromkunthong, W. Effects of hot-water extract from Sargassum sp. on antibacterial activity, non-specific immunity and TBARs production on Asian seabass (Lates calcarifer, Bloch). J. Fish. Environ. 2012, 36, 30–42. [Google Scholar]
- Yangthong, M.; Hutadilok-Towatana, N.; Thawonsuwan, J.; Phromkunthong, W. An aqueous extract from Sargassum sp. enhances the immune response and resistance against Streptococcus iniae in the Asian sea bass (Lates calcarifer Bloch). J. Appl. Phycol. 2016, 28, 3587–3598. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Firouzbakhsh, F.; Khalili, K.J. Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncohrynchus mykiss) infected with Yersinia rukeri. J. Appl. Phycol. 2018, 30, 2029–2037. [Google Scholar] [CrossRef]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 2005, 19, 457–472. [Google Scholar] [CrossRef]
- Wong, S.-L.; Gao, L.-H.; Chang, C.-C.; Cheng, W. The effect of hot-water extract of Sargassum cristaefolium on growth, innate immune responses and resistance of Grouper, Epinephelus coiodes. J. Fish. Soc. Taiwan 2013, 40, 11–26. [Google Scholar]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from brown seaweeds as a potential antimicrobial agent in animal feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, Z.M.; Manyelo, T.G.; Selaledi, L.; Mabelebele, M. The effects of tannins in monogastric animals with special reference to alternative feed ingredients. Molecules 2020, 25, 4680. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.P.; Fast, A. Biology of the spotted scat (Scatophagus argus) in the Philippines. Asian Fish. Sci. 1992, 5, 163–179. [Google Scholar]
- Sivan, G.; Radhakrishnan, C.K. Food, feeding habits and biochemical composition of Scatophagus argus. Turkish J. Fish. Aquat. Sci. 2011, 11, 603–608. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Wu, C.-H.; Gau, S.-Y.; Chien, S.-Y.; Hwang, D.-F. Antioxidant and immune-stimulating activities of hot-water extract from seaweed Sargassum hemiphyllum. J. Mar. Sci. Technol. 2010, 18, 5. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, R.; Li, M.; Zhou, Q.; Liang, X.; Elmada, Z.C. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2014, 41, 264–270. [Google Scholar] [CrossRef]
- Xin, L.; Bin, L.; Xiao-Lei, W.; Zhen-Liang, S.; Chang-Yun, W. Extraction, fractionation, and chemical characterisation of fucoidans from the brown seaweed Sargassum pallidum. Czech J. Food Sci. 2016, 34, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, J.; Wu, S. The growth performance and non-specific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary alginate oligosaccharide. 3 Biotech 2021, 11, 46. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Dawood, M.A.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Rev. Aquacult. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Reinecke, M. Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J. Fish Biol. 2010, 76, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, G.; Furukawa, S.; Kurosu, Y.; Yamada, T.; Takeshima, H.; Nishida, M.; Mitsuboshi, T.; Otaka, T.; Shirasu, K.; Koda, T.; et al. Correlation of accumulated mRNA levels of growth hormone receptor I and insulin-like growth factor I with the larval body size of F2 offspring derived from a fast-growing strain and wild fish of torafugu Takifugu rubripes. J. Fish Biol. 2011, 79, 854–874. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).