Comparative Evaluations to Enhance Chemical and Microbial Quality of Salted Grey Mullet Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Treatment Solutions
2.2.2. The Experimental Trials
2.2.3. Determination of Nanoemulsion Characterization
2.2.4. Salting of Grey Mullet (Mugilcephalus)
2.2.5. Determination of the Chemical Composition
2.2.6. Determination of Lipid Oxidation Changes
2.2.7. Determination of the Nitrogen Profile Changes
2.2.8. Microbial Analysis of Tested Samples
2.2.9. Estimation of Sensory Evaluations
2.2.10. Statistical Analysis
3. Results
3.1. Characterization of Nanoemulsion
3.2. Chemical Composition of Mullet
3.3. Lipid Oxidation Changes in Mullet Treatments
3.4. Total Volatile Nitrogen (TVN) and Non-Protein Nitrogen (NPN)
3.5. Microbial Analysis of Tested Mullet (Raw and Treated)
3.6. Sensory Evaluations of Treated Salted Mullet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farag, M.A.; Zain, A.E.; Hariri, M.L.; El Aaasar, R.; Khalifa, I.; Elmetwally, F. Potential food safety hazards in fermented and salted fish in Egypt (Feseekh, Renga, Moloha) as case studies and controlling their manufacture using HACCP system. J. Food Saf. 2022, 42, e12973. [Google Scholar] [CrossRef]
- Barat, J.M.; Rodríguez-Barona, S.; Andrés, A.; Fito, P. Cod salting manufacturing analysis. Food Res. Int. 2003, 36, 447–453. [Google Scholar] [CrossRef]
- Getu, A.; Misganaw, K.; Bazezew, M. Post-harvesting and major related problems of fish production. Fish. Aquac. J. 2015, 6, 1000154. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Bhandari, B.; Yang, C. Shelf life extension of aquatic products by applying nanotechnology: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1521–1535. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques. Am. J. Appl. Sci. 2010, 7, 859. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria–problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Chander, R.; Lewis, N.F. Effect of lysozyme and sodium EDTA on shrimp microflora. Eur. J. Appl. Microbiol. Biotechnol. 1980, 10, 253–258. [Google Scholar] [CrossRef]
- Levin, R.E. The effectiveness of EDTA as a fish preservative. J. Milk Food Technol. 1967, 30, 277–283. [Google Scholar] [CrossRef]
- Badr, A.N.; Youssef, M.; Abdel-Razek, A.G.; Shehata, M.G.; Hassanien, M.M.; Amra, H. Natural Antioxidants: Preservation Roles and Mycotoxicological Safety of Food. Egypt. J. Chem. 2021, 64, 285–298. [Google Scholar] [CrossRef]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; Alharthi, S.S.; Selim, K.A. Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites. Toxins 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Abdel-Salam, A.M.; Badr, A.N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef]
- Sharif, H.R.; Abbas, S.; Majeed, H.; Safdar, W.; Shamoon, M.; Khan, M.A.; Shoaib, M.; Raza, H.; Haider, J. Formulation, characterization and antimicrobial properties of black cumin essential oil nanoemulsions stabilized by OSA starch. J. Food Sci. Technol. 2017, 54, 3358–3365. [Google Scholar] [CrossRef]
- Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules 2022, 27, 3183. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-mahmood, S.M.A.; Doolaanea, A.A. Development and Validation of UV-Vis Spectrophotometric Method for Estimation of Black Seeds and Peppermint Oil in Emulsion. Anal. Chem. Lett. 2021, 11, 607–617. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Khojah, E.Y.; Badr, A.N.; Mohamed, D.A.; Abdel-Razek, A.G. Bioactives of Pomegranate By-Products and Barley Malt Grass Engage in Cereal Composite Bar to Achieve Antimycotic and Anti-Aflatoxigenic Attributes. Foods 2022, 11, 119. [Google Scholar] [CrossRef]
- Pearson, F.J., Jr.; Hanshaw, B.B. Sources of Dissolved Carbonate Species in Ground Water and Their Effects on Carbon-14 Dating. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/01/001/1001624.pdf (accessed on 10 July 2022).
- Egan, H.; Cox, H.E.; Pearson, D. Pearson’s Chemical Analysis of Foods; Churchill livingstone: London, UK, 1981. [Google Scholar]
- Faustman, C.; Specht, S.M.; Malkus, L.A.; Kinsman, D.M. Pigment oxidation in ground veal: Influence of lipid oxidation, iron and zinc. Meat Sci. 1992, 31, 351–362. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mohammadnabi, S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int. J. Biol. Macromol. 2017, 95, 769–777. [Google Scholar] [CrossRef]
- Jacobs, S. The determination of amino acids in food hydrolyzates with a stabilized indanetrione hydrate reagent. Microchem. J. 1965, 9, 87–397. [Google Scholar] [CrossRef]
- Anderson, M.E.; Marshall, R.T. Reducing microbial populations on beef tissues: Concentration and temperature of lactic acid. J. Food Saf. 1989, 10, 181–190. [Google Scholar] [CrossRef]
- Reynolds, A.E.; Harrison, M.A.; Rose-Morrow, R.; Lyon, C.E. Validation of dry cured ham process for control of pathogens. J. Food Sci. 2001, 66, 1373–1379. [Google Scholar] [CrossRef]
- Imai, I.; Ishida, Y.; Hata, Y. Killing of marine phytoplankton by a gliding bacterium Cytophaga sp., isolated from the coastal sea of Japan. Mar. Biol. 1993, 116, 527–532. [Google Scholar] [CrossRef]
- Collee, J.G.; Mackie, T.J.; McCartney, J.E. Mackie & McCartney Practical Medical Microbiology; Harcourt Health Sciences: New York, NY, USA, 1996. [Google Scholar]
- Eid, A.M.M.; Elmarzugi, N.; El-Enshasy, H.A. Preparation and evaluation of olive oil nanoemulsion using sucrose monoester. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 3), 434–440. [Google Scholar]
- Pando, D.; Beltrán, M.; Gerone, I.; Matos, M.; Pazos, C. Resveratrol entrapped niosomes as yoghurt additive. Food Chem. 2015, 170, 281–287. [Google Scholar] [CrossRef]
- Yue, P.-F.; Lu, X.-Y.; Zhang, Z.-Z.; Yuan, H.-L.; Zhu, W.-F.; Zheng, Q.; Yang, M. The study on the entrapment efficiency and in vitro release of puerarin submicron emulsion. AAPS PharmSciTech 2009, 10, 376–383. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Said, M.M.; El-Messery, T.M.; Abdel-Razek, A.G. Non-traditional oils encapsulation as novel food additive enhanced yogurt safety against aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef]
- Rosa, A.; Scano, P.; Atzeri, A.; Deiana, M.; Mereu, S.; Dessì, M.A. Effect of Storage Conditions on Lipid Components and Color of Mugil cephalus Processed Roes. J. Food Sci. 2012, 77, C107–C114. [Google Scholar] [CrossRef] [PubMed]
- Talab, A.S.; Ghanem, M.H. Effects of different salt concentrations on the quality alterations and shelf-life of the grey mullet fish. Egypt. J. Aquat. Biol. Fish. 2021, 25, 583–595. [Google Scholar] [CrossRef]
- El-Sherif, S.A.; Abd El-Ghafour, S. Investigation of the quality properties and nutritional values of four fish species from Lake Qaroun, Egypt. Int. J. Chem. Tech. Res. 2016, 9, 16–26. [Google Scholar]
- Hansen, L.T.; Gill, T.; Røntved, S.D.; Huss, H.H. Importance of autolysis and microbiological activity on quality of cold-smoked salmon. Food Res. Int. 1996, 29, 181–188. [Google Scholar] [CrossRef]
- Ali, M. Shelf life determination of the brined golden mullet Liza aurata during vacuum refrigerated storage using some quality aspect. Acta Sci. Pol. Technol. Aliment. 2012, 11, 37–43. [Google Scholar] [PubMed]
- Akonor, P.T.; Ofori, H.; Dziedzoave, N.T.; Kortei, N.K. Drying Characteristics and Physical and Nutritional Properties of Shrimp Meat as Affected by Different Traditional Drying Techniques. Int. J. Food Sci. 2016, 2016, 7879097. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, W.S.; Park, K.N. Antimicrobial activity of Caesalpina sappan L. extracts and its effect on preservation of ground meats. J.-Korean Soc. Food Sci. Nutr. 2000, 29, 888–892. [Google Scholar]
- Gould, G.W. Micro-Organisms in Foods 4. Application of the Hazard Analysis Critical Control Point (HACCP) System to Ensure Microbiological Safety and Quality: ICMSF, Blackwell Scientific Publications, London, 1988, 357 pp., ISBN 0-632-02181-0. Food Control 1990, 1, 246–247. [Google Scholar]
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin-producing fungi. Saudi J. Biol. Sci. 2010, 17, 167–175. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Chuang, T.-C.; Lin, H.-C.; Huang, Y.-R.; Lin, C.-M.; Kung, H.-F.; Tsai, Y.-H. Histamine content and histamine-forming bacteria in dried milkfish (Chanos chanos) products. Food Chem. 2009, 114, 933–938. [Google Scholar] [CrossRef]
- Kung, H.-F.; Chien, L.-T.; Liao, H.-J.; Lin, C.-S.; Liaw, E.-T.; Chen, W.-C.; Tsai, Y.-H. Chemical characterisation and histamine-forming bacteria in salted mullet roe products. Food Chem. 2008, 110, 480–485. [Google Scholar] [CrossRef]
- Ekici, K.; Alisarli, M. Histamine formation and microbiological changes in endemic Chalcalburnus tarichi Pallas 1811 (Inci Kefali) stored at 4 C. Arch. Med. Vet. 2008, 40, 95–98. [Google Scholar] [CrossRef][Green Version]
- Hwang, C.-C.; Lin, C.-M.; Kung, H.-F.; Huang, Y.-L.; Hwang, D.-F.; Su, Y.-C.; Tsai, Y.-H. Effect of salt concentrations and drying methods on the quality and formation of histamine in dried milkfish (Chanos chanos). Food Chem. 2012, 135, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Kaya, Y.; Erkoyuncu, İ.; SÖNmez, G. Chemical and microbiological qualities of dry-salted (lakerda) bonito (sarda sarda, bloch 1793). J. Food Qual. 2006, 29, 470–478. [Google Scholar] [CrossRef]
- Ogur, S.; Erkan, N. Microbiological and chemical quality of different types of salted pearl mullet (Chalcalburnus tarichi Pallas, 1811). J. Food Saf. 2020, 40, e12717. [Google Scholar] [CrossRef]
- Gram, L.; Trolle, G.; Huss, H.H. Detection of specific spoilage bacteria from fish stored at low (0 C) and high (20 C) temperatures. Int. J. Food Microbiol. 1987, 4, 65–72. [Google Scholar] [CrossRef]
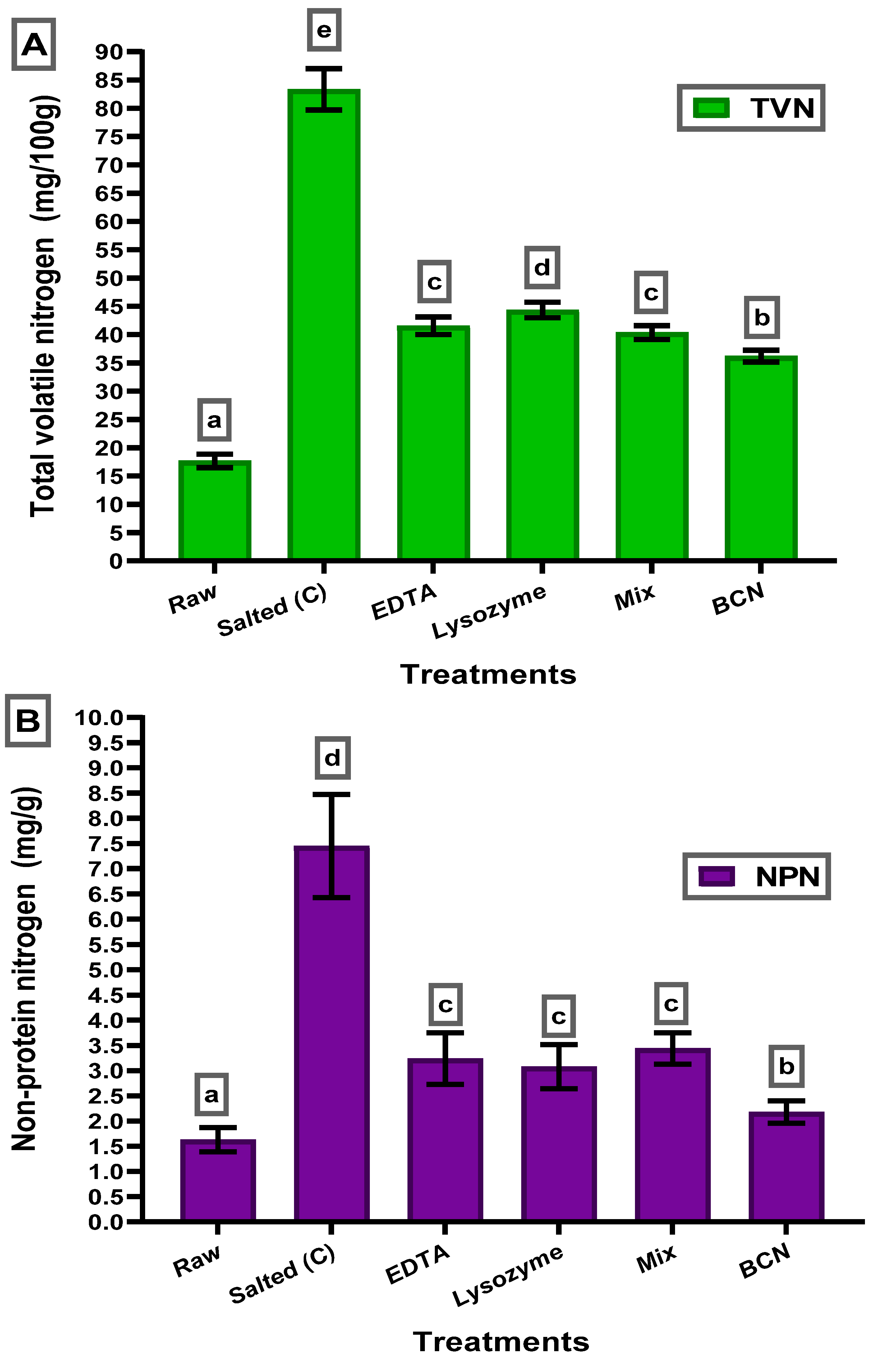
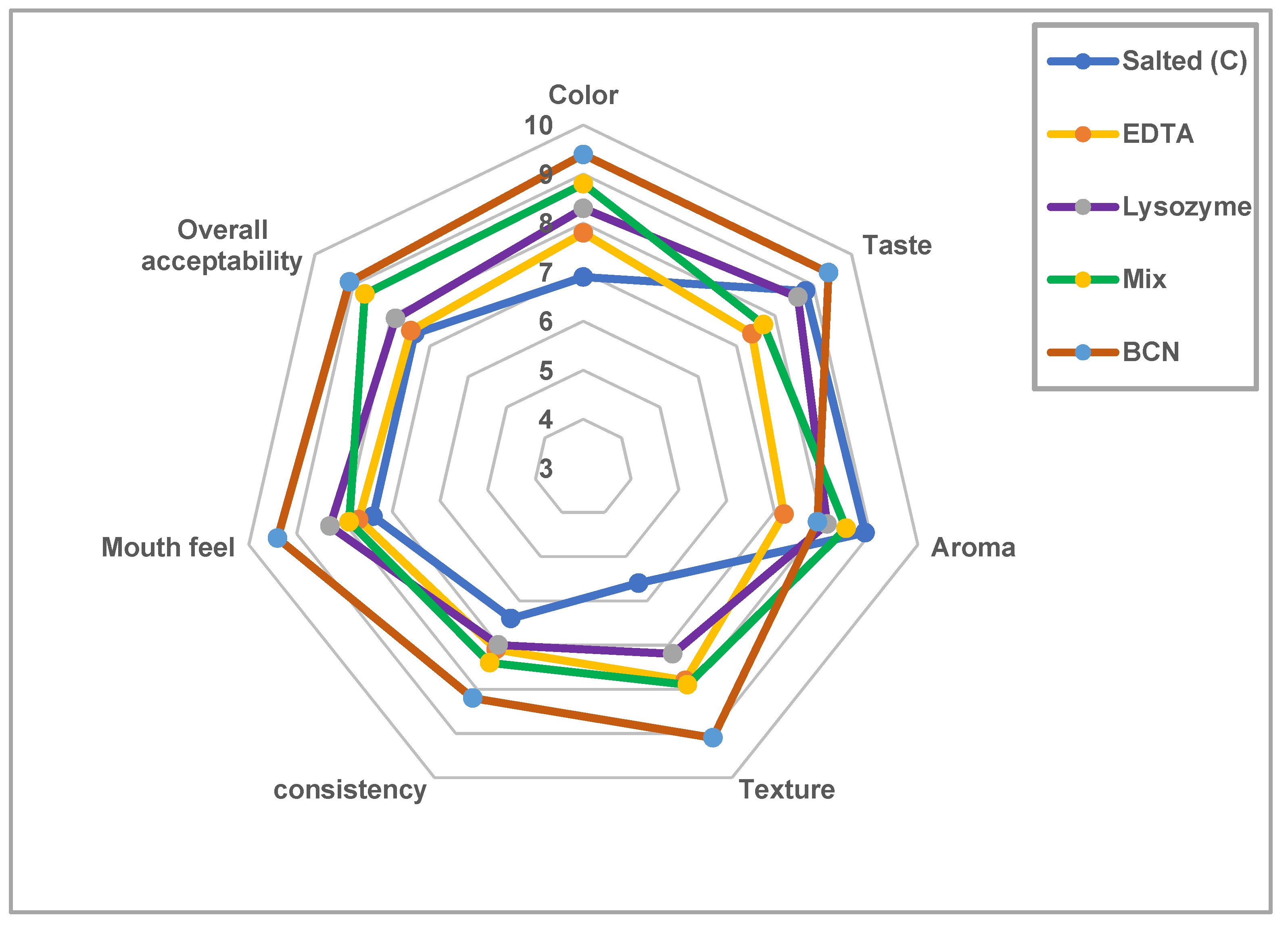
| Chemical Composition and Mullet Characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Protein (%) | Lipid (%) | Flesh-Salt (%) | pH | Water Activity (aW) | Ash (%) | |
| Raw | 72.27 ± 0.87 a | 22.41 ± 1.41 a | 6.81 ± 0.37 a | - | 6.24 ± 0.01 a | 0.779 ± 0.021 a | 24.51 ± 0.87 a |
| Salted (C) | 52.81 ± 1.74 d | 14.47 ± 1.27 c | 5.14 ± 0.31 d | 14.43 ± 0.45 a | 6.15 ± 0.05 b | 0.508 ± 0.015 b | 83.22 ±3.21 a |
| EDTA | 53.24 ± 0.84 c | 20.27 ± 0.94 b | 6.18 ± 0.22 b | 14.27 ± 0.71 a | 5.64 ± 0.02 c | 0.455 ± 0.018 c | 44.53 ± 1.74 c |
| Lysozyme | 52.42 ± 0.43 d | 20.25 ± 0.81 b | 5.87 ± 0.19 c | 12.31 ± 0.43 b | 5.47 ± 0.09 d | 0.461 ± 0.017 c | 50.42 ± 1.18 d |
| Mix | 53.21 ± 0.88 c | 20.45 ± 0.84 b | 5.81 ± 0.11 c | 12.05 ± 0.66 b | 5.53 ± 0.06 d | 0.447 ± 0.019 c | 40.89 ± 1.22 b |
| BCN | 54.94 ± 0.47 b | 21.08 ± 1.05 a | 5.98 ± 0.11 b | 11.11 ± 0.74 c | 5.31 ± 0.02 e | 0.424 ± 0.008 d | 40.55 ± 1.29 b |
| AV (mg/g Oil Extracted) | PV (meq/Kg) | TBA (mg MDA eq/Kg) | FFA (as % Oleic) | |
|---|---|---|---|---|
| Raw | 0.24 ± 0.005 a | 8.98 ± 1.02 a | 0.317 ± 0.074 a | 2.75 ± 0.49 a |
| Salted (C) | 2.79 ± 0.214 f | 35.71 ± 2.43 f | 2.191 ± 0.227 d | 7.95 ± 0.54 e |
| EDTA | 1.84 ± 0.184 e | 24.54 ± 1.47 e | 1.081 ± 0.218 b | 5.57 ± 0.41 c |
| Lysozyme | 1.37 ± 0.055 d | 20.25 ± 0.81 c | 1.487 ± 0.192 c | 6.71 ± 0.38 d |
| Mix | 1.09 ± 0.021 c | 21.45 ± 0.84 d | 1.211 ± 0.141 c | 5.64 ± 0.45 c |
| BCN | 0.97 ± 0.008 b | 14.28 ± 1.31 b | 0.684 ± 0.078 b | 3.78 ± 0.63 b |
| Bacterial Contamination | ||||||
|---|---|---|---|---|---|---|
| TPC/g | Coliform Group | E. coli | Staph. aureus | Salmonella sp. | Clostridium sp. | |
| Raw | 2.6 × 105 | 2.3 × 103 | 4.1 × 102 | 2.3 × 103 | ND | ND |
| Salted (C) | 6.13 × 105 | 0.31 × 103 | 0.05 × 102 | 4.54 × 104 | 0.02 × 102 | ND |
| EDTA | 1.24 × 106 | 0.05 × 102 | 0.01 × 102 | 1.37 × 104 | ND | ND |
| Lysozyme | 0.61 × 106 | ND | ND | 1.21 × 104 | ND | ND |
| Mix | 0.54 × 106 | ND | ND | 1.46 × 103 | ND | ND |
| BCN | 0.27 × 104 | ND | ND | ND | ND | ND |
| L* | a* | b* | ΔE Value | |
|---|---|---|---|---|
| Raw | 30.14 ± 0.02 | 0.27 ± 0.03 | 7.84 ± 0.05 | - |
| Salted (C) | 57.36 ± 0.03 | −1.24 ± 0.08 | −16.39 ± 0.07 | 28.54 ± 0.03 |
| EDTA | 40.34 ± 0.06 | 0.56 ± 0.04 | 10.21 ± 0.05 | 10.47 ± 0.01 |
| Lysozyme | 36.56 ± 0.07 | 0.84 ± 0.05 | 12.66 ± 0.08 | 8.05 ± 0.03 |
| Mix | 34.08 ± 0.05 | 0.69 ± 0.01 | 10.44 ± 0.03 | 3.33 ±0.01 |
| BCN | 32.25 ± 0.02 | 0.38 ± 0.03 | 8.27 ± 0.02 | 2.15 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifah, A.M.; Badr, A.N.; El-Khadragy, M.F.; Shehata, M.G.; Abdalla, S.A.; Yehia, H.M.; Ali, H.S. Comparative Evaluations to Enhance Chemical and Microbial Quality of Salted Grey Mullet Fish. Fishes 2022, 7, 175. https://doi.org/10.3390/fishes7040175
Khalifah AM, Badr AN, El-Khadragy MF, Shehata MG, Abdalla SA, Yehia HM, Ali HS. Comparative Evaluations to Enhance Chemical and Microbial Quality of Salted Grey Mullet Fish. Fishes. 2022; 7(4):175. https://doi.org/10.3390/fishes7040175
Chicago/Turabian StyleKhalifah, Ayman M., Ahmed N. Badr, Manal F. El-Khadragy, Mohammed G. Shehata, Sara A. Abdalla, Hany M. Yehia, and Hatem S. Ali. 2022. "Comparative Evaluations to Enhance Chemical and Microbial Quality of Salted Grey Mullet Fish" Fishes 7, no. 4: 175. https://doi.org/10.3390/fishes7040175
APA StyleKhalifah, A. M., Badr, A. N., El-Khadragy, M. F., Shehata, M. G., Abdalla, S. A., Yehia, H. M., & Ali, H. S. (2022). Comparative Evaluations to Enhance Chemical and Microbial Quality of Salted Grey Mullet Fish. Fishes, 7(4), 175. https://doi.org/10.3390/fishes7040175









